Abstract
Purposes
To investigate the value of prognostic nutritional index (PNI) in patients with upper tract urothelial carcinoma (UTUC) who underwent radical nephroureterectomy (RNU).
Patients and methods
A total of 717 patients were included in our study from 2003 to 2016. PNI was calculated as 10 × serum albumin level (g/dL) + 0.005 × total lymphocyte count (per mm3). Kaplan‐Meier analysis and Cox regression models were adapted to analyze the value of PNI on survival outcomes.
Results
The cutoff value of PNI was set as 46.91 and 298 patients (47.6%) had PNI <46.91. The median follow‐up was 50 months. The results suggested that low PNI was significantly associated with worse pathologic features (all P < 0.001). Multivariable Cox regression analysis revealed that PNI < 46.91 was an independent predictor of poor overall survival (Hazard ratios [HR] = 1.777, 95% CI = 1.383‐2.284, P < 0.001), cancer‐specific survival (HR = 1.850, 95% CI = 1.399‐2.445, P < 0.001), and recurrence‐free survival (HR = 1.554, 95% CI = 1.229‐1.964, P < 0.001).
Conclusions
Low preoperative PNI was associated with worse survival outcomes in patients with UTUC. PNI could be an easily assessed blood‐based biomarker to predict the prognosis in patients with UTUC treated with RNU.
Keywords: albumin, lymphocyte, prognostic nutritional index, radical nephroureterectomy, upper tract urothelial carcinoma
1. INTRODUCTION
Upper tract urothelial carcinoma (UTUC) is a relatively rare but potentially fatal disease, which occurs in the pyelocaliceal cavities or ureter. It accounts for 5%‐10% of urothelial carcinomas.1 Although the radical nephroureterectomy (RNU) with bladder cuff excision has been considered as the standard treatment for the patients with UTUCs, the prognosis remains poor with a potential of intravesical recurrence and distant metastasis.2 Despite the use of adjuvant chemotherapy, the overall survival (OS) of the patients has not been improved because of the complications.3, 4 Therefore, the identification of the prognostic factors is needed to improve therapies.
Till now, many preoperative and postoperative prognostic factors of UTUC have been indicated,5, 6 such as lymphovascular invasion (LVI), tumor stage, tumor grade, tumor size, and lymph node invasion,7, 8 which can be used to predict prognosis and adapt the treatment for the patients of UTUC. However, there are limited data about preoperative prognostic factor in UTUC. Recently, amounting evidence has suggested that patients' nutritional and immunologic conditions could influence the postoperative outcomes of malignant tumors, like breast cancer,9 nonsmall cell lung cancer,10 and colorectal cancer.11
The prognostic nutritional index (PNI), which was calculated based on serum albumin levels and total lymphocyte count, was first reported by Buzby and colleagues in 1980.12 To date, many studies have proved that PNI is a significant indicator for prognosis in patients with several malignancies, but the prognostic value of PNI has been poorly investigated in UTUC. Therefore, our study was designed to identify the impact of PNI on the survival and pathologic outcomes of patients with UTUC after RNU.
2. PATIENTS AND METHODS
2.1. Patient selection
A total of 806 patients with UTUC who underwent RNU from our institution were retrieved between January 2003 and December 2016. Patients with missing PNI data (n = 23), history of receiving preoperative chemotherapy or radiotherapy (n = 21), presence of inflammatory condition (n = 17), as well as those who were withdrawn within 3 months (n = 28) were excluded. Finally, 717 patients were included in the analyses. RNU was performed as standard procedure including the dissection of kidney with the entire part of ureter, and the bladder cuff resection. Lymphadenectomy was performed in the patients with enlarged lymph nodes which were indicated by preoperative radiology or intraoperative inspection.
2.2. Clinical and pathologic evaluation
Clinical features including patients' age, gender, surgical approach, smoking history, hydronephrosis, tumor size, and tumor side. Tumor stage was evaluated by the TNM classification system13 and tumor grade was assessed on the basis of the 1998 WHO consensus classification.14 LVI, multifocality, tumor architecture, and surgical margin status were reported by experienced urologic pathologists. The PNI data were extracted through the laboratory examination reports before surgery, which was calculated as 10 × serum albumin level (g/dL) + 0.005 × total lymphocyte count (per mm3).15
2.3. Follow‐up
Patients were assessed every 3 months for the first year and every 6 months for the second and third year after RNU. Then annually thereafter. Routine check‐ups included blood laboratory tests (blood routine examination, liver, and renal functions examination), medical history, cystoscopy, and imaging (chest/abdomen CT/MRI, carried out every year or if clinically indicated). Duration of follow‐up ranged from the date of operation to the latest follow‐up or death, which was defined as cancer related to the tumor or not.
2.4. Statistical analysis
All the patients were divided into two groups: patients with PNI ≥46.91 and patients with PNI <46.91. The cutoff value of PNI was defined as 46.91 according to the receiver operating characteristic (ROC) curves as well as Youden Index.16, 17 Student’s t test and chi‐squared test were adapted to analyze the continuous and categorical variables, respectively. Kaplan‐Meier curves were used to calculate cancer‐specific survival (CSS), recurrence‐free survival (RFS), and OS. The differences were assessed by using the log‐rank test. Univariable and multivariable Cox regression models were conducted to evaluate the risk factors for CSS, RFS, and OS, and those with P < 0.1 in the univariable model were accepted into the multivariable analyses. The multivariable Cox regression analysis was adjusted for tumor stage, tumor grade, tumor size, tumor architecture, surgical margin status, concomitant variant histology (CVH), lymph node status, LVI status, and PNI. Hazard ratios (HRs) were used to evaluate the strength of the variables with 95% CIs. The result of P < 0.05 was defined as statistical significance. All the analyses were conducted using SPSS 22.0 (IBM SPSS, Chicago, IL).
3. RESULTS
3.1. Characteristics of included patients
The characteristics of patients with UTUC in our study are presented in Table 1. Of all the 717 patients included, 298 were in PNI <46.91 group and 419 were in PNI ≥46.91 group. The cutoff of 46.91 was calculated by using the ROC curves (Figure 1). The median follow‐up duration was 50 months (interquartile range 28‐78 months). For the included patients, 408 (56.9%) were men and 309 (43.1%) were women. Four hundred and eighty‐four (67.5%) patients underwent open RNU and the remaining 233 (32.5%) patients underwent laparoscopic RNU. Among the patients, 205 (28.6%) had the tumor in the ureter, 385 (53.7%) had the tumor in the renal pelvis, and 127 (17.7%) had multifocal lesions. Pathological T stage was pTis/Ta/T1 in 221 cases (30.8%), pT2 in 145 (20.2%), pT3 in 248 (34.6%), and pT4 in 103 (14.4%). 71 (9.9%) patients were diagnosed with positive lymph nodes.
Table 1.
Demographics and clinicopathological characteristics of patients with urinary tract urothelial carcinoma included in present study
| Characteristic | Total | PNI <46.91 (n = 298, 47.6%) | PNI ≥ 46.91 (n = 419, 52.4%) | P |
|---|---|---|---|---|
| Gender (male vs female) | 408/309 | 172/126 | 236/183 | 0.710 |
| Age (>67 vs <67 years) | 354/363 | 160/138 | 194/225 | 0.051 |
| Body mass index (≥25 vs <25 kg/m2) | 188/529 | 70/228 | 118/301 | 0.161 |
| Smoking history (yes vs no) | 204/513 | 89/209 | 115/304 | 0.479 |
| Tumor side (right vs left) | 350/367 | 154/144 | 196/223 | 0.196 |
| Surgical approach, n (%) | 0.646 | |||
| Open RNU | 484 (67.5) | 204 (68.5) | 280 (66.8) | |
| Laparoscopic RNU | 233 (32.5) | 94 (31.5) | 139 (33.2) | |
| Hydronephrosis (Yes vs No) | 447/270 | 177/121 | 270/149 | 0.170 |
| Tumor location, n (%) | 0.013 | |||
| Pelvicalyceal | 385 (53.7) | 169 (56.7) | 216 (51.6) | |
| Ureteric | 205 (28.6) | 69 (23.2) | 136 (32.5) | |
| Both | 127 (17.7) | 60 (20.1) | 67 (16.0) | |
| Tumor grade (High vs Low) | 528/189 | 239/59 | 289/130 | 0.001 |
| Tumor stage, n (%) | <0.001 | |||
| Tis, Ta, T1 | 221 (30.8) | 74 (24.8) | 147 (35.1) | |
| T2 | 145 (20.2) | 56 (18.8) | 89 (21.2) | |
| T3 | 248 (34.6) | 107 (35.9) | 141 (33.7) | |
| T4 | 103 (14.4) | 61 (20.5) | 42 (10.0) | |
| Lymph node status, n (%) | 0.434 | |||
| pN0 | 90 (12.6) | 41 (13.8) | 49 (11.7) | |
| pNx | 556 (77.5) | 224 (75.2) | 332 (79.2) | |
| pN+ | 71 (9.9) | 33 (11.1) | 38 (9.1) | |
| LVI (positive vs negative) | 107/610 | 54/244 | 53/366 | 0.043 |
| Tumor size (>3 vs ≤3 cm) | 488/229 | 207/91 | 281/138 | 0.497 |
| Surgical margin status (positive vs negative) | 58/659 | 26/272 | 32/387 | 0.599 |
| Multifocality (present vs absent) | 119/598 | 45/253 | 74/345 | 0.364 |
| Sessile vs papillary | 492/225 | 223/75 | 269/150 | 0.002 |
| CVH (with vs without) | 165/552 | 80/218 | 85/334 | 0.040 |
| Bladder cancer status, n (%) | 0.930 | |||
| No | 616 (85.9) | 255 (85.6) | 361 (86.2) | |
| Previous | 22 (3.1) | 10 (3.4) | 12 (2.9) | |
| Concomitant | 79 (11.0) | 33 (11.1) | 46 (11.0) | |
| Adjuvant therapy (yes vs no) | 291/426 | 117/181 | 174/245 | 0.543 |
| Serum albumin (g/L) | 39.74 ± 5.03 | 35.53 ± 4.36 | 42.74 ± 2.85 | <0.001 |
| Lymphocyte count (109) | 1.73 ± 6.51 | 1.12 ± 0.43 | 2.17 ± 8.49 | 0.184 |
Abbreviations: RNU, radical nephroureterectomy; LVI, lymphovascular invasion; CVH, concomitant variant histology.
Figure 1.
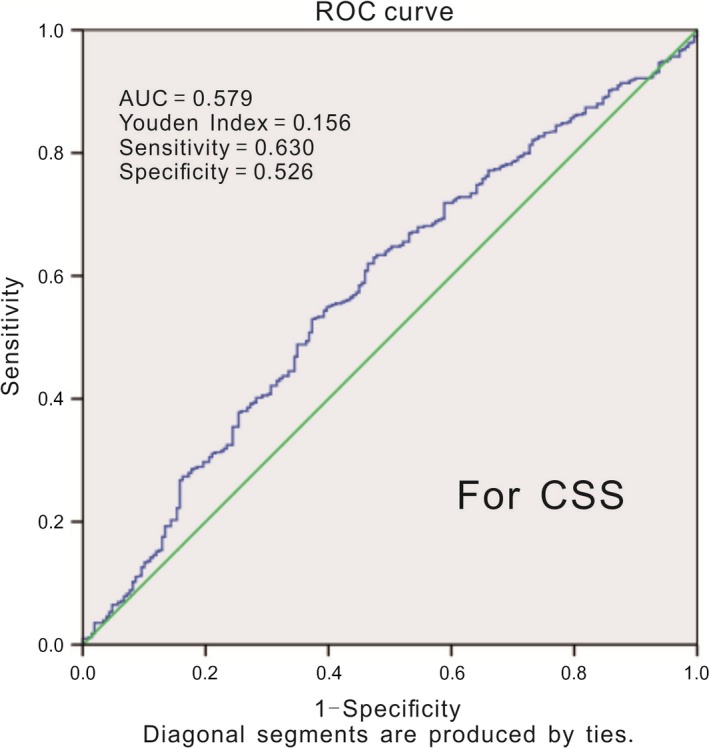
ROC curve of PNI of RFS in patients with UTUC and the cutoff of PNI was 46.91, with a sensitivity of 63% and a specificity of 52.6%
3.2. Low PNI (<46.91) independently predicted poor OS, RFS, and CSS
3.2.1. Low PNI and OS
During the follow‐up, 260 patients (36.3%) died of all causes, and the 3‐year and 5‐year OS were 70.8% and 63.3% for the high PNI group, as well as 53.8% and 40.6% for the low PNI group, respectively. Kaplan‐Meier survival analysis suggested that patients with low PNI had worse OS compared to those with high PNI (log‐rank test, P < 0.001) (Figure 2). Subsequently, our univariable analysis showed that patients with low PNI were statistically significantly correlated with worse OS (HR = 1.90, P < 0.001; Table 2). Meanwhile, multivariable analysis revealed that low PNI was a significant indicator of worse OS (HR = 1.78, P < 0.001; Table 3).
Table 2.
Univariable Cox regression analyses of survival outcomes in patients with UTUC
| Characteristic | Overall survival | Cancer‐specific survival | Recurrence‐free survival | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |
| Gender (male vs female) | 1.141 | 0.894‐1.457 | 0.290 | 1.214 | 0.925‐1.593 | 0.163 | 1.167 | 0.929‐1.466 | 0.184 |
| Age (>67 vs ≤67 years) | 1.020 | 0.800‐1.302 | 0.872 | 0.928 | 0.707‐1.217 | 0.588 | 0.938 | 0.747‐1.177 | 0.581 |
| BMI (≥25 vs < 25 kg/m2) | 0.953 | 0.726‐1.251 | 0.728 | 0.878 | 0.645‐1.195 | 0.409 | 0.978 | 0.760‐1.259 | 0.864 |
| Smoking history (yes vs no) | 0.901 | 0.683‐1.188 | 0.458 | 0.862 | 0.631‐1.177 | 0.350 | 0.884 | 0.682‐1.144 | 0.348 |
| Tumor side (right vs left) | 1.051 | 0.824‐1.341 | 0.687 | 1.089 | 0.830‐1.428 | 0.538 | 1.063 | 0.847‐1.333 | 0.601 |
| Surgical approach (open vs laparoscopic) | 0.724 | 0.541‐0.969 | 0.030 | 0.677 | 0.490‐0.934 | 0.018 | 0.869 | 0.672‐1.124 | 0.285 |
| Hydronephrosis (yes vs no) | 1.342 | 1.035‐1.740 | 0.026 | 1.249 | 0.938‐1.664 | 0.128 | 1.401 | 1.097‐1.788 | 0.007 |
| Tumor location, n (%) | 0.712 | 0.537 | 0.547 | ||||||
| Pelvicalyceal | 1 | Reference | 1 | Reference | 1 | Reference | |||
| Ureteric | 0.958 | 0.719‐1.277 | 0.771 | 1.012 | 0.737‐1.390 | 0.941 | 0.955 | 0.731‐1.249 | 0.738 |
| Both | 1.119 | 0.800‐1.565 | 0.510 | 1.224 | 0.849‐1.763 | 0.279 | 1.154 | 0.846‐1.574 | 0.366 |
| Tumor grade (High vs Low) | 2.832 | 1.991‐4.028 | <0.001 | 3.471 | 2.268‐5.313 | <0.001 | 2.276 | 1.679‐3.085 | <0.001 |
| Tumor stage, n (%) | <0.001 | <0.001 | <0.001 | ||||||
| Tis, Ta, T1 | 1 | Reference | 1 | Reference | 1 | Reference | |||
| T2 vs Tis, Ta, T1 | 1.621 | 1.040‐2.529 | 0.033 | 1.598 | 0.951‐2.686 | 0.077 | 1.499 | 1.011‐2.223 | 0.044 |
| T3 vs Tis, Ta, T1 | 3.279 | 2.266‐4.744 | <0.001 | 3.602 | 2.355‐5.511 | <0.001 | 2.836 | 2.041‐3.941 | <0.001 |
| T4 vs Tis, Ta, T1 | 7.984 | 5.374‐11.861 | <0.001 | 9.293 | 5.938‐14.544 | <0.001 | 6.974 | 4.857‐10.013 | <0.001 |
| Lymph node status, n (%) | <0.001 | <0.001 | <0.001 | ||||||
| pN0 | 1 | Reference | 1 | Reference | 1 | Reference | |||
| pNx vs pN0 | 1.524 | 0.995‐2.336 | 0.053 | 1.516 | 0.928‐2.478 | 0.097 | 1.517 | 1.022‐2.252 | 0.039 |
| pN+ vs pN0 | 5.392 | 3.295‐8.823 | <0.001 | 6.068 | 3.504‐10.509 | <0.001 | 5.496 | 3.467‐8.714 | <0.001 |
| LVI (positive vs negative) | 2.452 | 1.841‐3.265 | <0.001 | 2.666 | 1.950‐3.645 | <0.001 | 2.177 | 1.652‐2.869 | <0.001 |
| Tumor size (>3 vs ≤3 cm) | 1.988 | 1.494‐2.644 | <0.001 | 2.029 | 1.473‐2.796 | <0.001 | 1.862 | 1.433‐2.420 | <0.001 |
| Surgical margin status (positive vs negative) | 2.147 | 1.482‐3.111 | <0.001 | 2.370 | 1.591‐3.532 | <0.001 | 1.898 | 1.321‐2.728 | 0.001 |
| Multifocality (present vs absent) | 0.922 | 0.658‐1.291 | 0.635 | 1.012 | 0.704‐1.456 | 0.947 | 0.947 | 0.694‐1.294 | 0.734 |
| Sessile vs papillary | 2.968 | 2.151‐4.095 | <0.001 | 3.653 | 2.480‐5.380 | <0.001 | 2.536 | 1.906‐3.374 | <0.001 |
| CVH (with vs without) | 2.199 | 1.697‐2.850 | <0.001 | 2.357 | 1.770‐3.138 | <0.001 | 2.019 | 1.578‐2.584 | <0.001 |
| Bladder cancer status, n (%) | 0.136 | 0.203 | 0.376 | ||||||
| No | 1 | Reference | 1 | Reference | 1 | Reference | |||
| Previous | 0.297 | 0.074‐1.198 | 0.088 | 0.345 | 0.085‐1.391 | 0.134 | 0.903 | 0.425‐1.920 | 0.792 |
| Concomitant | 1.198 | 0.835‐1.719 | 0.327 | 1.205 | 0.809‐1.795 | 0.360 | 1.263 | 0.901‐1.770 | 0.176 |
| Adjuvant therapy (Yes vs No) | 0.858 | 0.671‐1.097 | 0.222 | 0.920 | 0.701‐1.209 | 0.551 | 1.090 | 0.868‐1.369 | 0.456 |
| PNI (<46.91 vs ≥46.91) | 1.895 | 1.485‐2.418 | <0.001 | 2.014 | 1.534‐2.643 | <0.001 | 1.635 | 1.302‐2.054 | <0.001 |
Abbreviations: RNU, radical nephroureterectomy; LVI, lymphovascular invasion; CVH, concomitant variant histology.
Table 3.
Multivariable Cox regression analysis of survival outcomes in patients with UTUC
| Characteristic | Overall survival | Cancer‐specific survival | Recurrence‐free survival | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |
| Tumor grade (high vs low) | 1.736 | 1.188‐2.537 | 0.004 | 1.944 | 1.235‐3.061 | 0.004 | 1.483 | 1.066‐2.064 | 0.019 |
| Tumor stage, n (%) | <0.001 | 0.001 | <0.001 | ||||||
| Tis, Ta, T1 | 1 | Reference | 1 | Reference | 1 | Reference | |||
| T2 vs Tis, Ta, T1 | 1.203 | 0.757‐1.914 | 0.434 | 1.101 | 0.641‐1.893 | 0.727 | 1.168 | 0.774‐1.763 | 0.459 |
| T3 vs Tis, Ta, T1 | 1.889 | 1.233‐2.895 | 0.003 | 1.860 | 1.142‐3.028 | 0.013 | 1.834 | 1.256‐2.679 | 0.002 |
| T4 vs Tis, Ta, T1 | 2.837 | 1.710‐4.705 | <0.001 | 2.723 | 1.536‐4.828 | 0.001 | 3.008 | 1.892‐4.780 | <0.001 |
| Lymph node status, n (%) | <0.001 | <0.001 | <0.001 | ||||||
| pN0 | 1 | Reference | 1 | Reference | 1 | Reference | |||
| pNx vs pN0 | 2.005 | 1.297‐3.098 | 0.002 | 1.995 | 1.212‐3.284 | 0.007 | 1.930 | 1.294‐2.880 | 0.001 |
| pN+ vs pN0 | 3.174 | 1.877‐5.367 | <0.001 | 3.460 | 1.933‐6.193 | <0.001 | 3.348 | 2.043‐5.486 | <0.001 |
| LVI (positive vs negative) | 1.079 | 0.783‐1.487 | 0.643 | 1.101 | 0.778‐1.560 | 0.586 | 0.961 | 0.702‐1.315 | 0.801 |
| Tumor size (>3 vs ≤3cm) | 1.717 | 1.273‐2.318 | <0.001 | 1.717 | 1.226‐2.404 | 0.002 | 1.603 | 1.220‐2.106 | 0.001 |
| Surgical margin status (positive vs negative) | 1.126 | 0.762‐1.662 | 0.552 | 1.191 | 0.785‐1.809 | 0.411 | 1.046 | 0.713‐1.533 | 0.819 |
| Sessile vs papillary | 1.520 | 1.043‐2.215 | 0.029 | 1.737 | 1.110‐2.718 | 0.016 | 1.415 | 1.011‐1.979 | 0.043 |
| CVH (with vs without) | 1.392 | 1.060‐1.827 | 0.017 | 1.435 | 1.064‐1.934 | 0.018 | 1.291 | 0.996‐1.674 | 0.054 |
| PNI (<46.91 vs ≥46.91) | 1.777 | 1.383‐2.284 | <0.001 | 1.850 | 1.399‐2.445 | <0.001 | 1.554 | 1.229‐1.964 | <0.001 |
Abbreviations: RNU, radical nephroureterectomy; LVI, lymphovascular invasion; CVH, concomitant variant histology.
3.2.2. Low PNI and RFS
The 3‐year and 5‐year RFS were 63.0% and 55.2% for the high PNI group, and 46.6% and 39.1% for the low PNI group, respectively. The Kaplan‐Meier curve proved that the rate of disease recurrence was higher in the low PNI group than that in the high PNI group (P < 0.001) (Figure 2). Moreover, univariable Cox regression analysis suggested that low PNI was significantly associated with the higher rate of disease recurrence (HR = 1.64, P < 0.001; Table 2). Low PNI was also indicated as a significant indicator of poor RFS through the multivariable Cox regression analysis (HR = 1.55, P < 0.001; Table 3).
Figure 2.
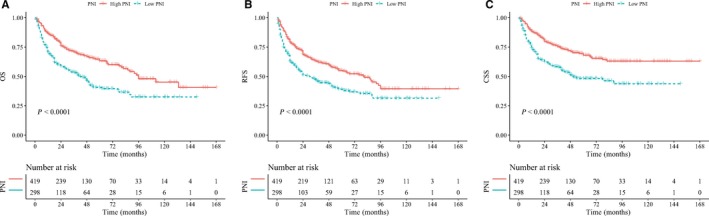
Kaplan‐Meier curves for OS (A), RFS (B), and CSS (C) which were performed according to PNI value for UTUC patients after RNU
3.2.3. Low PNI and CSS
A total of 209 patients (29.1%) died from cancer during follow‐up, and 3‐year and 5‐year CSS were 58.4% and 48.3% for the low PNI group and 75.5% and 68.1% for the high PNI group, respectively. Patients with low PNI had a significant worse CSS rate (P < 0.001) compared to the patients with high PNI according to the Kaplan‐Meier survival curve (Figure 2). Univariable analysis revealed that low PNI was significantly correlated with unfavorable CSS (HR = 2.01, P < 0.001). At the same time, multivariable analysis showed low PNI was a significant prognostic factor for poorer CSS (HR = 1.85, P < 0.001; Table 2).
Furthermore, our analysis also suggested that high tumor grade, tumor stage of T3 or T4, lymph node invasion, CVH, tumor size ≥3 cm, and sessile carcinoma also correlated with poor OS, RFS, and CSS (all P < 0.05; Table 3).
4. DISCUSSION
In our study, we found that PNI was a significant predictor for worse pathologic and oncologic outcomes in patients with UTUC. Comparing with the patients with high PNI, those with low PNI had decreased OS, RFS, and CSS. In the multivariable analysis, we found that PNI was an independent prognostic factor for OS, RFS, and CSS in UTUC.
PNI was first performed as a predictive indicator by Buzby and colleagues,12 who reported a complex formula as: PNI = 158‐0.78 × triceps skinfold (mm) – 16.6 × albumin (g/100 mL) – 5.8 × cutaneous delayed hypersensitivity – 0.20 × transferrin (mg/100 mL). In contrast, Onodera and coworkers15 calculated the PNI based on the total lymphocyte count and the serum albumin levels, which were more easily assessable. In our study, we used the latter method, and the ROC curve analysis suggested the cutoff value of PNI was 46.91. When the PNI was 46.91, the specificity and sensitivity for the 5‐year CSS were 52.6% and 63.0%, respectively.
PNI, a combination of serum albumin and lymphocyte count, has been reported as a useful predictor in several malignancies (eg. Lung cancer,10 breast cancer,9 colorectal cancer,11 and renal cell carcinoma 18). To date, we found that only a single study, which was conducted by Huang et al in 2017,19 had reported the prognostic value of PNI in UTUC. Four hundred and twenty‐five patients were included in their study and the results showed that PNI was a useful independent predictor for patients with UTUCs, which was consistent with our findings. In our analysis, we had a larger sample size and included more indicators, which was helpful for risk prediction in UTUCs.
Recently, a growing body of literature revealed that cancer‐related malnutrition had a negative influence on treatment outcomes, prognosis, and survival.20, 21 It is widely accepted that malnutrition takes a very important place in immune system, but malnutrition influences the immune functions which are fundamental to prevent infection or cancer through the cell‐mediated mechanism or other immune pathways.22, 23 Many studies have showed that preoperative malnutrition has a negative effect on the survival outcomes in patients with urologic carcinomas,20, 24 but few studies are performed to investigate the influence in UTUCs.25, 26
The PNI could be calculated by serum albumin level and lymphocyte count, both of which were routinely assessed and can be easily obtained by urologists before surgery. It is well accepted that lymphocyte plays an important role in cell‐mediated immunity in several cancers. As a result, the lymphocyte count could be a predictor of the survival. Serum albumin is also a simple marker for estimating the protein levels, which is usually used as a predictor of nutritional status. Gupta and colleagues27 investigated the connection between the serum albumin level and the treatment outcomes of patients with various cancers. Therefore, serum albumin levels are useful prognostic factors in malignant tumors.
In our study, the cutoff of PNI was calculated by the ROC curve analysis, and the mean value of PNI was lower than that in patients with renal cell carcinoma (RCC)18 but higher than that in patients with esophageal carcinoma.11 This finding shows that malnutrition is more common in gastrointestinal malignancy compared with UTUCs, and bad appetite and gastric obstruction may be the main reasons. As for the malnutrition in RCC is less common than that in UTUC, the age with the peak incidence in patients with UTUC is older than those with RCC might account for this.
In addition to PNI, the tumor stage, grade, size, architecture, variant histology, and lymph node invasion are proved as independent predictors in UTUC. Many of them have been recommended as prognostic factors by European Association of Urology guidelines and used for risk stratification except PNI.1 It may be because there are scarce studies concerning the prognostic value of PNI in UTUC. Even though the pathologic indicators, such as tumor stage, sessile carcinoma, positive lymph node, and CVH have higher HR than PNI, they just could be obtained via invasive therapy or after surgery. Conversely, we can calculate the PNI easily and rapidly from the preoperative laboratory examination results. Meanwhile, the blood test is cheaper than image examination, which could be used to estimate the tumor size. In addition, if the PNI could be recommended as a useful clinical reference, preoperative therapy such as neoadjuvant chemotherapy could be adopted to improve the outcomes. Therefore, we conducted this study to identify the independent predictors in UTUC, trying to provide more evidence for the risk stratification in UTUC.
A few limitations of our study should be noticed. First, it was a retrospective single center study, so the selection and information bias might not be avoided. Besides, some specific inflammatory indicator like cytokines and CRP were not routinely tested for the patients with UTUC, so we could not estimate their prognostic value. Furthermore, more high‐quality studies with long follow‐up time are still needed to provide more evidences for the prognostic value of PNI in patients with UTUC.
5. CONCLUSION
In conclusion, patients with low PNI had worse OS, CSS, and RFS. PNI is an independent predictor of oncologic outcomes in patients with localized UTUC after RNU. Therefore, we recommended that PNI could be incorporated in the traditional prognostic model, as an important predictor for the patients with UTUC.
CONFLICT OF INTEREST
None declared.
ETHICAL APPROVAL
All procedures conducted in our study involving human participants were consistent with the ethical standards of institutional and/or national research committee and with the Helsinki Declaration in 1964 and its subsequent amendments or similar ethical standards. For this type of research, there is no need for formal consent.
ACKNOWLEDGMENT
This program was supported by the National Key Research and Development Program of China (Grant No. SQ2017YFSF090096), the Prostate Cancer Foundation Young Investigator Award 2013, The National Natural Science Foundation of China (grants 81300627, 81370855, 81702536, 81770756), Programs from Science and Technology Department of Sichuan Province (grants 2018JY0089 and 2017HH0063), and Young Investigator Award of Sichuan University 2017. The funders had no role in designing the study, selecting patients, extracting data, statistical analysis or interpretation, writing or revising paper, or the decision to publish.
Xue W, Tan P, Xu H, Yang L, Wei Q. Impact of the preoperative prognostic nutritional index on survival outcomes in upper tract urothelial carcinomas. Cancer Med. 2019;8:2971–2978. 10.1002/cam4.2161
Wenbin Xue, Ping Tan, and Hang Xu contributed equally to this work.
Contributor Information
Lu Yang, Email: wycleflue@163.com.
Qiang Wei, Email: weiqiang933@126.com.
REFERENCES
- 1. Rouprêt M, Babjuk M, Compérat E, et al. European Association of Urology guidelines on upper urinary tract urothelial carcinoma: 2017 update. Eur Urol. 2018;73(1):111. [DOI] [PubMed] [Google Scholar]
- 2. Verhoest G, Shariat SF, Chromecki TF, et al. Predictive factors of recurrence and survival of upper tract urothelial carcinomas. World J Urol. 2011;29(4):495‐501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kaag MG, O'Malley RL, O'Malley P, et al. Changes in renal function following nephroureterectomy may affect the use of perioperative chemotherapy. Eur Urol. 2010;58(4):581‐587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lane BR, Smith AK, Larson BT, et al. Chronic kidney disease after nephroureterectomy for upper tract urothelial carcinoma and implications for the administration of perioperative chemotherapy. Cancer. 2010;116(12):2967‐2973. [DOI] [PubMed] [Google Scholar]
- 5. Novara G, Matsumoto K, Kassouf W, et al. Prognostic role of lymphovascular invasion in patients with urothelial carcinoma of the upper urinary tract: an international validation study. Eur Urol. 2010;57(6):1064‐1071. [DOI] [PubMed] [Google Scholar]
- 6. Sakano S, Matsuyama H, Kamiryo Y, et al. Risk group stratification based on preoperative factors to predict survival after nephroureterectomy in patients with upper urinary tract urothelial carcinoma. Ann Surg Oncol. 2013;20(13):4389‐4396. [DOI] [PubMed] [Google Scholar]
- 7. Shibing Y, Liangren L, Qiang W, et al. Impact of tumour size on prognosis of upper urinary tract urothelial carcinoma after radical nephroureterectomy: a multi‐institutional analysis of 795 cases. BJU Int. 2016;118(6):902‐910. [DOI] [PubMed] [Google Scholar]
- 8. Lee HY, Li CC, Huang CN, et al. Prognostic significance of lymphovascular invasion in upper urinary tract urothelial carcinoma is influenced by tumor location. Ann Surg Oncol. 2015;22(4):1‐9. [DOI] [PubMed] [Google Scholar]
- 9. Mohri T, Mohri Y, Shigemori T, Takeuchi K, Itoh Y, Kato T. Impact of prognostic nutritional index on long‐term outcomes in patients with breast cancer. World J Surg Oncol. 2016;14(1):1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shunsuke M, Noriyasu U, Koichi F, et al. The significance of the prognostic nutritional index in patients with completely resected non‐small cell lung cancer. PLoS ONE. 2015;10(9):e0136897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tokunaga R, Sakamoto Y, Nakagawa S, et al. Prognostic nutritional index predicts severe complications, recurrence, and poor prognosis in patients with colorectal cancer undergoing primary tumor resection. Dis Colon Rectum. 2015;58(11):1048. [DOI] [PubMed] [Google Scholar]
- 12. Giancarlo Maria L, Francesco B, Angela L, Pierluigi P, Gina R. Recommendations for the use of albumin and immunoglobulins. Blood Transfus. 2009;7(3):216‐234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gospodarowicz MK, Wittekind C, Sobin LH. TNM classification of malignant tumours. J Clin Pathol. 2012;51(1):84. [Google Scholar]
- 14. Epstein JI, Amin MB, Reuter VR, Mostofi FK. The World Health Organization/International Society of Urological Pathology consensus classification of urothelial (transitional cell) neoplasms of the urinary bladder. Bladder Consensus Conference Committee. Am J Surg Pathol. 1998;22(12):1435‐1448. [DOI] [PubMed] [Google Scholar]
- 15. Onodera T, Goseki N, Kosaki G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi. 1984;85(9):1001. [PubMed] [Google Scholar]
- 16. Hyun Hwan S, Hwang GJ, Byong Chang J, et al. Clinical significance of prognosis using the neutrophil‐lymphocyte ratio and erythrocyte sedimentation rate in patients undergoing radical nephroureterectomy for upper urinary tract urothelial carcinoma. BJU Int. 2015;115(4):587‐594. [DOI] [PubMed] [Google Scholar]
- 17. Azuma T, Matayoshi Y, Odani K, et al. Preoperative neutrophil‐lymphocyte ratio as an independent prognostic marker for patients with upper urinary tract urothelial carcinoma. Clin Genitourin Cancer. 2013;11(3):337‐341. [DOI] [PubMed] [Google Scholar]
- 18. Jeon HG, Choi DK, Sung HH, et al. Preoperative prognostic nutritional index is a significant predictor of survival in renal cell carcinoma patients undergoing nephrectomy. Ann Surg Oncol. 2016;23(1):321‐327. [DOI] [PubMed] [Google Scholar]
- 19. Huang J, Yuan Y, Wang Y, et al. Preoperative prognostic nutritional index is a significant predictor of survival in patients with localized upper tract urothelial carcinoma after radical nephroureterectomy. Urol Oncol. 2017;35(12). [DOI] [PubMed] [Google Scholar]
- 20. Morgan TM, Dominic T, Stratton KL, et al. Preoperative nutritional status is an important predictor of survival in patients undergoing surgery for renal cell carcinoma. Eur Urol. 2011;59(6):923‐928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ali E, Zohreh G, Mahdi AM, et al. Nutritional assessment of patients with acute leukemia during induction chemotherapy: association with hospital outcomes. Leuk Lymphoma. 2014;55(8):1743‐1750. [DOI] [PubMed] [Google Scholar]
- 22. Marcos A, Nova E, Montero A. Changes in the immune system are conditioned by nutrition. Eur J Clin Nutr. 2003;57(Suppl 1):S66‐69. [DOI] [PubMed] [Google Scholar]
- 23. Chandra RK. Nutrition and the immune system: an introduction. Am J Clin Nutr. 1997;66(2):460S. [DOI] [PubMed] [Google Scholar]
- 24. Gregg JR, Cookson MS, Phillips S, et al. Effect of preoperative nutritional deficiency on mortality after radical cystectomy for bladder cancer. J Urol. 2011;185(1):90‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ku JH, Kim M, Choi WS, Kwak C, Kim HH. Preoperative serum albumin as a prognostic factor in patients with upper urinary tract urothelial carcinoma. Int Braz J Urol. 2014;40(6):753‐762. [DOI] [PubMed] [Google Scholar]
- 26. Ho Won K, Hae Do J, Yun‐Sok H, et al. Preoperative underweight patients with upper tract urothelial carcinoma survive less after radical nephroureterectomy. J Korean Med Sci. 2015;30(10):1483‐1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: A systematic review of the epidemiological literature. Nutr J. 2010;9(1):69. [DOI] [PMC free article] [PubMed] [Google Scholar]


