Abstract
Background
A small proportion of patients with advanced esophageal squamous cell carcinoma (ESCC) could benefit from immune checkpoint inhibitors; however, reliable peripheral blood biomarkers for outcomes of anti‐PD‐1 immunotherapy in ESCC have not been identified.
Methods
The data of 43 patients in the ESCC cohort of a phase I trial at our center were retrospectively reviewed. All patients were administered intravenous camrelizumab (SHR‐1210), a novel anti‐PD‐1 antibody, at doses of 60 mg, 200 mg, or 400 mg (4‐week interval after first dose followed by a 2‐week schedule) until disease progression or intolerable toxicity. Associations between lactate dehydrogenase (LDH) and other peripheral blood biomarkers at baseline and the efficacy of camrelizumab were also investigated.
Results
After median follow‐up of 19.6 months, the overall response rate was 25.6% (11/43), including one complete response. Median progression‐free and overall survival rates were 2.0 and 8.0 months, respectively. Patients with an elevated baseline LDH had lower tumor response rates (P = 0.02) and shorter progression‐free (P = 0.002) and overall (P < 0.0001) survival than patients with normal LDH levels. An increase in LDH levels during treatment was significantly associated with disease progression. Multivariate Cox analysis identified LDH (hazard ratio [HR] 0.18), CRP (HR 0.27), the number of organs involved (HR 0.31), absolute monocyte count (HR 0.33), and Eastern Cooperative Oncology Group performance status (HR 0.36) as independent prognostic factors.
Conclusions
Serum LDH, which is readily available in routine clinical practice, is a potential marker for response and a powerful independent factor for survival in advanced ESCC patients treated with anti‐PD‐1 therapy.
Keywords: Esophageal squamous cell carcinoma, immune checkpoint inhibitor, lactate dehydrogenase, markers, programmed cell death‐1
Introduction
Esophageal carcinoma is the eighth most common cancer worldwide and the sixth leading cause of cancer‐related mortality.1 East Asia is one of the regions with the highest prevalence of esophageal cancer in the world. According to data released by the National Cancer Center in 2015, esophageal cancer is ranked third in incidence and fourth in mortality in China.2 Esophageal squamous cell carcinoma (ESCC) is the predominant histological subtype in East Asia and accounts for more than 90% of all esophageal carcinomas in China and Japan.3, 4, 5
Cytotoxic agents, such as fluorouracil, cisplatin, taxanes, and irinotecan, have proven active in patients with advanced or metastatic ESCC, either as monotherapy or in combined regimens. Nevertheless, the long‐term survival of these patients remains poor, with median overall survival (OS) of < 10 months.6, 7, 8, 9 Consequently, there remains an imperative need for effective alternatives, especially novel drugs.
Currently, immune checkpoint inhibitors (ICIs), particularly inhibitors of cytotoxic T‐lymphocyte antigen‐4 (CTLA‐4), PD‐1, and its associated ligand (PD‐L1), have been approved for the treatment of many advanced solid tumors.10 Several phase I/II studies have shown modest efficacy and tolerable toxicity in ESCC patients treated with anti‐PD‐1 antibodies.11, 12, 13 However, only a small proportion of advanced ESCC patients benefit from ICIs across all trials, and reliable peripheral blood biomarkers for the response and outcome of anti‐PD‐1 immunotherapy have not been defined in ESCC.
Elevated lactate dehydrogenase (LDH) has been demonstrated to predict poor prognosis in various malignancies.14 Recent studies have shown that elevated LDH levels at baseline and post‐treatment were associated with poor response and OS in melanoma patients treated with ipilimumab, pembrolizumab, and nivolumab.15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26 In a multivariable analysis conducted in melanoma patients treated with ipilimumab, low baseline LDH, low absolute monocyte counts (AMCs), high absolute eosinophil counts, relative lymphocyte counts, and frequencies of certain subsets of myeloid‐derived suppressor cells, as well as regulatory T cells, were associated with improved survival.22 Nevertheless, the predictive and prognostic roles of LDH in ESCC patients treated with ICIs have not been reported.
Therefore, the aim of this study was to explore whether the LDH level at baseline was associated with clinical outcome and whether the early increase in LDH level after camrelizumab (SHR‐1210) therapy, a novel humanized anti‐PD‐1 antibody, could predict disease progression. In addition, we identified prognostic markers for camrelizumab treatment in the ESCC cohort of a phase I trial, including baseline clinical characteristics and peripheral blood markers, such as serum LDH, complete blood count, and CRP.
Methods
Patients
The data of a consecutive series of patients with advanced ESCC who were enrolled in an open‐label, multicohort phase I trial and treated with camrelizumab between 11 May 2016 and 5 May 2017 at the Cancer Hospital, Chinese Academy of Medical Sciences were retrospectively analyzed. The clinical, pathological, and demographic characteristics of all patients were collected from electronic patient records, including age, gender, Eastern Cooperative Oncology Group performance status (ECOG PS), histologic grade, previous treatment, disease stage according to the 7th edition of the American Joint Committee on Cancer (AJCC) system, number of organs involved, and baseline LDH level.
Treatment and response evaluation
Camrelizumab was administered as monotherapy intravenously at an initial dose of 60 mg and repeated every two weeks, with subsequent dose escalation to 200 mg and 400 mg (4‐week interval after the first dose followed, by a 2‐week schedule), until disease progression, intolerable toxicity, or death. The objective tumor response was assessed according to Response Evaluation Criteria in Solid Tumors version 1.1 by computed tomography (CT) scan. Tumor imaging was performed at baseline and every eight weeks within the first six months, and then repeated every 12 weeks. The categories of response were: complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD). All efficacy data are reported using the intention‐to‐treat population. The overall response rate (ORR) was defined as the percentage of cases with the best overall response of CR or PR. Progression‐free survival (PFS) was calculated from the date of initial treatment with camrelizumab to the date of progression or death. The duration of response (DOR) was calculated from the date of the first documented response until the date of progression or death. OS was calculated from the date of initial treatment with camrelizumab to the date of death of any cause.
Identification of biomarkers
In an effort to identify associations of specific markers with clinical response and OS in patients treated with camrelizumab, the clinicopathologic and demographic characteristics, as well as peripheral blood samples for complete blood counts, LDH, and CRP tests were collected from all patients within two weeks before the first dose of camrelizumab and ± 1 day of subsequent doses. The patients were divided into two groups according to baseline LDH values (below or equal to the upper limit of normal [ULN] versus above the ULN). We calculated ORR, PFS, and OS stratified by baseline LDH in all patients. We also investigated whether changes in serum LDH prior to the first imaging assessment would predict the clinical response (non‐PD vs. PD). To this end, patients with serum LDH levels recorded at both baseline and at two weeks before the first radiological assessment were included. We calculated the relative increase or decrease in LDH values and assumed that an early increase in LDH could predict disease progression.
Statistical analysis
PFS, DOR, and OS were analyzed using the Kaplan–Meier method. Patients without progression and still alive at the time of analysis were censored. We also calculated ORR, PFS, and OS stratified by baseline LDH in all patients. The log‐rank test was used to compare survival between patients with different baseline LDH levels (≤ ULN vs. > ULN). Pearson's χ2 or Fisher's exact tests were used to analyze the relationship between the baseline LDH level and response. Differences in changes in LDH by response status were illustrated using box plots. Analysis of variance (unpaired t‐test) was used to compare the means between PD and non‐PD groups.
Cox proportional hazards models were applied to determine whether LDH and/or other baseline characteristics were associated with OS. Cox regression was performed as univariate or multivariate analyses. Hazard ratios (HRs) with 95% confidence intervals (CIs) were calculated to quantify the impact of a given factor on survival. P values were calculated based on Wald statistics.
Throughout the analysis, P < 0.05 was considered statistically significant. All analyses were performed using SPSS version 22.0 or GraphPad Prism version 6.01.
Results
Patient characteristics
Forty‐three patients with locally advanced or metastatic ESCC were included. The baseline characteristics are summarized in Table 1. A total of 95.3% of the patients were male, at a median age of 62 (range: 45–75) years. More than half of the patients were diagnosed with well or moderately differentiated ESCC (22/43); 62.8% of patients had received previous radiation therapy; and 55.8% of the patients had previously received at least two lines of chemotherapy (24/43). All but three patients had metastatic disease. Twelve of the 43 patients (27.9%) had an elevated LDH at baseline.
Table 1.
Patient characteristics
| Characteristic | Total (n = 43) | LDH normal (n = 31) | LDH elevated (n = 12) |
|---|---|---|---|
| Age (years) | |||
| Median (range) | 62 (45–75) | 63 (45–75) | 60 (52–72) |
| Gender | |||
| Male | 41 (95.3%) | 30 (96.8%) | 11 (91.7%) |
| Female | 2 (4.7%) | 1 (3.2%) | 1 (8.3%) |
| ECOG PS | |||
| 0 | 36 (83.7%) | 26 (83.9%) | 10 (83.3%) |
| 1 | 7 (16.3%) | 5 (16.1%) | 2 (16.7%) |
| Histologic grade | |||
| Well or moderately differentiated | 22 (51.2%) | 14 (45.2%) | 8 (66.7%) |
| Poorly differentiated | 19 (44.2%) | 15 (48.4%) | 4 (33.3%) |
| Unknown | 2 (4.7%) | 2 (6.5%) | 0 (0) |
| Previous line of chemotherapy | |||
| 1 | 19 (44.2%) | 15 (48.4%) | 4 (33.3%) |
| 2 | 14 (32.6%) | 9 (29.0%) | 5 (41.7%) |
| ≥ 3 | 10 (23.3%) | 7 (22.2%) | 3 (25.0%) |
| Previous surgery | 19 (44.2%) | 14 (45.1%) | 5 (41.7%) |
| Previous radiation | 27 (62.8%) | 21 (48.8%) | 6 (50.0%) |
| Disease stage | |||
| Locally advanced | 3 (7.0%) | 2 (6.5%) | 1 (8.3%) |
| Metastatic | 40 (93.0%) | 29 (93.5%) | 11 (91.7%) |
| Number of organs involved | |||
| 1 | 10 (23.3%) | 8 (25.8%) | 2 (16.7%) |
| 2 | 17 (39.5%) | 13 (41.9%) | 4 (33.3%) |
| ≥3 | 16 (37.2%) | 10 (32.3%) | 6 (50.0%) |
| Baseline LDH | |||
| Median (IQR) | 185 (156–233) | 165 (151–195) | 262 (237–408) |
LDH, lactate dehydrogenase; ECOG PS, Eastern Cooperative Oncology Group performance status; IQR, interquartile range.
Response and survival
As of June 2018, after a median follow‐up of 19.6 months, 8 (18.6%) patients were still alive and 40 of the 43 patients (93.0%) were assessable for response. Early death occurred in three patients before the first CT scan: gastrointestinal hemorrhage in two patients and rapid progression in one. No death was related to camrelizumab treatment. Objective responses to treatments were observed in 11 patients with an ORR of 25.6%, including one CR. The median time to response in these 11 patients was 56 days, with a median DOR of 6.5 (range: 1.8 to ≥ 18.0) months. The median PFS was 2.0 (95% CI 0–4.1) months, and the median OS was 8.0 (95% CI 7.2–8.8) months.
Lactate dehydrogenase (LDH) at baseline
Twelve of the 43 patients (27.9%) had an elevated serum LDH level at baseline. Of these 12 patients, the baseline LDH levels were elevated between the ULN and 2 × ULN in 75% (9/12) of patients and > 2 × ULN in 25% (3/12) of patients. The best overall responses stratified by LDH level at baseline are summarized in Table 2. The ORR was 32.3% for patients with normal serum LDH levels at baseline, whereas a significantly lower response rate (8.3%) was observed in patients with elevated LDH levels (P = 0.02). The median PFS was significantly longer in patients with elevated LDH levels (4.0 [95% CI 1.0–7.0] vs. 1.8 months [95% CI 1.5–2.0], HR 0.39, 95% CI 0.09–0.56; P = 0.002) (Fig 1a). Elevated baseline LDH levels were also associated with worse OS (median: 10.4 vs. 4.2 months, HR 0.22, 95% CI 0.01–0.17; P < 0.0001) (Fig 1b).
Table 2.
The correlation between baseline LDH level and best overall response
| Objective response | LDH normal (n = 31) | LDH elevated (n = 12) | P |
|---|---|---|---|
| CR + PR | 10 (32.3%) | 1 (8.3%) | 0.02* |
| SD | 10 (32.3%) | 1 (8.3%) | |
| PD | 9 (29.0%) | 9 (75.0%) | |
| Unassessable | 2 (6.5%) | 1 (8.3%) |
Tested by Fisher's exact test.
CR, complete remission; LDH, lactate dehydrogenase; PD, progressive disease; PR, partial remission; SD, stable disease.
Figure 1.
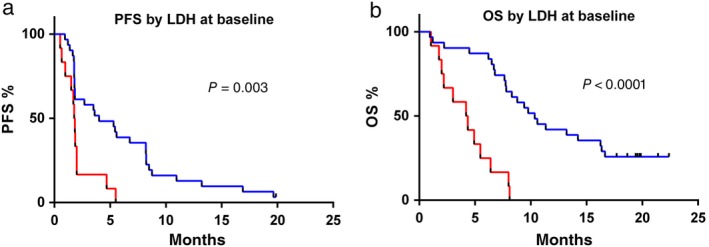
Kaplan–Meier curves of (a) progression‐free survival (PFS) and (b) overall survival (OS) of the entire cohort according to baseline lactate dehydrogenase (LDH) level. ( ) Normal LDH (n = 31), (
) Normal LDH (n = 31), ( ) LDH > ULN (n = 12).
) LDH > ULN (n = 12).
LDH changes and clinical response
Thirty‐nine patients who had serum LDH levels recorded both at baseline and two weeks before the first radiological assessment were included. The correlation between the changes in LDH and response before the first CT assessment is shown in Figure 2. Fourteen of the 21 patients who achieved disease control had a reduction in LDH compared to the baseline value (mean change −5.6%, standard deviation ± 15.7, range: 40.8–24.6%). In contrast, 13 out of the 18 patients with PD had an increase in serum LDH compared to their baseline value (mean change 22.3%, standard deviation ± 41.7, range: 30.0–144.9%). The differences in mean LDH change according to response (non‐PD vs. PD) were statistically significant by unpaired t‐test (P = 0.014).
Figure 2.
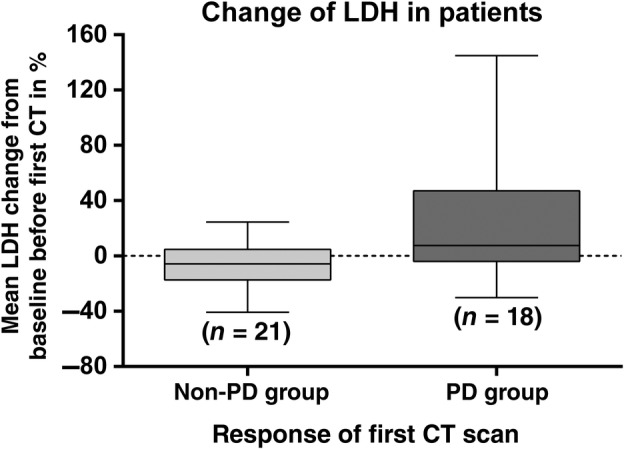
Correlation between changes in lactate dehydrogenase (LDH) level before the first computed tomography (CT) scan and tumor response. PD, progressive disease.
Identification of prognostic factors
In the initial step, we identified six significant or borderline significant factors that were associated with OS in the univariate analysis, including serum LDH, serum CRP, AMC, ECOG PS, number of organs involved, and liver metastasis (Table S1). Subsequently, these factors, along with age and prior lines of chemotherapy, were verified in the multivariate Cox regression model.
According to the Cox regression analysis, an elevated serum LDH level appeared to be the strongest independent factor (HR 0.18; P = 0.001) associated with OS, followed by an elevated CRP (HR 0.27; P = 0.002), involvement of one metastatic organ (HR 0.31; P = 0.045), AMC ≥ 650/μL (HR 0.33; P = 0.021), and ECOG PS = 1 (HR 0.36; P = 0.038), whereas other parameters were not associated with OS (Table 3).
Table 3.
Multivariate analysis of the associations between baseline patient characteristics and survival of patients in the entire cohort (n = 43)
| Parameter | HR | 95% CI | P |
|---|---|---|---|
| Age (< 65 vs. ≥ 65 years) | 0.73 | 0.31–1.75 | 0.483 |
| LDH (≤ ULN vs. > ULN) | 0.18 | 0.07–0.49 | 0.001 |
| CRP (≤ ULN vs. > ULN) | 0.27 | 0.12–0.62 | 0.002 |
| AMC (< 650/μL vs. ≥ 650/μL) | 0.33 | 0.13–0.84 | 0.021 |
| ECOG PS (0 vs. 1) | 0.36 | 0.14–0.94 | 0.038 |
| Number of organs involved (1 vs. ≥ 2) | 0.31 | 0.10–0.98 | 0.045 |
| Liver metastases | 0.70 | 0.28–1‐75 | 0.449 |
| Prior line of chemotherapy (1 vs. ≥ 2) | 0.71 | 0.31–1.60 | 0.405 |
AMC, absolute monocyte count; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio; LDH, lactate dehydrogenase; ULN, upper limit of normal.
Discussion
To our knowledge, this study is the first to demonstrate that a normal LDH level at baseline is associated with better response and OS in patients with ESCC treated with a PD‐1 inhibitors. We also found that an early increase in LDH level before the first radiological assessment might predict disease progression. Additionally, a panel of baseline peripheral blood biomarkers and clinical characteristics were described as independent factors associated with OS.
Nivolumab was the first PD‐1 antibody evaluated in patients with ESCC.11 After a median follow‐up duration of 10.8 months, the results showed an objective response of 17%, and median PFS and OS rates of 1.5 and 10.8 months, respectively. Additionally, pembrolizumab in 18 PD‐L1‐positive ESCC patients demonstrated a promising ORR of 28%, while in 23 patients, the median PFS and OS rates were 1.8 and 7.0 months, respectively, including squamous and adenocarcinoma histology.12 Moreover, we reported the safety and efficacy of treatment with a novel PD‐1 antibody, camrelizumab, from a phase I study.13 Continued follow‐up of our ESCC cohort verified encouraging ORRs and PFS, consistent with the results of previous reports, while the OS was different, mainly as a result of the variance in patient selection.11, 12
The durable responses observed in our study, as well as in other trials of ESCC patients treated with ICIs, are encouraging. However, the clinical benefit is restricted to only a fraction of patients, and biomarkers for both response and survival are under exploration. We previously reported that high PD‐L1 expression, mutation load, and potential mutation‐associated neoantigen count are associated with a better response.13 In the KEYNOTE‐028 trial, six‐gene interferon‐γ gene expression signature analysis indicated that higher interferon‐γ composite scores may predict delayed progression and an increased response.12 These preliminary results require further verification and are not readily applicable in real‐world clinical practice.
Changes in serum LDH at baseline and during treatment have been explored as a marker of prognosis in patients with advanced melanoma treated by ipilimumab, pembrolizumab, or nivolumab.15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26 Most trials have revealed the potential association between normal baseline LDH levels and better response or prolonged survival, except for one study in which the correlation was not statistically significant in patients treated with pembrolizumab.27 However, evidence regarding ESCC is lacking. A previous retrospective analysis of 906 patients demonstrated that a high LDH level was associated with shorter survival, a more advanced stage, and more distant metastasis in the era of chemotherapy.28 Our findings suggest a similar predictive role of baseline LDH in advanced ESCC patients treated with ICIs. An elevated LDH level is indicative of high tumor load and cell growth. Thus, we may infer that ESCC patients with lower tumor burdens or fewer metastases are more likely to benefit from anti‐PD‐1 therapy, whether in terms of response rate or long‐term survival. In clinical practice, a serum LDH test would be helpful to select patients before initiating ICI therapy, and has the advantages of lower expense and rapid results. In addition, by monitoring LDH levels during treatment, we found an association between post‐treatment increases in LDH and poor response. This correlation is of clinical value, as an early and marked increase in LDH level is suggestive of a radiological assessment ahead of schedule. In this case, alternative systemic therapy could be considered in patients with confirmed disease progression before further deterioration of their condition.
In our multivariate analysis, elevated LDH was the strongest independent factor of poor prognosis, which further verified the predictive value of LDH in ESCC before ICI treatment. Increased CRP also appeared to function as a poor prognostic factor, which was consistent with findings in melanoma patients treated with ipilimumab and interleukin‐2‐based immunotherapy.15, 29 A possible explanation is the increased systemic inflammation caused by the high tumor burden.26, 30, 31 However, the CRP level also reflects most forms of inflammatory conditions, such as infections, as a nonspecific acute phase response. The potential value of lymphocytes, such as the absolute/relative lymphocyte count and neutrophil‐to‐lymphocyte ratio as biomarkers for response to immunotherapy, has been explored in several studies,15, 19, 20, 21, 22, 23, 26, 30, 32, 33, 34, 35 but a significant association with OS was not observed in our analysis (Table S1). These results suggest that peripheral absolute/relative lymphocyte count may not directly reflect the level of intratumoral or intrastromal lymphocyte infiltration, although previous studies have established tumor‐infiltrating lymphocytes as a predictive marker for prognosis in a number of solid tumors.36, 37, 38
We are aware of the major limitations of the present study. There is the possibility of patient selection bias and heterogeneity because of the relatively small number of patients in our sample.
In conclusion, our study revealed that LDH serves as a potential marker for response and a powerful independent factor for survival in these patients. A normal LDH level at baseline and the change of LDH level during treatment correlate with the response or progression to camrelizumab therapy. In addition, our findings show that several baseline parameters used in clinical practice are independently associated with OS. Further prospective randomized trials are warranted to confirm our results.
Disclosure
No authors report any conflict of interest.
Supporting information
Supplementary Table S1. Univariate Cox regression analyses of clinical and laboratory parameters.
Acknowledgments
This study was partially supported by the National Key Basic Research Program of China (973 program, No. 2015CB553902).
This study was funded by Jiangsu Hengrui Medicine Co. Ltd., who also provided the study drug.
Contributor Information
Binghe Xu, Email: xubinghe@csco.org.cn.
Jing Huang, Email: huangjingwg@163.com.
References
- 1. Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet 2013; 381: 400–12. [DOI] [PubMed] [Google Scholar]
- 2. Chen W, Zheng R, Baade PD et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016; 66: 115–32. [DOI] [PubMed] [Google Scholar]
- 3. Dawsey SM, Lewin KJ, Wang GQ et al. Squamous esophageal histology and subsequent risk of squamous cell carcinoma of the esophagus. A prospective follow‐up study from Linxian, China. Cancer 1994; 74: 1686–92. [DOI] [PubMed] [Google Scholar]
- 4. Huang Q, Fang DC, Yu CG, Zhang J, Chen MH. Barrett's esophagus‐related diseases remain uncommon in China. J Dig Dis 2011; 12: 420–7. [DOI] [PubMed] [Google Scholar]
- 5. Lin Y, Totsuka Y, He Y et al. Epidemiology of esophageal cancer in Japan and China. J Epidemiol 2013; 23: 233–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huang J, Fan Q, Lu P et al. Icotinib in patients with pretreated advanced esophageal squamous cell carcinoma with EGFR overexpression or EGFR gene amplification: A single‐arm, multicenter phase 2 study. J Thorac Oncol 2016; 11: 910–7. [DOI] [PubMed] [Google Scholar]
- 7. Huang J, Zhou Y, Zhang H et al. A phase II study of biweekly paclitaxel and cisplatin chemotherapy for recurrent or metastatic esophageal squamous cell carcinoma: ERCC1 expression predicts response to chemotherapy. Med Oncol 2013; 30: 343. [DOI] [PubMed] [Google Scholar]
- 8. Ohashi S, Miyamoto S, Kikuchi O, Goto T, Amanuma Y, Muto M. Recent advances from basic and clinical studies of esophageal squamous cell carcinoma. Gastroenterology 2015; 149: 1700–15. [DOI] [PubMed] [Google Scholar]
- 9. Wang X, Wang X, Huang J. Irinotecan plus fluorouracil‐based regimen as second or third‐line chemotherapy for recurrent or metastatic esophageal squamous cell carcinoma. Thorac Cancer 2016; 7: 246–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gong J, Chehrazi‐Raffle A, Reddi S, Salgia R. Development of PD‐1 and PD‐L1 inhibitors as a form of cancer immunotherapy: A comprehensive review of registration trials and future considerations. J Immunother Cancer 2018; 6: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kudo T, Hamamoto Y, Kato K et al. Nivolumab treatment for oesophageal squamous‐cell carcinoma: An open‐label, multicentre, phase 2 trial. Lancet Oncol 2017; 18: 631–9. [DOI] [PubMed] [Google Scholar]
- 12. Doi T, Piha‐Paul SA, Jalal SI et al. Safety and antitumor activity of the anti‐programmed death‐1 antibody pembrolizumab in patients with advanced esophageal carcinoma. J Clin Oncol 2018; 36: 61–7. [DOI] [PubMed] [Google Scholar]
- 13. Huang J, Xu B, Mo H et al. Safety, activity, and biomarkers of SHR‐1210, an anti‐PD‐1 antibody, for patients with advanced esophageal carcinoma. Clin Cancer Res 2018; 24: 1296–304. [DOI] [PubMed] [Google Scholar]
- 14. Deme D, Telekes A. Prognostic importance of lactate dehydrogenase (LDH) in oncology. Orv Hetil 2017. (In Hungarian.); 158: 1977–88. [DOI] [PubMed] [Google Scholar]
- 15. Simeone E, Gentilcore G, Giannarelli D et al. Immunological and biological changes during ipilimumab treatment and their potential correlation with clinical response and survival in patients with advanced melanoma. Cancer Immunol Immunother 2014; 63: 675–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Valpione S, Martinoli C, Fava P et al. Personalised medicine: Development and external validation of a prognostic model for metastatic melanoma patients treated with ipilimumab. Eur J Cancer 2015; 51: 2086–94. [DOI] [PubMed] [Google Scholar]
- 17. Collins GS, Le Manach Y. Small data sets to develop and validate prognostic models are problematic. Eur J Cancer 2016; 54: 167–8. [DOI] [PubMed] [Google Scholar]
- 18. Diem S, Schmid S, Krapf M et al. Neutrophil‐to‐lymphocyte ratio (NLR) and platelet‐to‐lymphocyte ratio (PLR) as prognostic markers in patients with non‐small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer 2017; 111: 176–81. [DOI] [PubMed] [Google Scholar]
- 19. Khoja L, Atenafu EG, Templeton A et al. The full blood count as a biomarker of outcome and toxicity in ipilimumab‐treated cutaneous metastatic melanoma. Cancer Med 2016; 5: 2792–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Weide B, Martens A, Hassel JC et al. Baseline biomarkers for outcome of melanoma patients treated with pembrolizumab. Clin Cancer Res 2016; 22: 5487–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zaragoza J, Caille A, Beneton N et al. High neutrophil to lymphocyte ratio measured before starting ipilimumab treatment is associated with reduced overall survival in patients with melanoma. Br J Dermatol 2016; 174: 146–51. [DOI] [PubMed] [Google Scholar]
- 22. Martens A, Wistuba‐Hamprecht K, Geukes Foppen M et al. Baseline peripheral blood biomarkers associated with clinical outcome of advanced melanoma patients treated with ipilimumab. Clin Cancer Res 2016a; 22: 2908–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Delyon J, Mateus C, Lefeuvre D et al. Experience in daily practice with ipilimumab for the treatment of patients with metastatic melanoma: An early increase in lymphocyte and eosinophil counts is associated with improved survival. Ann Oncol 2013; 24: 1697–703. [DOI] [PubMed] [Google Scholar]
- 24. Dick J, Lang N, Slynko A et al. Use of LDH and autoimmune side effects to predict response to ipilimumab treatment. Immunotherapy 2016; 8: 1033–44. [DOI] [PubMed] [Google Scholar]
- 25. Kelderman S, Heemskerk B, van Tinteren H et al. Lactate dehydrogenase as a selection criterion for ipilimumab treatment in metastatic melanoma. Cancer Immunol Immunother 2014; 63: 449–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nakamura Y, Kitano S, Takahashi A et al. Nivolumab for advanced melanoma: Pretreatment prognostic factors and early outcome markers during therapy. Oncotarget 2016; 7: 77404–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ribas A, Puzanov I, Dummer R et al. Pembrolizumab versus investigator‐choice chemotherapy for ipilimumab‐refractory melanoma (KEYNOTE‐002): A randomised, controlled, phase 2 trial. Lancet Oncol 2015; 16: 908–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wei XL, Zhang DS, He MM et al. The predictive value of alkaline phosphatase and lactate dehydrogenase for overall survival in patients with esophageal squamous cell carcinoma. Tumour Biol 2016; 37: 1879–87. [DOI] [PubMed] [Google Scholar]
- 29. Tartour E, Blay JY, Dorval T et al. Predictors of clinical response to interleukin‐2‐based immunotherapy in melanoma patients: A French multiinstitutional study. J Clin Oncol 1996; 14: 1697–703. [DOI] [PubMed] [Google Scholar]
- 30. Ku GY, Yuan J, Page DB et al. Single‐institution experience with ipilimumab in advanced melanoma patients in the compassionate use setting: Lymphocyte count after 2 doses correlates with survival. Cancer 2010; 116: 1767–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wilgenhof S, Du Four S, Vandenbroucke F et al. Single‐center experience with ipilimumab in an expanded access program for patients with pretreated advanced melanoma. J Immunother 2013; 36: 215–22. [DOI] [PubMed] [Google Scholar]
- 32. Ferrucci PF, Gandini S, Battaglia A et al. Baseline neutrophil‐to‐lymphocyte ratio is associated with outcome of ipilimumab‐treated metastatic melanoma patients. Br J Cancer 2015; 112: 1904–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bagley SJ, Kothari S, Aggarwal C et al. Pretreatment neutrophil‐to‐lymphocyte ratio as a marker of outcomes in nivolumab‐treated patients with advanced non‐small‐cell lung cancer. Lung Cancer 2017; 106: 1–7. [DOI] [PubMed] [Google Scholar]
- 34. Bjoern J, Juul Nitschke N, Zeeberg Iversen T, Schmidt H, Fode K, Svane IM. Immunological correlates of treatment and response in stage IV malignant melanoma patients treated with ipilimumab. Oncoimmunology 2016; 5: e1100788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Martens A, Wistuba‐Hamprecht K, Yuan J et al. Increases in absolute lymphocytes and circulating CD4+ and CD8+ T cells are associated with positive clinical outcome of melanoma patients treated with ipilimumab. Clin Cancer Res 2016b; 22: 4848–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pages F, Berger A, Camus M et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med 2005; 353: 2654–66. [DOI] [PubMed] [Google Scholar]
- 37. Badoual C, Hans S, Rodriguez J et al. Prognostic value of tumor‐infiltrating CD4+ T‐cell subpopulations in head and neck cancers. Clin Cancer Res 2006; 12: 465–72. [DOI] [PubMed] [Google Scholar]
- 38. Piersma SJ, Jordanova ES, van Poelgeest MI et al. High number of intraepithelial CD8+ tumor‐infiltrating lymphocytes is associated with the absence of lymph node metastases in patients with large early‐stage cervical cancer. Cancer Res 2007; 67: 354–61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1. Univariate Cox regression analyses of clinical and laboratory parameters.


