Abstract
BACKGROUND
Several studies have explored the association between the use of proton pump inhibitors (PPIs) and the risk of developing hepatic encephalopathy (HE) in patients with advanced liver disease. However, the evidence-based conclusions are controversial. We hypothesized that using PPIs may increase the risk of HE in patients with advanced liver disease. If confirmed, clinicians must strictly adhere to the indications for PPI treatment in this population.
AIM
To evaluate the pooled risk of HE in patients with advanced liver disease who use PPIs.
METHODS
Three electronic databases (PubMed, EMBASE, and the Cochrane Library) were searched from the date of database inception through January 8, 2019 to identify comparative studies evaluating the association between PPI use and the risk of HE. Data from the included studies were extracted. The random-effects model was used for pooling risk estimates and the corresponding 95% confidence intervals (CIs). Subgroup and sensitivity analyses were also performed.
RESULTS
In total, 4342 patients from five case-control studies and 188053 patients from four cohort studies were included in this analysis. In patients with advanced liver disease, PPI use was associated with an elevated risk of developing HE, with significant heterogeneity. The pooled odds ratio for case-control studies was 2.58 (95%CI: 1.68-3.94, I2 = 72%). The pooled RR for cohort studies was 1.67 (95%CI: 1.30-2.14, I2 = 67%). The results of the subgroup analyses suggested that the heterogeneity may be the result of differences in the study designs and the definitions of PPI use. The sensitivity and subgroup analyses did not alter our findings.
CONCLUSION
In patients with advanced liver disease, PPI use is associated with an elevated risk of HE. Future large prospective studies are needed to confirm this association.
Keywords: Proton pump inhibitors, Cirrhosis, Hepatic encephalopathy, Systematic review, Meta-analysis
Core tip: Proton pump inhibitors (PPIs) are very commonly used in patients with advanced liver disease. Remarkably, previous studies have shown that approximately 50% of indications for PPIs treatment were unclear or inadequate in this special group of patients. All these may be because PPIs are generally considered safe. However, this meta-analysis shows that using PPIs is associated with an increased risk of hepatic encephalopathy (HE) in patients with advanced liver disease. This reminds clinicians that inappropriate use of PPIs may be not beneficial but put patients at an elevated risk of HE. These findings still need to be confirmed by more high-quality prospective studies because of the limitations of this meta-analysis.
INTRODUCTION
Hepatic encephalopathy (HE) is a serious neuropsychiatric syndrome usually identified in patients with advanced liver disease, which manifests along a spectrum spanning from minimal cognitive dysfunction to states of confusion and even coma[1,2]. HE can be subdivided into covert (minimal, grade 1) and overt (HE grades 2-4) according to the severity of the manifestations[2]. Minimal HE or covert HE is reported in 20%-80% of patients with cirrhosis, and the incidence of overt HE is 30%-40%[2]. These complications are associated with impaired quality of life and poor prognosis[2,3]. At present, several factors have been identified that induce the incidence of HE, such as infection, constipation, gastrointestinal bleeding, and the use of some nervous system drugs[2]. However, unknown influential factors still need to be explored to facilitate better management of patients with chronic liver disease.
With an excellent safety profile, proton pump inhibitors (PPIs) are commonly prescribed for peptic ulcer disease, reflux disease, esophagitis, nonvariceal upper gastrointestinal bleeding, etc[4]. PPIs are very commonly used by as many as 46%-78% of patients with cirrhosis, and the excessive use of PPIs leads to a poor prognosis[5-7]. By altering intestinal microflora and increasing bacterial proliferation, previous studies have reported that PPI treatment is associated with small intestinal bacterial overgrowth (SIBO), spontaneous bacterial peritonitis, and other bacterial infections in patients with cirrhosis[8-12]. In addition, there is growing evidence that PPI therapy is associated with HE in patients with advanced liver disease[13-18]. A previous meta-analysis evaluating the association between PPIs and HE included only three retrospective studies. Due to the limited number of studies, large heterogeneity, and lack of prospective studies, a definite conclusion could not be drawn[19]. To clarify the conclusions, this meta-analysis was performed to further examine the association between PPI use and the risk of HE in patients with advanced liver disease.
MATERIALS AND METHODS
Literature search
Our meta-analysis was conducted in line with the meta-analysis of observational studies in epidemiology guidelines[20]. Electronic databases including PubMed (from 1946 through January 8, 2019), EMBASE (from 1988 through January 8, 2019), Cochrane Central Register of Controlled Trials (CENTRAL) (from 1991through January 8, 2019), and Cochrane Database and Systematic Reviews (from 2005 through January 8, 2019) were searched by using subject headings and keywords. The search terms were proton pump inhibitor, proton pump inhibitors, PPI, PPIs, omeprazole, lansoprazole, pantoprazole, rabeprazole, esomeprazole, ilaprazole, hepatic encephalopathy, HE, and encephalopath*. No language restrictions were applied. Two researchers (Z.W. and L.Y.). independently completed the process of searching and screening. The titles and abstracts of the identified articles were assessed in the initial screening after duplicate citations were removed, and the full texts of potentially eligible studies were further evaluated to determine whether they should be included or not. In addition, additional studies were manually searched by reviewing the bibliographies of the included studies and the relevant review literature. ClinicalTrials.gov was also searched for unpublished studies. Disagreements between the researchers were resolved by discussing with a third researcher (J.W.).
Inclusion criteria
Randomized controlled trials, case-control studies, and cohort studies were included if meeting the following criteria: (1) The studies were performed in patients with advanced liver disease, including advanced fibrosis, cirrhosis (compensated or decompensated), and acute-on-chronic liver failure (ACLF); (2) The studies clearly clarified the definition of PPI exposure; (3) The studies evaluated the association between PPI use and the risk of HE, and reported a risk estimate and the corresponding 95% confidence interval (CI). The risk estimate included odds ratio (OR), relative risk (RR), and hazard ratio (HR). To reduce the impact of confounding factors, we only extracted the effect estimates adjusted for the greatest number confounding factors. If the same population was used in two or more articles, only the study with the most comprehensive information was included. The data from conference abstracts were included without distinction. However, due to the uncertain quality of abstracts, we conducted a sensitivity analysis excluding the data from conference abstracts. For articles with incomplete data and abstracts, we attempted to contact the corresponding author via email to request the relevant data.
Data extraction
Using a pre-established form, two researchers (Z.W. and L.Y.) independently completed data extraction. The collected data included the authors’ names, publication year, study location, study design, number of included patients, patient demographics (age and sex), type of advanced liver disease, outcomes analyzed, definition of PPI use, follow-up time, variables used for adjustment, risk estimates with 95% CIs, and information used for the quality assessment. If two researchers had some disagreements in the process of data extraction, a third reviewer (J.W.) would be invited to resolve the disagreements.
Quality assessment
Because all included studies were observational, the Newcastle-Ottawa scale (NOS) was used for quality assessment[20]. The NOS evaluates the quality of case-control or cohort studies from three aspects. The scale items include selection (four points), comparability (two points), and exposure/outcome (three points). The scale has a maximum possible score of 9 points; 7 or more points indicate high quality, 5-6 points indicate moderate quality, and four or fewer points indicate low quality. Due to incomplete information in the conference abstracts, we only conducted quality assessments of full-text articles. Two reviewers (C.X. and L.C.) independently completed the quality assessments, and any disagreements were discussed with a third reviewer (N.L.), with agreement determined by consensus.
Statistical analysis
The primary outcome of this meta-analysis was the pooled risk of HE in patients using PPIs. We only extracted the adjusted risk estimates instead of the raw data calculated by the events and the total number of patients. Using the random effects model and the inverse variance method, the risk estimates were pooled to obtain an overall effect estimate and the corresponding 95%CI. Because the incidence of HE is relatively low, different risk estimates (OR/RR/HR) were considered equivalent[21]. Tsai et al[16] reported the ORs for HE in a subgroup analysis of patients with different PPI use patterns, and we pooled these OR values and 95%CI using a random effects model to obtain an approximate overall OR value with the associated 95%CI[19,22]. Heterogeneity among the studies was tested by calculating Cochran’s Q (with P < 0.10 considered significant) and the I2 statistic. We considered the heterogeneity to be substantial when I2 was > 50%. A high degree of heterogeneity was further explored in the subgroup analyses. We estimated in advance that the heterogeneity was mainly the result of differences in study designs and definitions of PPI use. Pre-arranged subgroup analyses would be performed based on the study design, definition of PPI use, study location, type of advanced liver disease, outcomes analyzed, and quality of the research. We performed the sensitivity analysis by excluding the data from conference abstracts. To eliminate confounding by indication, we also performed a specific sensitivity analysis for patients without prior HE. According to the Cochrane Collaboration Handbook[22], it is not recommended to test for publication bias if the number of included studies is fewer than 10. We only explored publication bias by examining a funnel plot. All data analyses were performed by using Review Manager 5.3 (The Nordic Cochrane Centre, the Cochrane Collaboration, Copenhagen, 2014).
RESULTS
Literature search
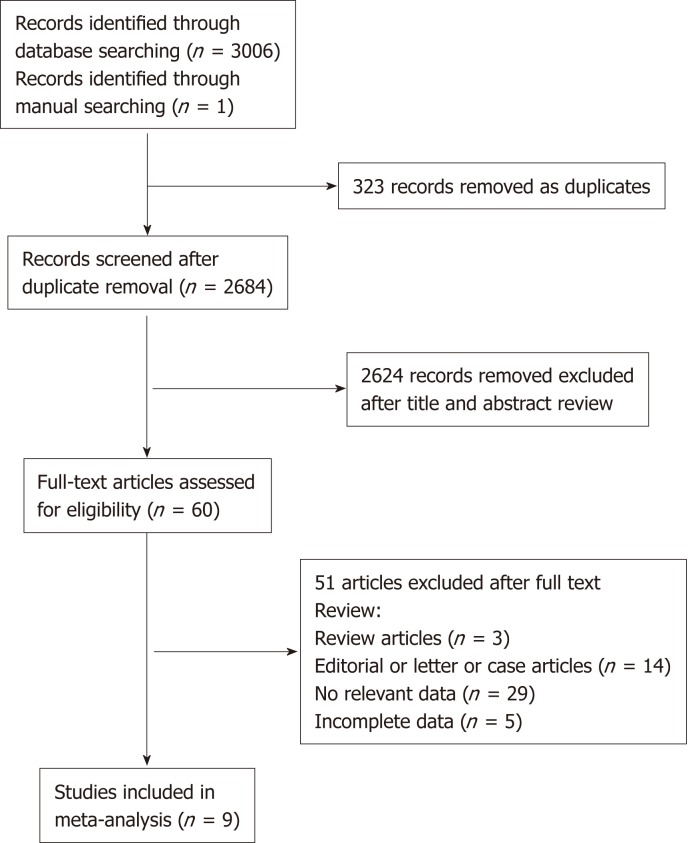
Of 3006 studies initially retrieved, 2578 were from EMBASE, 383 from PubMed, and 45 from the Cochrane Library. One study was identified through the manual search. After excluding duplicates, 2684 titles and abstracts were reviewed, and 60 studies were assessed for eligibility through full-text review. Based on the inclusion criteria, eight observational studies and six conference abstracts were identified. Of these, six observational studies (three case-control studies and three cohort studies)[13-18] and three conference abstracts[23-25] provided the necessary data and were ultimately included (Figure 1). Two cohort studies and three conference abstracts were excluded because of incomplete data. We tried to contact the corresponding authors via email. However, our requests did not receive responses.
Figure 1.
Flowchart of the literature search.
Study characteristics and quality assessment
Table 1 lists the characteristics of the included studies. Six observational studies (three case-control studies and three cohort studies)[13-18] and three conference abstracts (two case-control studies and one cohort study)[23-25] were included. In total, 4342 patients from the case-control studies and 188053 patients from the cohort studies were included in this analysis. Three studies from Asia used a case-control design[15,16,18], six studies were from Europe, and one study was from the United States[13,14,17,23-25]. Most of these studies included middle-aged and elderly individuals, and there were more men than women. Cirrhotic patients were analyzed in five studies[14-16,23,24], patients with cirrhosis with ascites in two studies[17,25], patients with cirrhosis with transjugular intrahepatic portosystemic shunt implantation in one study[13], and patients with hepatitis B virus-related ACLF in one study[18]. Seven studies provided outcomes regarding the risk of HE grades 1-4[13,15-17,23-25], three studies provided the risk of HE grades 2-4[14,17,18], and one study provided the risk of minimal HE[14]. With regard to the definition of PPI use, there was a clear difference among the included studies. Three studies adopted the definition of in-hospital PPI use as PPI exposure (in-hospital definition)[13,15,18], two studies adopted the definition of current PPI use as PPI exposure (current definition)[17,23], and the remaining studies specified the time of PPI exposure (≥ 2 weeks; > 30 cDDDs; ≥ 4 wk; > 90 d) (time-based definition)[14,16,24,25]. Five studies provided the follow-up time, and the approximate follow-up time ranged from 4 mo to 3 years. All the included studies adjusted for various confounding factors except for two abstracts, which reported adjusted OR values but lacked details regarding the variables used for the adjustment[23,25]. We assessed the quality of six full-text studies. Four studies scored 9 points[13,14,16,17], and two case-control studies scored 8 points (1 point was deducted for using hospital controls as a control group)[15,18]. All these studies were considered high quality because of their relatively low risk of bias.
Table 1.
Characteristics of included studies
| Author, Year | Location | Design | No. of cases/controls | Age of cases/controls | Sex (% male) cases/controls | Type of liver disease | Outcomes analyzed | Definition of PPI use | Follow-up time | Adjusted factors | Quality score |
| Lin et al[18], 2014 | China | Case-control | 55/110 | 46 (37-55)/43 (36-48) | 83.6/75.5 | HBV-related ACLF | Grades 2-4 HE | Patients using any PPI intravenously for at least 6 d before the occurrence of HE at the admission time | NA | Age, sex, MELD score, infection, hypokalemia, hyponatraemia, ascites, PTA, AFP, lactulose use, branched chain amino acids, and arginine hydrochloride | 8 |
| Dam et al[17], 2016 | Denmark | Cohort | 340/525 | 58 (50-64)/57 (51-64) | 68/69 | Cirrhosis with ascites | Grades 1-4 HE; grades 2-4 HE | A patient counted as a PPI user when he or she was using PPIs and as a nonuser when he or she was not | 148.2 person-years/186.1 person-years | Sex, age at inclusion, cirrhosis etiology, variceal bleeding, MELD score, serum sodium, albumin, and platelets; and lactulose use, spironolactone dose, furosemide dose, and potassiumsparing diuretic dose | 9 |
| Tsai et al[16], 2016 | Taiwan | Case-control | 1166/1166 | 53.09 ± 13.80/53.14 ± 13.78 | 74.2/74.2 | Cirrhosis | Grades 1-4 HE | PPI use was defined as > 30 cumulative defined daily doses (cDDDs); PPI non-use was defined as ≤ 30 cDDDs | 2.96 ± 3.40/2.87 ± 3.57 yr | Age, sex, income, level of urbanization, the use of PPIs in the past 6 mo before enrollment, Charlson Comobidity Index score, medical comorbidities, use of medication | 9 |
| Zhu et al[15], 2018 | China | Case-control | 128/128 | 58.34 ± 11.15/58.28 ± 10.97 | 63.3/63.3 | Cirrhosis | Grades 1-4 HE | PPI userswere defined as the patients who used PPIs during hospitalization | NA | Age, gender, Child-Pugh score, hemoglobin, gammaglutamyl transpeptidase, blood urea nitrogen, ammonia, international normalized ratio, and acute upper gastrointestinal bleeding | 8 |
| Nardelli et al[14], 2018 | Italy | Cohort | 125/185 | 61.5 ± 11.9/63.3 ± 11.6 | 74.1/67.2 | Cirrhosis | Minimal HE; grades 2-4 HE | Patients were considered PPIs users when the treatment started at least 4 wk prior to the admission | 14.1 ± 12.3 mo | MELD scores, MHE, previous overt HE, PPIs, age, albumin and sodium levels | 9 |
| Sturm et al[13], 2018 | Germany | Cohort | 303/94 | 59.2 ± 11.7/59.7 ± 10.2 | 67.7/69.1 | Cirrhosis with TIPS implantation | Grades 1-4 HE | PPI userswere defined as the patients who used PPIs during hospitalization | 116 ± 74 /135 ± 65 d | Age, etiology of liver disease, TIPS indication, acute variceal bleeding with early TIPS implantation, covering of the stent graft, portosystemic gradient before and after TIPS, HE before TIPS, MELD score, HE medication, and peri-interventional antibiotic treatment | 9 |
| Tapper et al[24], 2018 (abstract) | USA | Cohort | 186481 | 65 (57–73) | 55 | Cirrhosis | Grades 1-4 HE | Chronic use (> 90 d) | 542739 patient-years | Age, sex, race, etiology of cirrhosis, Medicaid coenrollment, hemodialysis, portal hyperten-sion (varices, ascites, TIPS placement), and management by a gastroenterologist | NA |
| Matei et al[25], 2017 (abstract) | Romania | Case-control | 436/327 | 60.41 (17–91) | 63.3 | Cirrhosis and ascites | Grades 1-4 HE | PPIs use was defined as the administration of at least 40 mg/day, for minimum 2 wk during the last 3 mo | NA | NA | NA |
| Shanab et al[23], 2018 (abstract) | UK | Case-control | 506/320 | 53.4 ± 12.0 | 66 | Cirrhosis | Grades 1-4 HE | Current PPI use | NA | NA | NA |
HBV: Hepatitis B virus; ACLF: Acute on chronic liver failure; HE: Hepatic encephalopathy; PPI: Proton pump inhibitor; cDDDs: Cumulative defined daily doses; PTA: Prothrombin activity; MELD: Model for end stage liver disease; AFP: Alpha-fetoprotein; TIPS: Transjugular intrahepatic portosystemic shunt; NA: not available.
HE risk in PPI users
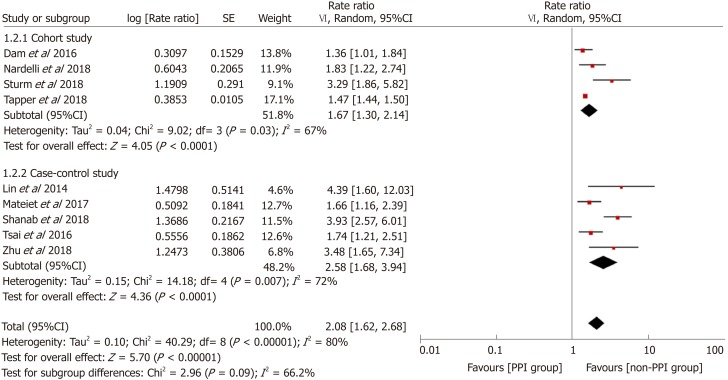
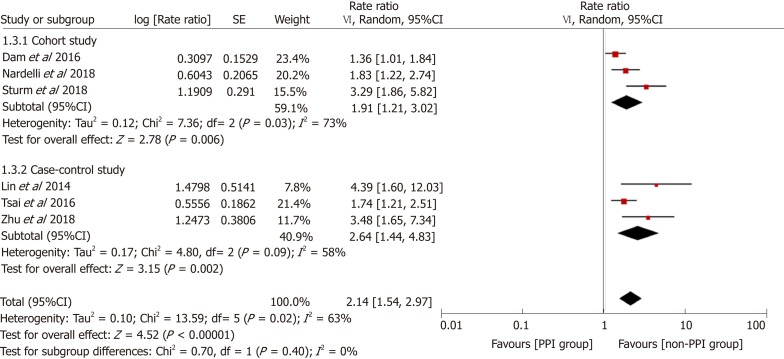
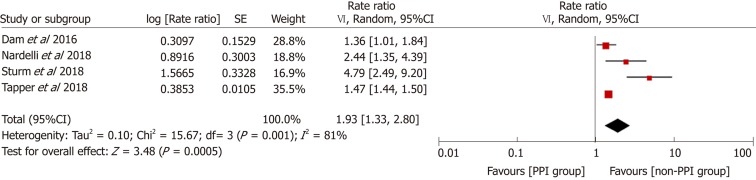
Figure 2 demonstrates that the risk of HE was elevated in PPI users. The pooled RR was 2.08 (95%CI: 1.62-2.68, Cochran Q test P < 0.001, I2 = 80%), indicating that compared with patients who did not use PPIs, PPI users had a 2.08-fold higher risk of developing HE, with significant heterogeneity. There was no significant difference based on study design in the effect on the risk of HE (χ2 = 2.96 and P = 0.09). The pooled RR for cohort studies was 1.67 (95%CI: 1.30-2.14, Cochran Q test P = 0.03, I2 = 67%), while the OR for case-control studies was 2.58 (95%CI: 1.68-3.94). In the sensitivity analysis with conference abstracts excluded, the pooled RR was 2.14 (95%CI: 1.54-2.97, Cochran Q test P = 0.02, I2 = 63%) (Figure 3). Two cohort studies excluded patients who had previous HE episodes[17,24], and the remaining two cohort studies provided supplementary data focusing on patients without past HE episodes[13,14]. In the sensitivity analysis of patients without prior HE episodes, the pooled RR was 1.93 (95%CI: 1.33-2.80, Cochran Q test P = 0.001, I2 = 81%) (Figure 4).
Figure 2.
Forest plot to evaluate the association between proton pump inhibitor use and hepatic encephalopathy with a subgroup analysis based on study design. CI: Confidence interval; PPI: Proton pump inhibitor.
Figure 3.
Sensitivity analysis excluding data from conference abstracts. CI: Confidence interval; PPI: Proton pump inhibitor.
Figure 4.
Sensitivity analysis focusing on patients without past hepatic encephalopathy episodes. CI: Confidence interval; PPI: Proton pump inhibitor.
Subgroup and stratified analyses
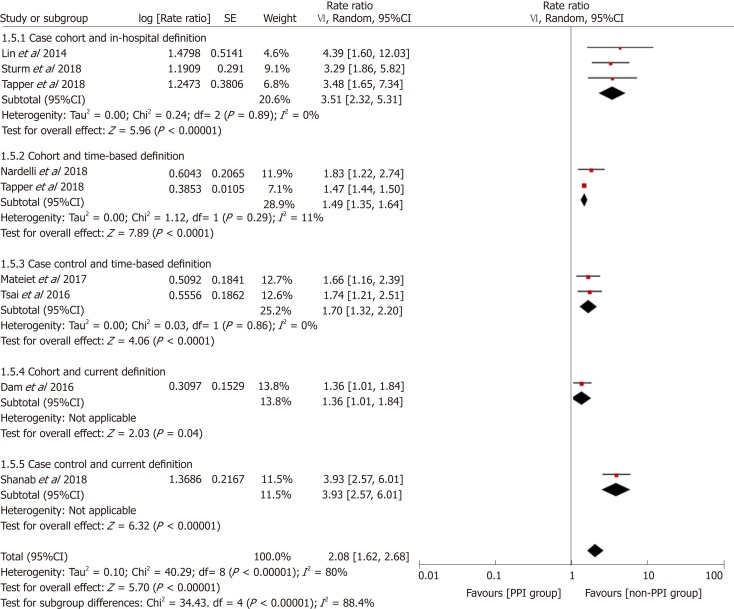
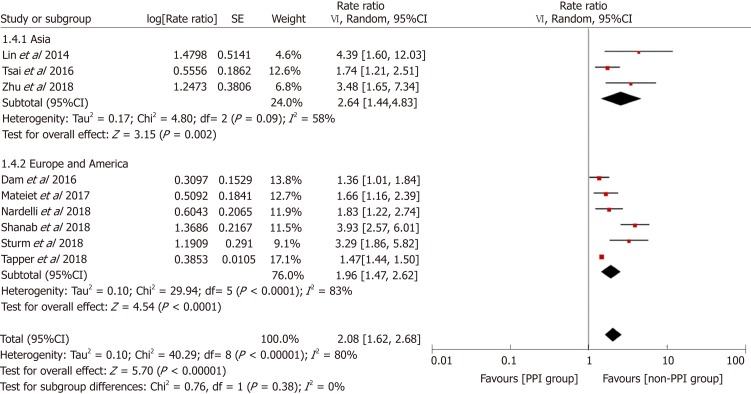
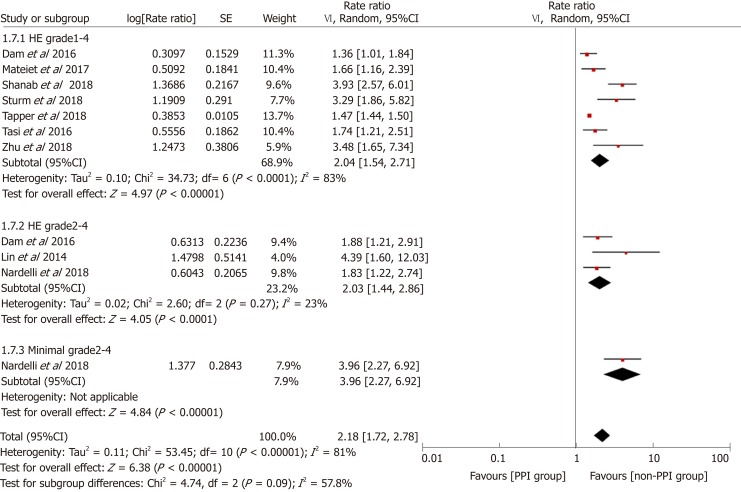
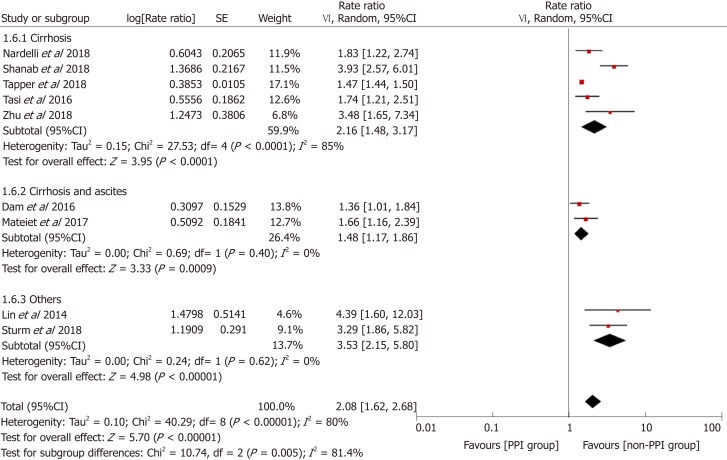
To explore the source of heterogeneity, we conducted subgroup and stratified analyses. Before performing this meta-analysis, we estimated that the sources of heterogeneity (if any) would mainly be differences in study designs and definitions of PPI use. We performed stratification analyses based on these two factors. The results showed that the heterogeneity was significantly reduced by each stratum, and the elevated risk of HE in the PPI group still existed (Figure 5). Furthermore, we performed subgroup analyses based on the study location, type of advanced liver disease, and outcomes analyzed, and the results were consistent among the different subgroups (Figures 6-8).
Figure 5.
Forest plot to evaluate the association between proton pump inhibitor use and hepatic encephalopathy with stratification analyses based on study design and definition of proton pump inhibitor use. CI: Confidence interval; PPI: Proton pump inhibitor.
Figure 6.
Forest plot to evaluate the association between proton pump inhibitor use and hepatic encephalopathy with a subgroup analysis based on the study location. CI: Confidence interval; PPI: Proton pump inhibitor.
Figure 8.
Forest plot to evaluate the association between proton pump inhibitor use and hepatic encephalopathy with a subgroup analysis based on the outcomes analyzed. CI: Confidence interval; PPI: Proton pump inhibitor.
Figure 7.
Forest plot to evaluate the association between proton pump inhibitor use and hepatic encephalopathy with a subgroup analysis based on the type of advanced liver disease. CI: Confidence interval; PPI: Proton pump inhibitor.
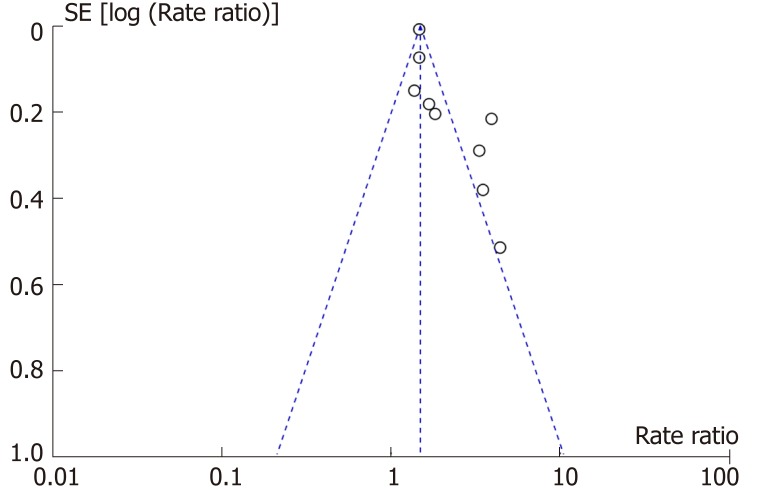
Publication bias
According to the Cochrane Collaboration Handbook[22], testing for publication bias is not recommended if the number of included studies is fewer than ten. True asymmetry and opportunity are indistinguishable because of the low test efficiency. However, a funnel plot generated from the nine studies revealed that it was visually asymmetrical (Figure 9).
Figure 9.
Funnel plot assessing publication bias.
DISCUSSION
In this meta-analysis, we found that PPI use was associated with a 2.08-fold higher risk of progression to HE. After excluding conference abstracts, the pooled results from the high-quality observational studies showed similar RR values and 95%CIs. There was a high degree of heterogeneity among studies, and the results of the subgroup analyses suggested that the heterogeneity may be the result of differences in the study designs and definitions of PPI use. The elevated risk of HE for PPI users was consistent regardless of the study design, definition of PPI use, study location, type of advanced liver disease, and outcomes analyzed. Previous studies revealed that HE was a predictor of PPI therapy[26,27], which suggests that the elevated risk of HE for PPI users may be affected by confounding by indication. However, the sensitivity analysis focusing on patients without past HE episodes showed a similar result. In addition, two of the included studies increased the persuasiveness of evidence by reporting significant dose-response relationships between PPI use and the risk of HE occurrence in patients with cirrhosis[13,16]. It is worth noting that the pooled effect size of the case-control design was greater than that of the cohort design. Case-control designs can generate an exaggerated risk estimate because they are susceptible to various biases[28]. Furthermore, ORs may overestimate the true effect of an exposure on the outcome of interest[29]. We also found that the pooled risk estimates differed based on study location and type of advanced liver disease. These differences may be mainly due to different study designs. In addition, infection and eradication status of H. pylori and the degree of gastric atrophy may affect expression of proton pump[30,31]. These factors largely influence gastric acid secretion and the effect of PPIs, thus causing differences in risk estimates in different locations. However, the impact of these factors cannot be analyzed because the data were not available. The study by Lin et al[18] was aimed to assess the role of PPI in a particular setting (ACLF), and sensitivity analysis by excluding the study showed that the pooled risk estimate was slightly altered. With respect to the outcomes analyzed, seven studies reported the results of HE grades 1-4, and three studies reported the results of HE grades 2-4. The results of these two outcomes were similar. Only one high-quality prospective cohort study evaluated the association between PPI use and minimal HE, and the risk estimate was higher than those for the other two groups. Because the diagnosis of minimal HE is difficult, most of the retrospective studies lack data for minimal HE. However, the incidence of minimal HE has been reported to range from 20% to 80% in patients with liver cirrhosis[2]. The lack of data regarding minimal HE may lead to an underestimation of the risk of overall HE (minimal HE and HE grades 1-4) in PPI users. Even so, the risk of HE was still high in our meta-analysis, which increases our confidence in our conclusion.
Previous studies showed that PPIs were used by as many as 46%-78% of cirrhotic patients[5,7], and the data from the six studies we included reported that the PPI use rate was 40%-76.3% in patients with advanced liver disease[13-18]. The above data indicate that PPIs are very commonly used by patients with advanced liver disease. PPIs are often overused in clinical practice. Data from five studies we included showed that the indication for PPI use was unclear or appropriate in 44%-62.2% of the patients[13-15,17,18]. Inappropriate use of PPIs may put patients at an elevated risk of HE. Although PPIs are generally considered safe, we should strictly adhere to the indications for PPI use in patients with advanced liver disease. The accumulated evidence also confirmed that PPIs were associated with diverse adverse effects and even an elevated mortality rate[7,32,33].
Bian et al[19] performed a meta-analysis evaluating the association between PPIs and HE. However, there were some shortcomings that limited the reliability of their conclusions. First, the deadline for the search was December 2016, and only three retrospective studies were included. Second, the heterogeneity among studies was substantial. Limited by the small number of included studies, they did not perform subgroup or sensitivity analysis. Third, they did not investigate in detail the characteristics of the included studies or evaluate the research quality. Considering the shortcomings of the previous meta-analysis, this meta-analysis included more high-quality studies, conducted subgroup and sensitivity analyses, and obtained more reliable results. Weersink et al[34] systematically reviewed the safety of PPIs in patients with cirrhosis and suggested that the use of PPIs in patients with cirrhosis should be carefully considered because of the risk of HE. The results of our quantitative analyses were consistent with the results of theirs, although four studies included in their analysis were excluded in this meta-analysis (no relevant data or incomplete data).
The impact of PPIs on the development of HE may be explained by changes in intestinal flora and bacterial translocation. Previous studies have reported a close association between PPI use and SIBO[35,36]. Moreover, recent studies showed that PPI use was associated with a less healthy gut microbiome, lower microbial diversity, and increased prevalence of Streptococcaceae[37,38]. Changes in gut flora have been found to be associated with the development of HE[39,40]. On the other hand, PPI use may predispose patients to bacterial infections by increasing bacterial proliferation and altering gastrointestinal motility[40-43]. Therefore, PPIs may increase the production and absorption of nitrogenous substances, thereby increasing the risk of HE. HE in patients with ACLF seems to be different from that of acute decompensation in the clinical and pathophysiological aspects, and the mechanism and classification are still unknown[44]. Systemic inflammation, impaired intestinal mucosal immunity, and changes of intestinal microbiota may increase the risk of bacterial translocation in patients with ACLF[45]. Using PPI in ACLF patients appears to further increase the risk of HE, which requires further research. Tsai et al[16] found that rabeprazole was not associated with an increased risk of HE. One reason may be that the sample size of rabeprazole users is too small, and another may be the metabolic difference among different types of PPIs. PPs are mainly metabolized in the liver by liver metabolizing isozyme CYP2C19. Based on different combinations of wild-type gene and mutated alleles, CYP2C19 genotypes can be classified as ultra-rapid metabolizer, rapid metabolizer, intermediate metabolizer, and poor metabolizer[46,47]. Different genotypes can influence pharmacokinetics and acid-suppressive effect. In addition, individual PPI has its own unique metabolic pathway[47]. These metabolic differences of PPIs may affect the occurrence of HE, and this deserves further research.
Our meta-analysis has some limitations. First, the number of studies included was small, and only six studies were included after the conference abstracts were excluded. Furthermore, only one study was a prospective cohort study, and the rest were retrospective cohort studies or case-control studies. Although these studies were of high quality, their inherent limitations based on design inhibited our ability to establish clear causality. Second, the heterogeneity among the included studies was significant. However, we performed subgroup analyses and found that the heterogeneity may stem from the differences in study designs and definitions of PPI use. When the heterogeneity was significantly reduced in the stratification analyses, the risk of HE was still present. Limited by the small number of studies, some subgroups only consisted of one or two studies. Third, our findings may have been affected by publication bias. Additional data from one study and three abstracts may be relevant, but we were unable to obtain the data even after attempting to contact the authors. Fourth, the definitions of PPI use differed substantially among the different studies. The studies included in our analysis defined a patient as a PPI user if the patient was using PPIs or had used PPIs for a short period of time in the past. During the progression of liver disease, the actual use of PPIs by individuals is unknown. However, the potential impact of this unknown factor can affect both the PPI and non-PPI groups. Fifth, although we performed the meta-analysis using adjusted estimates, the variables used for adjustment varied among the studies. We cannot exclude the possibility that these differences and other unknown factors may play roles in the progression to HE. The incidence of minimal HE was not assessed in most included studies, which might underestimate the risk of overall HE in PPI users. Some studies included special patients, which may lead to selection bias. Finally, due to insufficient data, we were unable to explore the association between the risk of HE and the type of PPI, indication for PPI treatment, time of PPI treatment, or method of PPI administration (oral/intravenous). We cannot predict whether the risk of HE changes after discontinuing the use of PPIs. These problems urgently need to be addressed in future studies.
In conclusion, the results of this meta-analysis of observational studies suggest that PPI use is significantly associated with the risk of progression to HE. Among patients with advanced liver disease, compared with nonusers, PPI users have a higher risk of HE. This important finding suggests that clinicians need to strictly adhere to the indications for PPI use in patients with advanced liver disease. Future large prospective studies and mechanistic studies are required to better understand the association between PPI use and the risk of HE.
ARTICLE HIGHLIGHTS
Research background
Given their safety profile, proton pump inhibitors (PPIs) are commonly prescribed for patients with advanced liver disease. Recent studies have reported that using PPIs may increase the risk of hepatic encephalopathy (HE) by increasing the gastric pH and bacterial translocation and changing intestinal flora. About the association between PPI use and HE, evidence-based conclusions need to be drawn.
Research motivation
PPIs are often overused in patients with advanced liver disease. Systematically reviewing the existing evidence on the association between PPI use and the risk of HE could help regulate clinical practice.
Research objectives
The aim of this meta-analysis was to analyze data on the association between PPI use and the risk of HE in patients with advanced liver disease.
Research methods
Electronic databases (PubMed, EMBASE, and the Cochrane Library) were searched for relevant articles meeting the inclusion criteria. We conducted a meta-analysis of all comparative studies that evaluated the association between PPI use and the risk of HE. The primary outcome was pooled risk estimates of HE in the PPI group and non-PPI groups. Subgroup analyses by different clinical and methodological characteristics were also performed.
Research results
We finally included nine observational studies (five case-control studies and four cohort studies). This analysis showed that PPI use was associated with an increased risk of developing HE regardless of the study design. The sensitivity analysis excluding conference abstracts or focusing on patients without prior HE showed a similar result. The results of subgroup analyses suggested that the heterogeneity may come from different study designs and definitions of PPI use.
Research conclusions
Compared with the non-PPI group, the PPI group has an elevated risk of HE in patients with advanced liver disease. This finding reminds clinicians that they should strictly adhere to the indications for PPI treatment in patients with advanced liver disease.
Research perspectives
To further explore the association between PPI therapy and the risk of HE, future studies need to refine the impact of the PPI types, time of PPI administration, and method of PPI administration on HE. In addition, more high-quality prospective studies and mechanistic studies are required to better understand the association between PPI use and the risk of HE.
Footnotes
Conflict-of-interest statement: The authors have no potential conflicts of interest to disclose.
PRISMA 2009 Checklist statement: The authors have read the PRISMA 2009 Checklist, and the manuscript was prepared and revised according to the PRISMA 2009 Checklist.
Peer-review started: March 13, 2019
First decision: April 11, 2019
Article in press: May 3, 2019
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Huang CC, Osawa H, Ridola L S-Editor: Yan JP L-Editor: Wang TQ E-Editor: Ma YJ
Contributor Information
Xin-Xing Tantai, Division of Gastroenterology, The Second Affiliated Hospital, Xi’an Jiaotong University, Xi’an 710004, Shaanxi Province, China.
Long-Bao Yang, Division of Gastroenterology, The Second Affiliated Hospital, Xi’an Jiaotong University, Xi’an 710004, Shaanxi Province, China.
Zhong-Cao Wei, Division of Gastroenterology, The Second Affiliated Hospital, Xi’an Jiaotong University, Xi’an 710004, Shaanxi Province, China.
Cai-Lan Xiao, Division of Gastroenterology, The Second Affiliated Hospital, Xi’an Jiaotong University, Xi’an 710004, Shaanxi Province, China.
Li-Rong Chen, Division of Gastroenterology, The Second Affiliated Hospital, Xi’an Jiaotong University, Xi’an 710004, Shaanxi Province, China.
Jin-Hai Wang, Division of Gastroenterology, The Second Affiliated Hospital, Xi’an Jiaotong University, Xi’an 710004, Shaanxi Province, China.
Na Liu, Division of Gastroenterology, The Second Affiliated Hospital, Xi’an Jiaotong University, Xi’an 710004, Shaanxi Province, China. liunafmmu@163.com.
References
- 1.Wijdicks EF. Hepatic Encephalopathy. N Engl J Med. 2016;375:1660–1670. doi: 10.1056/NEJMra1600561. [DOI] [PubMed] [Google Scholar]
- 2.Vilstrup H, Amodio P, Bajaj J, Cordoba J, Ferenci P, Mullen KD, Weissenborn K, Wong P. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60:715–735. doi: 10.1002/hep.27210. [DOI] [PubMed] [Google Scholar]
- 3.Prakash R, Mullen KD. Mechanisms, diagnosis and management of hepatic encephalopathy. Nat Rev Gastroenterol Hepatol. 2010;7:515–525. doi: 10.1038/nrgastro.2010.116. [DOI] [PubMed] [Google Scholar]
- 4.Barrison AF, Jarboe LA, Weinberg BM, Nimmagadda K, Sullivan LM, Wolfe MM. Patterns of proton pump inhibitor use in clinical practice. Am J Med. 2001;111:469–473. doi: 10.1016/s0002-9343(01)00901-9. [DOI] [PubMed] [Google Scholar]
- 5.Chavez-Tapia NC, Tellez-Avila FI, Garcia-Leiva J, Valdovinos MA. Use and overuse of proton pump inhibitors in cirrhotic patients. Med Sci Monit. 2008;14:CR468–CR472. [PubMed] [Google Scholar]
- 6.Kalaitzakis E, Björnsson E. Inadequate use of proton-pump inhibitors in patients with liver cirrhosis. Eur J Gastroenterol Hepatol. 2008;20:512–518. doi: 10.1097/MEG.0b013e3282f4aa01. [DOI] [PubMed] [Google Scholar]
- 7.Dultz G, Piiper A, Zeuzem S, Kronenberger B, Waidmann O. Proton pump inhibitor treatment is associated with the severity of liver disease and increased mortality in patients with cirrhosis. Aliment Pharmacol Ther. 2015;41:459–466. doi: 10.1111/apt.13061. [DOI] [PubMed] [Google Scholar]
- 8.Lewis SJ, Franco S, Young G, O'Keefe SJ. Altered bowel function and duodenal bacterial overgrowth in patients treated with omeprazole. Aliment Pharmacol Ther. 1996;10:557–561. doi: 10.1046/j.1365-2036.1996.d01-506.x. [DOI] [PubMed] [Google Scholar]
- 9.Tergast TL, Wranke A, Laser H, Gerbel S, Manns MP, Cornberg M, Maasoumy B. Dose-dependent impact of proton pump inhibitors on the clinical course of spontaneous bacterial peritonitis. Liver Int. 2018;38:1602–1613. doi: 10.1111/liv.13862. [DOI] [PubMed] [Google Scholar]
- 10.Lázaro-Pacheco IB, Servín-Caamaño AI, Pérez-Hernández JL, Rojas-Loureiro G, Servín-Abad L, Tijera FH. PROTON PUMP INHIBITORS INCREASE THE OVERALL RISK OF DEVELOPING BACTERIAL INFECTIONS IN PATIENTS WITH CIRRHOSIS. Arq Gastroenterol. 2018;55:28–32. doi: 10.1590/S0004-2803.201800000-09. [DOI] [PubMed] [Google Scholar]
- 11.Miura K, Tanaka A, Yamamoto T, Adachi M, Takikawa H. Proton pump inhibitor use is associated with spontaneous bacterial peritonitis in patients with liver cirrhosis. Intern Med. 2014;53:1037–1042. doi: 10.2169/internalmedicine.53.2021. [DOI] [PubMed] [Google Scholar]
- 12.Bulsiewicz WJ, Scherer JR, Feinglass JM, Howden CW, Flamm SL. Proton pump inhibitor (PPI) use is independently associated with spontaneous bacterial peritonitis (SBP) in cirrhotics with ascites. Gastroenterology. 2009;136:A11. [Google Scholar]
- 13.Sturm L, Bettinger D, Giesler M, Boettler T, Schmidt A, Buettner N, Thimme R, Schultheiss M. Treatment with proton pump inhibitors increases the risk for development of hepatic encephalopathy after implantation of transjugular intrahepatic portosystemic shunt (TIPS) United European Gastroenterol J. 2018;6:1380–1390. doi: 10.1177/2050640618795928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nardelli S, Gioia S, Ridola L, Farcomeni A, Merli M, Riggio O. Proton Pump Inhibitors Are Associated With Minimal and Overt Hepatic Encephalopathy and Increased Mortality in Patients With Cirrhosis. Hepatology. 2018 doi: 10.1002/hep.30304. [DOI] [PubMed] [Google Scholar]
- 15.Zhu J, Qi X, Yu H, Yoshida EM, Mendez-Sanchez N, Zhang X, Wang R, Deng H, Li J, Han D, Guo X. Association of proton pump inhibitors with the risk of hepatic encephalopathy during hospitalization for liver cirrhosis. United European Gastroenterol J. 2018;6:1179–1187. doi: 10.1177/2050640618773564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsai CF, Chen MH, Wang YP, Chu CJ, Huang YH, Lin HC, Hou MC, Lee FY, Su TP, Lu CL. Proton Pump Inhibitors Increase Risk for Hepatic Encephalopathy in Patients With Cirrhosis in A Population Study. Gastroenterology. 2017;152:134–141. doi: 10.1053/j.gastro.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 17.Dam G, Vilstrup H, Watson H, Jepsen P. Proton pump inhibitors as a risk factor for hepatic encephalopathy and spontaneous bacterial peritonitis in patients with cirrhosis with ascites. Hepatology. 2016;64:1265–1272. doi: 10.1002/hep.28737. [DOI] [PubMed] [Google Scholar]
- 18.Lin ZN, Zuo YQ, Hu P. Association of Proton Pump Inhibitor Therapy with Hepatic Encephalopathy in Hepatitis B Virus-related Acute-on-Chronic Liver Failure. Hepat Mon. 2014;14:e16258. doi: 10.5812/hepatmon.16258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bian J, Wang A, Lin J, Wu L, Huang H, Wang S, Yang X, Lu X, Xu Y, Zhao H. Association between proton pump inhibitors and hepatic encephalopathy: A meta-analysis. Medicine (Baltimore) 2017;96:e6723. doi: 10.1097/MD.0000000000006723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 21.Davies HT, Crombie IK, Tavakoli M. When can odds ratios mislead? BMJ. 1998;316:989–991. doi: 10.1136/bmj.316.7136.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.2011. Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. Available from: http://handbook.cochrane.org. [Google Scholar]
- 23.Shanab AA, Smith A, Abbas M, Iriarte R, Nightingale P, Gunson B, Holt AP, Rajoriya N. Proton pump inhibitors-a risk factor for hepatic encephalopathy in patients listed for liver transplantation? Hepatology. 2018;68:1176A. [Google Scholar]
- 24.Tapper EB, Henderson J, Baki J, Parikh ND, Lok AS. Incidence and predictors of hepatic encephalopathy in a population-based cohort of older americans with cirrhosis: Role of opiates, benzodiazepines and proton-pump inhibitors (PPIs) Hepatology. 2018;68:138A. [Google Scholar]
- 25.Matei D, Pasca S, David A, Procopet B, Stefanescu H, Vesa S, Andreica V, Tantau M. Proton pump inhibitors increase the risk for hepatic encephalopathy in patients with cirrhosis and ascites. J Hepatol. 2017;66:S136. [Google Scholar]
- 26.Cole HL, Pennycook S, Hayes PC. The impact of proton pump inhibitor therapy on patients with liver disease. Aliment Pharmacol Ther. 2016;44:1213–1223. doi: 10.1111/apt.13827. [DOI] [PubMed] [Google Scholar]
- 27.Schiavon LL, Silva TE, Fischer J, Narciso-Schiavon JL. Letter: Proton pump inhibitors and prognosis of cirrhosis - searching for the balance point. Aliment Pharmacol Ther. 2017;45:378–379. doi: 10.1111/apt.13873. [DOI] [PubMed] [Google Scholar]
- 28.Schulz KF, Grimes DA. Case-control studies: Research in reverse. Lancet. 2002;359:431–434. doi: 10.1016/S0140-6736(02)07605-5. [DOI] [PubMed] [Google Scholar]
- 29.Schmidt CO, Kohlmann T. When to use the odds ratio or the relative risk? Int J Public Health. 2008;53:165–167. doi: 10.1007/s00038-008-7068-3. [DOI] [PubMed] [Google Scholar]
- 30.Osawa H, Kita H, Ohnishi H, Hoshino H, Mutoh H, Ishino Y, Watanabe E, Satoh K, Sugano K. Helicobacter pylori eradication induces marked increase in H+/K+-adenosine triphosphatase expression without altering parietal cell number in human gastric mucosa. Gut. 2006;55:152–157. doi: 10.1136/gut.2005.066464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saha A, Hammond CE, Beeson C, Peek RM, Jr, Smolka AJ. Helicobacter pylori represses proton pump expression and inhibits acid secretion in human gastric mucosa. Gut. 2010;59:874–881. doi: 10.1136/gut.2009.194795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Corleto VD, Festa S, Di Giulio E, Annibale B. Proton pump inhibitor therapy and potential long-term harm. Curr Opin Endocrinol Diabetes Obes. 2014;21:3–8. doi: 10.1097/MED.0000000000000031. [DOI] [PubMed] [Google Scholar]
- 33.Tran-Duy A, Spaetgens B, Hoes AW, de Wit NJ, Stehouwer CD. Use of Proton Pump Inhibitors and Risks of Fundic Gland Polyps and Gastric Cancer: Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2016;14:1706–1719.e5. doi: 10.1016/j.cgh.2016.05.018. [DOI] [PubMed] [Google Scholar]
- 34.Weersink RA, Bouma M, Burger DM, Drenth JPH, Harkes-Idzinga SF, Hunfeld NGM, Metselaar HJ, Monster-Simons MH, van Putten SAW, Taxis K, Borgsteede SD. Safe use of proton pump inhibitors in patients with cirrhosis. Br J Clin Pharmacol. 2018;84:1806–1820. doi: 10.1111/bcp.13615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lo WK, Chan WW. Proton pump inhibitor use and the risk of small intestinal bacterial overgrowth: A meta-analysis. Clin Gastroenterol Hepatol. 2013;11:483–490. doi: 10.1016/j.cgh.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 36.Bajaj JS, Cox IJ, Betrapally NS, Heuman DM, Schubert ML, Ratneswaran M, Hylemon PB, White MB, Daita K, Noble NA, Sikaroodi M, Williams R, Crossey MM, Taylor-Robinson SD, Gillevet PM. Systems biology analysis of omeprazole therapy in cirrhosis demonstrates significant shifts in gut microbiota composition and function. Am J Physiol Gastrointest Liver Physiol. 2014;307:G951–G957. doi: 10.1152/ajpgi.00268.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Imhann F, Bonder MJ, Vich Vila A, Fu J, Mujagic Z, Vork L, Tigchelaar EF, Jankipersadsing SA, Cenit MC, Harmsen HJ, Dijkstra G, Franke L, Xavier RJ, Jonkers D, Wijmenga C, Weersma RK, Zhernakova A. Proton pump inhibitors affect the gut microbiome. Gut. 2016;65:740–748. doi: 10.1136/gutjnl-2015-310376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jackson MA, Goodrich JK, Maxan ME, Freedberg DE, Abrams JA, Poole AC, Sutter JL, Welter D, Ley RE, Bell JT, Spector TD, Steves CJ. Proton pump inhibitors alter the composition of the gut microbiota. Gut. 2016;65:749–756. doi: 10.1136/gutjnl-2015-310861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bajaj JS. The role of microbiota in hepatic encephalopathy. Gut Microbes. 2014;5:397–403. doi: 10.4161/gmic.28684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gupta A, Dhiman RK, Kumari S, Rana S, Agarwal R, Duseja A, Chawla Y. Role of small intestinal bacterial overgrowth and delayed gastrointestinal transit time in cirrhotic patients with minimal hepatic encephalopathy. J Hepatol. 2010;53:849–855. doi: 10.1016/j.jhep.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 41.van Vlerken LG, Huisman EJ, van Hoek B, Renooij W, de Rooij FW, Siersema PD, van Erpecum KJ. Bacterial infections in cirrhosis: Role of proton pump inhibitors and intestinal permeability. Eur J Clin Invest. 2012;42:760–767. doi: 10.1111/j.1365-2362.2011.02643.x. [DOI] [PubMed] [Google Scholar]
- 42.Bajaj JS, Ratliff SM, Heuman DM, Lapane KL. Proton pump inhibitors are associated with a high rate of serious infections in veterans with decompensated cirrhosis. Aliment Pharmacol Ther. 2012;36:866–874. doi: 10.1111/apt.12045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Merli M, Lucidi C, Di Gregorio V, Giannelli V, Giusto M, Ceccarelli G, Riggio O, Venditti M. The chronic use of beta-blockers and proton pump inhibitors may affect the rate of bacterial infections in cirrhosis. Liver Int. 2015;35:362–369. doi: 10.1111/liv.12593. [DOI] [PubMed] [Google Scholar]
- 44.Romero-Gómez M, Montagnese S, Jalan R. Hepatic encephalopathy in patients with acute decompensation of cirrhosis and acute-on-chronic liver failure. J Hepatol. 2015;62:437–447. doi: 10.1016/j.jhep.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 45.Fernández J, Acevedo J, Wiest R, Gustot T, Amoros A, Deulofeu C, Reverter E, Martínez J, Saliba F, Jalan R, Welzel T, Pavesi M, Hernández-Tejero M, Ginès P, Arroyo V European Foundation for the Study of Chronic Liver Failure. Bacterial and fungal infections in acute-on-chronic liver failure: Prevalence, characteristics and impact on prognosis. Gut. 2018;67:1870–1880. doi: 10.1136/gutjnl-2017-314240. [DOI] [PubMed] [Google Scholar]
- 46.Sahara S, Sugimoto M, Uotani T, Ichikawa H, Yamade M, Iwaizumi M, Yamada T, Osawa S, Sugimoto K, Umemura K, Miyajima H, Furuta T. Twice-daily dosing of esomeprazole effectively inhibits acid secretion in CYP2C19 rapid metabolisers compared with twice-daily omeprazole, rabeprazole or lansoprazole. Aliment Pharmacol Ther. 2013;38:1129–1137. doi: 10.1111/apt.12492. [DOI] [PubMed] [Google Scholar]
- 47.Ishizaki T, Horai Y. Review article: Cytochrome P450 and the metabolism of proton pump inhibitors--emphasis on rabeprazole. Aliment Pharmacol Ther. 1999;13 Suppl 3:27–36. doi: 10.1046/j.1365-2036.1999.00022.x. [DOI] [PubMed] [Google Scholar]