Abstract
Background
Chronic inflammation plays a significant role in the occurrence and development of non‐small cell lung cancer (NSCLC). Hydroxysafflor yellow A (HSYA), a chemical compound of the yellow color pigments extracted from the safflower, has been widely used in clinical treatment with positive antioxidation, anti‐inflammation, and antitumor effects. However, the role and underlying mechanisms of HYSA on development and progress in inflammation‐mediated NSCLC are unknown.
Methods
Cell counting kit‐8, colony formation, EdU, cell apoptosis, wound healing, Transwell migration and invasion, and enzyme‐linked immunosorbent assays; flow cytometry; and Western blotting were conducted using human NSCLC cell lines A549 and H1299.
Results
Lipopolysaccharide (LPS) significantly promoted the proliferation and enhanced colony formation of A549 and H1299 cells, while HYSA notably reversed the effects of LPS. HYSA induced apoptosis of LPS‐mediated A549 and H1299 cells in a dose dependent manner; and remarkably suppressed migration, invasion, and epithelial–mesenchymal transition (EMT), significantly regulated production of LPS‐induced inflammation cytokines, and downregulated protein expression of PI3K/Akt/mTOR and ERK/MAPK signaling pathways in LPS‐induced A549 and H1299 cells. Furthermore, PI3K (LY294002) and ERK (SCH772984) inhibitors remarkably inhibited proliferation, migration, invasion, and EMT, and induced apoptosis in LPS‐mediated A549 and H1299 cells. These effects were even more obvious in the presence of HYSA and LY294002 or SCH772984 compared to those of either agent alone.
Conclusion
HYSA suppressed LPS‐mediated proliferation, migration, invasion, and EMT in A549 and H1299 cells by inhibiting the PI3K/Akt/mTOR and ERK/MAPK signaling pathways, indicating that HYSA may be a potential candidate to treat inflammation‐mediated NSCLC.
Keywords: ERK/MAPK, Hydroxysafflor yellow A, lipopolysaccharide, non‐small cell lung cancer, PI3K/AKT/mTOR
Introduction
Primary bronchogenic carcinoma of the lung, referred to as lung cancer, is one of the most serious malignant tumors threatening human life.1, 2 Thus, determining the various methods to diagnose, prevent, monitor, and treat lung cancer are urgent issues worldwide at present. Epidemiological investigation has shown that despite continued research into the prevention, diagnosis, and treatment of lung cancer, the prognosis of lung cancer patients is still not optimistic. The mortality rate of lung cancer patients ranks first among malignant tumors in the world, with a five‐year survival rate of < 15%.3 According to pathological classification, lung cancer can be roughly divided into non‐small cell lung cancer (NSCLC) and small cell lung cancer (SCLC); NSCLC accounts for more than 85% of cases, three quarters of which are diagnosed at middle and advanced stages.4 Currently, the main treatment methods for lung cancer include surgical and non‐surgical treatment; non‐surgical treatment consists of radiotherapy, chemotherapy, and immunity and molecular targeted drugs. However, there are many defects in both individual and comprehensive treatment schemes.5, 6, 7 Therefore, new and targeted drug research is the current focus for the prevention and treatment of lung cancer.
An increasing number of studies have confirmed that chronic inflammation plays an important role in the occurrence and development of tumors.8, 9 The presence of inflammation, particularly chronic and low‐toxic inflammation, is closely related in a number of cancers, including gastric, colon, liver, prostate, and lung cancers.10, 11, 12 Escherichia coli stimulation can promote the invasion and migration of NSCLC cells, suggesting that gram‐negative bacillus transfection plays an essential role in the invasiveness of host NSCLC cells. The host NSCLC transfected with gram‐negative bacillus not only enhances invasion and migration abilities, but also promotes the malignant proliferation of NSCLC cells.13 Lipopolysaccharide (LPS) is a major component from the outer cell membrane of gram‐negative bacillus. LPS serves not only as a physical barrier to keep bacteria from invading the outside environment, but also as a biomarker for the immune system to identify pathogenic bacteria, playing a key role in the inflammatory immune response and endotoxic shock.14, 15, 16 Endotoxins released by bacteria can affect the proliferation of A549 cells in vitro, and LPS can strongly induce NSCLC cell proliferation in many animal models.17, 18, 19
In recent years, with the continuous development of traditional Chinese medicine (TCM), the national investment in TCM research has gradually increased and the anticancer role and underlying mechanisms of TCM have been elucidated to a certain extent. Active ingredients and natural Chinese herbal medicines play an important role in improving immune function, inhibiting cell proliferation and migration, promoting cell apoptosis, alleviating clinical symptoms, alleviating toxicity and the side effects of radiotherapy and chemotherapy, prolonging survival, reducing recurrence, and improving quality of life.20, 21, 22, 23 Many kinds of Chinese medicines or active ingredients have been discovered, such as vincristine and paclitaxel, which are widely used in clinical practice, and have been included in the recommended guidelines for the treatment of NSCLC.24, 25 Hydroxysafflor yellow A (HSYA), a chemical compound of the yellow color pigments extracted from the safflower, has been widely used in clinical treatment. Previous studies have shown that HSYA promotes blood circulation for removing blood stasis and positively affects antioxidant, anti‐inflammatory, and antitumor activities.26 Furthermore, HSYA can induce human gastric carcinoma BGC‐823 cell apoptosis by activating peroxisome proliferator‐activated receptor gamma (PPARγ), and suppress tumor capillary angiogenesis in transplanted human gastric adenocarcinoma BGC‐823 tumors in nude mice.27, 28 HSYA can also suppress adhesion, invasion, migration, and lung metastasis of hepatoma cells via the E‐cadherin/β‐catenin pathway, and inhibit angiogenesis of hepatocellular carcinoma by blocking the ERK/MAPK and NF‐κB signaling pathways in H22 tumor‐bearing mice.29, 30 These data indicate that HSYA plays a significant inhibitory role in tumors. However, relatively little is known concerning the therapeutic function of HYSA in NSCLC mediated with inflammation. Therefore, the present study was designed to explore the antitumor potential of HSYA and investigate the possible signaling pathways involved in NSCLC mediated with inflammation.
Methods
Cell lines and cell culture
Human NSCLC cell lines, including A549 and H1299, were purchased from the American Type Culture Collection (Manassas, VA, USA), and routinely cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 μg/mL streptomycin (Sigma‐Aldrich, St. Louis, MO, USA). Cells were then incubated in a humidified cell incubator maintained with 5% CO2 at 37°C.
Cell counting kit‐8 assay
The viabilities of A549 and H1299 cells were detected by cell counting kit‐8 (CCK‐8, Sigma‐Aldrich, St. Louis, MO, USA) assay. In brief, A549 and H1299 cells at a density of 1 × 104 cells/well were seeded in 96‐well plates. The cells were cultured in a humidified cell incubator maintained with 5% CO2 at 37°C for 24, 48, and 72 hours, respectively, after which 10 μL of CCK‐8 was added to each well and cells were incubated for another two hours at 37°C. The optical density (OD) was recorded at 450 nm using a microplate reader (Dojindo Molecular Technology, Rockville, MD, USA).
Colony formation assay
Colony formation assay was carried out to evaluate the role of HYSA in the proliferative potential of A549 and H1299 induced by LPS. Briefly, A549 and H1299 cells with a density of 1 × 103 cells/well were seeded in six‐well plates and cultured at 37°C with 5% CO2. The medium was replaced with fresh culture medium every two to three days for two weeks. Subsequently, cells were fixed with 4% paraformaldehyde for 20 minutes and stained using 10% crystal violet for 30 minutes.
EdU assay
EdU assay was conducted to assess the role of HYSA in the proliferation of A549 and H1299 cells. Cells were seeded in six‐well plates and incubated for 48 hours after different treatments in a humidified cell incubator maintained with 5% CO2 at 37°C. Following incubation, 100 μL of EdU (50 μM) was added to the culture medium for eight hours and the cells were then fixed with 4% paraformaldehyde for 20 minutes. Triton X‐100 was employed to permeabilize the nuclear membrane, and phosphate buffered saline (PBS) containing 10% goat serum was used for blocking for one hour at 25°C. Finally, cells were stained using a Cell‐hour Light EdU Apollo 488 in vitro Imaging Kit (Life Technologies, New York, NY, USA) according to the manufacturer's recommendations.
Cell apoptosis assay
Cell apoptosis was performed using the Annexin V Apoptosis Detection kit I (BD Biosciences, San Jose, CA USA). Briefly, the treated A549 and H1299 cells were digested with trypsin and washed in cold 1 × PBS twice at 4°C, followed by resuspension of the cell pellet with 300 μL of 1 × binding buffer. Next, 5 μL of Annexin V‐PE was added to the cell suspension for 15 minutes in the dark at room temperature, according to the manufacturer's instructions. Five minutes before flow cytometry analysis, 7‐AAD solution (5 μL) was added in the cell suspension and then 200 μL of 1 × binding buffer was added. FACS Calibur (BD Biosciences) was used to calculate the percentage of apoptotic cells.
Wound healing assay
Wound healing assay was used to assess the cell migration ability of A549 and H1299 cells in vitro. The cells were cultured into six‐well plates and incubated for 24 hours to full confluence. A scratch was then created using a sterile plastic tip, and the cells were incubated for 24 hours at 37°C. The closure of the scratch was analyzed under the microscope and images were captured using an Olympus light microscope (Olympus Corporation, Tokyo, Japan).
Transwell migration and invasion assays
Migration and invasion assays were performed using Transwell chambers (8 μm pore‐size; Corning Co., Tewksbury, MA, USA). In migration assay, A549 and H1299 cells at a density of 5 × 104 cells/well were added into the upper chamber. In invasion assay, Matrigel purchased from BD Biosciences was inoculated into the upper chamber to form a gel at 37°C, and then A549 and H1299 cells were seeded into the upper compartments at a density of 1 × 105 cells/well. For Transwell migration and invasion assays, the lower compartments were filled with 600 μL of medium with 20% FBS. After incubation for 48 hours, cells that had not migrated or invaded were removed from the upper surface while the cells that had migrated or invaded to the lower surface of the membrane were fixed with 4% paraformaldehyde and stained in 10% crystal violet.
Western blotting analysis
The treated A549 and H1299 cells were lysed with radioimmunoprecipitation assay lysis buffer according to the manufacturer's recommended protocol (Vazyme Biotech, Nanjing, China). Proteins (50 μg) were separated by 10% sodium dodecyl sulfate‐polyacrylamide gel electrophoresis and transferred onto polyvinylidene fluoride membranes blocked with 5% milk at room temperature for one hour. The blots were then incubated with primary antibodies at 4°C overnight. The membranes were then incubated with secondary antibodies for one hour at room temperature. Finally, the signals were detected using an electrochemiluminescence detection system and the protein levels were quantified using Image J software. Antibodies in Western blotting were purchased from Cell Signaling Technology (Beverly, MA, USA), including Bax (#5023), Bcl‐2 (#15071), cleaved‐caspase‐3 (#9662), cleaved‐caspase‐9 (#9502), E‐cadherin (#14472), vimentin (#5741), N‐cadherin (#13116), matrix metalloproteinase‐2 (MMP‐2, #40994), MMP‐9 (#13667), p‐phosphoinositide 3‐kinase (p‐PI3K, #4228), PI3K (#4249), p‐Akt (#4060), Akt (#4691), mTOR (#2983), and glyceraldehyde 3‐phosphate dehydrogenase (#5174).
Enzyme‐linked immunosorbent assay
The concentrations of cytokines in cell supernatant were determined by enzyme‐linked immunosorbent assay (ELISA) for human TNF‐α, IL‐6, IL‐1β, and IL‐10 (eBioscience, San Diego, CA, USA) following the manufacturer's instructions. The 96‐well microplates were read using a PowerWave X340 microplate reader (Bio‐Tek, Winooski, VT, USA).
Statistical analysis
All statistical analyses were carried out using SPSS version 19.0 and the data are presented as the mean ± standard deviation from three independent experiments. The difference between two groups of three independent experiments was analyzed by Student's t‐test, and one‐way analysis of variance was used to analyze the difference between more than two groups. For all tests, results were considered significant at P < 0.05.
Results
Effects of HYSA on proliferation in A549 and H1299 cells induced by LPS
Inflammation, especially chronic and low‐toxic inflammation, is closely associated with the development and progress of NSCLC.31 In the present study, CCK‐8 assay was performed to evaluate the role of HYSA in the viabilities of A549 and H1299 cells induced by LPS for 24, 48, and 72 hours, respectively. The results showed that LPS could significantly promote the cell proliferation of A549 and H1299 (Fig 1a). After treatment with HYSA (5, 10, and 20 μM), cell proliferation of A549 and H1299 was obviously suppressed in a dose and time dependent manner compared to the LPS group. Colony formation assay was carried out to determine whether HYSA could inhibit the abilities of colony formation in A549 and H1299 cells induced by LPS. Concentrations of 5, 10, and 20 μM HYSA were added to A549 and H1299 and the cells incubated for two weeks. LPS enhanced colony formation, while HYSA reduced colony formation of A549 and H1299 cells compared to the LPS group (Fig 1b). Moreover, EdU assay was used to detect the effects of HYSA on the proliferation of A549 and H1299 cells induced by LPS. After treatment with different concentrations of HYSA for 48 hours, LPS notably increased the percentage of EdU positive cells in A549 and H1299 compared to the control, while HYSA significantly reversed the effects of LPS on the cell proliferation of A549 and H1299 in a dose dependent manner (Fig 1c). These results suggest that HYSA plays a significant inhibitory role in cell proliferation of A549 and H1299 induced by LPS.
Figure 1.
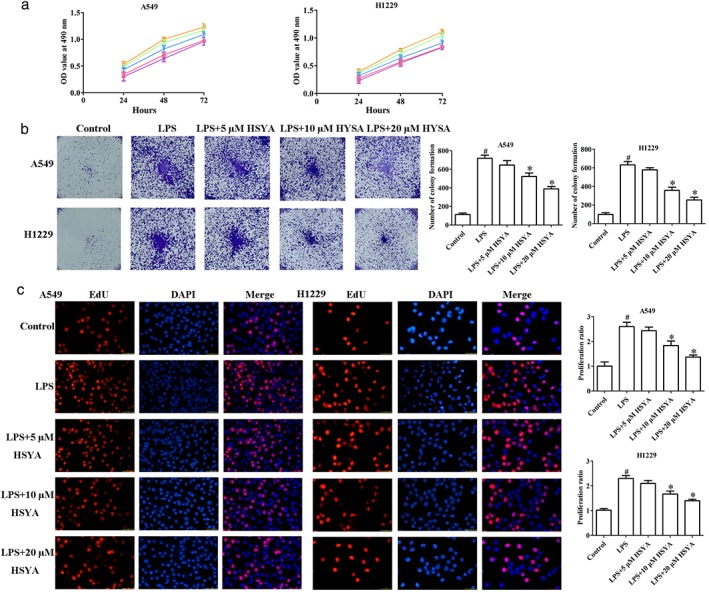
Effects of Hydroxysafflor yellow A (HYSA) on proliferation in A549 and H1299 cells induced by lipopolysaccharide (LPS). (a) The proliferation of LPS‐induced A549 and H1299 cells treated with HYSA (5, 10, and 20 μM) was evaluated at the indicated time points by cell counting kit‐8 assay. (b) The colony formation ability of LPS‐induced A549 and H1299 cells cultured with HYSA (5, 10, and 20 μM) for two weeks was detected by colony formation assay. (c) The percentage of EdU positive cells of LPS‐induced A549 and H1299 cells incubated with HYSA (5, 10, and 20 μM) was examined by EdU assay. OD, optical density. ( ) Control, (
) Control, ( ) LPS, (
) LPS, ( ) LPS + 5 μM HYSA, (
) LPS + 5 μM HYSA, ( ) LPS + 10 μM HYSA, and (
) LPS + 10 μM HYSA, and ( ) LPS + 20 μM HYSA.
) LPS + 20 μM HYSA.
Effects of HYSA on apoptosis in A549 and H1299 cells induced by LPS
To determine the effects of HYSA on the apoptosis of A549 and H1299 cells induced by LPS, flow cytometry with Annexin V‐FITC/propidium iodide staining was performed. After treatment with indicated concentrations of HYSA for 48 hours, the degree of apoptosis in the LPS group was lower than that in the control group, and HYSA could significantly promote the apoptosis of A549 and H1299 cells induced by LPS (Fig 2a). In addition, the levels of apoptosis‐related proteins, including cleaved‐caspase‐3, cleaved‐caspase‐9, Bax, and Bcl‐2, were examined by Western blot. As expected, LPS obviously increased the expression of Bcl‐2 and decreased the levels of cleaved‐caspase‐3, cleaved‐caspase‐9, and Bax compared to the control group, while HYSA could significantly restore the effects of LPS on the levels of apoptosis‐related proteins in A549 and H1299 cells (Fig 2b). These results indicate that HYSA has a key role in promoting the apoptosis of A549 and H1299 cells induced by LPS.
Figure 2.
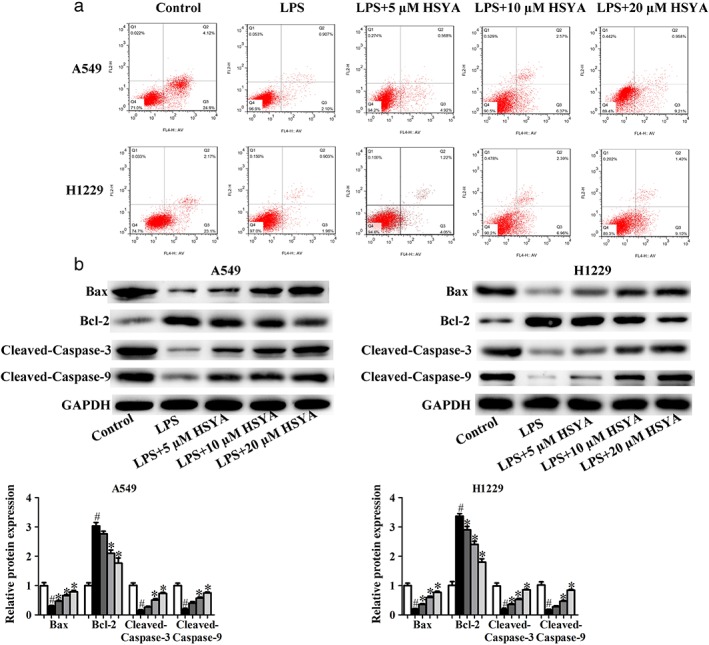
Effects of Hydroxysafflor yellow A (HYSA) on apoptosis in A549 and H1299 cells induced by lipopolysaccharide (LPS). (a) The apoptosis rate of LPS‐induced A549 and H1299 cells treated with HYSA (5, 10, and 20 μM) for 48 hours was analyzed via flow cytometry with Annexin V‐FITC/propidium iodide staining. (b) Bcl‐2, Bax, cleaved‐caspase‐3, and cleaved‐caspase‐9 expression in LPS‐induced A549 and H1299 cells treated with HYSA (5, 10, and 20 μM) for 48 hours was determined by Western blotting assay. The band intensity was quantified by Image J software. GAPDH, glyceraldehyde 3‐phosphate dehydrogenase. ( ) Control, (
) Control, ( ) LPS, (
) LPS, ( ) LPS + 5 μM HYSA, (
) LPS + 5 μM HYSA, ( ) LPS + 10 μM HYSA, and (
) LPS + 10 μM HYSA, and ( ) LPS + 20 μM HYSA.
) LPS + 20 μM HYSA.
Effects of HYSA on epithelial–mesenchymal transition (EMT) in A549 and H1299 cells induced by LPS
Epithelial–mesenchymal transition (EMT) is a biological phenomenon in which cells lose epithelial characteristics and acquire mesenchymal characteristics under certain conditions, and is closely related to in situ infiltration and distant metastasis of multiple tumors. The main molecular characteristics of EMT are the deletion of expression or function of E‐cadherin, Occludin, and other epithelial cell markers, and the upregulation of N‐cadherin, vimentin, and other interstitial cell markers. In this study, E‐cadherin, N‐cadherin, and vimentin were used as monitoring indicators to illustrate the occurrence of EMT.32, 33 Under the stimulation of LPS, E‐cadherin expression was significantly downregulated, and N‐cadherin and vimentin expression were significantly upregulated in A549 and H1299 cells, fully demonstrating that LPS can induce EMT in NSCLC cells (Fig 3). Interestingly, after treatment with HYSA, the expression of E‐cadherin was obviously increased and the levels of N‐cadherin and Vimentin were remarkably decreased in A549 and H1299 cells induced by LPS.
Figure 3.
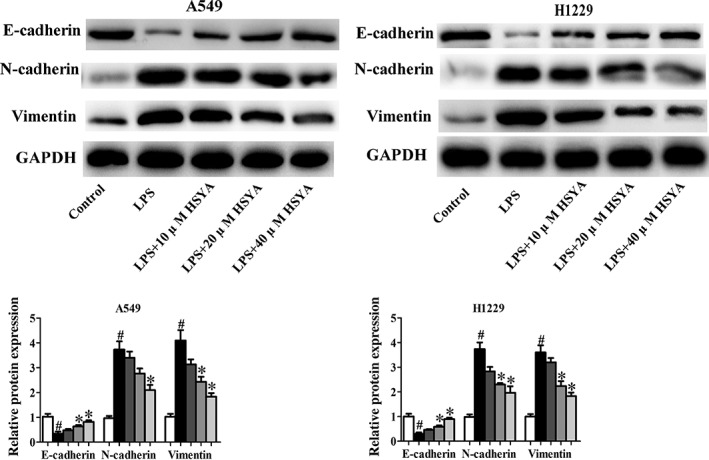
Effects of Hydroxysafflor yellow A (HYSA) on epithelial–mesenchymal transition (EMT) in A549 and H1299 cells induced by lipopolysaccharide (LPS). E‐cadherin, N‐cadherin, and vimentin expression in LPS‐induced A549 and H1299 cells treated with HYSA (5, 10, and 20 μM) for 48 hours was determined by Western blotting assay. The band intensity was quantified by Image J software. ( ) Control, (
) Control, ( ) LPS, (
) LPS, ( ) LPS + 5 μM HYSA, (
) LPS + 5 μM HYSA, ( ) LPS + 10 μM HYSA, and (
) LPS + 10 μM HYSA, and ( ) LPS + 20 μM HYSA.
) LPS + 20 μM HYSA.
Effects of HYSA on migration and invasion in A549 and H1299 cells induced by LPS
Studies have confirmed that LPS can increase the expression of COX‐2, and promote the release of inflammatory factors, such as IL‐8, which can enhance the neovascularization and metastasis of tumors and also upregulate the levels of MMP‐2 and MMP‐9 related to tumor invasion.34, 35 Therefore, LPS can promote the invasion and metastasis of cancer cells. In the present study, wound healing and Transwell migration and invasion assays were performed to evaluate the role of HYSA in migration and invasion in A549 and H1299 cells induced by LPS. As expected, LPS positively promoted migration and invasion compared to the control group, while HYSA significantly restored the effects of LPS on migration and invasion of A549 and H1299 cells in a dose dependent manner (Fig 4a–c). Western blotting analysis was used to determine the levels of metastasis‐related proteins, including MMP‐2 and MMP‐9. LPS obviously upregulated the expression of MMP‐2 and MMP‐9 compared to the control group, while HYSA significantly restored the effects of LPS on metastasis‐related protein expression in A549 and H1299 cells (Fig 4d). These data indicate that HYSA plays an essential role in suppressing the migration and invasion of A549 and H1299 cells induced by LPS.
Figure 4.
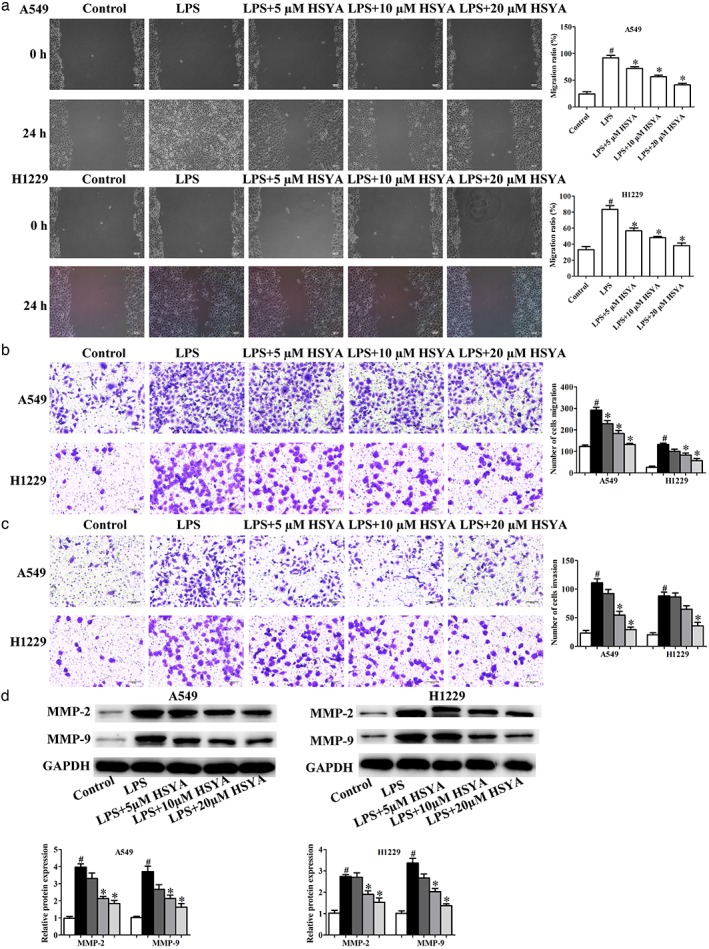
Effects of Hydroxysafflor yellow A (HYSA) on migration and invasion in A549 and H1299 cells induced by lipopolysaccharide (LPS). A549 and H1299 cells induced by LPS were incubated with HYSA (5, 10, and 20 μM) for 48 hours. In LPS‐induced A549 and H1299 cells, the migration abilities were examined by (a) wound healing and (b) transwell migration assays, and (c) the invasion abilities were evaluated by Transwell invasion assay. (d) Matrix metalloproteinase‐2 (MMP‐2) and MMP‐9 protein expression in LPS‐induced A549 and H1299 cells treated with HYSA (5, 10, and 20 μM) for 48 hours was determined by Western blotting assay. The band intensity was quantified by Image J software. GAPDH, glyceraldehyde 3‐phosphate dehydrogenase. ( ) Control, (
) Control, ( ) LPS, (
) LPS, ( ) LPS + 5 μM HYSA, (
) LPS + 5 μM HYSA, ( ) LPS + 10 μM HYSA, and (
) LPS + 10 μM HYSA, and ( ) LPS + 20 μM HYSA.
) LPS + 20 μM HYSA.
Effects of HYSA on production of inflammation cytokines in A549 and H1299 cells induced by LPS
We measured the production of inflammation cytokines in cell supernatant by ELISA, including TNF‐α, IL‐6, IL‐1β, and IL‐10. The results showed that LPS significantly promoted the production of TNF‐α, IL‐6, and IL‐1β in A549 and H1229, and inhibited the level of IL‐10. However, HYSA remarkably reversed the role induced by LPS (Fig 5).
Figure 5.
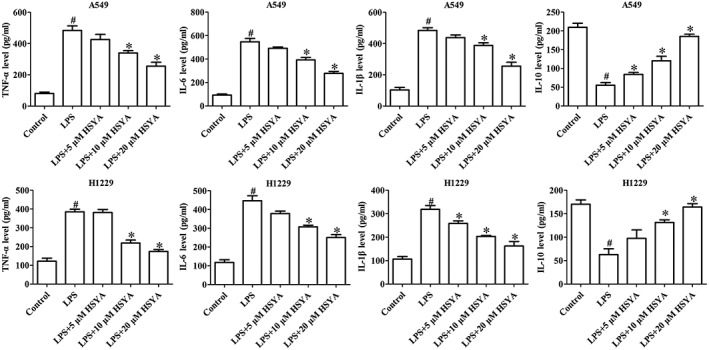
Effects of Hydroxysafflor yellow A (HYSA) on the production of inflammatory cytokines in A549 and H1299 cells induced by lipopolysaccharide (LPS). Enzyme‐linked immunosorbent assay was used to measure the production of inflammatory cytokines in cell supernatant, including TNF‐α, IL‐6, IL‐1β, and IL‐10.
Effects of HYSA on PI3K/Akt/mTOR and ERK/MAPK signaling pathways in A549 and H1299 cells induced by LPS
Advances in cancer research have revealed many signaling pathways closely related to tumorigenesis and development. The PI3K/Akt/mTOR and ERK/MAPK signaling pathways, as important intracellular signal transduction pathways, control the vital cellular biological processes in tumorigenesis and development by influencing the activation of many downstream effector molecules, including apoptosis, transcription, translation, metabolism, angiogenesis, and cell cycle regulation. In recent years, an increasing number of studies have investigated the PI3K/Akt/mTOR and ERK/MAPK signaling pathways in NSCLC.36, 37 However, whether HYSA suppresses the progress and development of NSCLC induced by LPS by regulating the PI3K/Akt/mTOR and ERK/MAPK signaling pathways remains unknown. In this study, Western blotting assay was performed to determine the effects of HYSA on the expression of PI3K/Akt/mTOR and ERK/MAPK signaling pathways related to proteins in A549 and H1299 cells induced by LPS. LPS could significantly increase the levels of PI3K/Akt/mTOR and ERK/MAPK signaling pathways, while HYSA treatment could obviously reverse the effects of LPS on the expression of PI3K/Akt/mTOR and ERK/MAPK signaling pathways related to proteins in A549 and H1299 cells induced by LPS (Fig 6).
Figure 6.
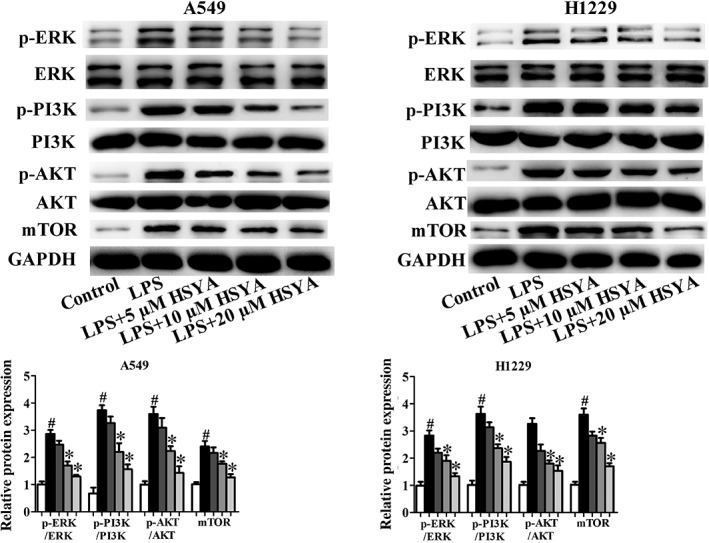
Effects of Hydroxysafflor yellow A (HYSA) on PI3K/Akt/mTOR and ERK/MAPK signaling pathways in A549 and H1299 cells induced by lipopolysaccharide (LPS). Expressions of p‐PI3K, PI3K, p‐Akt, Akt, mTOR, p‐Erk and Erk proteins were determined in LPS‐induced A549 and H1299 cells treated with HYSA (5, 10, and 20 μM) for 48 hours by Western blotting assay. The band intensity was quantified by Image J software ( ) Control, (
) Control, ( ) LPS, (
) LPS, ( ) LPS + 5 μM HYSA, (
) LPS + 5 μM HYSA, ( ) LPS + 10 μM HYSA, and (
) LPS + 10 μM HYSA, and ( ) LPS + 20 μM HYSA.
) LPS + 20 μM HYSA.
HYSA suppressed proliferation and induced apoptosis in LPS‐induced A549 and H1299 cells via PI3K/Akt/mTOR and ERK/MAPK signaling pathways
The results of our study have demonstrated that the levels of PI3K/Akt/mTOR and ERK/MAPK signaling pathways related to proteins in A549 and H1299 cells induced by LPS are significantly suppressed after treatment with HYSA (5, 10, and 20 μM). To further verify the involvement of PI3K/Akt/mTOR and ERK/MAPK signaling pathways in inhibiting proliferation and inducing apoptosis of A549 and H1299 cells induced by LPS, PI3K (LY294002) and ERK (SCH772984) inhibitors were employed to inhibit the PI3K/Akt/mTOR and ERK/MAPK signaling pathways related to proteins, respectively.38, 39 As expected, LY294002 and SCH772984 inhibited colony formation and promoted cell apoptosis of LPS‐induced A549 and H1299 cells. Furthermore, co‐treatment of HYSA and LY294002 or SCH772984 was superior for reducing colony formation and promoting apoptosis compared to either agent alone in LPS‐induced A549 and H1299 cells (Fig 7). These findings suggest that HYSA possibly works through the PI3K/Akt/mTOR and ERK/MAPK signaling pathways to inhibit proliferation and induce apoptosis of A549 and H1299 cells induced by LPS.
Figure 7.
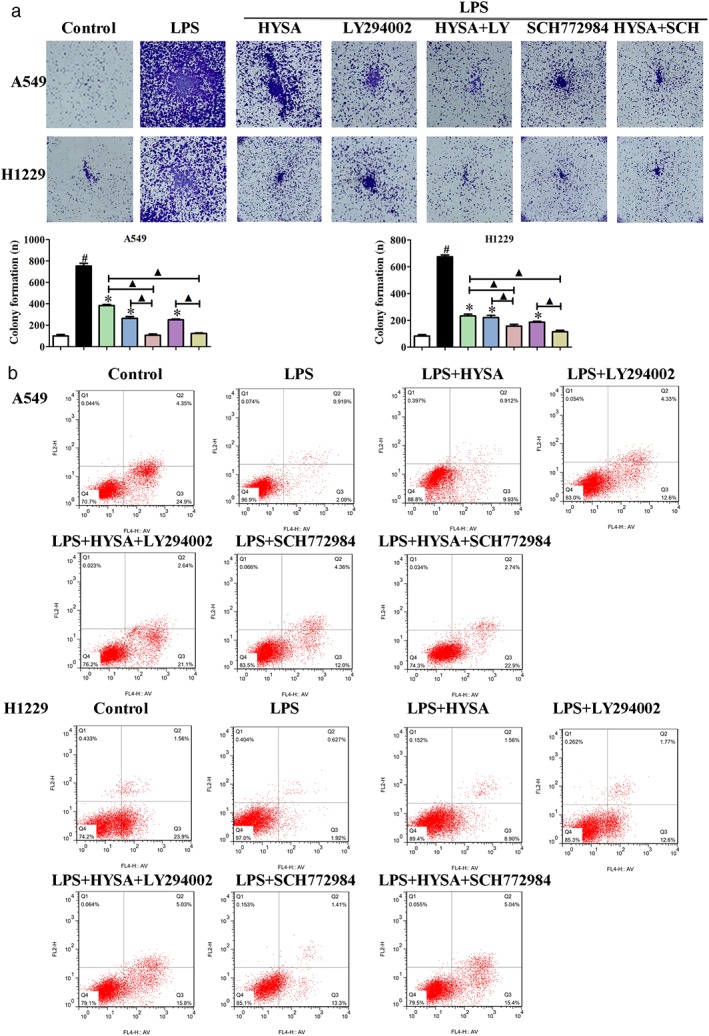
Hydroxysafflor yellow A (HYSA) suppressed proliferation and induced apoptosis in lipopolysaccharide (LPS)‐induced A549 and H1299 cells via the PI3K/Akt/mTOR and ERK/MAPK signaling pathways. (a) The colony formation ability of LPS‐induced A549 and H1299 cells cultured with HYSA and PI3K (LY294002) or ERK (SCH772984) inhibitors for two weeks was detected by colony formation assay. ( ) Control, (
) Control, ( ) LPS, (
) LPS, ( ) LPS + HYSA, (
) LPS + HYSA, ( ) LPS + LY294002, (
) LPS + LY294002, ( ) LPS + HYSA + LY294002, (
) LPS + HYSA + LY294002, ( ) LPS + SCH772984, and (
) LPS + SCH772984, and ( ) LPS + HYSA + SCH772984. (b) The apoptosis rate of LPS‐induced A549 and H1299 cells treated with HYSA and PI3K (LY294002) or ERK (SCH772984) inhibitors for 48 hours was analyzed via flow cytometry with Annexin V‐FITC/propidium iodide staining.
) LPS + HYSA + SCH772984. (b) The apoptosis rate of LPS‐induced A549 and H1299 cells treated with HYSA and PI3K (LY294002) or ERK (SCH772984) inhibitors for 48 hours was analyzed via flow cytometry with Annexin V‐FITC/propidium iodide staining.
HYSA suppressed migration, invasion, and EMT in LPS‐induced A549 and H1299 cells via PI3K/Akt/mTOR and ERK/MAPK signaling pathways
Transwell migration and invasion and Western blotting assays were performed. LY294002 and SCH772984 inhibited the migration and invasion, suppressed the expression of vimentin and N‐cadherin, and promoted the expression of E‐cadherin in A549 and H1299 cells induced by LPS (Fig 8). Interestingly, co‐treatment of HYSA and LY294002 or SCH772984 had a superior effect than treatment with either one alone. These data indicate that HYSA suppresses migration, invasion, and EMT of A549 and H1299 cells induced by LPS through the PI3K/Akt/mTOR and ERK/MAPK signaling pathways.
Figure 8.
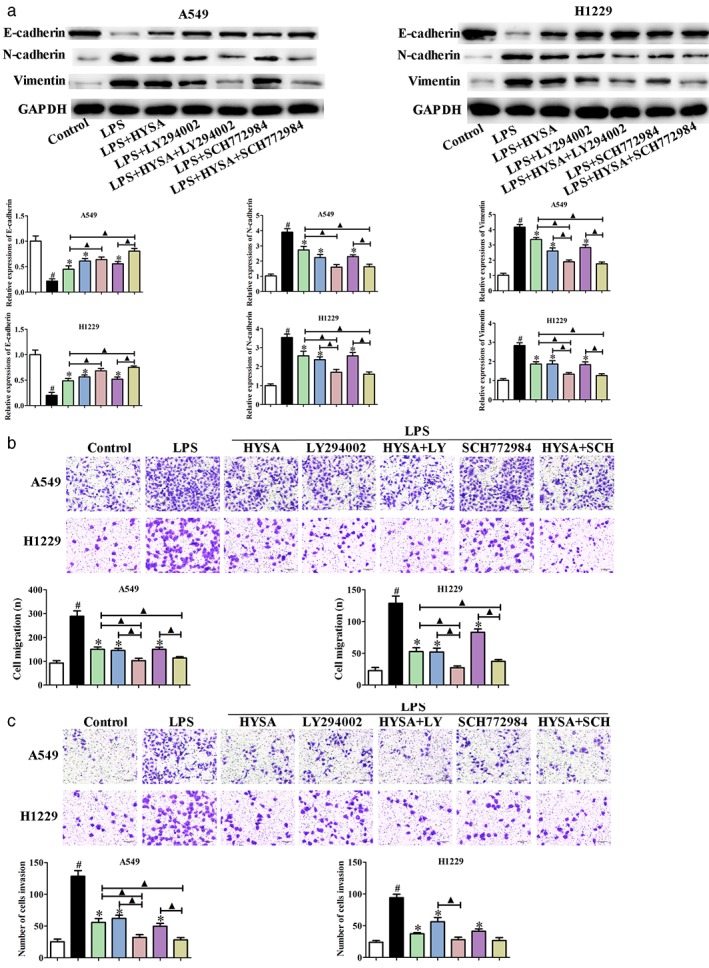
Hydroxysafflor yellow A (HYSA) suppressed migration, invasion and epithelial–mesenchymal transition (EMT) in lipopolysaccharide (LPS)‐induced A549 and H1299 cells via the PI3K/Akt/mTOR and ERK/MAPK signaling pathways. (a) E‐cadherin, N‐cadherin, and vimentin expression in LPS‐induced A549 and H1299 cells treated with HYSA and PI3K (LY294002) or ERK (SCH772984) inhibitors for 48 hours was determined by Western blotting assay. The band intensity was quantified by Image J software. (b) The migration and (c) invasion abilities of A549 and H1299 cells induced by LPS incubated with HYSA and PI3K (LY294002) or ERK (SCH772984) inhibitors for 48 hours were examined by Transwell migration assay. ( ) Control, (
) Control, ( ) LPS, (
) LPS, ( ) LPS + HYSA, (
) LPS + HYSA, ( ) LPS + LY294002, (
) LPS + LY294002, ( ) LPS + HYSA + LY294002, (
) LPS + HYSA + LY294002, ( ) LPS + SCH772984, and (
) LPS + SCH772984, and ( ) LPS + HYSA + SCH772984.
) LPS + HYSA + SCH772984.
Discussion
The tumor microenvironment plays a key role in tumorigenesis and development, and is an integral part of the physiology, structure, and function of tumors, providing a nutritional environment for tumorigenesis.40, 41 Furthermore, inflammation regulators and cytokines are important components of the local tumor microenvironment. In some tumors, inflammation often occurs before tumorigenesis, and in other tumors, changes in pro‐oncogenes can induce the inflammatory microenvironment to promote tumorigenesis. Regardless of the origin of inflammation, the presence of inflammation in the tumor microenvironment can promote the occurrence of tumors.42, 43 In fact, persistent inflammation caused by infection or injury can lead to the malignant transformation of normal cells. Chronic inflammation may promote DNA damage, proto‐oncogene activation, or tumor suppressor gene inactivation caused by genomic instability. On the other hand, the development of tumors may stimulate the production of the inflammatory microenvironment and promote the proliferation of cancer cells. Inflammation contributes to the proliferation and survival of tumor cells, promotes the angiogenesis and metastasis of tumors, and destroys the adaptive immune response.42 LPS, as a major component of the outer cell membrane of gram‐negative bacillus, plays an important role in the generation and development of the inflammatory response and the presence of inflammation, especially in chronic inflammation. LPS‐mediated changes in reactive oxygen species (ROS), reactive nitrogen, the NF‐kB signaling pathway, and bronchial EMT may be potential mechanisms for inducing tumorigenesis.44 At the same time, the inflammatory microenvironment around local inflammation can directly or indirectly promote the proliferation and angiogenesis of tumor cells, thus promoting the progress or metastasis of tumors. Studies have shown that the proliferation of A549 cells treated with low concentrations of LPS (0.1 μg/mL) is significantly higher than that of untreated cells, suggesting that low concentrations of LPS can promote the proliferation of A549 cells.45
Chemotherapy is one of the traditional methods to treat tumors, but its efficacy is limited. In addition, drug resistance and serious adverse reactions can place a psychological burden on patients, hinder the continuation of treatment, and impact the therapeutic effect of chemotherapy drugs.46, 47, 48 Therefore, there is an urgent need to develop new drugs to overcome drug resistance, improve the therapeutic effect, and reduce the side effects of cancer treatment. In recent years, TCM has been proven to have great potential in cancer treatment, and as such increasing attention has focused upon TCM.49, 50 Safflower has been cultivated and used in China for nearly 2000 years as a TCM for activating blood circulation to dissipate blood stasis. Modern medicine holds that safflower has the positive functions of anticoagulation, vasodilation, and promotion of microcirculation, and is also widely used in the treatment of cancers, including liver, rectal, and cervical cancers.51 HYSA, as a chalcone monomer, is the main component extracted from safflower. Previous studies have reported that HYSA induces human gastric carcinoma BGC‐823 cell apoptosis by activating PPARγ and suppresses angiogenesis in transplanted human gastric adenocarcinoma BGC‐823 tumors in nude mice.27, 28 HYSA inhibits the angiogenesis of hepatocellular carcinoma by blocking ERK/MAPK and NF‐κB signaling pathways in H22 tumor‐bearing mice and suppresses the adhesion, invasion, migration, and lung metastasis of hepatoma cells.29, 30 However, the role and underlying mechanisms of HYSA in LPS‐induced A549 and H1299 cells remains unknown. Therefore, this study was designed to explore the role and underlying mechanisms of HYSA in the proliferation, apoptosis, migration, and invasion of A549 and H1299 cells induced by LPS. We found that LPS could promote proliferation, suppress apoptosis, and enhance the migration and invasion of A549 and H1299 cells, which was consistent with previous reports. However, HYSA treatment could significantly reverse the effects of LPS on proliferation, apoptosis, migration, and invasion in a dose dependent manner. Furthermore, our data showed that HYSA could affect the expression of proteins related to apoptosis, migration, invasion, and EMT in A549 and H1299 cells induced by LPS. These results further confirm that HYSA had positive inhibitory activities on the development and progress of NSCLC.
Advances in cancer research have revealed many signaling pathways closely related to tumorigenesis. The PI3K/Akt/mTOR signaling pathway, as one of the important intracellular signal transduction pathways, controls the vital cellular biological processes in tumorigenesis and development, including apoptosis, transcription, translation, metabolism, angiogenesis, and cell cycle regulation by influencing the expression of many downstream effector molecules.52 Many studies have shown that the PI3K/Akt signaling pathway plays an important role in the growth of NSCLC, and 50–70% of NSCLC has Akt phosphorylation, which indicates that activation of the PI3K/Akt signaling pathway is common in NSCLC. The persistent activation of PI3K is the result of a series of factors, such as the alteration of upstream signal molecule genes, the mutation or amplification of PIK3CA, the deletion of PTEN, or the activation of downstream signal molecules.53 David et al. used an immunohistochemical method to analyze NSCLC specimens and found that p‐Akt was associated with the invasion and survival of tumors.54 Marinov et al. found persistent Akt activation and mTOR phosphorylation in 51% of NSCLC patients and 74% of NSCLC cell lines.55 In addition, statistical analysis showed that smoking, tumor size, lymph nodes, distant metastasis, tumor stage, and deletion of p‐Akt and PTEN were correlated with prognosis, while smoking, tumor stage, and PTEN expression were independent prognostic factors.56 It has been reported that relieving the regulation of the PI3K/Akt/mTOR signal transduction pathway can promote the occurrence and development of lung cancer, and the application of PI3K inhibitors such as LY294002 can promote the apoptosis of NSCLC cells and increase the sensitivity of chemotherapy.57 Therefore, the PI3K signaling pathway plays an important role in cell proliferation, survival, tumor progression, chemotherapy, radioresistance, and so on. In the present study, we found that HYSA could inhibit the PI3K/Akt/mTOR signaling pathway in A549 and H1299 cells induced by LPS. In eukaryotic cells, there are more than a dozen signaling pathways in MAPK involved in cell growth, differentiation, proliferation, and apoptosis. However, the main signaling pathways that have been identified include the ERK, JNK, and P38 pathways, of which the ERK transduction pathway is the main pathway that transmits extracellular signals to the nucleus, playing a major regulatory role in the process of cell proliferation, differentiation, and apoptosis.58, 59 ERK1/2, an extracellular regulated protein kinase, is mainly activated via phosphorylation by external stimulation, and is a key signaling molecule that determines cell proliferation and differentiation or participates in cell apoptosis.60, 61 Hauck et al. showed that PD98059, an ERK1/2 inhibitor, can significantly inhibit growth factor‐mediated cell migration by inhibiting the activation of ERK1/2 in lung adenocarcinoma A549 cells, indicating that ERK exhibits an essential role in the development and progress of NSCLC.62 In this study, the data showed that HYSA could significantly suppress the expression of the ERK/MAPK signaling pathway related to proteins in A549 and H1299 cells induced by LPS. To further confirm the HYSA exhibited positive activities on the development and progress of LPS‐induced A549 and H1299 cells through the signaling pathways, we used PI3K (LY294002) and ERK (SCH772984) inhibitors to inhibit the PI3K/Akt/mTOR and ERK/MAPK signaling pathways,38, 39 respectively, and the data showed that LY294002 and SCH772984 inhibited the proliferation, reduced colony formation, promoted apoptosis, inhibited migration and invasion, suppressed vimentin and N‐cadherin expression, and promoted the protein expression of E‐cadherin in A549 and H1299 cells induced by LPS. Further, co‐treatment of HYSA and LY294002 or SCH772984 had superior effects than treatment with either one alone.
To conclude, HYSA suppresses LPS‐mediated proliferation, migration, invasion, and EMT in A549 and H1299 cells by inhibiting the PI3K/Akt/mTOR and ERK/MAPK signaling pathways, indicating that HYSA may be a potential candidate to treat inflammation‐mediated NSCLC.
Disclosure
No authors report any conflict of interest.
Contributor Information
Ming Jiang, Email: xiaojiduoduo@163.com.
Qing An, Email: anqing8711@126.com.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018; 68: 7–30. [DOI] [PubMed] [Google Scholar]
- 2. Ferlay J, Colombet M, Soerjomataram I et al. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer 2018; 103: 356–87. [DOI] [PubMed] [Google Scholar]
- 3. Blandin Knight S, Crosbie PA, Balata H, Chudziak J, Hussell T, Dive C. Progress and prospects of early detection in lung cancer. Open Biol 2017; 7: 170070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Reck M, Heigener DF, Mok T, Soria JC, Rabe KF. Management of non‐small‐cell lung cancer: Recent developments. Lancet 2013; 382: 709–19. [DOI] [PubMed] [Google Scholar]
- 5. Du L, Morgensztern D. Chemotherapy for advanced‐stage non‐small cell lung cancer. Cancer J 2015; 21: 366–70. [DOI] [PubMed] [Google Scholar]
- 6. Karasaki T, Nagayama K, Kuwano H et al. An immunogram for the cancer‐immunity cycle: Towards personalized immunotherapy of lung cancer. J Thorac Oncol 2017; 12: 791–803. [DOI] [PubMed] [Google Scholar]
- 7. Kimura M, Yasue F, Usami E et al. Cost‐effectiveness and safety of the molecular targeted drugs afatinib, gefitinib and erlotinib as first‐line treatments for patients with advanced EGFR mutation‐positive non‐small‐cell lung cancer. Mol Clin Oncol 2018; 9: 201–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Landskron G, De la Fuente M, Thuwajit P et al. Chronic inflammation and cytokines in the tumor microenvironment. J Immunol Res 2014; 2014: 149185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wu Y, Antony S, Meitzler JL, Doroshow JH. Molecular mechanisms underlying chronic inflammation‐ associated cancers. Cancer Lett 2014; 345: 164–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hong JT, Son DJ, Lee CK, Yoon DY, Lee DH, Park MH. Interleukin 32, inflammation and cancer. Pharmacol Ther 2017; 174: 127–37. [DOI] [PubMed] [Google Scholar]
- 11. Diakos CI, Charles KA, McMillan DC et al. Cancer‐related inflammation and treatment effectiveness. Lancet Oncol 2014; 15: e493–503. [DOI] [PubMed] [Google Scholar]
- 12. Woller N, Kühnel F. Virus infection, inflammation and prevention of cancer. Recent Results Cancer Res 2014; 193: 33–58. [DOI] [PubMed] [Google Scholar]
- 13. Chow SC, Gowing SD, Cools‐Lartigue JJ et al. Gram negative bacteria increase non‐small cell lung cancer metastasis via Toll‐like receptor 4 activation and mitogen‐activated protein kinase phosphorylation. Int J Cancer 2015; 136: 1341–50. [DOI] [PubMed] [Google Scholar]
- 14. Cai KC, van Mil S, Murray E, Mallet JF, Matar C, Ismail N. Age and sex differences in immune response following LPS treatment in mice. Brain Behav Immun 2016; 58: 327–37. [DOI] [PubMed] [Google Scholar]
- 15. Sun LH, Pi DA, Zhao L et al. Response of selenium and selenogenome in immune tissues to LPS‐induced inflammatory reactions in pigs. Biol Trace Elem Res 2017; 177: 90–6. [DOI] [PubMed] [Google Scholar]
- 16. Neranjan Tharuka MD, Bathige SDNK, Oh M et al. Molecular characterization and expression analysis of big‐belly seahorse (Hippocampus abdominalis) interleukin‐10 and analysis of its potent anti‐inflammatory properties in LPS‐induced murine macrophage RAW 264.7 cells. Gene 2018; 685: 1–11. [DOI] [PubMed] [Google Scholar]
- 17. Yang N, Liang Y, Yang P, Ji F. Propofol suppresses LPS‐induced nuclear accumulation of HIF‐1α and tumor aggressiveness in non‐small cell lung cancer. Oncol Rep 2017; 37: 2611–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gökyildirim MY, Grandel U, Hattar K et al. Targeting CREB‐binding protein overrides LPS induced radioresistance in non‐small cell lung cancer cell lines. Oncotarget 2018; 9: 28976–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vlachostergios PJ, Gioulbasanis I, Ghosh S et al. Predictive and prognostic value of LPS‐stimulated cytokine secretion in metastatic non‐small cell lung cancer. Clin Transl Oncol 2013; 15: 903–9. [DOI] [PubMed] [Google Scholar]
- 20. Wang CY, Huang HS, Su YC, Tu CY, Hsia TC, Huang ST. Conventional treatment integrated with Chinese herbal medicine improves the survival rate of patients with advanced non‐small cell lung cancer. Complement Ther Med 2018; 40: 29–36. [DOI] [PubMed] [Google Scholar]
- 21. Lee IT, Lin CC, Yang CC et al. Resveratrol attenuates Staphylococcus Aureus‐induced monocyte adhesion through down‐regulating PDGFR/AP‐1 activation in human lung epithelial cells. Int J Mol Sci 2018; 19: E3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Han Y, Wang H, Xu W et al. Chinese herbal medicine as maintenance therapy for improving the quality of life for advanced non‐small cell lung cancer patients. Complement Ther Med 2016; 24: 81–9. [DOI] [PubMed] [Google Scholar]
- 23. Li L, Wang S, Yang X et al. Traditional Chinese medicine, Fuzheng Kang Ai decoction, inhibits metastasis of lung cancer cells through the STAT3/MMP9 pathway. Mol Med Rep 2017; 16: 2461–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gervais R, Le Caer H, Monnet I et al. Second‐line oral chemotherapy (lomustine, cyclophosphamide, etoposide) versus intravenous therapy (cyclophosphamide, doxorubicin, and vincristine) in patients with relapsed small cell lung cancer: A randomized phase II study of GFPC 0501. Clin Lung Cancer 2015; 16: 100–5. [DOI] [PubMed] [Google Scholar]
- 25. Liang J, Bi N, Wu S et al. Etoposide and cisplatin versus paclitaxel and carboplatin with concurrent thoracic radiotherapy in unresectable stage III non‐small cell lung cancer: A multicenter randomized phase III trial. Ann Oncol 2017; 28: 777–83. [DOI] [PubMed] [Google Scholar]
- 26. Xu R, Xu Z, Ge R. Effects of hydroxysafflor yellow A on the activity and mRNA expression of four CYP isozymes in rats. J Ethnopharmacol 2014; 151: 1141–6. [DOI] [PubMed] [Google Scholar]
- 27. Liu L, Si N, Ma Y et al. Hydroxysafflor‐yellow A induces human gastric carcinoma BGC‐823 cell apoptosis by activating peroxisome proliferator‐activated receptor gamma (PPARγ). Med Sci Monit 2018; 24: 803–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xi SY, Zhang Q, Liu CY et al. Effects of hydroxy safflower yellow‐A on tumor capillary angiogenesis in transplanted human gastric adenocarcinoma BGC‐823 tumors in nude mice. J Tradit Chin Med 2012; 32: 243–8. [DOI] [PubMed] [Google Scholar]
- 29. Ma L, Liu L, Ma Y et al. The role of E‐cadherin/β‐catenin in hydroxysafflor yellow A inhibiting adhesion, invasion, migration and lung metastasis of hepatoma cells. Biol Pharm Bull 2017; 40: 1706–15. [DOI] [PubMed] [Google Scholar]
- 30. Yang F, Li J, Zhu J, Wang D, Chen S, Bai X. Hydroxysafflor yellow A inhibits angiogenesis of hepatocellular carcinoma via blocking ERK/MAPK and NF‐κB signaling pathway in H22 tumor‐bearing mice. Eur J Pharmacol 2015; 754: 105–14. [DOI] [PubMed] [Google Scholar]
- 31. Gomes M, Teixeira AL, Coelho A et al. The role of inflammation in lung cancer. Adv Exp Med Biol 2014; 816: 1–23. [DOI] [PubMed] [Google Scholar]
- 32. Rahman MA, Barger JF, Lovat F, Gao M, Otterson GA, Nana‐Sinkam P. Lung cancer exosomes as drivers of epithelial mesenchymal transition. Oncotarget 2016; 7: 54852–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tsoukalas N, Aravantinou‐Fatorou E, Tolia M et al. Epithelial‐mesenchymal transition in non small‐cell lung cancer. Anticancer Res 2017; 37: 1773–8. [DOI] [PubMed] [Google Scholar]
- 34. Liu X, Liang J, Li G. Lipopolysaccharide promotes adhesion and invasion of hepatoma cell lines HepG2 and HepG2.2.15. Mol Biol Rep 2010; 37: 2235–9. [DOI] [PubMed] [Google Scholar]
- 35. Zhang ZH, Xie DD, Xu S et al. Total glucosides of paeony inhibits lipopolysaccharide‐induced proliferation, migration and invasion in androgen insensitive prostate cancer cells. PLoS One 2017; 12: e0182584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fumarola C, Bonelli MA, Petronini PG, Alfieri RR. Targeting PI3K/AKT/mTOR pathway in non small cell lung cancer. Biochem Pharmacol 2014; 90: 197–207. [DOI] [PubMed] [Google Scholar]
- 37. Cao Q, Mao ZD, Shi YJ et al. MicroRNA‐7 inhibits cell proliferation, migration and invasion in human non‐small cell lung cancer cells by targeting FAK through ERK/MAPK signaling pathway. Oncotarget 2016; 7: 77468–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fan J, Wu M, Wang J, Ren D, Zhao J, Yang G. 1, 7‐Bis (4‐hydroxyphenyl)‐1, 4‐heptadien‐3‐one induces lung cancer cell apoptosis via the PI3K/Akt and ERK1/2 pathways. J Cell Physiol 2018; 234: 6336–49. [DOI] [PubMed] [Google Scholar]
- 39. Iezzi A, Caiola E, Scagliotti A, Broggini M. Generation and characterization of MEK and ERK inhibitors‐resistant non‐small‐cells‐lung‐cancer (NSCLC) cells. BMC Cancer 2018; 18: 1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang X, Nie D, Chakrabarty S. Growth factors in tumor microenvironment. Front Biosci (Landmark Ed) 2010; 15: 151–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mbeunkui F, Johann DJ Jr. Cancer and the tumor microenvironment: A review of an essential relationship. Cancer Chemother Pharmacol 2009; 63: 571–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer‐related inflammation. Nature 2008; 454: 436–44. [DOI] [PubMed] [Google Scholar]
- 43. Rajput S, Wilber A. Roles of inflammation in cancer initiation, progression, and metastasis. Front Biosci (Schol Ed) 2010; 2: 176–83. [DOI] [PubMed] [Google Scholar]
- 44. Rook GA, Dalgleish A. Infection, immunoregulation, and cancer. Immunol Rev 2011; 240: 141–59. [DOI] [PubMed] [Google Scholar]
- 45. Hattar K, Savai R, Subtil FS et al. Endotoxin induces proliferation of NSCLC in vitro and in vivo: Role of COX‐2 and EGFR activation. Cancer Immunol Immunother 2013; 62: 309–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Boos G, Stopper H. Genotoxicity of several clinically used topoisomerase II inhibitors. Toxicol Lett 2000; 116: 7–16. [DOI] [PubMed] [Google Scholar]
- 47. Longley DB, Allen WL, Johnston PG. Drug resistance, predictive markers and pharmacogenomics in colorectal cancer. Biochim Biophys Acta 1766; 2006: 184–96. [DOI] [PubMed] [Google Scholar]
- 48. Nakanishi T. Drug transporters as targets for cancer chemotherapy. Cancer Genomics Proteomics 2007; 4: 241–54. [PubMed] [Google Scholar]
- 49. Ji HF, Li XJ, Zhang HY. Natural products and drug discovery. Can thousands of years of ancient medical knowledge lead us to new and powerful drug combinations in the fight against cancer and dementia? EMBO Rep 2009; 10: 194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhao J, Jiang P, Zhang W. Molecular networks for the study of TCM pharmacology. Brief Bioinform 2010; 11: 417–30. [DOI] [PubMed] [Google Scholar]
- 51. Yao D, Wang Z, Miao L. Effects of extracts and isolated compounds from safflower on some index of promoting blood circulation and regulating menstruation. J Ethnopharmacol 2016; 191: 264–72. [DOI] [PubMed] [Google Scholar]
- 52. Vivanco I, Sawyers CL. The phosphatidylinositol 3‐kinase AKT pathway in human cancer. Nat Rev Cancer 2002; 2: 489–501. [DOI] [PubMed] [Google Scholar]
- 53. Solomon B, Pearson RB. Class IA phosphatidylinositol 3‐kinase signaling in non‐small cell lung cancer. J Thorac Oncol 2009; 4: 787–91. [DOI] [PubMed] [Google Scholar]
- 54. David O, Jett J, Lebeau H et al. Phospho‐Akt overexpression in non‐small cell lung cancer confers significant stage‐independent survival disadvantage. Clin Cancer Res 2004; 10: 6865–71. [DOI] [PubMed] [Google Scholar]
- 55. Marinov M, Fischer B, Arcaro A. Targeting mTOR signaling in lung cancer. Crit Rev Oncol Hematol 2007; 63: 172–82. [DOI] [PubMed] [Google Scholar]
- 56. Tang JM, He QY, Guo RX, Chang XJ. Phosphorylated Akt overexpression and loss of PTEN expression in non‐small cell lung cancer confers poor prognosis. Lung Cancer 2006; 51: 181–91. [DOI] [PubMed] [Google Scholar]
- 57. Pisick E, Jagadeesh S, Salgia R. Receptor tyrosine kinases and inhibitors in lung cancer. Scientific World Journal 2004; 4: 589–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sun Y, Liu WZ, Liu T, Feng X, Yang N, Zhou HF. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J Recept Signal Transduct Res 2015; 35: 600–4. [DOI] [PubMed] [Google Scholar]
- 59. Niaudet C, Bonnaud S, Guillonneau M et al. Plasma membrane reorganization links acid sphingomyelinase/ceramide to p38 MAPK pathways in endothelial cells apoptosis. Cell Signal 2017; 33: 10–21. [DOI] [PubMed] [Google Scholar]
- 60. Jung O, Lee J, Lee YJ et al. Timosaponin AIII inhibits migration and invasion of A549 human non‐small‐cell lung cancer cells via attenuations of MMP‐2 and MMP‐9 by inhibitions of ERK1/2, Src/FAK and β‐catenin signaling pathways. Bioorg Med Chem Lett 2016; 26: 3963–7. [DOI] [PubMed] [Google Scholar]
- 61. Lin K, Yang R, Zheng Z et al. Sulforaphane‐cysteine‐induced apoptosis via phosphorylated ERK1/2‐mediated maspin pathway in human non‐small cell lung cancer cells. Cell Death Discov 2017; 3: 17025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hauck CR, Sieg DJ, Hsia DA et al. Inhibition of focal adhesion kinase expression or activity disrupts epidermal growth factor‐stimulated signaling promoting the migration of invasive human carcinoma cells. Cancer Res 2010; 61: 7079–90. [PubMed] [Google Scholar]


