Abstract
Background:
The optimal timing of renal replacement therapy (RRT) initiation in critically ill patients with acute kidney injury (AKI) remains controversial.
Objective:
In critically ill patients with AKI, to determine whether the accelerated initiation of RRT reduces mortality compared to a strategy of standard RRT initiation whereby RRT is initiated if urgent complications of AKI arise or based on clinician judgment.
Design:
Pragmatic allocation-concealed open-label randomized controlled trial.
Setting:
Up to 170 centers in Australia, Austria, Belgium, Brazil, Canada, China, France, Germany, Ireland, Italy, Finland, New Zealand, Switzerland, the United Kingdom, and the United States.
Patients:
We will enroll at least 2,866 critically ill patients with AKI stages 2 or 3 (defined as doubling of serum creatinine from baseline or serum creatinine ≥354 µmol/L with increase of ≥27 µmol/L from baseline or urine output <6 mL/kg in preceding 12 hours). Patients will be excluded if 1 or more of the following is/are present: potassium >5.5 mmol/L; bicarbonate <15 mmol/L; concomitant intoxication necessitating RRT; philosophy of care precluding escalation to RRT; any RRT in preceding 2 months; kidney transplant within the past year; preexisting estimated glomerular filtration rate <20 mL/min/1.73 m2; AKI etiology attributable to obstruction, glomerulonephritis, vasculitis, microangiopathy, or acute interstitial nephritis; clinician opinion that urgent RRT is mandated; or clinician opinion that RRT must be deferred.
Methods:
Participants will be randomized to one of two strategies: accelerated RRT initiation, which entails the initiation of RRT within 12 hours of the patient fulfilling all eligibility criteria, or standard RRT initiation, whereby clinicians would be discouraged from initiating RRT unless a conventional trigger for RRT initiation arises or if AKI persists for ≥72 hours.
Measurements:
The primary outcome is all-cause mortality at 90 days following randomization. Key secondary outcomes include RRT dependence, residual kidney function, health services use, and health-related quality of life, all assessed at 90 days after randomization. In jurisdictions where it is feasible, participants will be followed through day 365 using linked administrative data.
Results:
Through March 18, 2019, we have recruited 2623 (92% of target) participants.
Limitations:
Reliance on physician declaration of equipoise may create heterogeneity across the trial population; open-label design may introduce bias and uneven postrandomization cointerventions; variations in practice (eg, choice of RRT modality and RRT prescription) likely exist across sites.
Conclusions:
Once complete, the STARRT-AKI trial will provide the most robust evidence to date to guide clinical practice on the optimal timing of RRT initiation among critically ill patients with AKI.
Trial registration:
Keywords: acute kidney injury, renal replacement therapy, intensive care unit, randomized trial
Abrégé
Contexte:
Le meilleur moment pour amorcer une thérapie de remplacement rénal (TRR) chez les patients en soins critiques atteints d’insuffisance rénale aiguë (IRA) ne fait toujours pas consensus.
Objectif:
Déterminer, dans une population de patients en soins critiques atteints d’IRA, si l’initiation accélérée de la TRR réduit le taux de mortalité par rapport à la stratégie standard qui consiste à amorcer la TRR en cas de complications de la maladie ou selon le jugement du clinicien.
Type d’étude:
Un essai pragmatique, contrôlé, ouvert et à répartition aléatoire dissimulée.
Cadre:
Jusqu’à 170 centers répartis à travers le monde: Australie, Autriche, Belgique, Brésil, Canada, Chine, France, Allemagne, Irlande, Italie, Finlande, Nouvelle-Zélande, Suisse, Royaume-Uni et États-Unis.
Sujets:
L’étude portera sur au moins 2866 patients en soins critiques et atteints d’IRA de stade 2 ou 3; ce dernier critère étant défini par le doublement du taux de créatinine sérique comparé à la valeur initiale ou par un taux de créatinine sérique d’au moins 354 µmol/L avec une hausse d’au moins 27 µmol/L par rapport à la valeur initiale ou une diurèse inférieure à 6 mL/kg dans les 12 heures précédentes. Les patients seront exclus si au moins un des éléments suivants est présent: taux de potassium supérieur à 5.5 mmol/L; taux de bicarbonate inférieur à 15 mmol/L; une intoxication concomitante nécessitant une TRR; une approche de soins préconisant une escalade vers la TRR; toute forme de TRR tentée dans les deux mois précédents; une greffe rénale dans l’année précédente; la préexistence d’un débit de filtration glomérulaire estimé inférieur à 20 mL/min/1.73 m2; étiologie de l’IRA attribuable à de l’obstruction, à une glomérulonéphrite, une vascularite, une microangiopathie ou une néphrite interstitielle aiguë; l’avis du clinicien jugeant qu’une TRR urgente est requise ou que celle-ci doit être reportée.
Méthodologie:
Les participants seront répartis aléatoirement à l’une ou l’autre des deux stratégies: (1) l’initiation accélérée d’une TRR, soit dans les 12 heures suivant la confirmation que le patient rencontre les critères d’admissibilité ou (2) l’initiation de la TRR selon l’approche standard, soit que le clinicien ne la jugerait pas nécessaire à moins que ne survienne un de ses événements déclencheurs classiques ou que l’épisode d’IRA persiste pendant au moins 72 heures.
Mesures:
Le principal critère de jugement est le taux de mortalité de toutes causes dans les 90 jours suivant la répartition des sujets. Les principaux critères secondaires incluent une dépendance à la TRR, une fonction rénale résiduelle, le recours à des services de santé et la qualité de vie en lien avec la santé, lesquels seront mesurés 90 jours après la répartition des sujets. Dans les régions où ce sera possible, les participants seront suivis sur une période de 365 jours par l’entremise des données administratives couplées.
Résultats:
En date du 18 mars 2019, 2623 participants (92 % de la cible) ont été recrutés.
Limites:
Le fait de s’appuyer sur l’évaluation du médecin traitant quant à l’équilibre clinique (« equipoise ») pourrait contribuer à une certaine hétérogénéité dans la population retenue. Le caractère ouvert de l’étude est susceptible d’introduire des biais et de révéler des divergences dans les cointerventions après la répartition des sujets. Enfin, des variations dans les pratiques, notamment dans le choix de la modalité de la TRR ou en regard de sa prescription, sont prévisibles d’un site à l’autre.
Conclusion:
Une fois complété, l’essai STARRT-AKI fournira les données probantes les plus robustes à ce jour pour guider les pratiques cliniques en ce qui a trait au meilleur moment pour amorcer une TRR chez les patients en soins critiques atteints d’IRA.
Introduction
The optimal timing of renal replacement therapy (RRT) initiation in critically ill patients with acute kidney injury (AKI) is a longstanding clinical dilemma. Among patients experiencing life-threatening complications associated with AKI, such as marked hyperkalemia, metabolic acidosis, and major volume overload, the decision to promptly initiate RRT is unequivocal.1 However, for critically ill patients with severe AKI but without an emergent indication for RRT initiation, the appropriate triggers to initiate RRT are unclear. The lack of definitive evidence has spawned two broad philosophies of care: an early or preemptive approach to initiation of RRT versus a strategy of watchful waiting, wherein RRT is deferred until confronted with life-threatening complications of AKI.
There are plausible benefits underlying a preemptive strategy for RRT initiation in critically ill patients with AKI. Earlier RRT initiation can facilitate reliable extracorporeal ultrafiltration and proactively counter the fluid accumulation frequently observed in critically ill patients with AKI. Similarly, earlier RRT initiation may promote clinical recovery through the removal of solutes that putatively mediate the toxicity of AKI. RRT-delivered solute clearance mediated by diffusion, convection, and membrane adsorption may mitigate the inflammatory milieu that is frequently observed in AKI, though the precise mechanisms remain unclear. RRT is an effective means of countering the metabolic acidosis that accompanies AKI thereby preventing the downstream hemodynamic consequences of acidemia. The benefits of preemptive RRT are supported by observational data2,3 and a recently published single-center randomized controlled trial (RCT).4
Notwithstanding these potential advantages, the uncritical adoption of preemptive RRT into clinical practice based on the current evidence base would be premature. The provision of RRT and the dedicated central venous access for RRT both carry the risk of complications. Although difficult to predict, some patients with severe AKI may survive and recover kidney function without having received RRT. As a result, a routine strategy of preemptive RRT might expose some critically ill patients, perhaps unnecessarily, to greater risk relative to benefit while using greater resources. Moreover, two recent multicenter RCTs did not show clinical benefit with earlier initiation.5,6
The effect of the timing of RRT initiation on clinical outcomes has been identified as a top priority for research in the fields of nephrology and critical care.7 The Kidney Disease Improving Global Outcomes clinical practice guidelines for AKI recognized the uncertainty in this area and recommended a definitive trial.8 The National Institute for Health and Care Excellence in the United Kingdom also explicitly called for a trial that would randomize patients with severe AKI and no urgent indications to immediate or deferred initiation of RRT.9 Recently completed trials in this area were likely underpowered to detect a realistic treatment effect favoring any RRT initiation strategy and were all limited to one country.4-6 Moreover, these trials predicated enrollment on the achievement of guideline-defined criteria for AKI without considering whether eligible patients would be conceivable candidates for RRT in the context of usual care. Accordingly, we aimed to conduct a pragmatic RCT to definitively answer whether, in critically ill patients with severe AKI, accelerated RRT initiation confers a reduction in all-cause mortality as compared to a standard strategy, whereby RRT is initiated if urgent complications of AKI arise or based on clinician judgment.
Preparatory Work
The ongoing STandard versus Accelerated initiation of Renal Replacement Therapy in Acute Kidney Injury (STARRT-AKI) trial represents the culmination of a research program comprising observational studies describing prevailing clinical practice,10,11 a Canada-wide practice survey,12 and a systematic review of studies comparing earlier versus delayed strategies of RRT initiation in critically ill patients with AKI.13 We also completed a pilot RCT that confirmed the feasibility of patient recruitment, implementation of the protocol, and participant follow up.14,15 The experiences learned while conducting the pilot RCT informed protocol modifications in the main phase of the study as reported herein.
We describe the STARRT-AKI protocol according to the guidelines set out in the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) 2013 Statement.16
Methods
Trial Design
STARRT-AKI is a multi-national pragmatic open-label RCT of critically ill patients with severe AKI comparing a preemptive strategy (accelerated arm) versus a strategy of watchful waiting and RRT initiation guided by AKI-related complications and clinician judgment (standard arm). The protocol was finalized on October 5, 2015, and remains in effect without intervening amendments.
Trial Oversight
The trial is approved by the Research Ethics Boards at the University of Alberta (file number: Pro00063318), St. Michael’s Hospital (file number: 16-009), and the affiliated institutional research boards of all participating sites. The trial is governed by an international steering committee (Supplementary Material Appendix 1).
The trial is registered at ClinicalTrials.gov (NCT02568722; October 6, 2015).
Population and Eligibility
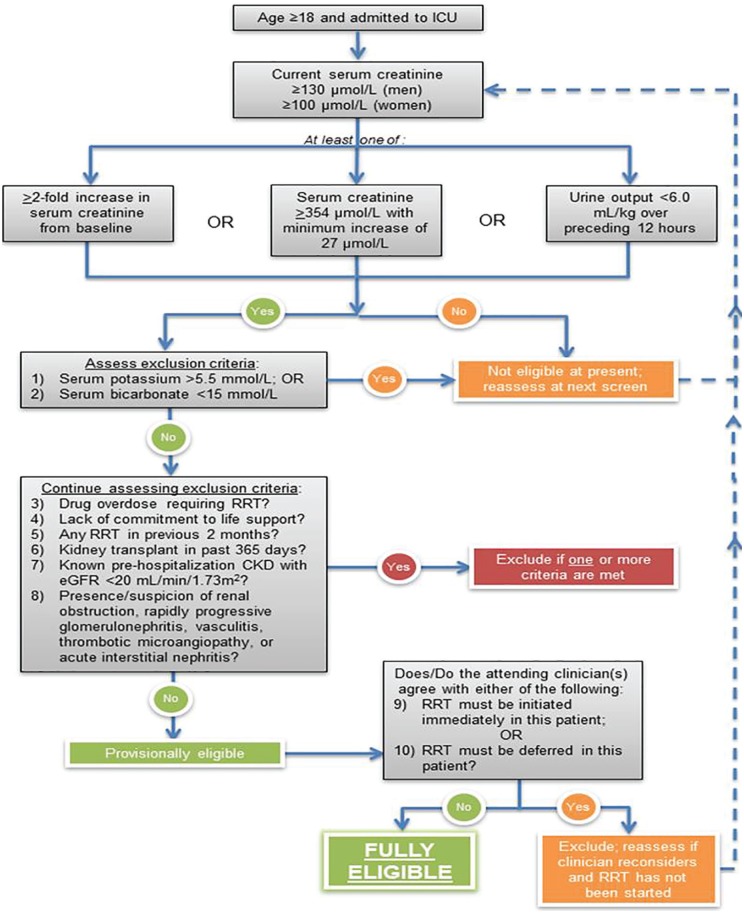
The STARRT-AKI eligibility criteria are shown in Table 1. After meeting all the inclusion criteria and elimination of the first 8 exclusions, a patient would be considered provisionally eligible. Achievement of full eligibility requires confirmation of equipoise by the attending clinicians (intensivist as well as the nephrologist at centers where nephrologists manage RRT orders) caring for the patient. Equipoise will be determined when attending clinicians agree that there is neither an urgent reason to immediately commence RRT (exclusion 9) nor is it mandated to defer RRT (exclusion 10). Once a patient is found to be fully eligible (ie, all inclusion criteria met and no exclusion criteria applicable), there is a 12-hour window, during which consent must be obtained (or deferred/waived consent invoked, as approved by local research ethics boards). If consent cannot be secured during this time window, the patient can no longer be considered for participation in the trial. The screening process is depicted in Figure 1. A flow diagram will be reported according to the Consolidated Standards of Reporting Trials (CONSORT) recommendations for reporting randomized trials.
Table 1.
Eligibility Criteria for Enrollment in the STARRT-AKI Trial.
| The following inclusion criteria have been
established, all of which must be fulfilled: 1. Age ≥18 years, and 2. Admission to a critical care unit, defined as any unit capable of providing invasive mechanical ventilation, and 3. Evidence of kidney dysfunction, defined as a serum creatinine ≥100 μmol/L in women and ≥130 μmol/L in men that has not declined by ≥27 μmol/L compared to the highest value recorded in the preceding 48 hours, and 4. Evidence of severe AKI based on at least 1 of the following 3 criteria: a. ≥2-fold increase in serum creatinine from baseline, or b. serum creatinine ≥354 μmol/L, accompanied by evidence of a minimum increase of 27 μmol/L from the baseline serum creatinine, or c. urine output <6 mL/kg in preceding 12 hours The presence of 1 or more of the following exclusion criteria disqualifies a participant from participation: 1. Serum potassium concentration >5.5 mmol/L, based on last available bloodwork 2. Serum bicarbonate concentration <15 mmol/L, based on last available bloodwork 3. Presence of a drug overdose that necessitates initiation of RRT 4. Lack of commitment to provide RRT due to limitations on the escalation of life support measures 5. Any RRT within the previous 2 months 6. Kidney transplant within the past 365 days 7. Known pre-hospitalization advanced chronic kidney disease, defined by an estimated glomerular filtration rate <20 mL/min/1.73 m2, as measured by the Chronic Kidney Disease Epidemiology Collaboration equation, using an outpatient serum creatinine value obtained within 365 days of admission for the current hospitalization 8. Presence or strong clinical suspicion of renal obstruction, rapidly progressive glomerulonephritis, vasculitis, thrombotic microangiopathy (eg, thrombotic thrombocytopenic purpura, hemolytic uremic syndrome, malignant hypertension, scleroderma renal crisis) or acute interstitial nephritis If the patient fulfills all inclusion criteria and no exclusion criteria have been identified the patient is deemed to be provisionally eligible. The next step is to ascertain whether the most responsible clinician(s) (the attending critical care physician and where relevant, the attending nephrologist) are in a position of clinical equipoise with respect to the 2 RRT initiation strategies that the provisionally eligible patient may receive if he/she is randomized. This is performed in practice by ascertaining the presence of the following 2 exclusion criteria: 9. Clinician(s) caring for patient believe(s) that immediate RRT is absolutely mandated 10. Clinician(s) caring for patient believe(s) that deferral of RRT initiation is mandated A negative answer by all of the relevant clinicians to exclusions 9 and 10 formally transitions the patient’s status from provisional to full eligibility. The time of full eligibility is noted and marks the beginning of a 12-hour period, during which informed consent must be obtained (or alternate consent approaches invoked) and the participant randomized. If consent cannot be secured during the 12 hours after full eligibility is established, the patient is no longer eligible for participation. |
Note. STARRT-AKI = STandard versus Accelerated initiation of Renal Replacement Therapy in Acute Kidney Injury; RRT = renal replacement therapy.
Figure 1.
Summary of screening process for determination of eligibility.
Randomization
Participants will be randomized 1:1 to accelerated versus standard initiation of RRT with variable block sizes and stratified by center using a centralized concealed web-based randomization system that is managed at the Applied Health Research Center (AHRC) at St. Michael’s Hospital in Toronto (http://www.stmichaelshospital.com/research/ahrc/index.php).
Interventions
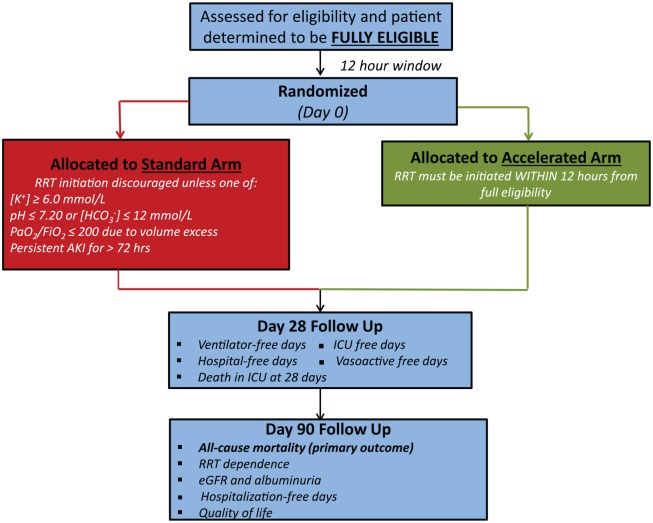
Accelerated RRT Initiation
Participants allocated to the accelerated RRT initiation strategy will initiate RRT within 12 hours of reaching full eligibility. This 12-hour window includes the time required to obtain consent (as described above), place a dialysis catheter, and initiate RRT.
Standard RRT Initiation
Clinicians caring for participants allocated to the standard arm of the trial will be discouraged from commencing RRT unless the following conditions are met: (1) persistent AKI, defined as a serum creatinine that remains >50% of the value recorded at randomization; and 2) one or more of the following indications for RRT initiation: (i) serum potassium ≥6.0 mmol/L; or (ii) pH ≤7.20 or serum bicarbonate ≤12 mmol/L; or (iii) evidence of severe respiratory failure, based on a PaO2/FiO2 ≤200 and clinical perception of volume overload; or (iv) persistent AKI for ≥72 hours from randomization. RRT may still be commenced in participants allocated to the standard RRT initiation strategy at any time based on the judgment of the attending clinician(s). In circumstances where RRT is commenced in the absence of meeting the trial-specified criteria, the clinician will be asked to specify the primary reason for initiating RRT. There is no obligation to initiate RRT in the standard arm, even among participants who fulfill one of the aforementioned conditions. For example, if a patient in the standard arm has a serum potassium concentration of 6.3 mmol/L, the clinician may opt to use medical interventions to promote potassium excretion and is not obligated to commence RRT. Among participants allocated to the standard strategy, most are expected to commence RRT; however, we anticipate that a significant proportion will not receive RRT due to either kidney recovery or death, as demonstrated in our pilot trial.14
RRT Delivery in the STARRT-AKI Trial
The decision regarding RRT modality will be made by the attending physician(s). RRT will be delivered using a set of recommended guidelines aligned with contemporary clinical practice, as described in the operations manual (Supplementary Material Appendix 2).
Criteria for Discontinuation of RRT
Once started in either treatment arm, RRT will continue until one of the following is encountered: (1) death; or (2) withdrawal of life-support interventions in the context of a change in the patient’s goals of care; or (3) kidney function recovery with no need for continued RRT as per the clinician’s judgment. If kidney function is deemed to be inadequate after a period of RRT discontinuation, RRT may be reinitiated at the discretion of the treating clinician.
Frequency and Duration of Follow-Up
Each participant is followed for a minimum of 90 days after randomization (Figure 2). In jurisdictions where this is feasible, follow up from day 91 to 365 will occur using linkages to administrative data.
Figure 2.
An overview of patient flow after randomization into STARRT-AKI.
Note. STARRT-AKI = STandard versus Accelerated initiation of Renal Replacement Therapy in Acute Kidney Injury.
We will monitor and collect data on all RRT that is administered during the first 14 days after randomization, as well as safety events that arise during this time period. As per recommendations for the follow up of patients surviving an episode of AKI, all participants who are alive and independent of RRT at 90 days following randomization will be asked to submit a blood sample for creatinine measurement (and estimation of glomerular filtration rate using the Chronic Kidney Disease Epidemiology Collaborative equation) and a spot urine sample for assessment of the urine albumin:creatinine ratio.8
Outcomes
Primary Outcome
The primary outcome is all-cause mortality within 90 days of randomization.
Secondary Outcomes
The secondary outcomes are:
RRT dependence at 90 days after randomization
Composite of all-cause mortality or RRT dependence at 90 days after randomization
Major adverse kidney events at 90 days after randomization, defined as the composite of death, RRT dependence, or sustained reduction in kidney function (defined as eGFR <75% of baseline eGFR)17,18
eGFR at 90 days after randomization
Albuminuria at 90 days after randomization
All-cause mortality in ICU, at 28 days and in hospital
RRT-free days through day 90
Mechanical ventilation-free days through day 28
Vasoactive therapy-free days through day 28
ICU length of stay and ICU-free days through 28 days
Hospital length of stay and hospitalization-free days through 90 days
Rehospitalization through 90 days
Health-related quality-of-life measured using the EuroQoL EQ-5D-5L at 90 days
All-cause mortality through 365 days (in jurisdictions where feasible)
RRT dependence at 365 days (in jurisdictions where feasible)
Health care costs through 365 days (in jurisdictions where feasible)
Safety and Adverse Events
All adverse events deemed to be related to one of the study interventions are being ascertained and recorded for the first 14 days after randomization. Specifically, events that are conceivably related to the insertion of a dialysis catheter, administration of RRT, or occurring as a complication of delaying RRT will be captured (Table 2).
Table 2.
Definitions of Adverse Events
| Event | Definition |
|---|---|
| RRT-associated | |
| Hypotension | A drop in blood pressure requiring one of: (1) Initiation of a vasopressor during RRT session or (2) A need to escalate dose of a vasopressor during the RRT session or (3) Premature discontinuation of RRT session due to hypotension |
| Arrhythmia | A new atrial (excluding sinus tachycardia or sinus arrhythmia) or ventricular arrhythmia that develops during RRT and was not present prior to initiation of RRT that requires treatment with any medication or cardioversion/defibrillation or decision to stop RRT prematurely as a result of arrhythmia |
| Seizure | A seizure that develops during RRT session and confirmed by the attending clinician |
| Major bleeding | (1) Life-threatening bleeding and associated hypovolemic
shock (eg, from ruptured abdominal aortic aneurysm or upper
or lower gastrointestinal hemorrhage) (2) Life-threatening bleeding at a critical site (eg, intracranial, retroperitoneal, and pericardial) (3) Overt, clinically important bleeding associated with 1 of the following within 24 hours of the bleed: decrease in hemoglobin >20 g/L or transfusion ≥ 2 packed red blood cells (4) Bleeding requiring an invasive intervention (eg, re-operation) |
| Allergic reaction | Clinician suspicion of allergic reaction to one of the components of the RRT apparatus |
| Hypophosphotemia | Serum phosphorus <0.5 mmol/L |
| Hypokalemia | Serum potassium <3.0 mmol/L |
| Hypocalcemia | Albumin-adjusted serum calcium <1.90 mmol/L or ionized calcium <0.90 mmol/L |
| Dialysis catheter-associated | |
| Pneumothorax | Air in the pleural space on routine chest x-ray that is performed following dialysis catheter insertion |
| Hemothorax | Blood in the pleural space following dialysis catheter insertion |
| Bleeding | Bleeding described by clinician inserting dialysis catheter requiring transfusion of ≥1 unit(s) of packed red blood cells and/or surgical intervention/repair within 12 hours following insertion |
| Thrombus | An ultrasound-confirmed occlusive or nonocclusive thrombus in the vein in which a dialysis catheter was placed (or remains in place) or in the venous system drained by the vein in which a dialysis catheter was placed |
| Arterial puncture | As document by the clinician placing the catheter |
| Bloodstream infection | Infection in 2 blood culture sets (one drawn from dialysis catheter and the other from another site) with no proven alternative source for bloodstream infection as per ICU attending OR culture-positive recovery of the same organism from the dialysis catheter upon removal |
| Air embolism | As documented in the medical chart |
| Other | Any other adverse event felt to be related to the patient’s participation in the trial including any event felt to be the consequence of the patient’s nonreceipt of RRT |
Note. RRT = renal replacement therapy.
A serious adverse event (SAE) includes any adverse event that meets at least 1 of the following conditions:
Is fatal
Is perceived to be life threatening
Requires in-patient hospitalization or prolongation of an existing hospitalization
Results in significant disability or incapacity
For this study, a reportable SAE must meet the definition noted above and also be considered:
An atypical event, defined as clinically significant and unexpected in the context of critical illness complicated by AKI; AND
An event that is at least possibly related to the study procedures.
Power and Sample Size
We anticipate a 90-day mortality rate of 40% in the standard arm. This mortality rate is compatible with 90-day mortality reported in contemporaneous cohorts of critically ill patients with severe AKI treated with RRT.19,20 There is no clear guidance on the estimated risk reduction afforded by accelerated RRT. We selected a relative risk reduction of 15% (absolute risk reduction 6%) as a plausible magnitude of effect that is clinically important. With a Type I error of 0.05 and power of 0.90, a sample size of 1359 participants/arm would be required (total 2718). In order to account for the interim analyses, the required sample size was increased to 2780. After estimating a combined rate of crossover and dropouts of 3% (as derived from the pilot phase),14 our target recruitment is at least 2866 participants.
Data Management
All data will be reviewed by dedicated managers at The George Institute in Sydney, Australia (for participants enrolled in Australia and China), the Medical Research Institute of New Zealand (for patients enrolled in New Zealand) and the Applied Health Research Centre in Toronto, Canada (for participants enrolled in all other countries). Managers will issue queries to participating centers regarding suspected data errors which will then require clarification before being considered resolved. Source data will be reviewed for randomly selected participants through on-site monitoring or submission of de-identified documentation.
Statistical Analyses
A detailed statistical analysis plan and proposed presentation of data will be published separately prior to completion of planned recruitment. In brief, the primary outcome of 90-day mortality will be evaluated using an intention-to-treat approach. A simple comparison of proportions will be performed using a chi-squared test. The risk ratio and risk difference will be estimated with 95% confidence intervals.
RRT dependence at 90 days is the most important secondary outcome and requires a more nuanced approach, as the noninclusion of participants who died might obviate the intergroup balance afforded by randomization. We will consider 2 complementary approaches to examine this question. First, we will develop a model for the primary outcome to estimate the probabilities of 90-day survival. We will then use the reciprocals of these probabilities as weights in a logistic regression for RRT dependence, resulting in an inverse probability weighted analysis. The second approach will employ a multinomial regression model to jointly consider the following states: dead at 90 days, alive at 90 days receiving RRT, and alive at 90 days and RRT-free. A similar approach will be used to estimate the patient’s status at 365 days. In addition, time-to-event models that incorporate competing risks or multiple outcomes may be considered.
Duration of ventilation, vasoactive therapy, intensive care unit (ICU) stay and hospitalization, and albuminuria at 90 days (expressed as the urinary albumin to creatinine ratio in mg/mmol) will be compared by means or medians using a t-test or Mann–Whitney U test, respectively, as appropriate. Finally, RRT dependence at 90 days, a composite of death or RRT dependence at 90 days, major adverse kidney events at 90 days (MAKE90), eGFR decline to <75% of baseline eGFR, death at all pre-specified time milestones, ICU readmission and rehospitalization within 90 days will be compared using chi-squared tests, respectively. Where appropriate, the inverse probability weighted approach will be used to ensure that “survivor-only” analyses are not misleading.
Interim analyses for efficacy based on the primary outcome will be performed when 25, 50, and 75% of participants have completed 90-day follow-up. Given the risk of false-positive results with early stopping for benefit, statistical significance will be declared using extreme P-values established by O’Brien-Fleming boundaries on the primary outcome (90-day mortality).21
Planned Subgroup Analyses
We will evaluate the effect of accelerated versus standard RRT on the primary outcome of 90-day mortality in the following a priori defined subgroups:
Patient sex (since sex may affect muscle mass which in turn affects serum creatinine, severity of AKI may be differentially perceived in men and women)
The presence of preexisting chronic kidney disease (based on the plausible modifying effect of preexisting chronic kidney disease on mortality and progression to long-term RRT dependence)
Baseline Simplified Acute Physiology Score (SAPS) II score (based on the possibility that the timing of RRT initiation may have a minimal impact on modifying mortality in patients with a low SAPS score who have a favorable prognosis as well as in individuals with a high SAPS score who have a poor prognosis)
Surgical (versus medical) status (based on the rationale that a recently completed trial that demonstrated lower mortality in patients who received earlier RRT initiation was conducted in a predominantly postsurgical population)4
Patients with sepsis and septic shock, as defined by the Sepsis-3 criteria22 (based on the rationale that earlier RRT, due to more aggressive removal of inflammatory mediators, might have a more prominent effect among patients with sepsis-associated AKI)
Geographic regions of the world: North America; France; United Kingdom; Europe (not including France or the United Kingdom); Australia/New Zealand; Asia and South America (based on rationale that regional RRT and/or critical care practices may modify the relationship between the timing of RRT initiation and the outcomes of interest)
Health Economic Evaluation
A cost-utility analysis will be performed comparing the two approaches to RRT initiation in critically ill patients with AKI. This will be part of a broader health economic evaluation to be reported after the publication of the main trial findings.
Co-Enrollment
Patients recruited to STARRT-AKI may be considered for co-enrollment in observational studies or clinical trials, provided those studies do not modify the STARRT-AKI interventions or have a plausible interaction with the timing of RRT initiation. Investigators and coordinators will routinely review trials that are concurrently operating in ICUs at participating centers. After reviewing the trial protocol, the co-principal investigators (R.W. or S.M.B.) will determine whether a trial is appropriate for co-enrollment.
Plasma-Lyte148 versUs Saline Study
The Plasma-Lyte148 versUs Saline Study (PLUS, ClinicalTrials.gov NCT02721654) recently commenced enrollment in Australia and New Zealand and is concurrently recruiting at several sites at which STARRT-AKI is active.23 PLUS is evaluating the effect of resuscitation with Plasma-Lyte148 compared with 0.9% saline on the primary outcome of 90-day mortality in 8800 critically ill patients. Since enrollment in PLUS occurs shortly following arrival in the ICU, recruitment into PLUS will generally take place before eligibility for STARRT-AKI is ascertained. Given the hypothesis that the PLUS intervention strategy may impact upon the risk of AKI progression and the initiation of RRT, efforts have been made to ensure that PLUS participants who enter STARRT-AKI are balanced across both RRT initiation strategies depending on the PLUS treatment allocation.
At centers that are participating in PLUS, randomization into STARRT-AKI will be further stratified according to nonparticipation in PLUS, receipt of PLUS-Plasma-Lyte148, and receipt of PLUS—0.9% saline. For participants who are enrolled in PLUS, the PLUS study bag number (which is linked to the blinded PLUS study intervention) will be entered into the STARRT-AKI database. There will be no specific reporting on the subgroup of STARRT-AKI participants co-enrolled in the PLUS trial.
Limitations
Embedding the attending clinicians’ declaration of equipoise into the eligibility criteria may create heterogeneity in the patients included within and across participating sites. We have not specifically protocolized any aspect of RRT delivery or critical care practice. There will be inevitable practice variability which should be balanced by randomization. However, the permitted flexibility in clinical practice in the context of an open-label design could introduce bias through the delivery of unbalanced cointerventions.
Trial Management
Coordination
The international coordinating and data management center is the Applied Health Research Center located at the Li Ka Shing Knowledge Institute (www.ahrconline.com) of St. Michael’s Hospital in Toronto, Canada. Coordination for sites in Australia and China is managed by The George Institute, Sydney, Australia. Coordination for sites in New Zealand is managed by the Medical Research Institute of New Zealand, Wellington, New Zealand.
Steering Committee
The Steering Committee has assumed overall responsibility for conduct of the trial worldwide (Supplementary Material Appendix 1). The committee is chaired by the co-principal investigators (R.W. and S.M.B.) and meets monthly via teleconference to review recruitment progress, protocol adherence, protocol violations, and overall trial operations and logistics. A current list of investigators, personnel, and sites can be found in the Supplementary Material Appendix 3.
Data Safety and Monitoring Board
An independent data safety and monitoring board (DSMB) comprised of experts in clinical trials, biostatistics, critical care, and nephrology have been appointed to monitor the trial. DSMB membership and the charter guiding DSMB operations are found in Supplementary Material Appendix 4. The DSMB meets to review safety data following the enrollment of every 300 patients, to review the results of interim analyses, and on an ad hoc basis as indicated. The DSMB chair will communicate with the co-principal investigators after each meeting and provide recommendations regarding continuation of the trial. The DSMB may request evaluation of available trial data at any time at its discretion.
Implications
STARRT-AKI will provide high-quality evidence to answer one of the most vexing questions in the area of critical care nephrology. The trial is appropriately powered to assess a potential benefit of accelerated RRT initiation that is both plausible and clinically meaningful. The eligibility criteria reflect patients experiencing AKI for whom the question of when to initiate RRT is relevant. Pragmatic deployment of the interventions and the multinational nature of the trial will ensure that the findings are widely applicable.
Supplemental Material
Supplemental material, Appendix_1,_2_,_3_and_4 for STandard versus Accelerated initiation of Renal Replacement Therapy in Acute Kidney Injury: Study Protocol for a Multi-National, Multi-Center, Randomized Controlled Trial by Ron Wald and Sean Bagshaw in Canadian Journal of Kidney Health and Disease
Acknowledgments
The authors greatly appreciate the thoughtful review of this manuscript by Drs Damon Scales and Constantine Karvellas (representing the Canadian Critical Care Trials Group Manuscript Review Committee) and Dr Rita Suri (representing the Canadian Nephrology Trials Network). The staff of the NIHR Clinical Research Network and the Northern Ireland Clinical Research Network support STARRT-AKI operations in the United Kingdom. Dr Bagshaw is supported by a Canada Research Chair in Critical Care Nephrology. Professor Nichol is supported by a Health Research Board of Ireland Clinical Trial Network award. Professor Udy is supported by a National Health and Medical Research Council of Australia Early Career Fellowship.
Footnotes
Ethics Approval and Consent to Participate: Acknowledging the variability in policy regarding consent mechanisms at different centers, regions, and countries, STARRT-AKI allows the application of any consent mechanism that is approved by local ethics boards and the regulatory authorities in that jurisdiction.
Consent for Publication: Members of the writing committee listed below approved the submission of this manuscript for publication.
Availability of Data and Materials: There are no current plans in place for data sharing.
Authors’ Note: Writing committee: Ron Wald, MDCM MPH (corresponding); Sean M Bagshaw, MD MSc (corresponding); Neill KJ Adhikari, MD MSc; Rinaldo Bellomo, MD PhD; Remi Bruyere, MD; Nikita Chavda, BSc; Didier Dreyfuss, MD; Erika Dempsey, MPP; Bin Du, MD; Martin Gallagher, MD; Stephane Gaudry, MD PhD; Gill Arbane, BSc MRES; Eric Hoste, MD PhD; Michael Joannidis, MD; Francois Lamontagne, MD MSc; Kathleen D Liu, MD; Jessica Marchese, BSc; Lauralyn McIntyre, MD; Shay McGuiness, MBBS; Danny McAuley, MBBS; Javier A Neyra, MD MSCS; Alistair Nichol, MB PhD; Marlies Ostermann, MD PhD; Paul Palevsky, MD; Ville Pettila, MD; Bertrand Pons, MD; Jean-Pierre Quenot, MD PhD; Haibo Qui, MD PhD; Bram Rochwerg, MD MS; Orla Smith, RN PhD; Antoine Schneider, MD PhD; Kevin Thorpe, MMath; Andrew Udy, MBChB PhD; Suvi Vaara, MD; Amanda Wang, MD; Matthew Weir, MD MSc; Paul Young, MD; Alexander Zarbock, MD; On behalf of the STARRT-AKI Investigators, the Canadian Critical Care Trials Group, the Canadian Nephrology Trials Network, the Australia and New Zealand Intensive Care Society Clinical Trials Group, the United Kingdom Critical Care Research Group and the Irish Critical Care Clinical Trials Network. Drs. Wald and Bagshaw contributed equally to this manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study is funded by the following sources: Canadian Institutes of Health Research (Open Operating Grant MOP142296); Canadian Institutes of Health Research in partnership with Baxter Healthcare (Industry-Partnered Operating Grant IPR 139081); Canadian Institutes of Health Research (2017 Project Grant 389635); the National Health and Medical Research Council of Australia (2016 Project Grant 1127121); the Health Research Council of New Zealand (2017 Project Grant 17/204), and the National Institutes of Health Research Health Technology Assessment Program (England) (2018 Reference Number: 17/42/74). In 2017, the STARRT-AKI was adopted by the National Institutes for Health Research (NIHR) (England) as a portfolio study. The funding organizations and partners were not involved in the design, implementation, or management of the trial. All analyses will be undertaken independent of the funding organizations and partners. All manuscripts relating to the trial will be written by the investigators, and the decisions to submit for publication will be independent from the funding organizations and partners.
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Tolwani A. Continuous renal-replacement therapy for acute kidney injury. N Engl J Med. 2012;367:2505-2514. [DOI] [PubMed] [Google Scholar]
- 2. Liu KD, Himmelfarb J, Paganini E, et al. Timing of initiation of dialysis in critically ill patients with acute kidney injury. Clin J Am Soc Nephrol. 2006;1(5):915-919. [DOI] [PubMed] [Google Scholar]
- 3. Vaara ST, Reinikainen M, Wald R, Bagshaw SM, Pettila V. Timing of RRT based on the presence of conventional indications. Clin J Am Soc Nephrol. 2014;9(9):1577-1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zarbock A, Kellum JA, Schmidt C, et al. Effect of early vs delayed initiation of renal replacement therapy on mortality in critically ill patients with acute kidney injury: the ELAIN randomized clinical trial. JAMA. 2016;315(20):2190-2199. [DOI] [PubMed] [Google Scholar]
- 5. Gaudry S, Hajage D, Schortgen F, et al. Initiation strategies for renal-replacement therapy in the intensive care unit. N Engl J Med. 2016;375(2):122-133. [DOI] [PubMed] [Google Scholar]
- 6. Barbar SD, Clere-Jehl R, Bourredjem A, et al. Timing of renal-replacement therapy in patients with acute kidney injury and sepsis. N Engl J Med. 2018;379:1431-1442. [DOI] [PubMed] [Google Scholar]
- 7. Kellum JA, Mehta RL, Levin A, et al. Development of a clinical research agenda for acute kidney injury using an international, interdisciplinary, three-step modified Delphi process. Clin J Am Soc Nephrol. 2008;3(3):887-894. [DOI] [PubMed] [Google Scholar]
- 8. Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int. 2012;2(Suppl):1-138. [Google Scholar]
- 9. National Clinical Guideline Centre. NICE Clinical Guideline 169. Acute kidney injury: prevention, detection and management of acute kidney injury up to the point of renal replacement therapy; 2013. https://www.nice.org.uk/guidance/cg169. [PubMed] [Google Scholar]
- 10. Bagshaw SM, Wald R, Barton J, et al. Clinical factors associated with initiation of renal replacement therapy in critically ill patients with acute kidney injury—a prospective multicenter observational study. J Crit Care. 2012;27(3):268-275. [DOI] [PubMed] [Google Scholar]
- 11. Clark E, Wald R, Levin A, et al. Timing the initiation of renal replacement therapy for acute kidney injury in Canadian intensive care units: a multicentre observational study. Can J Anaesth. 2012;59(9):861-870. [DOI] [PubMed] [Google Scholar]
- 12. Clark E, Wald R, Walsh M, Bagshaw SM. Timing of initiation of renal replacement therapy for acute kidney injury: a survey of nephrologists and intensivists in Canada. Nephrol Dial Transplant. 2012;27(7):2761-2767. [DOI] [PubMed] [Google Scholar]
- 13. Karvellas CJ, Farhat MR, Sajjad I, et al. A comparison of early versus late initiation of renal replacement therapy in critically ill patients with acute kidney injury: a systematic review and meta-analysis. Crit Care. 2011;15:R72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wald R, Adhikari NK, Smith OM, et al. Comparison of standard and accelerated initiation of renal replacement therapy in acute kidney injury. Kidney Int. 2015;88(4):897-904. [DOI] [PubMed] [Google Scholar]
- 15. Smith OM, Wald R, Adhikari NK, Pope K, Weir MA, Bagshaw SM. Standard versus accelerated initiation of renal replacement therapy in acute kidney injury (STARRT-AKI): study protocol for a randomized controlled trial. Trials. 2013;14:320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chan AW, Tetzlaff JM, Altman DG, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. 2013;158:200-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Grams ME, Sang Y, Coresh J, et al. Candidate surrogate end points for ESRD after AKI. J Am Soc Nephrol. 2016;27(9):2851-2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Billings FT, IV, Shaw AD. Clinical trial endpoints in acute kidney injury. Nephron Clin Pract. 2014;127(1-4):89-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vaara ST, Pettila V, Reinikainen M, Kaukonen KM. Population-based incidence, mortality and quality of life in critically ill patients treated with renal replacement therapy: a nationwide retrospective cohort study in Finnish intensive care units. Crit Care. 2012;16(1):R13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hoste EA, Bagshaw SM, Bellomo R, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 2015;41(8):1411-1423. [DOI] [PubMed] [Google Scholar]
- 21. O’Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics. 1979;35:549-556. [PubMed] [Google Scholar]
- 22. Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315:801-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hammond NE, Bellomo R, Gallagher M, et al. The Plasma-Lyte 148 v Saline (PLUS) study protocol: a multicentre, randomised controlled trial of the effect of intensive care fluid therapy on mortality. Crit Care Resusc. 2017;19(3):239-246. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Appendix_1,_2_,_3_and_4 for STandard versus Accelerated initiation of Renal Replacement Therapy in Acute Kidney Injury: Study Protocol for a Multi-National, Multi-Center, Randomized Controlled Trial by Ron Wald and Sean Bagshaw in Canadian Journal of Kidney Health and Disease