Abstract
Background:
Improving medication appropriateness is a priority of French national campaigns in nursing homes. A pilot study was conducted to evaluate the impact of a medication review in a French nursing home with a 3-month follow up.
Method:
A medication review was conducted in 2015 using version 2 STOPP and START criteria. The number and type of drugs meeting a STOPP that were reintroduced and the number and type of drug meeting a START that were stopped during follow up were measured. An expert committee adjudicated whether 3-month hospitalizations and deaths were related to medication review. The impact of medication review on the cost related to drug consumption was calculated for 3 months.
Results:
The 52 residents (age 84 ± 9 years, 83% female) fulfilled, on average, 2 ± 1.4 of the STOPP criteria and 0.7 ± 0.6 of the START criteria. A total of 101 drugs were stopped and 34 drugs were started. Five deaths occurred during follow up and were judged as not related to medication review. Five drugs stopped were reintroduced in five residents for a rebound effect or a symptom occurrence and one resident had stopped a START medication (aspirin) for a minor adverse drug reaction. At 3 months, a gain of 20.21 ± 31.34 euros per resident was observed.
Conclusion:
The medication review using version 2 STOPP and START criteria and involving the physician in charge seems useful for detecting and correcting inappropriate prescribing in a nursing home.
Keywords: inappropriate prescribing, nursing homes, older people, STOPP–START criteria
Introduction
Inappropriate prescribing is common for older people living in nursing homes in all Western countries1,2 including France.3–5 It encompasses both potentially inappropriate medications (PIMs, e.g. drugs or association of drugs with an unfavorable risk/benefit ratio) and potential prescription omissions (PPOs). The use of PIMs in residents was found to be associated with subsequent serious adverse outcomes (hospitalization, emergency visits and deaths).6,7 A recent systematic review found that the prevalence of PIM use varied widely from 18.5% to 82.6% of residents and PPOs from 30.5% to 74%, even among studies with similar characteristics. The authors concluded that ‘future policies should promote systematically executed medication reviews to make them standard practice in residential long-term care facilities.’8
In France, a program of the French National Authority for Health that related to the quality and safety of drug prescription in older people was developed in collaboration with the French Geriatrics Society.9 This program was influenced by the publication of the Assessing Care of Vulnerable Elders criteria,10 and consisted of providing tools to physicians, whatever their setting of practice, to perform a medication review in multimorbid older people. This medication review is recommended annually or in the occurrence of a new medical problem.
Indeed, medication review may help professionals to identify, to resolve drug issues and finally, to improve medication appropriateness.1 The Screening Tool of Older Person’s Prescriptions (STOPP) and Screening Tool to Alert doctors to Right Treatment (START) criteria aim at identifying PIMs and PPOs.11 This set of European criteria, based on current clinical evidence and consensus opinions, is currently considered the most relevant, explicit tool for identifying inappropriate prescribing.12–14 After a medication review using version 1 STOPP and START criteria, Frankenthal and colleagues15 found a significant reduction in both PIMs and PPOs which was sustained at 6 and 12 months. In 2014, the version 2 of the STOPP and START criteria was published, differing substantially from the version 1.16 To our knowledge, there is no published study which assesses whether its use in a nursing home is associated with a sustained impact on inappropriate prescribing.
In 2015, we decided to use version 2 STOPP and START criteria to perform the annual medication review recommended by the French National Authority for Health. A pilot study was conducted to check sustainability of the STOPP and START criteria at 3-month follow up.
Methods
Setting and participants
A prospective observational study was conducted in a French urban publicly funded nursing home attached to the Hospital of Montfermeil. This nursing home has 53 beds, including an Alzheimer unit with 10 beds. Two geriatricians (AS, RA) have the residents in charge and the hospital pharmacist (WT) analyzed drug prescription after each therapeutic change with a clinical decision support system. All prescriptions are computerized. Drug dispensing is weekly, automated, and nominative.
Medication review
To improve professional practices, a drug prescription review is implemented every year since 2015. This medication review is conducted by an expert geriatrician (MLGD) with one of the two physicians (AS, RA) and the pharmacist (WT) in charge of the residents. The expert geriatrician is not usually in charge of the residents. The resident’s nurse is interviewed prior to the review about medication administration: help with taking medication, crushing medication, and resident comments about their medication. This interview is qualitative. The medication review is performed from the electronic medical record of each resident using version 2 STOPP and START criteria. For each detected criterion, the team composed of the expert geriatrician, one of the physicians and the pharmacist confirmed, collegially, the presence or not of inappropriate prescribing. In a second step, medication prescription could be also improved out of the STOPP and START criteria using the expert clinical knowledge of the team. Once a year, the team meets 1 hour each week and examines, on average, six prescriptions. The medication changes are decided collegially during the review and proposed to the residents by the geriatrician in charge of them. Palliative care residents are excluded from the medication review.
In 2015, the medication reviews were prospectively conducted from 14 January 2015 to 18 March 2015. A pilot study was conducted from 14 January 2015 to 17 June 2015 to evaluate the impact of the medication review 3 months afterward. Opposition of the resident (or of his legal representative) to the collection of his personal data was recorded.
Version 2 STOPP and START criteria
Version 2 STOPP and START criteria includes 115 criteria; 81 STOPP criteria (classified in 14 sections) and 34 START criteria (classified in 9 sections). The STOPP criteria refer to PIMs, and START criteria refer to PPOs in patients aged 65 years and older. Each criterion is named by the letter of the section followed by its number.16
Baseline data collection
Data were collected anonymously from the medical record by the pharmacist (WT) after the medication review in a Microsoft Excel 97–2003® (Microsoft, Redmond, WA) table and included: age, sex, chronic diseases meeting STOPP and START criteria, number of medications per prescription, prescribed medications [International Nonproprietary Name and Anatomical Therapeutic Chemical (ATC) code], number of psychotropic drugs (see Table A1 in the Appendix) per prescription, the number and type of STOPP and START criteria and the associated therapeutic changes (stop, withdrawal and start of drugs). Others drug changes following medication review but not related to STOPP and START criteria were also collected in order to present all therapeutic changes resulting from medication review. The geriatrician (MLGD) checked the data collection for accuracy or any data entry error.
Primary outcome measures
Outcome measures included the number and type of drugs meeting a STOPP at baseline and reintroduced at 3 months and the number and type of drugs meeting a START at baseline and stopped during follow up. These outcomes were measured at exactly 3 months of the medication review for each resident using the electronic medical record. The reasons for changes were collected through the medical record and interview of the physician in charge of the resident. The maintenance of drugs changes following medication review but not associated with STOPP and START criteria was also assessed.
Secondary outcome measures
Secondary outcome measures were:
(1) The number of drug adverse events, deaths and hospitalizations occurring during the 3 months follow up that could be related to drug changes performed during the medication review. The clinical outcomes were collected retrospectively by the physician in charge and the expert geriatrician from the resident medical record. Then, an expert committee with geriatricians (AS, DBZ, MLGD, RA) and pharmacists (FF, WT) judged whether drug adverse events, deaths and hospitalizations occurring before the 3-month reassessment were related to drug changes resulting from medication review (distinguishing those resulting from correction of STOPP and START criteria and those resulting from other drug changes). Three types of conclusion could be made: not related, related or impossible to conclude (doubtful association or paucity of source data). The expert committee had access to the resident electronic medical record and to their hospitalization reports, including the hospitalization report during which the death occurred. On this committee, two geriatricians (DBZ, MLGD) and one pharmacist (FF) did not have residents in charge; one geriatrician (DBZ) and one pharmacist (FF) did not participate in the medication review.
(2) The cost at 3 months resulting from the use of STOPP and START screening tools in the nursing home. This cost was calculated by the pharmacist taking into account the daily cost of the stopped drugs (STOPP criteria), the daily cost of the introduced drugs (START criteria), the resumption date of the STOPPs and the stop date of the STARTs. The daily cost for each drug was the drug cost negotiated by the national central purchasing agency for hospitals and nursing homes. The cost of each drug is entered in the software PHARMA® (Computer Engineering version 5.4.40526, Paris, France) per dose. For each resident and for each stopped or introduced drug, the pharmacist has researched its cost per dose and its cost per day; then she calculated the sum of the stopped drugs over 3 months by taking into account its cost per day and its possible date of resumption; she calculated the sum of the introduced drugs over 3 months by taking into account its cost per day and its possible date of stop. The cost saving was the difference between the sum of the stopped drugs and the sum of the introduced drugs for 3 months.
Statistical analysis
The data was collected and analyzed in a Microsoft Excel 97-2003® table. Categorical variables are expressed as numbers and percentages. Quantitative variables are expressed as means and standard deviation.
Ethics
Ethical approval is not required for such studies at our institution.
The study objectives, procedures, and the observational design have been explained in lay language to potential participants or their carer (in case of severe dementia). In particular, they have been informed that they could refuse to participate. The nonopposition of the patient or their carer to participate was notified in the medical chart.
Results
Study population
The flow chart of the study is presented in figure 1 Fifty-three residents were living in the nursing home at the time of the study; one resident died before his medication review. No resident (or legal representative) was opposed to the collection of his personal data. A total of 52 residents were included: 43 women and 9 men, mean age 84 ± 9 years (minimum–maximum: 59–102). Their baseline medical characteristics are presented in Table 1.
Figure 1.
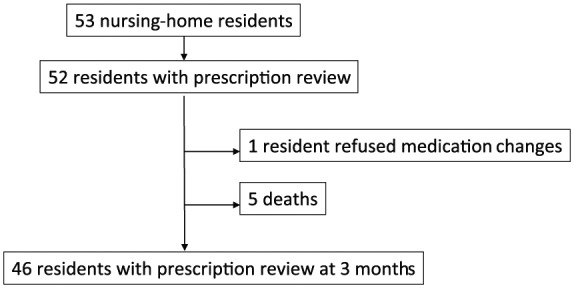
Study flow chart.
Table 1.
Baseline characteristics of residents.
| n (%) | |
|---|---|
| Age | 84 ± 9 years |
| Female | 43 (83%) |
| Chronic diseases (meeting STOPP and START criteria): | |
| Current depression | 37 (71%) |
| Dementia | 33 (63%) |
| Chronic constipation | 33 (63%) |
| Hypertension | 29 (56%) |
| Osteoporosis | 18 (35%) |
| Osteoarthritis | 12 (23%) |
| Atrial fibrillation | 10 (19%) |
| Dyslipidemia | 10 (19%) |
| Diabetes mellitus | 7 (13%) |
| Ischemic stroke | 7 (13%) |
| Ischemic heart disease | 6 (12%) |
| Renal failure (15 < Cockcroft–Gault estimate clearance < 30 ml/min) | 6 (12%) |
| Chronic obstructive pulmonary disease | 6 (12%) |
| Hypothyroidism | 6 (12%) |
| Venous insufficiency | 5 (10%) |
| Active cancer | 5 (10%) |
| Parkinson disease | 4 (8%) |
| Gastroesophageal reflux | 4 (8%) |
| Congestive heart failure | 3 (6%) |
| Peripheral arterial disease | 3 (6%) |
| Epilepsy | 2 (4%) |
| Current pulmonary embolism | 2 (4%) |
| Psychosis | 1 (2%) |
| Prostate adenoma | 1 (2%) |
| Aortic aneurysm | 1 (2%) |
| Polyneuropathy | 1 (2%) |
| Psoriasis | 1 (2%) |
START, Screening Tool to Alert doctors to Right Treatment; STOPP, Screening Tool of Older Person’s Prescriptions.
Baseline prescriptions
Their medications are presented in Table 2.
Table 2.
Medications of residents.
| Anatomical Therapeutic Chemical (ATC) class | International Nonproprietary Name (ATC code) | n (%) |
|---|---|---|
| Nervous system | 186 (42%) | |
| Analgesics | 55 | |
| Opioids | Oxycodone (N02AA05), fentanyl (N01AH01), tramadol (N02AX02), codeine (N02AJ01) | 15 |
| Other analgesics and antipyretics | Paracetamol (N02BE01) | 40 |
| Antiepileptics | Valproic acid (N03AG01), pregabalin (N03AX16), levetiracetam (N03AX14) | 12 |
| Anti-Parkinson drugs | Levodopa (N04BA01), trihexyphenidyl (N04AA01), tropatepine (N04AA12) | 6 |
| Psycholeptics | 39 | |
| Antipsychotics | Haloperidol (N05AD01), tiapride (N05AL03), loxapine (N05AH01), cyamemazine (N05AA06) | 10 |
| Anxiolytics | Oxazepam (N05BA04), alprazolam (N05BA12), lorazepam (N05BA06), bromazepam (N05BA08), prazepam (N05BA11), hydroxyzine (N05BB01) | 13 |
| Hypnotics and sedatives | Zopiclone (N05CF01), zolpidem (N05CF02), midazolam (N05CD08), scopolamine (N05CM05) | 16 |
| Psychoanaleptics | 74 | |
| Antidepressants | Mianserin (N06AX03), escitalopram (N06AB10), paroxetine (N06AB05), sertraline (N06AB06), fluoxetine (N06AB03), venlafaxine (N06AX16) | 53 |
| Drugs used to treat dementia | Memantine (N06DX01), rivastigmine (N06DA03), donepezil (N06DA02) | 21 |
| Cardiovascular system | 75 (17%) | |
| Cardiac therapy | Amiodaron (C01BD01), digoxin (C01AA05), nicorandil (C01DX16), glyceryl trinitrate (C01DA02) | 11 |
| Diuretics | Furosemide (C03CA01), chlorothiazide (C03AA04) | 17 |
| Beta-blocking agents | Bisoprolol (C07AB07) | 12 |
| Calcium-channel blockers | Amlodipine (C08CA01), nicardipine (C08CA04), diltiazem (C08DB01) | 9 |
| Agents acting on the renin–angiotensin system | Irbesartan (C09CA04), ramipril (C09AA05), candesartan (C09CA06), lisinopril (C09AA03), perindopril (C09AA04), valsartan (C09CA03) | 19 |
| Lipid-modifying agents | 7 | |
| HMG-CoA reductase inhibitors | Atorvastatin (C10AA05), simvastatin (C10AA01), pravastatin (C10AA03), rosuvastatin (C10AA07) | 6 |
| Fibrates | Fenofibrate (C10AB05) | 1 |
| Alimentary tract and metabolism | 118 (27%) | |
| Drugs for acid-related disorders | Esomeprazole (A02BC05), antiacid with sodium bicarbonate (A02AH) | 35 |
| Drugs for functional gastrointestinal disorders | Phloroglucinol (A03AX12) | 2 |
| Drugs for constipation | Lactulose (A06AD11), macrogol (A06AD15), liquid paraffin (A06AA01) | 45 |
| Drugs used in diabetes | Insulin glargine (A10AE04), insulin aspart (A10AB05), metformin (A10BA02), sitagliptin (A10BH01), gliclazide (A10BB09) | 12 |
| Vitamin D and analogs | Vitamin D (A11CC) | 8 |
| Mineral supplements | Potassium (A12BA), calcium (A12AA) | 16 |
| Blood and blood-forming organs | 30 (7%) | |
| Antithrombotic agents | 24 | |
| Vitamin K antagonists | Fluindione (B01AA12), warfarin (B01AA03) | 3 |
| Direct factor Xa inhibitors | Rivaroxaban (B01AF01), dabigatran (B01AE07) | 9 |
| Platelet aggregation inhibitors | Acetylsalicylic acid (B01AC06), clopidogrel (B01AC04) | 12 |
| Antihemorrhagics | Tranexamic acid (B02AA02) | 1 |
| Antianemic preparations | Oral iron (B03AA02), cyanocobalamin (B03BA01), folic acid (B03BB01) | 5 |
| Musculoskeletal system | 7 (2%) | |
| Muscle relaxants | Baclofen (M03BX01) | 1 |
| Antigout preparations | Allopurinol (M04AA01), colchicine (M04AC01) | 3 |
| Drugs for treatment of bone diseases | Alendronic acid (M05BA04) | 3 |
| Respiratory system | 12 (3%) | |
| Drugs for obstructive airway disease | Salbutamol (R03AC02), formoterol (R03AC13), salmeterol and fluticasone (R03AK06), beclomethasone (R03BA01) | 7 |
| Antihistamines for systemic use | Cetirizine (R06AE07) | 5 |
| Systemic hormonal preparations, excluding sex hormones and insulins | 7 (2%) | |
| Corticosteroids for systemic use | Prednisone (H02AB07) | 1 |
| Thyroid therapy | Levothyroxine sodium (H03AA01) | 6 |
| Antineoplastic and immunomodulating agents | 1 (<1%) | |
| Endocrine therapy | Bicalutamide (L02BB03) | 1 |
| Genito-urinary system and sex hormones | 2 (<1%) | |
| Sex hormones and modulators of the genital system | Cyproterone (G03HA01) | 1 |
| Urologicals | Finasteride (G04CB01) | 1 |
| Sensory organs | 1 (<1%) | |
| Ophthalmologicals | Timolol (S01ED01) | 1 |
| Total | 439 | |
HMG-CoA, beta-hydroxy beta-methylglutaryl coenzyme A.
A mean of 8.5 ± 3.5 medications (minimum–maximum: 2–18) were prescribed per resident and 39 (75%) residents were taking 7 medications or more.
A mean of 1.6 ± 1.2 psychotropic drugs (minimum–maximum: 0–4) were prescribed per resident and 12 (23%) residents were taking 3 psychotropic drugs or more.
Inappropriate prescribing at baseline according to STOPP and START criteria
STOPP criteria
The residents fulfilled on average 2 ± 1.4 STOPP criteria (minimum–maximum: 0–6).
A total of 45 (86.5%) residents fulfilled at least 1 STOPP criterion: 15 (28.8%) residents fulfilled 1 STOPP criterion, 14 (26.9%) residents fulfilled 2 STOPP criteria, 8 (15.4%) residents fulfilled 3 STOPP criteria, 5 (9.6%) residents fulfilled 4 STOPP criteria, 2 (3.8%) residents fulfilled 5 STOPP criteria and 1 (1.9%) resident fulfilled 6 STOPP criteria.
The most frequent STOPP criteria were the drug prescribed beyond the recommended duration (A2), the drug prescribed without indication (A1), and duplication in a drug class (A3). Type, prevalence, and drugs meeting the criteria are detailed Table 3.
Table 3.
STOPP criteria at baseline: type, prevalence and drugs meeting the criteria.
| STOPP criteria | Prevalence, n (%) | Drugs implicated |
|---|---|---|
| A1: any drug prescribing without an evidence-based clinical indication | 26 (50%) | Proton-pump inhibitor (n =
15) Memantine (n = 4) Antidepressant (n = 2) Acetylsalicylic acid (n = 1) Beta blocker (n = 2) Calcium supplement (n = 1) Aluminum antacid (n = 1) |
| A2: any drug prescribed beyond the recommended duration, where treatment duration is well defined | 33 (63%) | Calcium supplement (n = 4) H1 antagonist (n = 4) Memantine (n = 5) Potassium supplement (n = 3) Vasodilator drugs (n = 3) Acetylcholinesterase inhibitor (n = 2) Beta blocker (n = 2) Paracetamol (n = 2) Alprazolam (n = 1) Vitamin B9 supplement (n = 1) Fenofibrate (n = 1) Bisphosphonate (n = 1) Tranexamic acid (n = 1) Nifedipine (n = 1) Nicardipine (n = 1) Colchicine (n = 1) |
| A3: any duplicate drug class | 18 (35%) | Duplication of antidepressants (n =
10) Duplication of laxative drugs (n = 5) Duplication of vitamin D supplements (n = 1) Duplication of antiarrhythmic drugs (digoxin and beta blocker n = 1) Duplication of antiacid drugs (n = 1) |
| B1: digoxin for heart failure with normal systolic ventricular function | 1 (2%) | Digoxin (n = 1) |
| B7: loop diuretic for dependent ankle edema without clinical, biochemical evidence or radiological evidence of heart failure, liver failure, nephrotic syndrome or renal failure | 3 (6%) | Furosemide (n = 3) |
| D5: benzodiazepine for ⩾4 weeks | 2 (4%) | Lorazepam (n = 2) |
| D8: anticholinergics in patients with delirium or dementia | 1 (2%) | Hydroxyzine (n = 1) |
| D9: neuroleptic antipsychotic in patients with behavioral and psychological symptoms of dementia (unless symptoms are severe and other treatments have failed) | 3 (6%) | Haloperidol (n = 1) Loxapine (n = 1) Tiapride (n = 1) |
| F3: drugs likely to cause constipation in patients with chronic constipation where nonconstipating alternatives are appropriate | 5 (10%) | Aluminum antacid (n = 3) Oral iron (n = 2) |
| J1: sulfonylureas with a long duration of action with type 2 diabetes mellitus | 2 (4%) | Gliclazide (n = 2) |
| K1: benzodiazepines | 1 (2%) | Oxazepam (n = 1) |
| K2: neuroleptic drugs | 3 (6%) | Haloperidol (n = 1) Loxapine (n = 1) Cyamemazine (n = 1) |
| K4: hypnotic Z drugs | 4 (8%) | Zolpidem (n = 4) |
| L1: use of oral or transdermal strong opioids as first-line therapy for mild pain | 1 (2%) | Tramadol (n = 1) |
STOPP, Screening Tool of Older Person’s Prescriptions.
One hundred and three drugs met a STOPP criterion. The most frequently met drug classes were: nervous system (n = 39), alimentary tract and metabolism (n = 34) and cardiovascular system (n = 14).
START criteria
The residents fulfilled on average 0.7 ± 0.6 START criteria (minimum–maximum: 0–2).
A total of 30 (57.7%) residents had at least 1 START criterion: 26 (50%) residents had 1 START criterion and 4 (7.7%) residents had 2 START criteria.
The most frequent START criteria were the lack of vitamin D supplement (E5) in almost half of cases and lack of antihypertensive therapies despite proven hypertension (A4). See details in Table 4.
Table 4.
START criteria at baseline: type, prevalence and drugs meeting the criteria.
| START criteria | Prevalence, n (%) | Drugs implicated |
|---|---|---|
| E5: vitamin D supplement in older people who are housebound or experiencing falls or with osteopenia | 28 (54%) | Vitamin D supplement |
| A4: antihypertensive therapy where systolic blood pressure consistently > 160 mmHg or diastolic blood pressure consistently > 90 mmHg; if systolic blood pressure > 140 mmHg or diastolic blood pressure > 90 mmHg if diabetic | 3 (6%) | Perindopril (n = 2) Ramipril (n = 1) |
| A6: angiotensin-converting enzyme inhibitor with systolic heart failure or documented coronary artery disease | 1 (2%) | Ramipril (n = 1) |
| A3: antiplatelet therapy with a documented history of coronary, cerebral or peripheral vascular disease | 1 (2%) | Aspirin (n = 1) |
| C2: non-TCA antidepressant drug in the presence of persistent major depressive symptoms | 1 (2%) | Mianserin (n = 1) |
TCA, tricyclic antidepressant; START, Screening Tool to Alert doctors to Right Treatment.
Drug changes following medication review
Drug changes according to STOPP and START criteria
One resident refused to stop two drugs meeting STOPP criteria (proton-pump inhibitor and nifedipine).
All the others drugs meeting STOPP criteria were stopped [n = 101, 34 (34%) drugs classified in the ‘alimentary tract and metabolism’ ATC class, 40 (40%) in ‘nervous system,’ 15 (15%) in ‘cardiovascular system,’ 5 (5%) in ‘respiratory system,’ 4 (4%) in ‘blood and blood-forming organs,’ 3 (3%) in ‘musculoskeletal system’] or were prescribed a decreased dosage in the case of a weaning-off perspective (n = 7, 4 hypnotic Z drugs, 1 benzodiazepine and 2 neuroleptic drugs).
According to START criteria, 34 drugs were started: 28 (82%) vit D3 supplements, 4 (12%) antihypertensive drugs, 1 (3%) low-dose aspirin and 1 (3%) antidepressant.
Drug changes according to medication review out of STOPP and START criteria
Additional drug changes were prescribed following medication review out of the correction of STOPP and START criteria (see Table 5).
Table 5.
Drug changes following medication review out of the correction of STOPP and START criteria.
| Type of drug change | Details |
|---|---|
| Drug switches (n = 2) | – Stop clopidogrel and start aspirin according to
indication (aortic aneurysm) – Stop NOVOMIX30® and start LEVEMIR® to reduce the risk of hypoglycemia in a frail elder |
| Change in dose according to indication (n = 3) | – Esomeprazole from 40 mg per day to 20 mg in
gastroesophageal reflux disease – Aspirin from 160 mg per day to 75 mg in ischemic heart disease – Escitalopram from 10 mg per day to 5 mg per day in anxiety |
| Change in dose according to weight (n = 1) | – Paracetamol from 3000 mg per day to 2000 mg per day |
| Change in dose according to creatinine clearance (n = 3) | – Lisinopril from 20 mg per day to 10 mg per
day – Allopurinol from 100 mg per day to 100 mg every 2 days – Sitagliptin from 100 mg per day to 50 mg per day |
| Change in dose after clinical evaluation (n = 5) | – Mianserin from 10 mg per day to 30 mg per day in the
presence of depressive symptoms (n =
2) – Mianserin from 45 mg per day to 60 mg per day in the presence of depressive symptoms – Furosemide from 40 mg per day to 20 mg per day in the absence of congestive symptoms (n = 2) |
| Change in medication schedule in frail elders (n = 2) | – Insulin glargine injection from evening to morning to
reduce the risk of hypoglycemia – Irbesartan from morning to evening to reduce the risk of orthostatic hypotension |
START, Screening Tool to Alert doctors to Right Treatment; STOPP, Screening Tool of Older Person’s Prescriptions.
Maintenance of drug changes at 3 months
Among the remaining 46 patients, 5 drugs stopped were reintroduced in 5 residents at 3 months due to rebound effect or symptom occurrence:
(1) Digestive symptoms 1 week after stopping proton-pump inhibitor treatment (n = 1), and 4 weeks after stopping sodium alginate treatment (n = 1);
(2) Pain 12 weeks after stopping paracetamol (n = 1);
(3) Sleep disorder 6 weeks after stopping mianserin (n = 1);
(4) Hypokalemia 6 weeks after the stop of potassium supplement (n = 1).
Only one resident had stopped a START medication at 3 months: aspirin was stopped 2 weeks after its start due to adverse drug reaction (rectal bleeding in the context of hemorrhoids) that then resolved once stopped.
Four other drug changes were not maintained: decreased dosage of proton-pump-inhibitor treatment and of loop diuretic were not maintained due to rebound effects after 1 week and 2 weeks, respectively. The two increased dosages of mianserin were not maintained due to swallowing disorders in one case, and palliative care with impossible oral medication administration for the other case.
Adverse outcomes during follow up
Five residents died before the 3-month re-evaluation: three died in the nursing home (two in the context of palliative care, and one from pneumonia) and two residents died during an emergency hospitalization (one for stroke, one for pneumonia). There was no other resident hospitalization during the follow up. The characteristics of the residents who died are detailed in the Table 6. The expert committee concluded that no death was related to drug changes induced by the baseline medication review.
Table 6.
Characteristics of residents who died.
| Date of medication review | Date of death | Cause of death | STOPP criteria | START criteria |
|---|---|---|---|---|
| 28 January 2015 | 16 April 2015 | Palliative care context | A1 (proton-pump inhibitor) | E5 (vitamin D supplement) |
| 28 January 2015 | 15 March 2015 | Pneumonia | A2 (colchicine) J1 (gliclazide) A2 (memantine) |
|
| 11 February 2015 | 24 February 2015 | Pneumonia | A3 (duplication of antidepressants) | |
| 18 February 2015 | 7 April 2015 | Palliative care context | A 2 (nicardipine) | E5 (vitamin D supplement) |
| 18 February 2015 | 5 April 2015 | Stroke | A2 (vasodilator drug) B1 (digoxin) A2 (alprazolam) A3 (antiacid) |
START, Screening Tool to Alert doctors to Right Treatment; STOPP, Screening Tool of Older Person’s Prescriptions.
Evolution of drug-consumption-related cost at 3 months
At 3 months, the cost saving was 20.21 ± 31.34 euros per resident with a total cost saving of 949.78 euros at the nursing-home level.
Discussion
Among the prescriptions of 52 consecutive residents of a French hospital-based nursing home, the prevalence of version 2 STOPP and START criteria assessed during a pharmacist- and geriatrician-conducted comprehensive review were high: 86.5% of the residents fulfilled at least one STOPP criterion, the two most frequent being a proton-pump inhibitor prescribed without an evidence-based clinical indication and the duplication of antidepressants; and 57.7% of the residents fulfilled at least one START criterion, the two most frequent being the lack of vitamin D supplement and the lack of antihypertensive therapies despite proven hypertension. According to the medication review, 101 drugs were stopped (mostly those related to alimentary tract and central nervous system) and 34 started (mostly 25-OH vitamin D3 supplements and antihypertensive therapies). At 3 months, these changes were mainly maintained: only five drugs among those stopped (4.9%) were reintroduced, due to rebound effect or symptom occurrence, and one aspirin among drugs started (2.9%) was stopped due to nonserious hemorrhage.
The prevalence of PIMs and PPOs were higher than those described in previous studies conducted in nursing homes. According to the most recent reviews,2,8,17 the rate of residents with at least one STOPP varied between 23.7% and 79.8%, with an average around 60% versus 86.5% in our study, and the rate of residents with at least one START varied between 30.5% and 74%, with an average around 49% versus 57.7% in our study. There are a few possible explanations. First, the studies included in these reviews were conducted using version 1 STOPP and START criteria, which differ widely from the version 2. Indeed, 12 STOPP criteria were removed from version 1, and 16 were added in the second version.16 A study comparing assessment of the prevalence of PIMs according to version 1 versus version 2 has found that the level of agreement between them was moderate; the sensitivity to detect PIMs being significantly improved in the updated version.18 Thus, our PIM prevalence is close to those found in 57 Belgian nursing homes using the same version (88.3%).19 Regarding START criteria, 3 were removed, and 15 were added in the second version that could explain how our prevalence of PPOs was often higher than those previously described with version 1; however, it remained lower than that described in Belgian nursing homes (57.7% versus 85%).19 Second, the prevalence of inappropriate prescribing assessed by STOPP and START differed between countries, with European countries having higher prevalence than those elsewhere and from the period of study, and with higher prevalence in recent studies.2 Finally, the method of medication review may influence the number of detected STOPP and START criteria. However, our medication review was based mainly on medical records whose use is usually associated with a lower detection of STOPP and START criteria comparatively with review performed without.20
Considering the drugs and type implicated in inappropriate prescribing, PIMs without indication were previously described as one of the most frequent PIMs in nursing homes.8,17 Inappropriate use of psychotropic drugs has also been described as a major problem in all studies conducted in nursing homes. However, it was usually the benzodiazepines first meeting STOPP criteria.8,17,19 Surprisingly, benzodiazepines were only the ‘second class’ of psychotropic drugs meeting STOPP criteria in our study (8/103 STOPP), antidepressants being the first (12/103) due mainly to duplication of antidepressants. Antidementia drugs were also almost twice as involved in the inappropriate prescriptions than neuroleptics (11/103 versus 6/103). These results could be explained by the fact that the national health plan is focused on the reduction of benzodiazepines and neuroleptics, especially in older people and in demented people,9 with specific actions led in nursing homes by the French national health insurance. The reduction in the use of these two drug classes could have led to switches toward antidepressants and antidementia drugs as described previously,21,22 explaining why inappropriate prescribing of antidepressants and antidementia became more frequent in residents. Regarding the START criteria, we found the lack of vitamin D supplements was commonly described as the major PPO in previous studies conducted in nursing homes.
Concerning the maintenance of drug change following medication review using STOPP and START criteria, previous studies suggested that significant improvements in prescribing appropriateness were sustained during follow up varying from 6 to 24 months.15,23–25 However, inappropriate prescribing was assessed using version 1 STOPP and START criteria in all these studies; only one was performed in nursing homes15,23 and medication review was performed by a pharmacist,23 a physician,24 or a geriatric team25 making recommendations to the physician in charge who could accept/reject the drug changes. Our study is the first to investigate the maintenance of drug changes following a medication review using version 2 STOPP and START criteria and performed by a team integrating the physician in charge of the residents. The low rate of STOPP- and START-related drugs, respectively, restarted (4.9%) or stopped (2.9%) at 3 months in our study suggesting that the large majority of the drug changes performed were pertinent. This is reinforced by the lack of adverse outcomes related to these drug changes during follow up, with the exception of one nonserious bleeding related to aspirin start. However, the pertinence of starting new preventative drugs (e.g. vitamin D and antihypertensives) could be questioned in these older people who usually have limited life expectancy. The STOPPFrail criteria published in 2017, dedicated to assessment of inappropriate prescribing in frailer older people with poor 1-year survival prognosis26,27 and applicable to the majority of patients awaiting long-term care28 are a promising tool for drug optimization in nursing homes. Further studies are needed to demonstrate if their use in residents is associated with less adverse clinical outcomes (hospitalizations, emergency visits, falls) but also with better cognitive function and quality of life.
Another interesting point is that the large majority (57.3%) of the PIMs detected in our study was related to the first two STOPP criteria (any drug prescribing without an evidence-based clinical indication and any drug prescribed beyond the recommended duration) which are more implicit than explicit criteria. This finding reinforces the need for a medication review based on medical records and performed with the physician in charge of the residents. At least, our economic analysis tended to demonstrate the effectiveness of the version 2 STOPP and START criteria, as it was previously demonstrated for version 1 of the tool. Further studies are needed to explore this health economic relevance by considering direct and indirect health costs.
Our study has several strengths: a prospective design with no loss to follow up; an original medication review (using version 2 STOPP and START criteria and associating nurse and physician in charge of the patients); and a follow up that included analysis of adverse patient outcomes and their relationship to drug changes at medication review. The limits of our study are: a small number of residents in a single center, limiting the generalizability of the findings; a short follow up; a restricted economic analysis (focused only on costs related to drug consumption); and the lack of analysis of the impact of the medication review on the quality of life of residents.
Conclusion
The findings of our study confirm that inappropriate prescribing is highly prevalent in nursing homes. It is suggested that medication review using version 2 STOPP and START criteria is useful for detecting and correcting inappropriate prescribing to that which would be sustainable and efficient. However, the most frequent STOPP criteria were closely related to drug indication and duration, suggesting that medication review should be based on the medical record and performed in association with the physician in charge of residents.
Appendix
Table A1.
Psychotropic drugs with ATC code.
| Antipsychotics | N05AA06 Cyamemazine |
| N05AD01 Haloperidol | |
| N05AH01 Loxapine | |
| N05AL03 Tiapride | |
| Antidepressants | N06AB03 Fluoxetine |
| N06AB05 Paroxetine | |
| N06AB06 Sertraline | |
| N06AB10 Escitalopram | |
| N06AX03 Mianserin | |
| N06AX16 Venlafaxine | |
| Anxiolytics, Hypnotics and other drugs used as anxiolytics and hypnotics | N05BA04 Oxazepam |
| N05BA06 Lorazepam | |
| N05BA08 Bromazepam | |
| N05BA11 Prazepam | |
| N05BA12 Alprazolam | |
| N05BB01 Hydroxyzine | |
| N05CF01 Zopiclone | |
| N05CF02 Zolpidem |
ATC, Anatomical Therapeutic Chemical Classification System.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Marie-Line Gaubert-Dahan, CMLS Les Ormes, Groupe Hospitalier Intercommunal Le Raincy-Montfermeil, 13 Place Jean Mermoz 93 370 Montfermeil, France.
Abdellatif Sebouai, Groupe Hospitalier Intercommunal Le Raincy-Montfermeil, Pôle Gériatrie-SSR, Montfermeil, France.
Wafaa Tourid, Groupe Hospitalier Intercommunal Le Raincy-Montfermeil, Service de Pharmacie, Montfermeil, France.
Francis Fauvelle, Groupe Hospitalier Intercommunal Le Raincy-Montfermeil, Service de Pharmacie, Montfermeil, France.
Raoul Aikpa, Groupe Hospitalier Intercommunal Le Raincy-Montfermeil, Pôle Gériatrie-SSR, Montfermeil, France.
Dominique Bonnet-Zamponi, Observatoire du Médicament des Dispositifs Médicaux et de l’Innovation Thérapeutique, Paris, France; Département Biostatistique Santé Publique et Information Médicale, Centre de Pharmaco-épidémiologie (Cephepi), Paris, France.
References
- 1. Alldred DP, Kennedy MC, Hughes C, et al. Interventions to optimise prescribing for older people in care homes. Cochrane Database Syst Rev 2016; 2: CD009095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Morin L, Laroche ML, Texier G, et al. Prevalence of potentially inappropriate medication use in older adults living in nursing homes: a systematic review. J Am Med Dir Assoc 2016; 17: 862.e1–e9. [DOI] [PubMed] [Google Scholar]
- 3. Cool C, Cestac P, Laborde C, et al. Potentially inappropriate drug prescribing and associated factors in nursing homes. J Am Med Dir Assoc 2014; 15: 850.e1–e9. [DOI] [PubMed] [Google Scholar]
- 4. Beuscart JB, Genin M, Dupont C, et al. Potentially inappropriate medication prescribing is associated with socioeconomic factors: a spatial analysis in the French Nord-Pas-de-Calais region. Age Ageing 2017; 46: 607–613. [DOI] [PubMed] [Google Scholar]
- 5. Rousseau A, Rybarczyk-Vigouret MC, Vogel T, et al. Inappropriate prescription and administration of medications in 10 nursing homes in Alsace, France. Revue d’Epidémiologie et de Santé Publique 2016; 64: 95–101. [DOI] [PubMed] [Google Scholar]
- 6. Lau DT, Kasper JD, Potter DE, et al. Hospitalization and death associated with potentially inappropriate medication prescriptions among elderly nursing home residents. Arch Intern Med 2005; 165: 68–74. [DOI] [PubMed] [Google Scholar]
- 7. Perri M, III, Menon AM, Deshpande AD, et al. Adverse outcomes associated with inappropriate drug use in nursing homes. Ann Pharmacother 2005; 39: 405–411. [DOI] [PubMed] [Google Scholar]
- 8. Storms H, Marquet K, Aertgeerts B, et al. Prevalence of inappropriate medication use in residential long-term care facilities for the elderly: a systematic review. Eur J Gen Pract 2017; 23: 69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. French National Authority for Health (Haute Autorité de Santé, HAS). Programmes pilotes: prescription médicamenteuse chez le sujet agé (PMSA), http://www.has-sante.fr/portail/jcms/j_5/accueil (accessed 1 November 2005).
- 10. Shekelle PG, MacLean CH, Morton SC, et al. Assessing care for vulnerable elders: methods for developing quality indicators. Ann Intern Med 2001; 135: 647–652. [DOI] [PubMed] [Google Scholar]
- 11. Gallagher P, Ryan C, Byrne S, et al. STOPP (Screening Tool of Older Person’s Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment). Consensus validation. Int J Clin Pharmacol Ther 2008; 46: 72–83. [DOI] [PubMed] [Google Scholar]
- 12. Page RL, Linnebur SA, Bryant LL, et al. Prescribing in the hospitalized elderly patients: defining the problem, evaluation tools, possible solutions. Clin Interv Aging 2010; 5: 75–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hamilton H, Gallagher P, Ryan C, et al. Potentially inappropriate medications defined by STOPP criteria and the risk of adverse drug events in older hospitalized patients. Arch Intern Med 2011; 171: 1013–1019. [DOI] [PubMed] [Google Scholar]
- 14. Levy HB, Marcus EL, Christen C. Beyond the Beers criteria: a comparative overview of explicit criteria. Ann Pharmacother 2010; 44: 1968–1975. [DOI] [PubMed] [Google Scholar]
- 15. Frankenthal D, Lerman Y, Kalendaryev E, et al. Intervention with the screening tool of older persons potentially inappropriate prescriptions/screening tool to alert doctors to right treatment criteria in elderly residents of a chronic geriatric facility: a randomized clinical trial. J Am Geriatr Soc 2014; 62: 1658–1665. [DOI] [PubMed] [Google Scholar]
- 16. O’Mahony D, O’Sullivan D, Byrne S, et al. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing 2015; 44: 213–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Thomas RE. Assessing medication problems in those ⩾ 65 using the STOPP and START criteria. Curr Aging Sci 2016; 9: 150–158. [DOI] [PubMed] [Google Scholar]
- 18. Blanco-Reina E, García-Merino MR, Ocaña-Riola R, et al. Assessing potentially inappropriate prescribing in community-dwelling older patients using the updated version of STOPP-START criteria: a comparison of profiles and prevalences with respect to the original version. PLoS One 2016; 11: e0167586. eCollection 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Anrys PMS, Strauven GC, Foulon V, et al. Potentially inappropriate prescribing in Belgian nursing homes: prevalence and associated factors. J Am Med Dir Assoc 2018; 19: 884–890. [DOI] [PubMed] [Google Scholar]
- 20. Ryan C, O’Mahony D, O’Donovan DÓ, et al. A comparison of the application of STOPP/START to patients’ drug lists with and without clinical information. Int J Clin Pharm 2013; 35: 230–235. [DOI] [PubMed] [Google Scholar]
- 21. Nørgaard A, Jensen-Dahm C, Gasse C, et al. Time trends in antipsychotic drug use in patients with dementia: a nationwide study. J Alzheimers Dis 2015; 49: 211–220. [DOI] [PubMed] [Google Scholar]
- 22. Breining A, Bonnet-Zamponi D, Zerah L, et al. Exposure to psychotropics in the French older population living with dementia: a nationwide population-based study. Int J Geriatr Psychiatry 2017; 32: 750–760. [DOI] [PubMed] [Google Scholar]
- 23. Frankenthal D, Lerman Y, Kalendaryev E, et al. Intervention with the screening tool of older persons potentially inappropriate prescriptions/screening tool to alert doctors to right treatment criteria in elderly residents of a chronic geriatric facility: a randomized clinical trial. J Am Geriatr Soc 2014; 62: 1658–1665. [DOI] [PubMed] [Google Scholar]
- 24. Gallagher PF, O’Connor MN, O’Mahony D. Prevention of potentially inappropriate prescribing for elderly patients: a randomized controlled trial using STOPP/START criteria. Clin Pharmacol Ther 2011; 89: 845–854. [DOI] [PubMed] [Google Scholar]
- 25. Dalleur O, Boland B, Losseau C, et al. Reduction of potentially inappropriate medications using the STOPP criteria in frail older inpatients: a randomised controlled study. Drugs Aging 2014; 31: 291–298. [DOI] [PubMed] [Google Scholar]
- 26. Lavan AH, Gallagher P, Parsons C, et al. STOPPFrail (Screening Tool of Older Persons Prescriptions in frail adults with limited life expectancy): consensus validation. Age Ageing 2017; 46: 600–607. [DOI] [PubMed] [Google Scholar]
- 27. Curtin D, Dukelow T, James K, et al. Deprescribing in multi-morbid older people with polypharmacy: agreement between STOPPFrail explicit criteria and gold standard deprescribing using 100 standardized clinical cases. Eur J Clin Pharmacol 2019; 75: 427–432. [DOI] [PubMed] [Google Scholar]
- 28. Lavan AH, O’Mahony D, Gallagher P. STOPPFrail (Screening Tool of Older Persons’ Prescriptions in frail adults with a limited life expectancy) criteria: application to a representative population awaiting long-term nursing care. Eur J Clin Pharmacol. Epub ahead of print 26 January 2019. DOI: 10.1007/s00228-019-02630-3. [DOI] [PubMed] [Google Scholar]


