Abstract
B cells are not only producers of antibodies, but also contribute to immune regulation or act as potent antigen-presenting cells. The potential of B cells for cellular therapy is still largely underestimated, despite their multiple diverse effector functions. The CD40L/CD40 signaling pathway is the most potent activator of antigen presentation capacity in B lymphocytes. CD40-activated B cells are potent antigen-presenting cells that induce specific T-cell responses in vitro and in vivo. In preclinical cancer models in mice and dogs, CD40-activated B cell-based cancer immunotherapy was able to induce effective antitumor immunity. So far, there have been only few early-stage clinical studies involving B cell-based cancer vaccines. These trials indicate that B cell-based immunotherapy is generally safe and associated with little toxicity. Furthermore, these studies suggest that B-cell immunotherapy can elicit antitumor T-cell responses. Alongside the recent advances in cellular therapies in general, major obstacles for generation of good manufacturing practice-manufactured B-cell immunotherapies have been overcome. Thus, a first clinical trial involving CD40-activated B cells might be in reach.
Keywords: B-cell therapy, Antigen presentation, Cellular therapy
Introduction
B cells are best known for their role as producers of antibodies. Over recent decades, it has become clear that B cells serve much more diverse functions than just antibody production. B cells are an important source of cytokines and chemokines and thus contribute to the regulation of immune responses. Depending on the mode of activation, the subtype involved, or the microenvironment, B cells either contribute to upregulation of T-cell responses or they can exert immunoregulatory functions and participate in the downregulation of T-cell immunity [reviewed in 1].
In the 1980s, the ability of B cells to act as antigen-presenting cells (APCs) became increasingly appreciated. However, concurrently dendritic cells (DCs) were characterized as potent professional APCs. Due to their potent antigen-presenting capacity, DCs were regarded as the primary APCs for the induction of T-cell immunity and became the main focus for further development of cellular cancer vaccines. However, DCs possess several important drawbacks as APCs for cellular cancer vaccines. It is difficult and relatively expensive to generate sufficient amounts of DCs for repeated vaccinations. Furthermore, there are a large variety of protocols using different cytokine cocktails to generate DCs for immunotherapeutic purposes. Little is known about which protocol is optimal. Therefore, several research groups have investigated alternative cellular adjuvants.
Activated B cells become potent professional APCs only when appropriately activated. Soon after CD40 and its ligand CD40L (also named CD154) were first described, it became clear that CD40L/CD40 signaling was among the most potent stimuli for the activation of B cells [2, 3]. Classically, CD40L is expressed on activated CD4+ T cells and, thus, is essential for a thymus-dependent B-cell response and for the development of a humoral and cellular immune response. CD40L is a type II transmembrane protein, which exists as a trimer, inducing oligomerization of CD40 upon binding [4], a process that is critical for signaling via the CD40 receptor and likely accounts for the diverse biologic activities induced by different monoclonal antibodies [5]. CD40 acts a transmembrane signal transducer activating intracellular kinases and transcription factors within the cell. More specifically, recruitment of TRAF proteins to the cytoplasmic tail of CD40 activates the canonical and noncanonical NFκB pathways, MAP kinases, phosphoinositide 3-kinases, and the phospholipase Cγ pathway [reviewed in 6]. Independent of TRAF proteins, Janus family kinase 3 can directly bind to the cytoplasmic tail of CD40 inducing phosphorylation of STAT5 [7, 8]. These signaling cascades in B cells eventually promote germinal center formation, immunoglobulin isotype switch, somatic hypermutation, and formation of long-loved plasma cells or memory B cells [9, 10, 11, 12]. Moreover, the CD40L/CD40 interaction is involved in the cellular immune response by regulating the costimulatory activity of APCs [13] and thus influences T-cell priming and effector functions. This discovery resulted in the development of cell culture systems that allow the activation and expansion of B cells from peripheral blood [14]. In the late 1990s, Schultze et al. [15] proposed in vitro-generated CD40-activated B cells (CD40B cells) as an alternative to DCs as cellular adjuvant for cancer immunotherapy. Ex vivo-generated CD40B cells possess potent immunostimulatory properties and are capable of priming CD4 and CD8 T cells in vitro and in vivo [16, 17, 18]. Over the subsequent years, the antigen-presenting function of B cells was characterized in more detail and the concept of B cell-based cancer vaccines was increasingly refined. Several experimental studies in different tumor models confirmed that vaccination with CD40B cells could induce effective antitumor CD4 and CD8 T-cell responses.
In 2005, Biagi et al. [19] reported the first small clinical trial of a cancer vaccine that used CD40B cells as cellular adjuvant. They transduced autologous leukemic B cells isolated from patients with chronic lymphocytic leukemia (CLL) with an adenoviral vector that contained the human CD40L gene and reinfused these cells together with transduced autologous CLL cells that expressed interleukin (IL)-2. Three of 9 patients demonstrated a greater than 50% reduction in lymph node size. Unfortunately, the induced T-cell responses were only transient and unable to overcome tumor-induced immunosuppression in the long term. In spite of these disappointing results, this study provided a first proof-of-concept for B cell-based cancer immunotherapy and demonstrated that antitumor T-cell responses can be induced by activated antigen-presenting B lymphocytes.
Generation of Antigen-Presenting B Cells
Resting B lymphocytes are poor APCs and are unable to induce strong T-cell immunity [20]. B cells can be activated by a variety of stimuli to acquire immunostimulatory capacity, including B-cell receptor (BCR) binding to antigen and toll-like receptor-mediated signals. However, signals transmitted via CD40 have consistently been found to be the most potent inducer of many features of potent APCs [2]. Several strategies have been investigated to exploit CD40-CD40L interaction for the generation of antigen-presenting B cells (summarized in Table 1 for human B cells) [reviewed in 21]. These include the usage of recombinant soluble CD40L proteins [22, 23, 24, 25], triggering CD40 with agonistic monoclonal CD40 antibodies [26, 27], and CD40L-expressing feeder cells [28, 29, 30]. A number of factors affect the extent of B-cell activation by CD40-mediated signals. For instance, the effect of anti-CD40 antibodies on B-cell activation is determined by the exact location of their binding to CD40 [5]. Another factor that crucially determines the extent of B cell activation is the degree of CD40 crosslinking. It has long been established that optimal bioactivity is only observed when using a multimerized form of the CD40L homotrimer, thus allowing clustering on the cell surface [31, 32, 33]. Clustering of the CD40L is not elicited by monoclonal anti-CD40 antibodies, thus only inducing activation, but not proliferation of B cells [31, 32, 33]. CD40L- expressing feeder cells naturally provide a multimerized form of the CD40L, but to avoid xenogeneic components in clinical products recombinant soluble CD40L is the preferred choice for a clinical application of B cells.
Table 1.
Methods for generating human antigen-presenting B cells
| B-cell source | Activation | Antigen loading | Application |
| PBMCs or purified B cells | CD40L-expressing murine NIH3T3 cells + IL-4 + CsA | Peptide pulsing [15, 35, 43, 51]; RNA transfection [38]; N/A [16, 27, 4, 5, 6, 7, 64] | Generation of viral or tumor antigen-specific CD8+ T cells in vitro [15, 38]; generation of viral and tumor antigen-specific CD4 T cells in vitro [35, 43]; CD40B cells generated from PBMCs of cancer patients [51]; B-cell homing and T-cell chemotaxis [16]; induction of regulatory T cells [27, 47]; early activation of CD4+ T cells [46]; effect of immunosuppressive factors on CD40B cells [64]; influence of statins on antigen presentation by CD40B cells [45] |
| PBMCs or purified B cells | Soluble CD40L + IL-4 | Pulsing with tumor cell lysate transfection [37]; Antigen pulsing [24, 49]; N/A [22] | Generation of tumor antigen-specific CD4+ T cells in vitro [37]; stimulation of CD8+ and/or CD4+ T cells [22, 24, 49] |
| PBMCs | CD40L-expressing murine L cells + IL-4 and CsA | Peptide pulsing [36, 48] | Expansion of rare antigen-specific CD8+ T cells [48]; long-term CD40B culture and generation of antigen-specific CD8+ T cells in vitro [36] |
| PBMCs | Anti-CD40 antibody (Mab89) + IL-4 | N/A | Cultivation of primary B cells in vitro [14] |
| PBMCs from CLL patients | Transduction with adeno-hCD40L vector + hIL-2 vector | N/A | Treatment of 9 CLL patients [19] |
| Purified B cells | CD40L-expressing stromal cells + IL-2, IL-4, IL-21 and BAFF | Antigen pulsing [17] | Stimulation of antigen-specific CD4+ T cells [17] |
| Allogeneic lymphocytes | Allogeneic B cells from healthy donors fused with tumor cells | N/A | Treatment of 11 RCC patients [78]; treatment of 16 melanoma patients [79] |
| Purified CD27+ B cells | CpG + soluble CD40L + IL-2 +IL-10 +IL-15 | N/A | Generation of FCRL4-positive B cells [23] |
| Purified B cells | Anti-CD40 antibody (CP-870,893) | N/A | Stimulation of allogeneic CD4+ T cells [26] |
| Purified B cells | CD40L-expressing Schneider 2 cells + IL-4 | N/A | Stimulation of allogeneic T cells [28] |
| Purified B cells | CD40L-expression human 293 cells + IL-4 + IL-10 | N/A | Stimulation of allogeneic or autologous T cells [30] |
| PBMCs | CD40L-expressing murine fibroblasts (LTK-CD40L) + IL-4 + CsA | Transfusion of with antigen-encoding plasmids [44] | Stimulation of tumor-specific CD4 T cells [44] |
| Purified B cells | Bacterial stimuli + IL-2 | N/A | Induction of CD4+ T-cell anergy and apoptosis [66] |
IL, interleukin; CLL, chronic lymphocytic leukemia; CsA, cyclosporin A; PBMC, peripheral blood mononuclear cells; RCC, renal cell carcinoma.
Typically, human peripheral blood mononuclear cells (PBMCs) or purified B cells are cultured for a period of at least 14 days in the presence of the soluble CD40L and IL-4 [22, 23], in which the addition of IL-4 is necessary for B-cell proliferation [34]. These culture conditions result in a profound polyclonal activation of B cells that leads to an approximately 20-fold expansion [15, 35, 36] and the acquisition of an antigen-presenting phenotype [15, 37, 38]. When PBMCs are used as the starting material, typically B-cell purities of more than 95% can be achieved. Throughout the culture period, B cells acquire a memory-like state that represents an intermediary stage between naïve B cells and plasma cells [39]. B cells that are stimulated for at least 3 days by the CD40L show a high expression of MHC class I and MHC class II molecules, the costimulatory markers CD80, CD83, and CD86, and the adhesion molecules CD54 and CD58, which remains stable throughout the subsequent culture period [15, 18, 35, 37, 38]. Combination of CD40L stimulation with CpG as proposed by some studies [18, 40] has no further impact on the expression of activation markers or proliferation of B cells, while additional stimulation with LPS further increases the activation of B cells [18, 40]. When normalized relative to cell size, expression levels of activation molecules on the cell surface of CD40B cells are equivalent to CD40L/IFN-γ or TNF-α-matured DCs [35].
Increased expression of MHC and costimulatory molecules on CD40B cells correlates with the acquisition of antigen-presenting functions. CD40 activation results in improved antigen processing and presentation [41], typically via the classical MHC class II pathway [18, 42], but also a distinct nonclassical, cytosolic MHC class II pathway [43]. Becker et al. [43] demonstrated that presentation of the model antigen CMV pp65 by CD40B cells was limited when using the proteasome inhibitor epoxomicin resulting in reduced T-cell activation and IFN-γ production. However, epoxomicin sensitivity was not observed in DCs, suggesting an antigen-processing mechanism unique to CD40B cells.
The ability of human CD40B cells to expand antigen-experienced CD4+ T cells, but also to prime naïve CD4+ T cells was demonstrated in several studies [15, 17, 22, 24, 26, 35, 37, 44, 45]. Lapointe et al. [37] showed that when pulsed with tumor lysates, CD40B cells expanded and activated tumor antigen-specific memory CD4+ T cells from the blood of cancer patients. Our group demonstrated that responses of naïve CD4+ T cells against MCH class II-restricted neoantigens could be induced when using CD40B cells as sole APCs [42]. In addition, expression of CD107a and CD40L was detected in CD4+ T cells early after activation with CD40B cells [46].
Human CD40B cells also cross-present antigen via MHC class I pathways and, thus, where shown to induce naïve and memory CD8+ T-cell responses [47, 48, 49]. Similar to the system used for CD4+ T cells, CD40B cells were used as APCs to expand antigen-specific CD8+ T cells from healthy donors and cancer patients [15, 35, 36, 45, 48, 49, 50]. Specific T-cell responses where not only detected against the memory antigens influenza A MP58, MART-1, and hTERT, but also the neoantigen RTpol from HIV [35], thus again demonstrating the ability of CD40B cells to induce naïve T-cell responses.
B Cells for Immunotherapy
CD40B cells fulfill crucial requirements for their use as APCs in cancer immunotherapy: (1) they can be consistently generated from peripheral blood, (2) they are relatively insensitive towards tumor-derived immunosuppressive mechanisms, (3) they do not induce tolerance by themselves, and (4) they are well tolerated upon infusion in terms of toxic side effects.
From a practical view, CD40B cells offer several potential advantages over DCs. From a small amount of peripheral blood, one can usually obtain sufficient numbers (approximately 1 × 105 to 1 × 107 cells/kg body weight) of activated antigen-presenting B cells [35, 51], whereas the generation of DCs typically requires a leukapheresis [52, 53, 54, 55]. It has been shown that this is even feasible in cancer patients [35, 51]. This aspect is particularly important considering that cancer patients typically are frequently lymphocytopenic due to the underlying disease and/or prior chemotherapy. Furthermore, the culture system for generating CD40B cells is relatively easy and inexpensive.
Tumor-derived factors mediating immunosuppression in the tumor microenvironment, such as prostaglandin E2 [56], TGF-β [57, 58], VEGF [59, 60] or IL-10 [61, 62], act in part by inhibiting DC differentiation, maturation, trafficking, and antigen presentation [62, 63]. Therefore, one might suppose that they have similar effects on antigen-presenting B cells. However, activated B cells turned out to be relatively resistant to inhibition by tumor-associated immunosuppressive molecules. In vitro, neither migration nor activation of CD40B cells was inhibited by these immunosuppressive factors, nor did they influence the ability of CD40B cells to induce proliferation of CD4+ or CD8+ T cells [64]. TGF-β and VEGF had no effect on the proliferation of CD40B cells, while IL-10 even increased their expansion. On the contrary, TGF-β actually enhances BCR-mediated antigen presentation [65]. Concerning the induction of tolerance by administration of activated B cells, the mode of activation is of considerable importance. While human B cells that were activated by bacterial stimuli induced anergy and apoptosis of CD4+ T cells in an IL-2-dependent manner [66], CD40B cells were shown to activate T cells in the presence of IL-2 besides the fact that they express CD25 [37, 44, 67]. Toxic side effects of CD40B-cell administration were not observed in in vivo in studies with mice or dogs. Wild-type mice received autologous CD40B cells in different injection routes (intravenous, subcutaneous, and intraperitoneal) and two different high concentrations (40 × 106 and 40 × 107 cells/kg). Body weight and survival remained unchained under all tested conditions. No abnormal lymphocytic infiltration, structural tissue injury, or indications of inflammation could be detected in histological analyses of heart, lung, liver, spleen, and kidney [68]. These results are in line with a study where administration of RNA-loaded CD40B cells was well tolerated by dogs with non-Hodgkin's lymphoma and no long-term complications were observed in the follow-up [69].
Only few studies investigated antigen presentation by CD40B cells or the influence of their administration on tumor growth in vivo (summarized in Table 2). Sorenmo et al. [69] published results of a study using tumor RNA-loaded CD40B cells as cellular adjuvant in dogs with non-Hodgkin's lymphoma. The authors reported positive specific immune responses as detected by IFN-γ ELISPOTs, but could not detect a statistically significant correlation between the immunological response and the clinical outcome. However, they detected a significant improvement in the rate of durable second remission and survival between vaccinated and nonvaccinated groups. Since dogs are a widely accepted animal model to evaluate safety and efficacy before proceeding to a clinical trial, this study was an important step towards a clinical application of CD40B cells.
Table 2.
Preclinical and clinical studies involving activated B cells
| Species | Tumor entity | Therapy | Outcome | Ref. |
|---|---|---|---|---|
| C57BL/6 mice | LL-LCMV s.c. tumors | Vaccination with LCMV antigen-pulsed CD40B cells or LPS-activated B cells | Delay in tumor growth by CD40B cells but not LPS-activated B cells | 70 |
| C57BL7/6 mice | B16.F10 melanomas | Therapeutic application of RNA-transfected (of antigen and costimulatory molecules) CD40B cells | No delay of tumor growth | 71 |
| C57BL7/6 mice | E.G7 lymphoma | Therapeutic application of OVA antigen-pulsed CD40B cells | No delay of tumor growth | 72 |
| C57BL7/6 mice | E.G7 lymphoma | Therapeutic application of OVA antigen-transfected CD40B cells | Protection against tumor growth | 72 |
| C57BL7/6 mice | MCA205 pulmonary metastases; MCA205 or D5G6 s.c. tumors | Adoptive transfer of B and T cells from TDLN after CD40-activation; B cells in combination with TBI or chemotherapy | Combination of B and T cells led to regression of metastases; B cells alone in combination with TBI or chemotherapy inhibited s.c. tumors | 73 |
| Balb/C mice | 4T1 mammary carcinoma | Adoptive transfer of B cells from TDLN; CD40 activation | Reduction of spontaneous metastases | 74 |
| C57BL7/6 mice | Pulmonary metastases after i.v. injection of HEL-expressing B16 melanoma | Therapeutic application of HEL-specific B cells, in vitro stimulation with IL-4, IL-21, CD40L, and BAFF-expressing feeder cells | Regression of metastases | 75 |
| C57BL/6 mice | E.G7 lymphomas | Vaccination with OVA antigen-pulsed CD40B cells | Significant delay of tumor growth | 68 |
| C57BL/6 mice | B16.F10 melanomas | Vaccination with TRP2 antigen-pulsed CD40B cells | Significant delay of tumor growth | 68 |
| C57BL7/6 mice | EG.7 lymphoma | Therapeutic application of tumor antigen-specific CD40B cells + plasma cells | Significant delay of tumor growth and increased survival | 76 |
| C57BL7/6 mice | Panc02-OVA | Therapeutic application of tumor antigen-specific CD40B cells + plasma cells | Significant delay of tumor growth and increased survival | 76 |
| Dogs | Non-Hodgkin's lymphoma | Tumor RNA-loaded CD40B cells | Induction of specific immune response; improvement in survival | 69 |
| Humans | CLL | CD40L-transduced CLL cells + IL-2-transduced CLL cells | Reduction in lymph node size in 3 of 9 patients | 19 |
| Humans | Renal cell carcinoma | Allogeneic B cells from healthy donors fused with autologous tumor cells | Two complete and 2 partial remissions out of 11 patients | 78 |
| Humans | Metastatic melanoma | Allogeneic B cells from healthy donors fused with autologous tumor cells | One complete and 1 partial remission; 5 stable disease out of 16 patients | 79 |
| Humans | Patients after allogeneic stem cell transplantation | Adoptive transfer of CD19+-selected B cells | No acute adverse reactions or chronic GvHD; mobilization of plasma blasts after revaccination | 81 |
IL, interleukin; CD40B cells, CD40-ativated B cells; TDLN, tumor-draining lymph node; CLL, chronic lymphocytic leukemia; s.c., subcutaneous; i.v., intravenous; TBI, total body irradiation.
More promising results in terms of cancer treatment were reported in mice. Vaccination of wild-type mice with LCMV-antigen pulsed CD40B cells, but not LPS-activated B cells, significantly reduced growth of LL-LCMV subcutaneous tumors [70]. In two studies, which applied RNA-transfected or OVA antigen-pulsed CD40B cells in a therapeutic setting, treatment did not result in delayed tumor growth of B16.F10 melanomas or E.G7 lymphomas, respectively [71, 72]. In two studies, B cells were isolated from tumor-draining lymph nodes (TDLN) of wild-type mice with MCA205, D5G6, or 4T1 tumors [73, 74]. After activation with anti-CD40 antibodies, they were adoptively transferred into syngeneic tumor-bearing mice. In combination with activated T cells, CD40B-cell administration resulted in the reduction of spontaneous metastases. Moreover, combining adoptive transfer of B cells with chemotherapy or total body irradiation significantly inhibited tumor growth. The generated B cells were shown to produce tumor antigen-specific IgG antibodies, indicating specificity for tumor antigens presented by B cells isolated from TDLN. However, these studies used soluble anti-CD40 antibodies for the activation of B cells, which was demonstrated to result in weaker CD40 stimulation than activation by CD40L-expressing feeder cells [21, 31]. When using CD40L-expressing feeder cells for activation, vaccination with tumor antigen-pulsed CD40B cells before B16 melanomas or E.G7 lymphomas were injected [68] resulted in significantly delayed growth in both tumor models. The rate of tumor control by CD40B cell vaccination was comparable to that induced by DCs. Using tumor antigen-specific B cells for immunotherapy seems to further improve the observed antitumor efficacy. Moutai et al. [75] isolated HEL-specific B cells and stimulated them with a combination of CD40L/BAFF-expressing feeder cells, IL-4, and IL-21. Therapeutic administration of these iGC-termed B cells resulted in the regression of pulmonary metastases of HEL-expressing B16 melanomas. These results are in line with a more recent study using tumor antigen-specific CD40B cells for therapeutic treatment of EG.7 lymphoma or Panc02OVA tumor-bearing mice [76]. This study exploited the advantage of antigen-specific B cells to take up and process antigen more efficiently via the specific BCR than polyclonal B cells do via BCR-independent mechanisms such as pinocytosis [77]. Antigen-specific B cells more efficiently induced antigen-specific T-cell responses in vitro and in vivo than polyclonal CD40B cells, subsequently resulting in complete remission in 60% of mice [76]. In addition, B cells were differentiated into antibody-secreting plasma cells supporting the antitumor immune response induced by CD40B cells.
Clinical Application of B Cell-Based Cancer Vaccines
The preclinical experiments described above provide a strong rational for the clinical application of CD40B cells as a cellular cancer vaccine. The proof-of-principle studies in several distinct murine cancer models and the more genetically diverse canine tumors demonstrate the potential of B cell-based cancer vaccines for the therapeutic treatment of established tumors. Apart from the above-mentioned clinical study of a CD40-activated B cell vaccine by Biagi et al. [19], there are only few clinical studies that assessed the use of B cells for cancer immunotherapy.
In two small clinical trials, B cells were used as part of a hybrid cell vaccination approach, in which allogeneic B cells from PBMCs of healthy donors were fused with autologous tumor cells. In the first study in patients with renal cell carcinoma, two complete and two partial responses were observed out of 11 patients. Most patients at least showed an initial response and the vaccination was well tolerated [78]. The second study was conducted in patients with metastatic melanoma. The vaccination with the hybrid vaccine induced T-cell relocation into the tumor nodules. Out of 16 patients, 1 complete and 1 partial remission and 5 cases of stable disease were observed. The vaccination proved to be safe as only minor side effects occurred [79].
Another study in humans using adoptive B-cell transfer rather focused on the ability of memory B cells to differentiate into plasma cells. Winkler and colleagues [80] developed a method to produce good manufacturing practice (GMP)-conforming purified human B cells for the treatment of patients after allogeneic stem cell transplantation to restore humoral immunity. They initiated a first-in-man phase I/IIa clinical trial to evaluate safety and tolerability of adoptively transferred donor B cells in a dose escalation study [81] (ClinicalTrials.gov identifier: NCT02007811). B cells were isolated under GMP-conditions from donor leukapheresis products in two separation steps in the CliniMACS® System including the depletion of CD3+ T cells followed by positive selection of CD19+ B cells. When the first results were reported (in 2016 at the ASH conference), the lower doses of 0.5 × 106, 1 × 106, and 2 × 106 B cells were well tolerated without any acute adverse reactions or chronic GvHD reactions during the observation period of 4 month. As secondary endpoints, the activity of the infused donor memory B cells was evaluated. Preliminary results suggested a significant mobilization of plasma blasts in some of the patients after revaccination with a pentavalent vaccine.
The recent development of a GMP-grade CD40-activating reagent has been one of the important steps towards the clinical testing of a CD40B cell-based cancer vaccine [22]. This B cell-activating reagent has overcome some of the problems described above with other activating reagents, i.e., xenogeneic components or poor proliferation.
Apart from the effective activation and expansion of immunostimulatory B cells, the process of loading B cells with antigen is crucial for the successful application of B cell-based cancer vaccines. BCR-mediated antigen uptake is the most efficient way of antigen acquisition and leads to highly efficient antigen processing and presentation [77, 82, 83]. Other modes of antigen uptake such as pinocytosis are less effective. Therefore, several different strategies for antigen delivery to B cells have been explored. A promising approach of antigen delivery to B cells is via the targeting of antigens to CD19 [84]. Szeto et al. [85] recently reported another interesting approach using a microfluidic device for antigen delivery to B cells through a process termed mechanoporation. In this microfluidic device, B cells are passed through narrow channels. The passage through the narrow channel causes the transient formation of pores in the B-cell membrane that facilitate the intracellular uptake of proteins from the surrounding medium.
A possible alternative to the use of polyclonal B cells that have to be loaded with tumor antigens is the isolation of B cells with tumor antigen-specific BCRs from the patients' blood or tumor tissue. Since antigen-uptake through the BCR is highly specific and results in rapid and effective antigen processing and presentation, one can circumvent the need for antigen-loading prior to reinfusion of the B-cell vaccine. At least in mice, the use of antigen-specific CD40B cells for immunotherapy was highly efficient in inducing a strong antitumor immune response resulting in complete remission [76]. However, like the use of polyclonal B cells, this method requires the prior choice of a defined tumor antigen.
Another interesting antigen-agnostic strategy for the generation of tumor antigen-specific immunostimulatory B cells is the use of B cells that were isolated from the patient's tumor or TDLN. In murine experiments this approach proved to be successful at inducing antitumor immunity [74]. These results are in line with an in vitro study where B cells isolated from TDLN of patients with esophageal-gastric cancer or colorectal cancer were partially specific for the tumor antigens NY-ESO-1 or CEA, respectively, and induced antigen-specific T-cell responses in vitro [76].
Future Perspectives
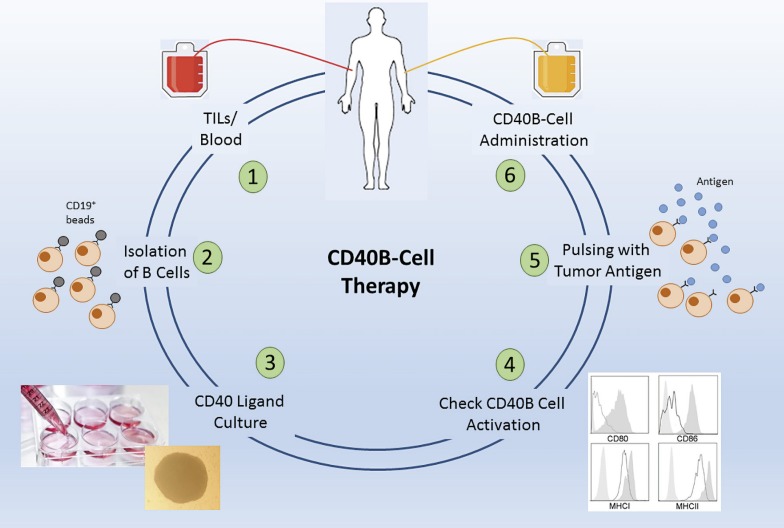
A possible first study with CD40B cells should strive to include the aspect of antigen specificity, to ensure that their full potential is exploited. However, since patient material is limited and isolation of antigen-specific B cells by antigen-tetramers is complex and costly to be developed in GMP-grade, isolation of the whole B-cell population from TILs offers the most promising option. This B-cell population contains B cells specific for tumor antigens, is presumably loaded with tumor antigen already, but can also be further stimulated with the CD40L [76]. Thus, a tumor entity should be chosen where TILs are easily assessable, i.e., surgery is part of the standard procedure, a possible tumor antigen for pulsing is known, and where there is a great clinical need. Manufacturing of B cells under GMP-conditions comprises no obstacles anymore after today's experience with CAR trials [86] and B cell-adoptive transfer [81], and suitable tumor antigens for pulsing have been discovered in many solid tumor entities [87]. The whole isolation and activation process of CD40B cells in general would also be suitable for an automated manufacturing process, e.g., in the CliniMACS Prodigy (Miltenyi Biotec) [88]. The most straightforward approach would thus include isolation of B cells from TILs by CD19+ microbeads, activation and expansion with the CD40L, loading with antigen after control of the activation status, and reinjection into the patient (Fig. 1). The primary objectives would of course be the feasibility, safety, and toxicity of a CD40B-cell vaccination, but surely the induction of an immune response, persistence of transfused CD40B cells, and evidence of disease control would be equally exciting secondary objectives.
Fig. 1.
Concept of a possible CD40B-cell study. B cells can be isolated from tumor-infiltrating lymphocyte tumors (TIL) or alternatively from peripheral blood (1) by CD19 microbeads (2). After cultivation and expansion in the CD40L culture (3), the activation status is checked by determining the expression of the activation markers, CD80, CD86, MHC class I, and MHC class I, which are usually highly upregulated after CD40L stimulation (4). After pulsing with a suitable tumor antigen (5), CD40B cells are reinjected into the patient (6).
Even though cancer vaccination has long been regarded as a promising approach for cancer immunotherapy, the sobering results of early clinical trials and the economic failure of the few approved cancer vaccines have led to reduced interest in the further development of cellular cancer vaccines. However, the current excitement about the success of immune checkpoint blockade in the treatment of a broad range of malignancies has sparked a renaissance of cancer vaccines [52]. Currently, several clinical trials are investigating the combination of DC vaccines with checkpoint inhibitors. Tumor-induced T-cell dysfunction seems to be the major immunologic mechanism that limits the ability of cellular vaccines to elicit an antitumor immune response [89, 90]. Thus, combining B-cell immunotherapy with drugs that reverse T-cell dysfunction appear to be a plausible future line of investigation.
In particular, combination of CD40B-cell vaccination and checkpoint inhibition represents a promising combination approach to further enhance the activity of B cell-based cancer immunotherapy. There are already several checkpoint inhibitors that are approved for clinical use and preclinical studies demonstrate that a dual strategy of active tumor vaccination and checkpoint blockade can overcome tumor-induced immune escape [91]. Furthermore, it can be expected that in the near future additional drugs that reverse T-cell dysfunction become available [92].
Taken together, the work of recent years that we summarized here strongly highlight the potential of B cells for immunotherapy and their applicability in a clinical setting. The most challenging obstacles for the use of CD40B cells in humans have been overcome in the meantime. The CD40-activation culture system is a versatile tool for the generation of activated B cells that can be used for immunotherapeutic purposes. The current success of chimeric antigen-receptor T cells will lead to a more widespread establishment of the infrastructure required for the clinical application of cellular therapies. In addition, technological advances such as small, automated, closed system cell manufacturing platforms that enable the decentralized “point-of-care” generation of cellular therapies will further ease the clinical testing of cellular immunotherapies such as CD40B-cell cancer vaccines [88]. Therefore, it can be expected that the near future will see the first clinical trials of B cell-based cancer vaccines. These trials will show if B cells deserve a place in the oncologist's toolbox.
References
- 1.Lund FE, Randall TD. Effector and regulatory B cells: modulators of CD4+ T cell immunity. Nat Rev Immunol. 2010 Apr;10((4)):236–47. doi: 10.1038/nri2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Belle K, Herman J, Boon L, Waer M, Sprangers B, Louat T. Comparative In Vitro Immune Stimulation Analysis of Primary Human B Cells and B Cell Lines. J Immunol Res. 2016;2016:5281823. doi: 10.1155/2016/5281823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kornbluth RS, Stempniak M, Stone GW. Design of CD40 agonists and their use in growing B cells for cancer immunotherapy. Int Rev Immunol. 2012 Aug;31((4)):279–88. doi: 10.3109/08830185.2012.703272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karpusas M, Hsu YM, Wang JH, Thompson J, Lederman S, Chess L, et al. 2 A crystal structure of an extracellular fragment of human CD40 ligand. Structure. 1995 Oct;3((10)):1031–9. doi: 10.1016/s0969-2126(01)00239-8. [DOI] [PubMed] [Google Scholar]
- 5.Barr TA, Heath AW. Functional activity of CD40 antibodies correlates to the position of binding relative to CD154. Immunology. 2001 Jan;102((1)):39–43. doi: 10.1046/j.1365-2567.2001.01148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bishop GA, Moore CR, Xie P, Stunz LL, Kraus ZJ. TRAF proteins in CD40 signaling. Adv Exp Med Biol. 2007;597:131–51. doi: 10.1007/978-0-387-70630-6_11. [DOI] [PubMed] [Google Scholar]
- 7.Säemann MD, Kelemen P, Zeyda M, Böhmig G, Staffler G, Zlabinger GJ. CD40 triggered human monocyte-derived dendritic cells convert to tolerogenic dendritic cells when JAK3 activity is inhibited. Transplant Proc. 2002 Aug;34((5)):1407–8. doi: 10.1016/s0041-1345(02)02907-x. [DOI] [PubMed] [Google Scholar]
- 8.Säemann MD, Diakos C, Kelemen P, Kriehuber E, Zeyda M, Böhmig GA, et al. Prevention of CD40-triggered dendritic cell maturation and induction of T-cell hyporeactivity by targeting of Janus kinase 3. Am J Transplant. 2003 Nov;3((11)):1341–9. doi: 10.1046/j.1600-6143.2003.00225.x. [DOI] [PubMed] [Google Scholar]
- 9.Danese S, Sans M, Fiocchi C. The CD40/CD40L costimulatory pathway in inflammatory bowel disease. Gut. 2004 Jul;53((7)):1035–43. doi: 10.1136/gut.2003.026278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawabe T, Naka T, Yoshida K, Tanaka T, Fujiwara H, Suematsu S, et al. The immune responses in CD40-deficient mice: impaired immunoglobulin class switching and germinal center formation. Immunity. 1994 Jun;1((3)):167–78. doi: 10.1016/1074-7613(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 11.Castigli E, Alt FW, Davidson L, Bottaro A, Mizoguchi E, Bhan AK, et al. CD40-deficient mice generated by recombination-activating gene-2-deficient blastocyst complementation. Proc Natl Acad Sci USA. 1994 Dec;91((25)):12135–9. doi: 10.1073/pnas.91.25.12135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Renshaw BR, Fanslow WC, 3rd, Armitage RJ, Campbell KA, Liggitt D, Wright B, et al. Humoral immune responses in CD40 ligand-deficient mice. J Exp Med. 1994 Nov;180((5)):1889–900. doi: 10.1084/jem.180.5.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caux C, Massacrier C, Vanbervliet B, Dubois B, Van Kooten C, Durand I, et al. Activation of human dendritic cells through CD40 cross-linking. J Exp Med. 1994 Oct;180((4)):1263–72. doi: 10.1084/jem.180.4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Banchereau J, de Paoli P, Vallé A, Garcia E, Rousset F. Long-term human B cell lines dependent on interleukin-4 and antibody to CD40. Science. 1991 Jan;251((4989)):70–2. doi: 10.1126/science.1702555. [DOI] [PubMed] [Google Scholar]
- 15.Schultze JL, Michalak S, Seamon MJ, Dranoff G, Jung K, Daley J, et al. CD40-activated human B cells: an alternative source of highly efficient antigen presenting cells to generate autologous antigen-specific T cells for adoptive immunotherapy. J Clin Invest. 1997 Dec;100((11)):2757–65. doi: 10.1172/JCI119822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.von Bergwelt-Baildon M, Shimabukuro-Vornhagen A, Popov A, Klein-Gonzalez N, Fiore F, Debey S, et al. CD40-activated B cells express full lymph node homing triad and induce T-cell chemotaxis: potential as cellular adjuvants. Blood. 2006 Apr;107((7)):2786–9. doi: 10.1182/blood-2004-01-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Su KY, Watanabe A, Yeh CH, Kelsoe G, Kuraoka M. Efficient Culture of Human Naive and Memory B Cells for Use as APCs. J Immunol. 2016 Nov;197((10)):4163–76. doi: 10.4049/jimmunol.1502193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mathieu M, Cotta-Grand N, Daudelin JF, Boulet S, Lapointe R, Labrecque N. CD40-activated B cells can efficiently prime antigen-specific naïve CD8+ T cells to generate effector but not memory T cells. PLoS One. 2012;7((1)):e30139. doi: 10.1371/journal.pone.0030139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Biagi E, Rousseau R, Yvon E, Schwartz M, Dotti G, Foster A, et al. Responses to human CD40 ligand/human interleukin-2 autologous cell vaccine in patients with B-cell chronic lymphocytic leukemia. Clin Cancer Res. 2005 Oct;11((19 Pt 1)):6916–23. doi: 10.1158/1078-0432.CCR-05-0484. [DOI] [PubMed] [Google Scholar]
- 20.Evans DE, Munks MW, Purkerson JM, Parker DC. Resting B lymphocytes as APC for naive T lymphocytes: dependence on CD40 ligand/CD40. J Immunol. 2000 Jan;164((2)):688–97. doi: 10.4049/jimmunol.164.2.688. [DOI] [PubMed] [Google Scholar]
- 21.Néron S, Nadeau PJ, Darveau A, Leblanc JF. Tuning of CD40-CD154 interactions in human B-lymphocyte activation: a broad array of in vitro models for a complex in vivo situation. Arch Immunol Ther Exp (Warsz) 2011 Feb;59((1)):25–40. doi: 10.1007/s00005-010-0108-8. [DOI] [PubMed] [Google Scholar]
- 22.Garcia-Marquez MA, Shimabukuro-Vornhagen A, Theurich S, Kochanek M, Weber T, Wennhold K, et al. A multimerized form of recombinant human CD40 ligand supports long-term activation and proliferation of B cells. Cytotherapy. 2014 Nov;16((11)):1537–44. doi: 10.1016/j.jcyt.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 23.Jourdan M, Robert N, Cren M, Thibaut C, Duperray C, Kassambara A, et al. Characterization of human FCRL4-positive B cells. PLoS One. 2017 Jun;12((6)):e0179793. doi: 10.1371/journal.pone.0179793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naito M, Hainz U, Burkhardt UE, Fu B, Ahove D, Stevenson KE, et al. CD40L-Tri, a novel formulation of recombinant human CD40L that effectively activates B cells. Cancer Immunol Immunother. 2012;••• doi: 10.1007/s00262-012-1331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fournel S, Wieckowski S, Sun W, Trouche N, Dumortier H, Bianco A, et al. C3-symmetric peptide scaffolds are functional mimetics of trimeric CD40L. Nat Chem Biol. 2005 Dec;1((7)):377–82. doi: 10.1038/nchembio746. [DOI] [PubMed] [Google Scholar]
- 26.Carpenter EL, Mick R, Rüter J, Vonderheide RH. Activation of human B cells by the agonist CD40 antibody CP-870,893 and augmentation with simultaneous toll-like receptor 9 stimulation. J Transl Med. 2009 Nov;7((1)):93. doi: 10.1186/1479-5876-7-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tu W, Lau YL, Zheng J, Liu Y, Chan PL, Mao H, et al. Efficient generation of human alloantigen-specific CD4+ regulatory T cells from naive precursors by CD40-activated B cells. Blood. 2008 Sep;112((6)):2554–62. doi: 10.1182/blood-2008-04-152041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoon SH, Cho HI, Kim TG. Activation of B cells using Schneider 2 cells expressing CD40 ligand for the enhancement of antigen presentation in vitro. Exp Mol Med. 2005 Dec;37((6)):567–74. doi: 10.1038/emm.2005.70. [DOI] [PubMed] [Google Scholar]
- 29.Schultze JL, Cardoso AA, Freeman GJ, Seamon MJ, Daley J, Pinkus GS, et al. Follicular lymphomas can be induced to present alloantigen efficiently: a conceptual model to improve their tumor immunogenicity. Proc Natl Acad Sci USA. 1995 Aug;92((18)):8200–4. doi: 10.1073/pnas.92.18.8200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ivanov R, Aarts T, Hagenbeek A, Hol S, Ebeling S. B-cell expansion in the presence of the novel 293-CD40L-sCD40L cell line allows the generation of large numbers of efficient xenoantigen-free APC. Cytotherapy. 2005;7((1)):62–73. doi: 10.1080/14653240510018055. [DOI] [PubMed] [Google Scholar]
- 31.Fanslow WC, Srinivasan S, Paxton R, Gibson MG, Spriggs MK, Armitage RJ. Structural characteristics of CD40 ligand that determine biological function. Semin Immunol. 1994 Oct;6((5)):267–78. doi: 10.1006/smim.1994.1035. [DOI] [PubMed] [Google Scholar]
- 32.Morris AE, Remmele RL, Jr, Klinke R, Macduff BM, Fanslow WC, Armitage RJ. Incorporation of an isoleucine zipper motif enhances the biological activity of soluble CD40L (CD154) J Biol Chem. 1999 Jan;274((1)):418–23. doi: 10.1074/jbc.274.1.418. [DOI] [PubMed] [Google Scholar]
- 33.Haswell LE, Glennie MJ, Al-Shamkhani A. Analysis of the oligomeric requirement for signaling by CD40 using soluble multimeric forms of its ligand, CD154. Eur J Immunol. 2001 Oct;31((10)):3094–100. doi: 10.1002/1521-4141(2001010)31:10<3094::aid-immu3094>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 34.Tadmori W, Lee HK, Clark SC, Choi YS. Human B cell proliferation in response to IL-4 is associated with enhanced production of B cell-derived growth factors. J Immunol. 1989 Feb;142((3)):826–32. [PubMed] [Google Scholar]
- 35.von Bergwelt-Baildon MS, Vonderheide RH, Maecker B, Hirano N, Anderson KS, Butler MO, et al. Human primary and memory cytotoxic T lymphocyte responses are efficiently induced by means of CD40-activated B cells as antigen-presenting cells: potential for clinical application. Blood. 2002 May;99((9)):3319–25. doi: 10.1182/blood.v99.9.3319. [DOI] [PubMed] [Google Scholar]
- 36.Wiesner M, Zentz C, Mayr C, Wimmer R, Hammerschmidt W, Zeidler R, et al. Conditional immortalization of human B cells by CD40 ligation. PLoS One. 2008 Jan;3((1)):e1464. doi: 10.1371/journal.pone.0001464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lapointe R, Bellemare-Pelletier A, Housseau F, Thibodeau J, Hwu P, Cells ST, et al. CD40-stimulated B lymphocytes pulsed with tumor antigens are effective antigen-presenting cells that can generate specific T cells. Cancer Res. 2003 Jun;63((11)):2836–43. [PubMed] [Google Scholar]
- 38.Coughlin CM, Vance BA, Grupp SA, Vonderheide RH. RNA-transfected CD40-activated B cells induce functional T-cell responses against viral and tumor antigen targets: implications for pediatric immunotherapy. Blood. 2004 Mar;103((6)):2046–54. doi: 10.1182/blood-2003-07-2379. [DOI] [PubMed] [Google Scholar]
- 39.Upadhyay M, Priya GK, Ramesh P, Madhavi MB, Rath S, Bal V, et al. CD40 signaling drives B lymphocytes into an intermediate memory-like state, poised between naïve and plasma cells. J Cell Physiol. 2014 Oct;229((10)):1387–96. doi: 10.1002/jcp.24572. [DOI] [PubMed] [Google Scholar]
- 40.Hawkins ED, Turner ML, Wellard CJ, Zhou JH, Dowling MR, Hodgkin PD. Quantal and graded stimulation of B lymphocytes as alternative strategies for regulating adaptive immune responses. Nat Commun. 2013;4((1)):2406. doi: 10.1038/ncomms3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Faassen AE, Dalke DP, Berton MT, Warren WD, Pierce SK. Faassen a E, Dalke DP, Berton MT, Warren WD, Pierce SK: CD40-CD40 ligand interactions stimulate B cell antigen processing. Eur J Immunol. 1995;25((12)):3249–55. doi: 10.1002/eji.1830251208. [DOI] [PubMed] [Google Scholar]
- 42.von Bergwelt-Baildon M, Schultze JL, Maecker B, Menezes I, Nadler LM. Correspondence re R. Lapointe et al., CD40-stimulated B lymphocytes pulsed with tumor antigens are effective antigen-presenting cells that can generate specific T cells. Cancer Res. 2003;63:2836–2843. Cancer Res. 2004 Jun; 64(11): 4055–6. [PubMed] [Google Scholar]
- 43.Becker HJ, Kondo E, Shimabukuro-Vornhagen A, Theurich S, von Bergwelt-Baildon MS. Processing and MHC class II presentation of exogenous soluble antigen involving a proteasome-dependent cytosolic pathway in CD40-activated B cells. Eur J Haematol. 2015;••• doi: 10.1111/ejh.12699. [DOI] [PubMed] [Google Scholar]
- 44.Fujiwara H, Melenhorst JJ, El Ouriaghli F, Kajigaya S, Grube M, Sconocchia G, et al. In vitro induction of myeloid leukemia-specific CD4 and CD8 T cells by CD40 ligand-activated B cells gene modified to express primary granule proteins. Clin Cancer Res. 2005 Jun;11((12)):4495–503. doi: 10.1158/1078-0432.CCR-04-2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shimabukuro-Vornhagen A, Zoghi S, Liebig TM, Wennhold K, Chemitz J, Draube A, et al. Inhibition of protein geranylgeranylation specifically interferes with CD40-dependent B cell activation, resulting in a reduced capacity to induce T cell immunity. J Immunol. 2014 Nov;193((10)):5294–305. doi: 10.4049/jimmunol.1203436. [DOI] [PubMed] [Google Scholar]
- 46.Theurich S, Malcher J, Becker HJ, Chemnitz JM, Liebig TM, Shimabukuro-Vornhagen A, et al. Activated primary human B cells efficiently induce early CD40L and CD107a expression in CD4+ T cells. Blood. 2011 Nov;118((22)):5979–80. doi: 10.1182/blood-2011-05-356683. [DOI] [PubMed] [Google Scholar]
- 47.Zheng J, Liu Y, Qin G, Chan PL, Mao H, Lam KT, et al. Efficient induction and expansion of human alloantigen-specific CD8 regulatory T cells from naive precursors by CD40-activated B cells. J Immunol. 2009 Sep;183((6)):3742–50. doi: 10.4049/jimmunol.0901329. [DOI] [PubMed] [Google Scholar]
- 48.Zentz C, Wiesner M, Man S, Frankenberger B, Wollenberg B, Hillemanns P, et al. Activated B cells mediate efficient expansion of rare antigen-specific T cells. Hum Immunol. 2007 Feb;68((2)):75–85. doi: 10.1016/j.humimm.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 49.Wu C, Liu Y, Zhao Q, Chen G, Chen J, Yan X, et al. Soluble CD40 ligand-activated human peripheral B cells as surrogated antigen presenting cells: A preliminary approach for anti-HBV immunotherapy. Virol J. 2010 Dec;7((1)):370. doi: 10.1186/1743-422X-7-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wan Y, Ma X, Li X, Yi J. A novel immunotherapy to hepatocellular carcinoma: CD40-activated B lymphocytes transfected with AFPmRNA. Med Hypotheses. 2009 Nov;73((5)):835–7. doi: 10.1016/j.mehy.2008.12.050. [DOI] [PubMed] [Google Scholar]
- 51.Kondo E, Gryschok L, Klein-Gonzalez N, Rademacher S, Weihrauch MR, Liebig T, et al. CD40-activated B cells can be generated in high number and purity in cancer patients: analysis of immunogenicity and homing potential. Clin Exp Immunol. 2009 Feb;155((2)):249–56. doi: 10.1111/j.1365-2249.2008.03820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Garg AD, Coulie PG, Van den Eynde BJ, Agostinis P. Integrating Next-Generation Dendritic Cell Vaccines into the Current Cancer Immunotherapy Landscape. Trends Immunol. 2017 Aug;38((8)):577–93. doi: 10.1016/j.it.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 53.Nava S, Dossena M, Pogliani S, Pellegatta S, Antozzi C, Baggi F, et al. An optimized method for manufacturing a clinical scale dendritic cell-based vaccine for the treatment of glioblastoma. PLoS One. 2012;7((12)):e52301. doi: 10.1371/journal.pone.0052301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nguyen XD, Eichler H, Sucker A, Hofmann U, Schadendorf D, Klüter H. Collection of autologous monocytes for dendritic cell vaccination therapy in metastatic melanoma patients. Transfusion. 2002 Apr;42((4)):428–32. doi: 10.1046/j.1525-1438.2002.00067.x. [DOI] [PubMed] [Google Scholar]
- 55.Svensson A, Adamson L, Pisa P, Petersson M, Hansson M. Monocyte enriched apheresis for preparation of dendritic cells (DC) to be used in cellular therapy. Transfus Apheresis Sci. 2005 Oct;33((2)):165–73. doi: 10.1016/j.transci.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 56.Harizi H, Grosset C, Gualde N. Prostaglandin E2 modulates dendritic cell function via EP2 and EP4 receptor subtypes. J Leukoc Biol. 2003 Jun;73((6)):756–63. doi: 10.1189/jlb.1002483. [DOI] [PubMed] [Google Scholar]
- 57.Yang L. TGFbeta, a potent regulator of tumor microenvironment and host immune response, implication for therapy. Curr Mol Med. 2010 Jun;10((4)):374–80. doi: 10.2174/156652410791317039. [DOI] [PubMed] [Google Scholar]
- 58.Geissmann F, Revy P, Regnault A, Lepelletier Y, Dy M, Brousse N, et al. TGF-beta 1 prevents the noncognate maturation of human dendritic Langerhans cells. J Immunol. 1999 Apr;162((8)):4567–75. [PubMed] [Google Scholar]
- 59.Johnson BF, Clay TM, Hobeika AC, Lyerly HK, Morse MA. Vascular endothelial growth factor and immunosuppression in cancer: current knowledge and potential for new therapy. Expert Opin Biol Ther. 2007 Apr;7((4)):449–60. doi: 10.1517/14712598.7.4.449. [DOI] [PubMed] [Google Scholar]
- 60.Gabrilovich DI, Chen HL, Girgis KR, Cunningham HT, Meny GM, Nadaf S, et al. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat Med. 1996 Oct;2((10)):1096–103. doi: 10.1038/nm1096-1096. [DOI] [PubMed] [Google Scholar]
- 61.Sabat R, Grütz G, Warszawska K, Kirsch S, Witte E, Wolk K, et al. Biology of interleukin-10. Cytokine Growth Factor Rev. 2010 Oct;21((5)):331–44. doi: 10.1016/j.cytogfr.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 62.Steinbrink K, Jonuleit H, Müller G, Schuler G, Knop J, Enk AH. Interleukin-10-treated human dendritic cells induce a melanoma-antigen-specific anergy in CD8(+) T cells resulting in a failure to lyse tumor cells. Blood. 1999 Mar;93((5)):1634–42. [PubMed] [Google Scholar]
- 63.Gabrilovich D. Mechanisms and functional significance of tumour-induced dendritic-cell defects. Nat Rev Immunol. 2004 Dec;4((12)):941–52. doi: 10.1038/nri1498. [DOI] [PubMed] [Google Scholar]
- 64.Shimabukuro-Vornhagen A, Draube A, Liebig TM, Rothe A, Kochanek M, von Bergwelt-Baildon MS. The immunosuppressive factors IL-10, TGF-β, and VEGF do not affect the antigen-presenting function of CD40-activated B cells. J Exp Clin Cancer Res. 2012 May;31((1)):47. doi: 10.1186/1756-9966-31-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Arai C, Ichijo T, Tanaka Y, Okada Y, Umeda M, Uchida T, et al. Selective enhancement of B cell antigen receptor-mediated antigen presentation by treatment with transforming growth factor-beta. Eur J Immunol. 2003 Jul;33((7)):1806–15. doi: 10.1002/eji.200324018. [DOI] [PubMed] [Google Scholar]
- 66.Tretter T, Venigalla RK, Eckstein V, Saffrich R, Sertel S, Ho AD, et al. Induction of CD4+ T-cell anergy and apoptosis by activated human B cells. Blood. 2008 Dec;112((12)):4555–64. doi: 10.1182/blood-2008-02-140087. [DOI] [PubMed] [Google Scholar]
- 67.Shimabukuro-Vornhagen A, Kondo E, Liebig T, von Bergwelt-Baildon M. Activated human B cells: stimulatory or tolerogenic antigen-presenting cells? Blood. 2009 Jul;114((3)):746–7. doi: 10.1182/blood-2009-03-212886. [DOI] [PubMed] [Google Scholar]
- 68.Wennhold K, Weber TM, Thelen M, Garcia-Marquez M, Chakupurakal G, Klein-Gonzalez N, et al. CD40-activated B cells induce anti-tumor immunity in vivo. Oncotarget. 2016;••• doi: 10.18632/oncotarget.7720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sorenmo KU, Krick E, Coughlin CM, Overley B, Gregor TP, Vonderheide RH, et al. CD40-activated B cell cancer vaccine improves second clinical remission and survival in privately owned dogs with non-Hodgkin's lymphoma. PLoS One. 2011;6((8)):e24167. doi: 10.1371/journal.pone.0024167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ritchie DS, Yang J, Hermans IF, Ronchese F. B-Lymphocytes activated by CD40 ligand induce an antigen-specific anti-tumour immune response by direct and indirect activation of CD8(+) T-cells. Scand J Immunol. 2004 Dec;60((6)):543–51. doi: 10.1111/j.0300-9475.2004.01517.x. [DOI] [PubMed] [Google Scholar]
- 71.Lee J, Dollins CM, Boczkowski D, Sullenger BA, Nair S. Activated B cells modified by electroporation of multiple mRNAs encoding immune stimulatory molecules are comparable to mature dendritic cells in inducing in vitro antigen-specific T-cell responses. Immunology. 2008 Oct;125((2)):229–40. doi: 10.1111/j.1365-2567.2008.02833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Guo S, Xu J, Denning W, Hel Z. Induction of protective cytotoxic T-cell responses by a B-cell-based cellular vaccine requires stable expression of antigen. Gene Ther. 2009 Nov;16((11)):1300–13. doi: 10.1038/gt.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li Q, Lao X, Pan Q, Ning N, Yet J, Xu Y, et al. Adoptive transfer of tumor reactive B cells confers host T-cell immunity and tumor regression. Clin Cancer Res. 2011 Aug;17((15)):4987–95. doi: 10.1158/1078-0432.CCR-11-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li Q, Teitz-Tennenbaum S, Donald EJ, Li M, Chang AE. In vivo sensitized and in vitro activated B cells mediate tumor regression in cancer adoptive immunotherapy. J Immunol. 2009 Sep;183((5)):3195–203. doi: 10.4049/jimmunol.0803773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Moutai T, Yamana H, Nojima T, Kitamura D. A novel and effective cancer immunotherapy mouse model using antigen-specific B cells selected in vitro. PLoS One. 2014 Mar;9((3)):e92732. doi: 10.1371/journal.pone.0092732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wennhold K, Thelen M, Schlößer HA, Haustein N, Reuter S, Garcia-Marquez M, et al. Using Antigen-Specific B Cells to Combine Antibody and T Cell-Based Cancer Immunotherapy. Cancer Immunol Res. 2017 Sep;5((9)):730–43. doi: 10.1158/2326-6066.CIR-16-0236. [DOI] [PubMed] [Google Scholar]
- 77.Batista FD, Neuberger MS. Affinity dependence of the B cell response to antigen: a threshold, a ceiling, and the importance of off-rate. Immunity. 1998 Jun;8((6)):751–9. doi: 10.1016/s1074-7613(00)80580-4. [DOI] [PubMed] [Google Scholar]
- 78.Kugler A, Seseke F, Thelen P, Kallerhoff M, Müller GA, Stuhler G, et al. Autologous and allogenic hybrid cell vaccine in patients with metastatic renal cell carcinoma. Br J Urol. 1998 Oct;82((4)):487–93. doi: 10.1046/j.1464-410x.1998.00794.x. [DOI] [PubMed] [Google Scholar]
- 79.Trefzer U, Weingart G, Chen Y, Herberth G, Adrian K, Winter H, et al. Hybrid cell vaccination for cancer immune therapy: first clinical trial with metastatic melanoma. Int J Cancer. 2000 Mar;85((5)):618–26. doi: 10.1002/(sici)1097-0215(20000301)85:5<618::aid-ijc4>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 80.Tittlbach H, Schneider A, Strobel J, Zimmermann R, Maas S, Gebhardt B, et al. GMP-production of purified human B lymphocytes for the adoptive transfer in patients after allogeneic hematopoietic stem cell transplantation. J Transl Med. 2017 Nov;15((1)):228. doi: 10.1186/s12967-017-1330-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Winkler J, Tittlbach H, Roesler W, Strobel J, Zimmermann R, Maas S, et al. Adoptive Transfer of Purified Donor-B-Lymphocytes after Allogeneic Stem Cell Transplantation: Results from a Phase I/IIa Clinical Trial. Blood. 2016:128. [cited 2018 Jun 22]; Available from: http://www.bloodjournal.org/content/128/22/502?sso-checked=true. [Google Scholar]
- 82.Rodríguez-Pinto D, Moreno J. B cells can prime naive CD4+ T cells in vivo in the absence of other professional antigen-presenting cells in a CD154-CD40-dependent manner. Eur J Immunol. 2005 Apr;35((4)):1097–105. doi: 10.1002/eji.200425732. [DOI] [PubMed] [Google Scholar]
- 83.Liljedahl M, Winqvist O, Surh CD, Wong P, Ngo K, Teyton L, et al. Altered antigen presentation in mice lacking H2-O. Immunity. 1998 Feb;8((2)):233–43. doi: 10.1016/s1074-7613(00)80475-6. [DOI] [PubMed] [Google Scholar]
- 84.Ma Y, Xiang D, Sun J, Ding C, Liu M, Hu X, et al. Targeting of antigens to B lymphocytes via CD19 as a means for tumor vaccine development. J Immunol. 2013 Jun;190((11)):5588–99. doi: 10.4049/jimmunol.1203216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Szeto GL, Van Egeren D, Worku H, Sharei A, Alejandro B, Park C, et al. Microfluidic squeezing for intracellular antigen loading in polyclonal B-cells as cellular vaccines. Sci Rep. 2015 May;5((1)):10276. doi: 10.1038/srep10276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Köhl U, Arsenieva S, Holzinger A, Abken H. CAR T Cells in Trials: Recent Achievements and Challenges that Remain in the Production of Modified T Cells for Clinical Applications. Hum Gene Ther. 2018 May;29((5)):559–68. doi: 10.1089/hum.2017.254. [DOI] [PubMed] [Google Scholar]
- 87.Finn OJ. Human Tumor Antigens Yesterday, Today, and Tomorrow. Cancer Immunol Res. 2017 May;5((5)):347–54. doi: 10.1158/2326-6066.CIR-17-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mock U, Nickolay L, Philip B, Cheung GW, Zhan H, Johnston IC, et al. Automated manufacturing of chimeric antigen receptor T cells for adoptive immunotherapy using CliniMACS prodigy. Cytotherapy. 2016 Aug;18((8)):1002–11. doi: 10.1016/j.jcyt.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 89.Saxena M, Bhardwaj N. Re-Emergence of Dendritic Cell Vaccines for Cancer Treatment. Trends Cancer. 2018 Feb;4((2)):119–37. doi: 10.1016/j.trecan.2017.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Thommen DS, Schumacher TN. T Cell Dysfunction in Cancer. Cancer Cell. 2018 Apr;33((4)):547–62. doi: 10.1016/j.ccell.2018.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Duraiswamy J, Kaluza KM, Freeman GJ, Coukos G. Dual blockade of PD-1 and CTLA-4 combined with tumor vaccine effectively restores T-cell rejection function in tumors. Cancer Res. 2013 Jun;73((12)):3591–603. doi: 10.1158/0008-5472.CAN-12-4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zarour HM. Reversing T-cell Dysfunction and Exhaustion in Cancer. Clin Cancer Res. 2016 Apr;22((8)):1856–64. doi: 10.1158/1078-0432.CCR-15-1849. [DOI] [PMC free article] [PubMed] [Google Scholar]