Abstract
Genome damage and defective DNA repair are etiologically linked to several neurodegenerative disorders, including fused in sarcoma (FUS)–associated amyotrophic lateral sclerosis (ALS). However, the underlying mechanisms remain enigmatic, which is a roadblock for exploiting genome repair-targeted therapies. Our recent studies identified defects in DNA nick ligation and oxidative damage repair caused by mutations in the RNA/DNA-binding protein FUS in familial ALS patients. In healthy neurons, FUS protects the genome by facilitating PARP1-dependent recruitment of XRCC1/DNA Ligase IIIα (LigIII) to oxidized genome sites and activating LigIII via direct interaction. This is a critical step in the repair of oxidative genome damage, a foremost challenge for postmitotic neurons due to their high oxygen consumption. We discovered that mutant FUS significantly inhibited the recruitment of XRCC1/LigIII to DNA strand breaks, causing defects in DNA ligation during the repair of oxidative DNA damage, which contributed to neurodegeneration. While the FUS loss of function was responsible for the repair defects, increased oxidative genome damage due to mutant FUS aggregation could exacerbate the phenomenon. We highlight how these new molecular insights into previously undescribed DNA repair defect linked to FUS-associated neurodegeneration could provide an important opportunity for exploring DNA repair-based therapeutic avenues.
Keywords: amyotrophic lateral sclerosis, FUS, DNA ligase, DNA repair defects, neurodegeneration
Comment on: Wang H, Guo W, Mitra J, et al. Mutant FUS causes DNA ligation defects to inhibit oxidative damage repair in amyotrophic lateral sclerosis. Nat Commun. 2018;9:3683. doi: 10.1038/s41467-018-06111-6. PubMed PMID: 30206235; PMCID: PMC6134028. https://www.ncbi.nlm.nih.gov/pubmed/30206235.
Amyotrophic lateral sclerosis (ALS), also known as Lou Gehrig’s disease, is a progressive motor neuron disease that typically begins with muscle weakness due to the loss of upper and lower motor neurons, leading to patient death within 2 to 5 years after the onset of symptoms. Unfortunately, there are very few FDA (Food and Drug Administration) approved medications that can slow the progress of ALS and a cure is not yet available. Although ~90% of ALS is sporadic ALS (SALS), in 10% of patients there is a clear family history, which is referred to as familial ALS (FALS). Notably, many cases of ALS show pathological overlap with frontotemporal lobar degeneration (FTLD), a disorder characterized by cognitive, behavioral, and linguistic dysfunction,1 which indicates a likely common molecular basis between motor neuron diseases and cognitive deficits.
The etiopathology of ALS is complex, and several endogenous and exogenous risk factors are believed to be responsible for the initiation and development of ALS, including elevated reactive oxygen species (ROS) and exposure to smoking and radiation. Extensive investigations have been conducted to uncover the molecular mechanisms in ALS, and mutations in a dozen genes have been identified in different ALS cases. These genes include redox regulator Cu-Zn superoxide dismutase 1 (SOD1), DNA/RNA-binding proteins fused in sarcoma (FUS) and TAR DNA-binding protein 43 (TDP-43), ubiquilin 2 (UBQLN2), optoneurin (OPTN), and C9orf72.1 One common feature among all ALS cases is the misfolding or cytoplasmic accumulation/aggregation of the proteins encoded by the mutated genes in ALS patient cells, which results in their loss of function and/or gain of toxicity in motor neurons. For example, mutation-induced misfolding of SOD1 is associated with mitochondrial dysfunction, impaired axonal transport, and glutamate excitotoxicity.1 The discoveries a decade ago implicating the RNA/DNA-binding proteins TDP-43 and FUS in ALS and FTLD2–5 triggered a flurry of research that led to the discovery of FUS mutations in subsets of familial (~5%-10%) and sporadic (~1%) ALS and FTLD patients.1,6 The common autosomal dominant missense point mutations in FUS (eg, R521H and P525L) are clustered in the gene segment encoding the nuclear localization sequence (NLS) in its C-terminus and induce nuclear depletion and cytosolic aggregation.1,7 However, how FUS pathology triggers neuronal apoptosis remains unclear.
Both FUS and TDP-43 play roles in RNA processing, including splicing, transcription, and transport. Mutation of FUS or TDP-43 induces cytoplasmic inclusion and causes dysfunction of the protein in RNA processing. Interestingly, involvement of FUS and TDP-43 in the cellular genome damage response was discovered recently.6,8,9 Although multifunctional FUS has emerged as the latest member of RNA-binding proteins to be associated with genome repair, its precise mechanistic role in the DNA damage response (DDR) is not completely understood. The lack of precise mechanistic understanding of FUS’ involvement in neuronal genome maintenance has hindered the potential utilization of “DNA repair rescue” as a therapeutic target. The linkage of DNA damage repair deficiency/genome instability and neurodegenerative disorders including ALS has been demonstrated by multiple studies in both cellular and animal models.1 Our recent studies6 identified a novel function of FUS in regulating DNA Ligase IIIα (LigIII) and demonstrated a connection between FUS toxicity (loss of function and/or dominant negative mutation) with a specific DNA nick ligation defect in oxidative genome damage repair, which also depends on PARP1’s PARylation activity. These novel findings provide insight into a previously undescribed DNA repair defect in FUS-associated neurodegeneration, and raise the question whether rescuing DNA ligation defects is a promising avenue to develop neuroprotective therapies, which will be highlighted in this commentary.
Mutations Implicated in FUS-ALS
FUS is a member of the TET (TAF15, EWS, and TLS) family of multifunctional heterogeneous nuclear ribonucleoproteins (hnRNP), which are implicated in multiple aspects of RNA metabolism. In healthy neurons, FUS is predominantly localized in the nucleus, but it can shuttle between the nucleus and cytosol in response to various stimuli.6 The FUS protein contains an SYGO-rich region, 3 RGG boxes, an RRM motif, a ZnF motif, and an NLS domain.1,9 FUS is able to bind with RNA and single- or double-strand DNA. FUS is ubiquitously expressed in both the nucleus and cytoplasm in many cell types but predominantly localized in the nucleus in glial and neuronal cells. FUS was initially identified as an oncogene due to its fusion with transcription repressor C/EBP homologous protein 10 (CHOP) in myxoid liposarcomas, but subsequently the mutation of FUS was detected in approximately 5% of FALS patients and around 1% of SALS cases, and most missense point mutations are clustered in the NLS in the C-terminus and induce cytosolic aggregation of FUS. Among the mutations identified so far, R521 is the most commonly mutated amino acid site in the NLS, and P525L mutation can induce severe nuclear clearance and is highly associated with juvenile ALS.6
FUS’ Multifaceted Role in DDR
Growing evidence shows that FUS functions in the maintenance of genome integrity by its involvement in DDR. In response to DNA damage-inducing agents, FUS is phosphorylated by ataxia-telangiectasia mutated (ATM) and DNA-dependent protein kinase (DNA-PK), 2 central DDR factors that are required for successful double-strand break (DSB) repair. FUS interacts with histone deacetylase 1 (HDAC1), which may indirectly affect DSB repair insistently; inhibition of FUS was shown to induce non-homology end joining (NHEJ)-mediated and homologous-recombination (HR)-mediated DSB repair deficiency in cell and animal models; and DNA damage accumulation is observed in FUS-ALS patient tissues. These studies suggest a complex role of FUS in DDR; however, the precise mechanism, as well as relative contribution of loss of function vs gain of toxicity, was unclear.6
Nature of genomic damage in FUS knockout neurons
Although FUS was linked to multiple DNA repair pathways including DSB repair, quantification of the level of DNA single-strand breaks (SSBs) vs DSBs in FUS knockdown (KD) and knockout (KO) cells by comet analysis under alkaline vs neutral conditions revealed that most unrepaired DNA strand breaks that accumulated after loss of FUS were alkali-labile lesions or SSBs. The small increase in DSB level could be a secondary result of closely spaced alkali-labile lesions and/or SSBs. Impaired oxidative DNA damage repair due to FUS deficiency also correlated with the increased sensitivity of the neurons to oxidative stress.
Mutant FUS-induced DNA ligation defects
To elucidate the mechanism underlying FUS’ role in DDR, we performed a comprehensive investigation using multiple in vitro and in vivo model systems, including CRISPR/Cas9-mediated FUS KO HEK293 cells, FALS patient-derived fibroblast with FUS mutations (R521H and P525L) and induced pluripotent stem cells (iPSCs) induced therefrom, motor neurons differentiated from the iPSCs, and spinal cord tissue with FUS pathology from ALS patients. We identified that FUS protects the genome by facilitating PARP1-dependent recruitment of XRCC1/LigIII to oxidized genome sites, and the loss of FUS or its mutations in ALS causes significant defects in DNA nick ligation and oxidative damage repair. Oxidative DNA damage, including DNA SSB, is continuously generated by ROS and repaired by base excision repair (BER). BER is a process that involves multiple DNA repair factors and 4 basic steps: repair initiation by DNA glycosylases, DNA end processing by polynucleotide kinase or endonuclease, DNA gap filling by DNA polymerase, and DNA ligation by a DNA ligase. Notably, PARP1 plays an essential role in BER. In response to DNA damage, PARP1 is self-activated and creates long and branched poly(ADP-ribose) on target DNA repair proteins to facilitate their recruitment; for example, this is the mechanism of PARP1-dependent recruitment of XRCC1/LigIII.6,10
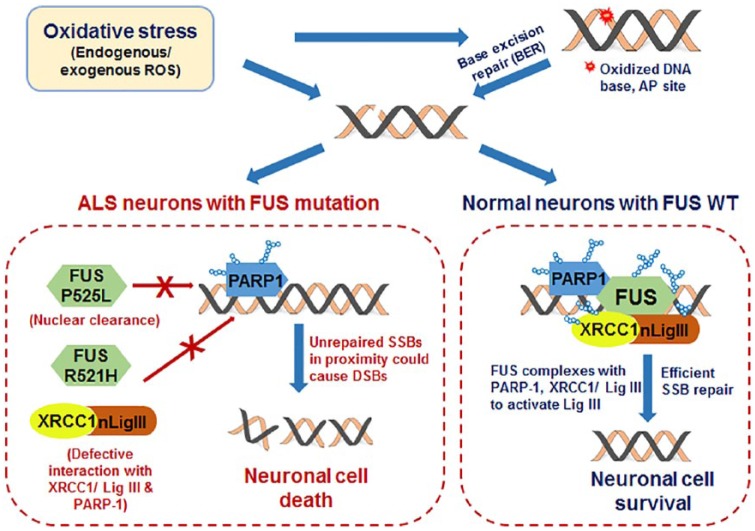
FUS is able to form a complex with PARP1, XRCC1, and LigIII in response to oxidative stress and facilitate optimal recruitment of XRCC1/LigIII at oxidatively damaged DNA in a PARP1 activity-dependent manner. Depletion of FUS is linked to SSB accumulation from SSB repair deficiency due to a DNA nick ligation defect, which can be rescued by introducing FUS. We also found that FUS directly interacts with LigIII and enhances its ligation activity. DNA ligation defects and loss of DNA integrity were seen in spinal cord tissue from ALS patients with FUS pathology and iPSC-derived motor neurons from SALS patients with an R521H or P525L FUS mutation. Correction of FUS mutations in mutant FUS iPSC lines and motor neurons by Crisper/Cas9 technology rescued cytoplasmic accumulation and DNA ligation defects. The R521H FUS mutation, with reduced recruitment at DNA damage sites and defective repair complex formation, confers dominant negative activity (Figure 1). Furthermore, FUS regulates PARP1’s PARylation activity in motor neurons and thus could affect neuronal energy metabolism by uncoupling NAD+/NADH levels.
Figure 1.
Schematic summarization of our recent studies,6 which provided new molecular insights on the role of FUS in nuclear genome maintenance and implications of FUS mutations in ALS. Fused in sarcoma is required for optimal DNA nick ligation in healthy neurons to facilitate efficient oxidative genome damage repair. However, how loss of FUS or its familial mutations in ALS causes DNA nick ligation defects, which could contribute neurodegeneration, is unknown. ALS indicates amyotrophic lateral sclerosis; FUS, fused in sarcoma; ROS, reactive oxygen species.
Importantly, our studies revealed that both loss of function and the dominant nature of toxic gain of function of FUS mutants contribute to genome instability. This is consistent with recent in vivo FUS KO and mutant transgenic mice studies. However, an FUS KO mouse was viable and did not develop a strong ALS-like phenotype,11 and FUS mutant transgene expression in mice induced selective motor neuron degeneration.1
Unanswered Questions and Future Perspectives
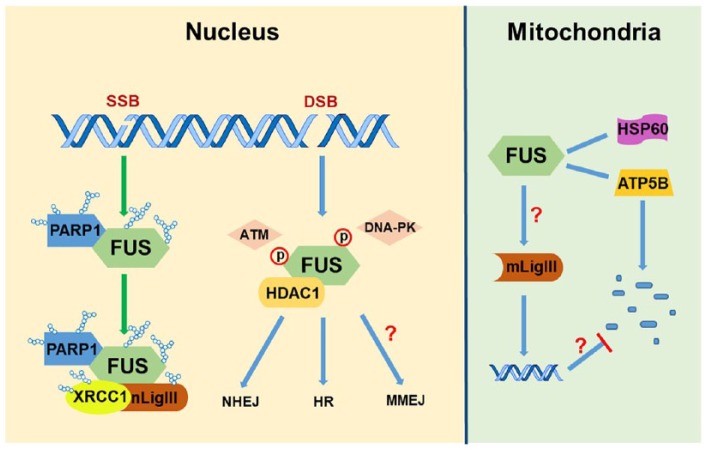
These new molecular insights into the involvement of specific DNA ligation defects in ALS-FUS raised several questions that need to be addressed to develop DNA repair-based interventions for ALS. These are as follows: (1) Does FUS play a role in mitochondrial genome together with mitochondrial LigIII? Mitochondrial LigIII is the only DNA ligase in the mitochondria that supports both their replication and repair. This suggests a broader impact of FUS mutations in mitochondria. Few studies have established the relationship between FUS and mitochondrial function. Deng et al found that FUS interacts with 2 mitochondrial proteins, HSP60 and ATP synthase beta subunit ATP5B, and overexpression of FUS WT or FUS P525L mutant induces mitochondrial fragmentation and cellular apoptosis. Down-regulation of HSP60 and ATP5B is able to rescue these mitochondrial abnormalities in human cells and neurodegenerative phenotypes in FUS transgenic flies.12,13 Nakaya and Maragkakis14 found that expression of human FUS R495X in mouse embryonic stem cell–differentiated neurons disturbs the translation efficiency of mitochondria-associated genes and induces mitochondrial shortening. However, although the dysfunction of mitochondria has been linked with ALS-FUS, the role of FUS in mitochondrial genome integrity has not been explored. (2) Does the FUS-PARP1-XRCC1/LigIII axis play a role in microhomology-mediated end joining (MMEJ) in ALS neurons? LigIII, together with XRCC1 and PARP1, also participates in the MMEJ-mediated DSB repair pathway,15 the role of which in primary neurons is unknown. The contribution of MMEJ to the repair of DSBs in motor neurons and how loss of FUS affects MMEJ and contributes to genomic instability are important unexplored questions, which we are currently pursuing. (3) What is the effect of mutant FUS on genome integrity in astrocytes? Recent studies have underscored the importance of abnormalities in the non-neuronal milieu in the central nervous system (CNS) in ALS. It will therefore be critical to investigate FUS toxicity-induced PARP1 and LigIII defects in astrocytes and their collateral influence on motor neurons. (4) Does lack of back-up DNA ligase I (LigI) in the CNS make them selectively vulnerable to FUS-mediated LigIII defects? One of the confounding challenges is to understand why neurons are selectively vulnerable to FUS-mediated LigIII defects in ALS. Mammalian cells have 3 DNA ligases, namely, DNA ligase IV (LigIV), which exclusively participates in NHEJ-mediated DSB repair; LigI, which is primarily involved with replicating the genome6; and LigIII, which has nuclear and mitochondrial isoforms. Nuclear and mitochondrial LigIII are very similar with ~98% homology, except that the mitochondrial isoform has an additional mitochondrial targeting signal in its N-terminus. LigI has been shown to functionally overlap with LigIII for both nuclear genome BER/single strand break repair (SSBR) and MMEJ, and loss of LigIII in proliferating cells has very little effect on DNA damage sensitivity, likely due to back-up functions of LigI. However, the level of LigI expression in non-cycling, postmitotic cells like neurons is negligible due to lack of replication-associated repair. Key findings of our study and their possible implications have been schematically described in Figure 2.
Figure 2.
A model of FUS’ multifaceted involvement in the DNA damage response and potential implications of FUS toxicity-mediated DNA repair defects in neurodegeneration. In the nucleus, FUS is recruited to DNA damage sites in a PARP1 activity-dependent manner in response to DNA SSB, and then facilitates recruitment of XRCC1 and nuclear LigIII (nLigIII) to regulate the ligation activity of LigIII for an optimal BER. In response to DSB, FUS is phosphorylated by ATM and DNA-PK. Fused in sarcoma also interacts with HDAC1, which may indirectly affect NHEJ- and HR-mediated DSB repair. But the precise role of FUS in NHEJ and HR is not clear. Furthermore, because of its functional regulation of XRCC1/LigIII, it is likely that FUS could affect MMEJ-mediated DSB repair, which has not been investigated. In mitochondria, FUS interacts with HSP60 and ATP5B, which is related to mitochondria abnormalities. Because LigIII is the only DNA ligase in mammalian mitochondria, FUS is likely to have a major influence on protecting genome integrity, a subject for future investigation. ATM indicates ataxia-telangiectasia mutated; BER, base excision repair; DNA-PK, DNA-dependent protein kinase; DSB, double-strand break; FUS, fused in sarcoma; HDAC, histone deacetylase; MMEJ, microhomology-mediated end joining; NHEJ, non-homology end joining; SSB, single-strand break; HR, homologous recombination.
Addressing these questions should unravel clinically relevant ways to rescue and boost the specific DNA repair defect and thus provide new perspective on DNA repair-based therapeutic approach for ALS and other neurodegenerative diseases.
Acknowledgments
The authors thank Drs Ludo Van Den Bosch (U. Belgium), A. Tomkinson (The University of New Mexico), and S. Mitra (Houston Methodist Research Institute) for their collaboration in the original study. Authors also thank Dr G. Talakatta for assisting in preparation of an illustration.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The research in the authors’ laboratory is supported by USPHS Grant NINDS R01NS088645 and Houston Methodist Research Institute funds.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: HW and MLH conceived and wrote the commentary.
ORCID iD: Muralidhar L Hegde  https://orcid.org/0000-0001-7333-8123
https://orcid.org/0000-0001-7333-8123
References
- 1. Guerrero EN, Wang H, Mitra J, et al. TDP-43/FUS in motor neuron disease: complexity and challenges. Prog Neurobiol. 2016;145-146:78–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Neumann M, Sampathu DM, Kwong LK, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. [DOI] [PubMed] [Google Scholar]
- 3. Kwiatkowski TJ, Jr, Bosco DA, Leclerc AL, et al. Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science. 2009;323:1205–1208. [DOI] [PubMed] [Google Scholar]
- 4. Vance C, Rogelj B, Hortobagyi T, et al. Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science. 2009;323:1208–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mackenzie IR, Rademakers R, Neumann M. TDP-43 and FUS in amyotrophic lateral sclerosis and frontotemporal dementia. Lancet Neurol. 2010;9:995–1007. [DOI] [PubMed] [Google Scholar]
- 6. Wang H, Guo W, Mitra J, et al. Mutant FUS causes DNA ligation defects to inhibit oxidative damage repair in amyotrophic lateral sclerosis. Nat Commun. 2018;9:3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dormann D, Haass C. Fused in sarcoma (FUS): an oncogene goes awry in neurodegeneration. Mol Cell Neurosci. 2013;56:475–486. [DOI] [PubMed] [Google Scholar]
- 8. Mitra J, Guerrero EN, Hegde PM, et al. Motor neuron disease-associated loss of nuclear TDP-43 is linked to DNA double-strand break repair defects [published online ahead of print February 15, 2019]. Proc Natl Acad Sci U S A. doi: 10.1073/pnas.1818415116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sama RR, Ward CL, Bosco DA. Functions of FUS/TLS from DNA repair to stress response: implications for ALS [published online ahead of print June 1, 2014]. ASN Neuro. doi: 10.1177/1759091414544472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang H, Dharmalingam P, Vasquez V, et al. Chronic oxidative damage together with genome repair deficiency in the neurons is a double whammy for neurodegeneration: is damage response signaling a potential therapeutic target? Mech Ageing Dev. 2017;161:163–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kino Y, Washizu C, Kurosawa M, et al. FUS/TLS deficiency causes behavioral and pathological abnormalities distinct from amyotrophic lateral sclerosis. Acta Neuropathol Commun. 2015;3:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Deng J, Wang P, Chen X, et al. FUS interacts with ATP synthase beta subunit and induces mitochondrial unfolded protein response in cellular and animal models. Proc Natl Acad Sci U S A. 2018;115:E9678–E9686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Deng J, Yang M, Chen Y, et al. FUS interacts with HSP60 to promote mitochondrial damage. PLoS Genet. 2015;11:e1005357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nakaya T, Maragkakis M. Amyotrophic lateral sclerosis associated FUS mutation shortens mitochondria and induces neurotoxicity. Sci Rep. 2018;8:15575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dutta A, Eckelmann B, Adhikari S, et al. Microhomology-mediated end joining is activated in irradiated human cells due to phosphorylation-dependent formation of the XRCC1 repair complex. Nucleic Acids Res. 2017;45:2585–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]