Abstract
Background
Whether postoperative radiotherapy is beneficial in the treatment of esophageal squamous cell carcinoma with one or two regional lymph node (LN) metastases (pN1) after esophagectomy is uncertain. This study aimed to explore the effect of postoperative radiotherapy (PORT) on survival.
Methods
Propensity score‐matching (PSM) analysis was conducted to balance the two arms (surgery only [S] or surgery plus postoperative radiotherapy [PORT]). The survival rate was calculated by the Kaplan‐Meier method and analyzed using the log‐rank test.
Results
A total of 992 cases confirmed positive for one or two regional LN metastases were eligible. After PSM, 622 patients were reviewed. Each group consisted of 311 cases. The median follow‐up was 80.7 months. For the overall cohort, the one‐, three‐ and five‐year overall survival (OS) were 90.6%, 51.9% and 38.2%, respectively. Disease‐free survival (DFS) was 76.0%, 41.4% and 32.1%, respectively. The five‐year OS and DFS were 45.0% and 39.8% for PORT, which was significantly higher than the S group (31.3% and 24.2%, both P < 0.001). On subgroup analysis, PORT was associated with improved OS and DFS for patients with pathological stage pT3–4N1M0, compared with S group (five‐year OS 41.3% vs. 23.5%, P < 0.001; five‐year DFS 35.8% vs. 18.8%, P < 0.001). However, for pT1–2N1M0 patients, PORT did not benefit OS and DFS compared with S (P = 0.063).
Conclusions
In summary, the addition of PORT after esophagectomy was associated with a statistically significant improvement in OS and DFS for patients with pathological one or two lymph‐node positive pathology, in particular for stage pT3–4N1M0 patients.
Keywords: Adjuvant therapy, esophageal neoplasm, overall survival, radiotherapy, surgery
Introduction
Although adjuvant postoperative radiotherapy (PORT) is not recommended for esophageal squamous cell carcinoma after R0 resection in the 2018 version of the National Comprehensive Cancer Network (NCCN) guidelines, previous prospective stratified study results by Xiao et al. 1, 2 showed that PORT could decrease locoregional recurrence rates and improve the overall survival rate for patients with lymph node (LN) positive disease and stage III disease. Several large retrospective studies have since confirmed these results. However, in the studies by Chen et al. 2 and Xiao et al. 3 adjuvant radiotherapy was not shown to benefit patients whose postoperative stage confirmed one or two LN metastases.We retrospectively analyzed the effects of PORT on survival, using propensity score‐matching with cases from two cancer hospitals.
Methods
Eligibility
Included cases were those (i) having undergone radical esophagectomy with two‐ or three‐field lymphadenectomy; (ii) with pathologically‐confirmed esophageal squamous cell carcinoma; (iii) with one or two pathologically defined LN metastases; (iv) having received surgery alone or PORT following surgery without neoadjuvant chemotherapy or chemoradiotherapy; and (v) with Karnofsky performance status ≥70.
Surgery
Details of the operation are described elsewhere.1, 4 At the Fujian Provincial Cancer Hospital, all included patients received radical esophagectomy and three‐field lymphadenectomy (neck, right chest and upper abdomen) with cervical anastomosis. In the Cancer Hospital Chinese Academy of Medical Sciences, the surgical approach and procedure were determined by the tumor location. Right thoracotomy was the most common surgical approach for upper thoracic esophageal carcinoma. Left thoracotomy was the most common surgical approach for middle and lower thoracic esophageal carcinoma. Radical surgical resection consisted of a transthoracic subtotal esophagectomy, including abdominal and mediastinal lymphadenectomy. A gastric tube through the posterior mediastinal route was then used as a substitute for the resected esophagus to restore the continuity of the alimentary tract. Pathology and staging were according to the 8th American Joint Committee on Cancer (AJCC) cancer staging criteria. Regional lymph nodes (N) were defined as those extending from the periesophageal cervical nodes to the celiac nodes. N1 indicated metastasis in one or two regional LNs.
Postoperative radiotherapy
Radiotherapy was initiated four to six weeks after surgery and performed according to procedures described previously.5, 6 Patients received conventional, three‐dimensional conformal or intensity‐modulated radiation therapy. The clinical target volume encompassed the bilateral supraclavicular area, drainage areas of the LN mediastinum, and primary esophageal tumor bed or left gastric artery (distal lesion). A total dose of 50–60 Gy was delivered in 25–30 fractions at 1.8–2.0 Gy per fraction, five days per week. The maximum tolerated doses to critical normal structures were: spinal cord: <40 Gy; total lung: V20 < 30%; and stomach: V40 < 50%.
Follow‐up
Follow‐up ended in July 2017. Patients were instructed to return periodically for follow‐up evaluations every three months for the first year, every six months for the next two years, then annually thereafter. Computed tomography (CT) of the neck, thorax and upper abdomen with contrast, ultrasonography of neck and upper abdomen, nuclear bone scanning, conventional blood and biochemistry studies were performed at each follow‐up, as well as gastric endoscopy, positron emission tomography, or cytologic puncture if needed.
The definition of recurrence and metastasis were as follows: (i) confirmation by pathology or cytology; (ii) diagnosis by imaging: dynamic change observed at follow‐up, such as CT revealing that locoregional LNs were obviously enlarged, increased or newly appeared; and (iii) simultaneous recurrence: the interval between the two recurrent sites was within one month. Only the first recurrence or metastasis site was recorded. If the interval between two recurrence sites was more than one month, or the second recurrence site occurred after palliative treatment, the second recurrence site would not be counted again.
Mediastinal LN metastasis, recurrence of primary esophageal tumor bed or anastomotic recurrence were defined as intrathoracic failure. Regional LNs included supraclavicular and celiac axis LNs, including left gastric, hepatic artery, splenic hilar and celiac artery LNs.
Hematogenous metastasis included liver, lung, bone, pleura, subcutaneous metastasis and other nonregional LN metastasis such as axillary and inguinal LNs.
Statistical analyses
We evaluated the endpoints of overall survival (OS) and disease‐free survival (DFS). OS was measured from the date of thoracic esophageal squamous cell carcinoma (TESCC) surgery to the date of death, or the last follow‐up date if censored. DFS duration was calculated from day 1 of surgery to the date of event occurrence. Actuarial survival analysis was performed using the Kaplan–Meier method and differences between groups were compared using the log‐rank test. A P‐value of less than 0.05 was considered to indicate statistical significance. Univariate and multivariate analyses were performed using a Cox proportional hazard regression model. Statistical analysis was performed using Statistical Package for Social Sciences (SPSS) 24.0.
Stata SE 12.0 was used for propensity score‐matching (1:1) to reduce the possibility of selection bias and to balance the baseline characteristics between the two groups. Univariate analysis showed that the treatment regimen and pathological tumor stage were the prognostic factors. So the propensity scores of the patients were estimated using a logistic regression model based on the following six variables: sex, age, vascular carcinomatous thrombi, differentiation degree, tumor location and pathological tumor stage, all of which are previously reported independent prognostic factors.5, 7 Based on estimated propensity scores, one patient in the PORT group was matched (best match) to one patient in the surgery only (S) group using a caliper of 0.01. The selection process was conducted without duplication so that each patient was selected only once.
Results
Patient characteristics
From January 1993 to December 2012, the two centers had 3811 patients diagnosed with TESCC who underwent radical surgery. Of those, 2683 cases were excluded with N0 (1771), N2 (668), and N3 (244). Thirty‐three patients who received postoperative chemotherapy alone and 103 patients who had postoperative chemoradiotherapy were also excluded. Eventually, a total of 992 cases confirmed as N1 were eligible, of whom 605 patients underwent surgery alone and 387 patients received PORT. After PSM, each group had 311 patients (S vs. PORT), with the groups well balanced for clinical and pathological characteristics (Fig 1 and Table 1). This study was approved by the ethics committee of our institution.
Figure 1.
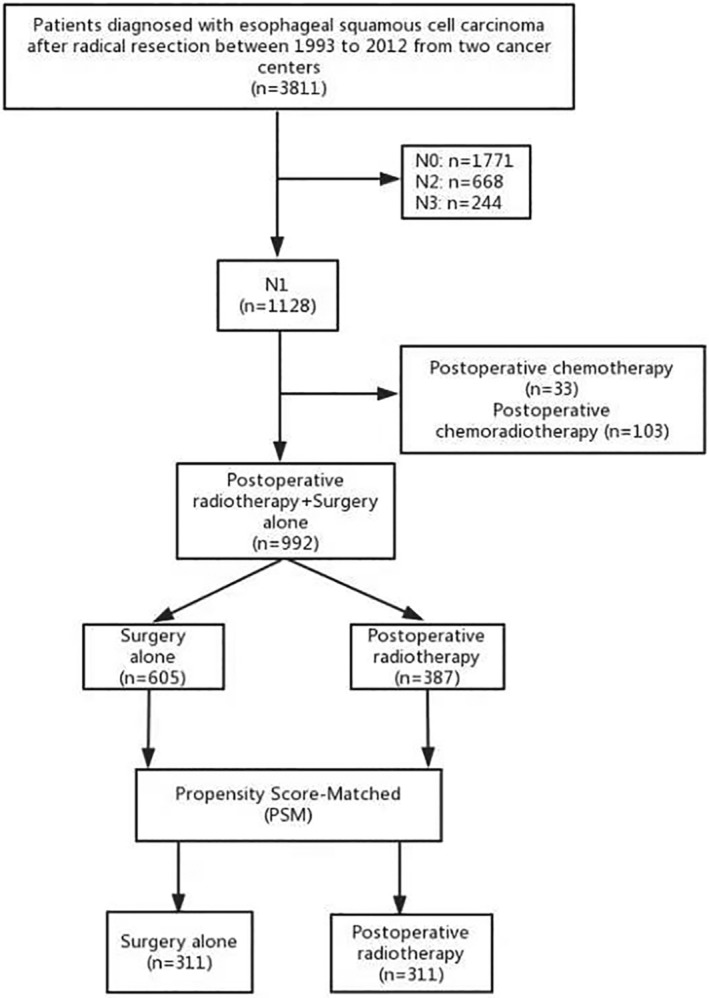
Trial profile.
Table 1.
Baseline characteristics of patients before and after propensity score‐matched cohort
| Before PSM | P | After PSM | P | |||
|---|---|---|---|---|---|---|
| S n = 605 (%) | PORT n = 387 (%) | S n = 311 (%) | PORT n = 311 (%) | |||
| Sex | 0.361 | 0.316 | ||||
| Female | 140 (23.1) | 80 (20.7) | 57 (18.3) | 67 (21.5) | ||
| Male | 465 (76.9) | 307 (79.3) | 254 (81.7) | 244 (78.5) | ||
| Age (years) | <0.001 | 0.868 | ||||
| ≤60 | 297 (49.1) | 268 (69.3) | 199 (64) | 197 (63.3) | ||
| >60 | 308 (50.9) | 119 (30.7) | 112 (36) | 114 (36.7) | ||
| Vascular carcinomatous thrombi | 0.277 | 0.204 | ||||
| No | 527 (87.1) | 346 (89.4) | 262 (84.2) | 273 (87.8) | ||
| Yes | 78 (12.9) | 41 (10.6) | 49 (15.8) | 38 (12.2) | ||
| Differentiation | 0.077 | 0.767 | ||||
| Well | 89 (14.7) | 62 (16.0) | 46 (14.8) | 46 (14.8) | ||
| Moderate | 375 (62.0) | 213 (55.0) | 168 (54.0) | 176 (56.6) | ||
| Poor | 141 (23.3) | 112 (29.0) | 97 (31.2) | 89 (28.6) | ||
| Tumor location | <0.001 | 0.864 | ||||
| Upper | 42 (6.9) | 64 (16.5) | 36 (11.6) | 36 (11.6) | ||
| Medium | 366 (60.5) | 223 (57.6) | 179 (57.6) | 185 (59.5) | ||
| Lower | 197 (32.6) | 100 (25.8) | 96 (30.9) | 90 (28.9) | ||
| T stage | <0.001 | 0.86 | ||||
| T1 | 35 (5.8) | 29 (7.5) | 31 (10.0) | 26 (8.4) | ||
| T2 | 103 (17.0) | 74 (19.1) | 60 (19.3) | 63 (20.3) | ||
| T3 | 445 (73.6) | 236 (61.0) | 203 (65.3) | 202 (65.0) | ||
| T4a | 22 (3.6) | 48 (12.4) | 17 (5.5) | 20 (6.4) | ||
| T stage category | 0.173 | 0.86 | ||||
| T1–2 | 138 (22.8) | 103 (26.6) | 91 (29.3) | 89 (28.6) | ||
| T3–4a | 467 (77.2) | 284 (73.4) | 220 (70.7) | 222 (71.4) | ||
| N = 1 | <0.001 | 0.334 | ||||
| T1 | 30 (7.5) | 15 (6.5) | 29 (13.4) | 15 (8.0) | ||
| T2 | 72 (17.9) | 53 (22.8) | 43 (19.8) | 44 (23.5) | ||
| T3 | 287 (71.4) | 136 (58.6) | 135 (62.2) | 118 (63.1) | ||
| T4a | 13 (3.2) | 28 (12.1) | 10 (4.6) | 10 (5.3) | ||
| N = 2 | 0.001 | 0.211 | ||||
| T1 | 5 (2.5) | 14 (9) | 2 (2.1) | 11 (8.9) | ||
| T2 | 31 (15.3) | 21 (13.5) | 17 (18.1) | 19 (15.3) | ||
| T3 | 158 (77.8) | 100 (64.5) | 68 (72.3) | 84 (67.7) | ||
| T4a | 9 (4.4) | 20 (12.9) | 7 (7.4) | 10 (8.1) | ||
| Eighth AJCC stage | 0.35 | 0.771 | ||||
| IIB (T1N1) | 35 (5.8) | 29 (7.5) | 31 (10.0) | 26 (8.4) | ||
| IIIA (T2N1) | 103 (17.0) | 74 (19.1) | 60 (19.3) | 63 (20.3) | ||
| IIIB (T3–4N1) | 467 (77.2) | 284 (73.4) | 220 (70.7) | 222 (71.4) | ||
Survival analyses
The median follow‐up time was 80.7 months in the PSM cohort. For all patients, the median and one‐, three‐, and five‐year OS were 37.7 months and 90.6%, 51.9%, and 38.2%, respectively. The median and one‐, three‐, and five‐year DFS were 26.6 months and 76.0%, 41.4%, and 32.1%, respectively. The treatment intervention analysis gave a five‐year OS of 45.0% in the PORT group compared with 31.3% for the S group, which was a significant difference (P < 0.001). This benefit persisted for DFS, with five‐year DFS 39.8% versus 24.2% for PORT versus S (P < 0.001; Fig 2).
Figure 2.
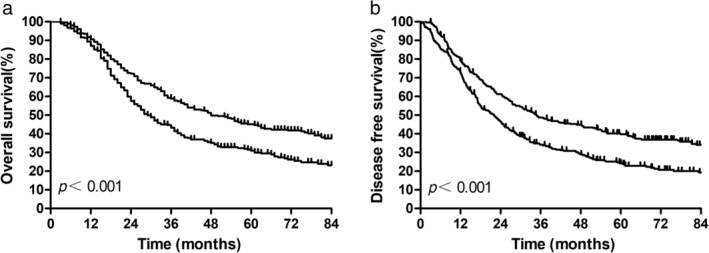
Overall survival and disease‐free survival for all patients after propensity score matching. ( ) PORT, and (
) PORT, and ( ) Surgery alone.
) Surgery alone.
Effect of PORT on overall survival according to primary tumor status
Survival outcomes stratified by subgroup are summarized in Figure 3a,b. On subgroup analysis, PORT was shown to significantly improve OS for pT3–4N1M0 compared with the surgery alone, and five‐year OS was 41.3% versus 23.5%, respectively (P < 0.001). This difference continuously occurred in pT3–4 patients with one positive LN (N = 1) and two positive LNs (N = 2). For stage pT3–4 N = 1 M0 cases, the five‐year OS for PORT was 45.5% and 23.2% for surgery alone (P < 0.001), and for stage pT3–4 N = 2 M0 cases was 35.7% for PORT and 23.8% for surgery alone (P = 0.012). However, there was no difference in OS and DFS associated with PORT for pT1–2N1M0 cases. The five‐year OS in the PORT group was 54.0%, which was similar to that in the S group (49.6%; P = 0.201). The five‐year DFS in the PORT group was 49.7%, compared with 36.8% in the S group (P = 0.063).
Figure 3.
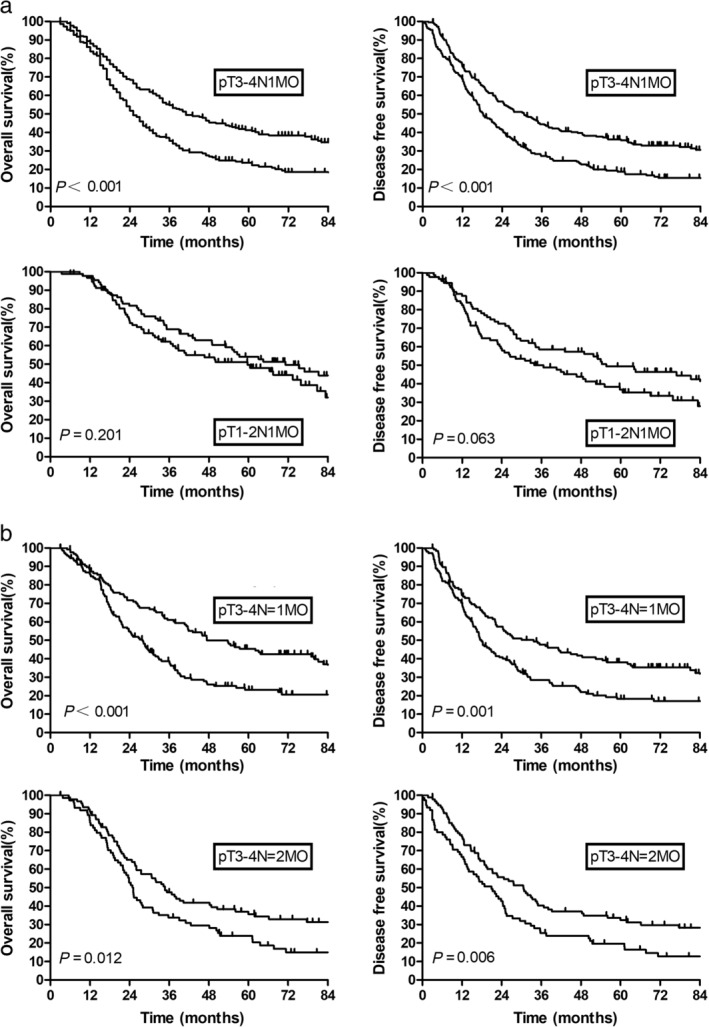
(a) Overall survival and disease‐free survival by pathological stage; (b) Overall survival and disease‐free survival for pT3–4N1M0 subgroup. ( ) PORT, and (
) PORT, and ( ) Surgery alone.
) Surgery alone.
The benefit of adjuvant radiotherapy to OS and DFS was consistent across subgroups, without any significant interaction identified. Hazard ratios for the subgroup effects for baseline covariates are shown in Figure 4.
Figure 4.
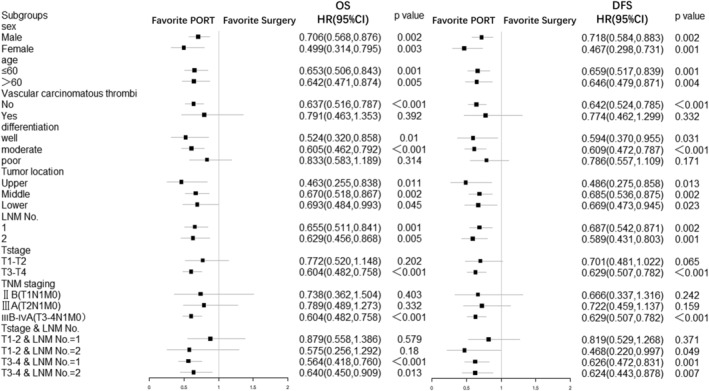
Hazard ratios for death and recurrence, according to subgroup characteristics. This forest plot shows univariate hazard ratios for death and recurrence and 95% confidence intervals for 622 patients with esophageal cancer, according to baseline characteristics. CI, Confidence interval; DFS, disease‐free survival; HR, hazard ratio; LNM, lymph node metastasis; OS, overall survival; PORT, postoperative radiotherapy.
Univariate and multivariate analyses for overall survival and disease‐free survival in propensity score‐matched cohort
On multivariate analysis, PORT was associated with improved OS, with a hazard ratio (HR) of 0.644 (95% confidence interval [CI], 0.527–0.785; P < 0.001). Age and primary tumor stage were also independent prognostic factors for OS and DFS. Further details are available in Table 2 and Figure 5a.
Table 2.
Univariate analyses for overall survival and disease‐free survival after PSM
| HR (95% CI) | P | HR (95% CI) | P | |
|---|---|---|---|---|
| Sex (female vs. male) | 1.261 (0.978–1.625) | 0.074 | 1.313 (1.028–1.675) | 0.029 |
| Age (>60 vs. ≤ 60) | 1.283 (1.052–1.565) | 0.014 | 1.232 (1.018–1.491) | 0.032 |
| Vascular carcinomatous thrombi (yes vs. no) | 1.179 (0.886–1.569) | 0.259 | 1.162 (0.884–1.527) | 0.283 |
| Differentiation | ||||
| Moderate vs. well | 0.859 (0.653–1.130) | 0.278 | 0.921 (0.707–1.201) | 0.544 |
| Poor vs. well | 0.981 (0.728–1.322) | 0.898 | 1.032 (0.773–1.377) | 0.833 |
| Tumor location | ||||
| Medium vs. upper | 1.006 (0.736–1.376) | 0.97 | 1.001 (0.743–1.349) | 0.994 |
| Lower vs. upper | 1.035 (0.739–1.448) | 0.842 | 1.023 (0.742–1.410) | 0.89 |
| T stage category (T3–4a vs. T1–2) | 1.663 (1.325–2.086) | <0.001 | 1.584 (1.277–1.966) | <0.001 |
| N stage category (N = 2 vs. N = 1) | 1.123 (0.918–1.373) | 0.259 | 1.067 (0.879–1.294) | 0.512 |
| Treatment (PORT vs. S) | 0.654 (0.537–0.795) | <0.001 | 0.654 (0.542–0.790) | <0.001 |
| TNM stage | ||||
| IIIA vs. IIB | 1.103 (0.721–1.688) | 0.651 | 1.102 (0.735–1.652) | 0.639 |
| IIIB vs. IIB | 1.777 (1.228–2.572) | 0.002 | 1.692 (1.188–2.409) | 0.004 |
Figure 5.
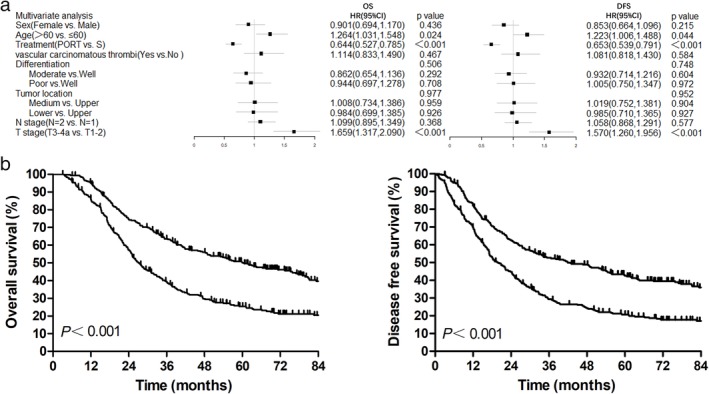
(a) Multivariate analyses for overall survival and disease‐free survival; (b) Overall survival and disease‐free survival based on recursive partitioning analysis ( ) class 1, and (
) class 1, and ( ) class 2. DFS, disease‐free survival; HR, hazard ratio; OS, overall survival; PORT, postoperative radiotherapy; RPA, recursive partitioning analysis; S, surgery only.
) class 2. DFS, disease‐free survival; HR, hazard ratio; OS, overall survival; PORT, postoperative radiotherapy; RPA, recursive partitioning analysis; S, surgery only.
A recursive partitioning analysis (RPA) model was used to predict the OS based on the three independent prognostic factors. RPA resulted in a two‐class stratification: class 1, pT1–2N1M0 or pT3–4N1M0 with PORT and age ≤60 years; and class 2, pT3–4N1M0 with surgery alone or pT3–4N1M0 with PORT and age >60 years. Five‐year OS and DFS in class 1 were significantly higher than in class 2 (five‐year OS 50.0% vs. 25.4%, P < 0.001; five‐year DFS 42.5% vs. 20.8%, P < 0.001; Fig 5b)
Recurrent patterns
Patterns of treatment failure are shown in Table 3. PORT significantly decreased local regional recurrence, with an absolute difference of about 26.0% (P < 0.001), including recurrence in supraclavicular and mediastinum. However, hematogenous metastasis was the main pattern of failure for the PORT group (32.9% vs. 24.5%, P = 0.042). For pT3–4N1M0, PORT also decreased supraclavicular and mediastinum LN metastasis compared with surgery alone (both P < 0.001). For stage pT1–2N1M0 patients, PORT resulted in a decrease in the absolute local regional recurrence rate of 20.0%, and the difference was statistically significant (P = 0.011). However, the recurrence rate was lower in both the supraclavicular and upper abdominal LNs in the surgery group, a result that may be attributable to the small sample size.
Table 3.
The patterns of failure for patients
| All patients | pT3‐4N1M0 | pT1‐2N1M0 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| S 229 | PORT 246 | P | S 149 | PORT 171 | P | S 80 | PORT 75 | P | |
| Locoregional recurrence | 116 (50.7%) | 61 (24.8%) | <0.001 | 80 (53.7%) | 42 (24.6%) | <0.001 | 36 (45%) | 19 (25.3%) | 0.011 |
| Supraclavicular | 32 (14%) | 10 (4.1%) | <0.001 | 25 (16.8%) | 7 (4.1%) | <0.001 | 7 (8.8%) | 3 (4%) | 0.229 |
| Anastomotic | 9 (3.9%) | 8 (3.3%) | 0.691 | 8 (5.4%) | 6 (3.5%) | 0.417 | 1 (1.3%) | 2 (2.7%) | 0.522 |
| Mediastinum | 75 (32.8%) | 34 (13.8%) | <0.001 | 56 (37.6%) | 21 (12.3%) | <0.001 | 19 (23.8%) | 13 (17.3%) | 0.324 |
| Celiac | 18 (7.9%) | 15 (6.1%) | 0.450 | 9 (6%) | 12 (7%) | 0.725 | 9 (11.3%) | 3 (4%) | 0.091 |
| Hematogenous metastasis | 56 (24.5%) | 81 (32.9%) | 0.042 | 37 (24.8%) | 64 (37.4%) | 0.016 | 19 (23.8%) | 17 (22.7%) | 0.873 |
Toxicity
Complications related to radiotherapy were mild. Leukocytopenia and radiation esophagitis were common, and grade 1–2 toxicity was 47.2% (147/311) and 33.4% (104/311), respectively. Grade 3 leukocytopenia was 3.2% (10/311). No grade 4 complications or deaths related to radiotherapy occurred (Table 4).
Table 4.
Toxicity related to radiotherapy
| Gastrointestinal | Leukocytopenia | Anemia | Thrombocytopenic | Radiation esophagitis | Radiation tracheitis | Radiodermatitis | |
|---|---|---|---|---|---|---|---|
| Grade 1–2 | 20 (6.4%) | 147 (47.2%) | 40 (12.9%) | 14 (4.5%) | 104 (33.4%) | 50 (16.1%) | 29 (9.3%) |
| Grade 3 | 0 (0%) | 10 (3.2%) | 3 (1.0%) | 2 (0.6%) | 3 (1%) | 0 (0%) | 1 (0.3%) |
| Grade 4 | 0 (0%) | 0 (0%) | 1 (0.3%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
Discussion
The number of positive LNs was an independent predictor of survival in TESCC. With an increase in LN numbers, OS sharply decreased,2, 3, 8, 9 and locoregional recurrence and hematogenous metastasis obviously increased.2 Pathological metastasis in one or two regional LNs was defined as N1 according to the eighth AJCC criteria because of different OS and recurrence with N2–3. Several researchers have revealed that adjuvant radiotherapy improves survival for stage III or pathological LN involvement, especially for more than three positive LNs.1, 2, 3, 5, 10, 11, 12 However, whether patients with pathological N1 esophageal squamous cell carcinoma after esophagectomy could benefit from PORT is unclear.
The intrathoracic failure rate has been reported as 35.9% and the supraclavicular recurrence rate as 19.7% following surgery alone in patients with pathological positive LNs.2 The relapse rate in our study was still high, up to 32.8% and 14.0%, respectively, for intrathoracic failure and supraclavicular recurrence. Other studies have similarly reported a locoregional recurrence rate in the surgery arm of between 23.8% and 41.8%.13, 14, 15, 16, 17, 18 In our study, PORT significantly reduced the in‐field recurrence rate after PSM, suggesting that PORT is of value for these patients. However, we found that hematological metastasis was high, up to 24.5%–32.9% for both the surgery alone group and the PORT group. The CROSS study showed that preoperative chemoradiotherapy could obviously reduce distant metastasis.19 A prospective randomized controlled trial would validate whether adjuvant radiotherapy with concurrent chemotherapy can decrease hematological metastasis.
Two previous studies demonstrated no improvement in OS with PORT in pN1 patients.2, 3 However, in both studies, sample size was small. In our large two‐hospital study, the use of PORT after esophagectomy was associated with OS benefit compared with esophagectomy alone. Although in our cohort five‐year OS was similar to previous studies (45.1%–50.7%),2, 3 the difference was statistically significant (P < 0.001), for which there are a number of possible reasons. First, in our study, PSM was used to balance the characteristics of the two groups. Second, our cohort originated from two separate institutions providing a large sample size. Third, with advances in radiation technology, most patients received 3D‐CRT or intensity‐modulated radiation therapy.
Significant differences in survival rates of patients according to differing depth of cancer invasion have previously been established,20 and primary tumor stage is an independent prognostic factor.9 This is consistent with our study's multivariate analysis result that pT3–4 survival rate was lower than that of pT1–2 (HR: 1.659, 95% CI 1.313–2.090, P < 0.001). In the subset analysis, PORT was still associated with improvement in OS and DFS for pT3–4 compared with surgery alone, irrespective of the number of positive LNs. This may be because PORT significantly decreased the locoregional recurrence rate by 29.0%, eventually conferring survival benefits. However, a prospective randomized clinical trial exploring the value of PORT in this patient group is still warranted.
In the pT1–2N1M0 group, the use of PORT reduced the locoregional failure rate. However, the adjuvant radiotherapy did not achieve this benefit to OS compared with surgery alone. The reason for this result is unclear at present but is possibly due to the small sample size. A further stratified analysis with an expanded sample size is recommended.
Side effects associated with PORT are also important when considering patient benefit. In 1993, Fok and colleagues21 reported on fatal hemorrhage from gastric ulcer secondary to radiotherapy, which may have been due to the delivery of large fractional doses (3.5 Gy per fraction). Other studies have reported grade 1–2 hematological toxicity ranging from 23.7% to 34.0%, and radiation esophagitis at 13.7%–28.0% after PORT.3, 6, 22 In our study, a conventional radiation dose of 2 Gy was delivered. Grade 3 side effects associated with PORT were reported in less than 5.0% of cases. Many patients experienced grade 1–2 complications, including 47.2% with leukocytopenia and 33.4% with radiation esophagitis, similar to the previously mentioned figures. The acute side effects of postoperative radiotherapy can therefore be considered as tolerable.
Conclusion
In conclusion, postoperative radiotherapy obviously reduced in‐field recurrence of esophageal cancer, improving OS and DFS of patients with one or two pathologically positive LNs, especially in stage pT3–4N1M0 patients.
A limitation was that the study was a retrospective analysis. Additionally, there were some differences in the surgical procedure and the target volume delineation of radiotherapy between the two institutions; thus, results need further confirmation from a prospective randomized controlled study.
Disclosures
The authors have no conflicts of interest to declare.
Acknowledgments
The authors acknowledge Fujian Cancer Hospital for providing clinical data. This work was supported by the Capital Fund for Health Improvement and Research (grant number 2016‐2‐4021), Capital Clinical Characteristic Application Research of China (grant number Z121107001012004) and Beijing Hope Run Special Fund of Cancer Foundation of China (grant number LC2016L04).
Contributor Information
Junqiang Chen, Email: junqiangc@163.com.
Zefen Xiao, Email: xiaozefen@sina.com.
References
- 1. Xiao ZF, Yang ZY, Liang J et al. Value of radiotherapy after radical surgery for esophageal carcinoma: a report of 495 patients. Ann Thorac Surg. 2003; 75 (2): 331–6. [DOI] [PubMed] [Google Scholar]
- 2. Xiao Z, Yang Z, Miao Y et al. Influence of number of metastatic lymph nodes on survival of curative resected thoracic esophageal cancer patients and value of radiotherapy: Report of 549 cases. Int J Radiat Oncol Biol Phys. 2005; 62 (1): 82–90. [DOI] [PubMed] [Google Scholar]
- 3. Chen J, Pan J, Zheng X et al. Number and location of positive nodes, postoperative radiotherapy, and survival after esophagectomy with three‐field lymph node dissection for thoracic esophageal squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2012; 82 (1): 475–82. [DOI] [PubMed] [Google Scholar]
- 4. Chen J, Liu S, Pan J et al. The pattern and prevalence of lymphatic spread in thoracic oesophageal squamous cell carcinoma. Eur J Cardiothorac Surg. 2009; 36 (3): 480–6. [DOI] [PubMed] [Google Scholar]
- 5. Chen J, Zhu J, Pan J et al. Postoperative radiotherapy improved survival of poor prognostic squamous cell carcinoma esophagus. Ann Thorac Surg. 2010; 90 (2): 435–42. [DOI] [PubMed] [Google Scholar]
- 6. Zhang W, Liu X, Xiao Z et al. Postoperative intensity‐modulated radiotherapy improved survival in lymph node‐positive or stage III thoracic esophageal squamous cell carcinoma. Oncol Res Treat. 2015; 38 (3): 97–102. [DOI] [PubMed] [Google Scholar]
- 7. Zhang W, Zhu H, Liu X et al. Epidermal growth factor receptor is a prognosis predictor in patients with esophageal squamous cell carcinoma. Ann Thorac Surg. 2014; 98 (2): 513–9. [DOI] [PubMed] [Google Scholar]
- 8. Rice TW, Rusch VW, Apperson‐Hansen C et al. Worldwide esophageal cancer collaboration. Dis Esophagus. 2009; 22 (1): 1–8. [DOI] [PubMed] [Google Scholar]
- 9. Shimada H, Okazumi S, Matsubara H et al. Impact of the number and extent of positive lymph nodes in 200 patients with thoracic esophageal squamous cell carcinoma after three‐field lymph node dissection. World J Surg. 2006; 30 (8): 1441–9. [DOI] [PubMed] [Google Scholar]
- 10. Worni M, Martin J, Gloor B et al. Does surgery improve outcomes for esophageal squamous cell carcinoma? An analysis using the surveillance epidemiology and end results registry from 1998 to 2008. J Am Coll Surg. 2012; 215 (5): 643–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xu Y, Liu J, Du X et al. Prognostic impact of postoperative radiation in patients undergoing radical esophagectomy for pathologic lymph node positive esophageal cancer. Radiat Oncol. 2013; 8: 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shridhar R, Weber J, Hoffe SE, Almhanna K, Karl R, Meredith K. Adjuvant radiation therapy and lymphadenectomy in esophageal cancer: A SEER database analysis. J Gastrointest Surg. 2013; 17 (8): 1339–45. [DOI] [PubMed] [Google Scholar]
- 13. Hsu P, Wang B, Huang C, Wu Y, Hsu W. Prognostic factors for post‐recurrence survival in esophageal squamous cell carcinoma patients with recurrence after resection. J Gastrointest Surg. 2011; 15 (4): 558–65. [DOI] [PubMed] [Google Scholar]
- 14. Miyata H, Yamasaki M, Kurokawa Y et al. Survival factors in patients with recurrence after curative resection of esophageal squamous cell carcinomas. Ann Surg Oncol. 2011; 18 (12): 3353–61. [DOI] [PubMed] [Google Scholar]
- 15. Lu J, Tao H, Song D, Chen C. Recurrence risk model for esophageal cancer after radical surgery. Chin J Cancer Res. 2013; 25 (5): 549–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu Q, Cai XW, Wu B, Zhu ZF, Chen HQ, Fu XL. Patterns of failure after radical surgery among patients with thoracic esophageal squamous cell carcinoma: implications for the clinical target volume design of postoperative radiotherapy. PLoS One. 2014; 9 (5): e97225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Oppedijk V, van der Gaast A, van Lanschot JJB et al. Patterns of recurrence after surgery alone versus preoperative chemoradiotherapy and surgery in the CROSS Trials. J Clin Oncol. 2014; 32 (5): 385–91. [DOI] [PubMed] [Google Scholar]
- 18. Kim KH, Chang JS, Cha JH et al. Optimal adjuvant treatment for curatively resected thoracic esophageal squamous cell carcinoma: A radiotherapy perspective. Cancer Res Treat. 2017; 49 (1): 168–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shapiro J, van Lanschot J, Hulshof M et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long‐term results of a randomised controlled trial. Lancet Oncol. 2015; 16 (9): 1090–8. [DOI] [PubMed] [Google Scholar]
- 20. Isono K, Sato H, Nakayama K. Results of a nationwide study on the three‐field lymph node dissection of esophageal cancer. Oncology. 1991; 48 (5): 411–20. [DOI] [PubMed] [Google Scholar]
- 21. Fok M, Sham JS, Choy D, Cheng SW, Wong J. Postoperative radiotherapy for carcinoma of the esophagus: a prospective, randomized controlled study. Surgery. 1993; 113 (2): 138–47. [PubMed] [Google Scholar]
- 22. Zhang W, Liu X, Xiao Z et al. Efficacy of intensity‐modulated radiotherapy for resected thoracic esophageal squamous cell carcinoma. Thorac Cancer. 2015; 6 (5): 597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]


