Abstract
Background:
Adjuvant bisphosphonates reduce the rate of breast cancer recurrence in the bone and improve breast cancer survival. However, the risk of adverse events associated with bisphosphonate therapy for breast cancer remains poorly defined.
Methods:
A literature search was conducted using the PubMed, EMBASE, Cochrane and Web of Science libraries. Risk ratio (RR) was calculated to evaluate the adverse events of the meta-analytic results. Osteonecrosis of the jaw (ONJ) incidence was calculated using the random effect model (D+L pooled) for meta-analysis.
Results:
A total of 47 studies comprising 20,607 patients were included; 23 randomized controlled studies (RCTs) provided data of adverse events for bisphosphonate therapy versus without bisphosphonates. Bisphosphonates were significantly associated with influenza-like illness (RR = 4.52), fatigue (RR = 1.08), fever (RR = 1.82), dyspepsia (RR = 1.25), anorexia (RR = 1.29), and urinary tract infection (RR = 1.32). No differences were observed in other adverse events. We combined the incidence of ONJ in 24 retrospective studies to analyze the incidence of ONJ using bisphosphonates. The pooled probability of ONJ toxicity in the bisphosphonates group was 2%.
Conclusions:
Bisphosphonates were significantly associated with influenza-like illness, fatigue, fever, dyspepsia, anorexia, and urinary tract infection. Furthermore, bisphosphonates increase the risk of ONJ toxicity.
Keywords: adverse events, bisphosphonates, breast cancer, neoadjuvant therapy, osteonecrosis of the jaw
Introduction
Breast cancer is the most common malignant cancer among women worldwide, in both developing and developed countries. The survival rate of breast cancer is highly correlated with the degree of disease at the time of diagnosis, with early stage disease conferring superior survival rates. However, the majority of patients with advanced breast cancer develop bone metastases.1 Some studies have reported that increased osteoclast activity in patients with breast cancer and breast cancer bone metastases leads to skeletal-related events (SREs), which include bone fractures, hypercalcemia, nerve compression, and severe pain.2 These skeletal complications, in turn, increase the need for palliative radiation or surgery to bone, limit functional independence, adversely affect quality of life, and continue to cause morbidity of the affected patients.3,4
Bisphosphonates are effective inhibitors of osteoclast-mediated bone resorption and have been approved for the prevention and treatment of postmenopausal osteoporosis and corticosteroid-induced bone metastases.5–7 Bisphosphonates are analogues to pyrophosphates, which inhibit bone resorption by interfering with osteoclast activation and promoting osteoclast apoptosis.8,9 Due to their antiresorptive properties, bisphosphonates are used to prevent excessive activation of osteoclasts due to cancer cells in the bone. Therefore, by blocking the vicious circle, bisphosphonates can prevent bone complications caused by tumor-induced osteolysis.10,11 Zoledronic acid (ZOL), ibandronate, pamidronate, and clodronate are approved for the prevention of skeletal complications in breast cancer patients with bone metastases.12–14 In the randomized, double-blind ProBONE II trial, a 2-year treatment with ZOL 4 mg intravenous every 3 months prevented cancer-treatment-induced bone loss in premenopausal women with breast cancer, and maintained bone mass density (BMD) up to 3 years post-treatment.15 At present, bisphosphonates are the standard treatment for the prevention of SREs, especially for patients with breast cancer and metastatic bone disease.16
Although bisphosphonates can be effective in the prevention of SREs in patients with breast cancer and breast cancer bone metastases, they have been associated with an increased risk of adverse events such as renal toxicity,17,18 acute-phase reactions, osteonecrosis of the jaw (ONJ), and intravenous administration.19 However, these are rare events among patients enrolled in clinical trials, and individual studies designed to demonstrate efficacy of each of these agents have been ineffective in detecting statistically significant differences in the incidence of adverse events. In addition, it is difficult to determine which of these adverse events are caused by bisphosphonates and which are caused by cancer. Since the clinical use of bisphosphonate therapy should be based on a balance between efficacy and safety, a solid understanding of the safety profile of these drugs is of critical clinical value. To determine the risk of adverse events associated with these agents, we conducted a systematic review and meta-analysis of the literature to determine the incidence and risk of adverse events induced by bisphosphonates in patients with breast cancer.
Methods
Search strategy
This study was reported in accordance with both the PRISMA statement for reporting systematic reviews and meta-analyses.20 A systematic literature search of the PubMed, EMBASE, Cochrane and Web of Science databases up to November 2018 was conducted by two study investigators (YYL and XRL) independently. The following search terms, treated as free text or mesh terms, were used: ‘bisphosphonates,’ ‘alendronate,’ ‘clodronate,’ ‘ibandronate,’ ‘pamidronate’, ‘risedronate,’ ‘zoledronic acid,’ combined with ‘breast neoplasms’ or ‘breast cancer,’ or ‘breast tumor.’ The search was restricted to human studies. The title and abstract of studies identified in the search were reviewed by two authors (YYL and XRL) independently to exclude studies that did not address the research question of interest. References of identified articles were also retrieved to assess potentially eligible studies. Inclusion was not restricted on the basis of language of publication.
Selection criteria
Two authors (YYL and XRL) independently extracted data from selected studies. Disagreements between the two reviewers were resolved by consensus, or by deference to a third author (XZJ) where needed. Reasons for exclusion were documented. If there were multiple reports of the same trial, we included data from the most up-to-date reference possible.
We classified studies on bisphosphonates, focusing on adverse events of using bisphosphonates in the treatment of breast cancer. Adverse events studies were RCT studies. The studies corresponding to ONJ were retrospective studies. Therefore, we conducted a separate meta-analysis of ONJ studies.
Inclusion criteria were formed using the participants, intervention, control, outcomes, and study designs (PICOS) strategy.21 For the two meta-analyses in this paper, the inclusion criteria were different:
(1) RCT studies on adverse events after treatment of breast cancer patients with/without the use of bisphosphonates. RCT studies should fulfill following prespecified PICOS criteria. P (participants): breast cancer patients; I (intervention): intravenous bisphosphonates; C (control): without bisphosphonates; O (outcomes): incidence of adverse events; S (study designs): RCT.
(2) For the ONJ studies in breast cancer patients after treatment with bisphosphonates, eligible trials had to satisfy the following prespecified PICOS criteria. P (participants): patients with solid tumors; I (intervention): with bisphosphonates; C (control): without bisphosphonates; O (outcomes): incidence of ONJ.
Assessment of risk of bias
The Cochrane Risk of Bias tool was used to assess the quality of RCTs. Two reviewers (YJH and XZJ) extracted data and assessed risk of bias in the included studies independently. Disagreements were resolved via the consensus of the two reviewers.
Data extraction and analysis
We selected 28 adverse events, and, for each adverse event, we combined the risk ratio (RR) of the intervention group and the control group. For analysis by ONJ, we extracted data related to breast cancer, did not adopt data related to ONJ incidence of other types of cancer, and conducted a merge to achieve overall incidence of ONJ.
Statistical methods
We combined the RR (m-h, Fixed, 95% CI) and heterogeneity test (I2) of each adverse event, and drew a forest plot of the combined risk ratio of 28 adverse events. Of the 23 studies, 28 important adverse events were selected for the combined analysis. It is worth pointing out that the number of studies included in each adverse event was different, and that there is the possibility of bias between studies.
We combined the ONJ incidence of 24 studies and the heterogeneity test (I2), and we used the random effects model (D+L pooled) for meta-analysis. In addition, in these 24 studies, we extracted data only of breast cancer patients. Data synthesis and graphical representation were performed using Review Manager 5, version 5.3., and Stata14.
Results
Eligible studies
A search in PubMed, EMBASE, Cochrane and Web of Science databases identified 5125 unique records. After eligibility assessment, 47 studies comprising a total of 20,607 patients were included (Figure 1). Of these 47 studies, 2 evaluated alendronate, 3 clodronate, 2 ibandronate, 2 pamidronate, 2 risedronate, and 12 zoledronic, while 24 studies evaluated ONJ.
Figure 1.

Flowchart of selection of studies for inclusion in meta-regression.
Description of studies
The publications included in the analyses comprised 23 RCTs and 24 retrospective studies. The characteristics of these studies are summarized in Table 1.
Table 1.
Summary of studies included in the meta-analysis of adverse events except ONJ.
| Agent | Number of studies | Patients in treatment groups | Patients in control groups |
|---|---|---|---|
| Alendronate | 222,23 | 78 | 284 |
| Clodronate | 314,24,25 | 1216 | 1233 |
| Ibandronate | 213,26 | 1911 | 1004 |
| Pamidronate | 227,28 | 941 | 944 |
| Risedronate | 229,30 | 161 | 160 |
| Zoledronic | 1212,15,31–40 | 2177 | 1973 |
| Total | 23 | 6484 | 5598 |
ONJ, osteonecrosis of the jaw.
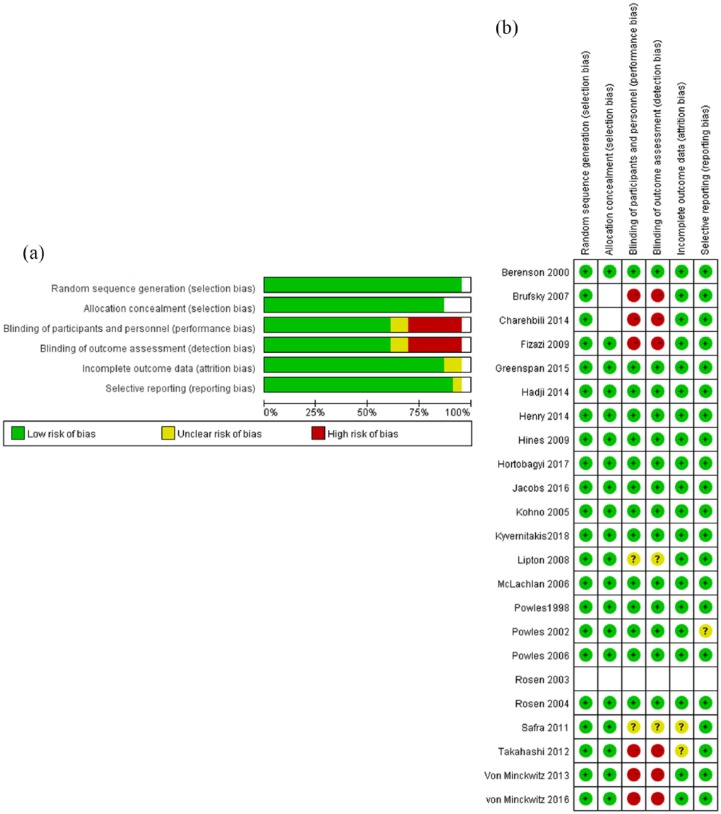
Among the studies selected, 23 RCTs aimed to explore adverse events after treatment of breast cancer patients with/without the use of bisphosphonates. We classified bisphosphonates into different categories. The number of studies, patients in treatment groups and patients in control groups involved in trials were shown in Table 1. We evaluated 28 adverse events and compared the RR of adverse events in the bisphosphonate intervention group versus the control group. The quality of the 23 RCT studies was assessed by the modified Cochrane risk of bias tool (Figure 2); all studies were randomized and a few studies were unblinded. In our study, all six included studies were blinded. Most studies had prospective adverse event monitoring using well-described, objective criteria, although the types of adverse events studied and their definition varied between trials. Therefore, we classified the adverse events to minimize the risk of such bias. The second meta-analysis included 24 retrospective studies related to incidence of ONJ in breast cancer after treatment with bisphosphonates (Table 2). We combined the incidence of ONJ in these 24 retrospective studies to analyze the incidence of ONJ using bisphosphonates and assess the hazard ratio compared with the control group.
Figure 2.
(a) Risk of bias graph: review authors’ judgments about each risk of bias item presented as percentages across all included randomized controlled trial (RCT) studies. (b) Risk of bias summary: review authors’ judgements about each risk of bias item for each included RCT study.
Table 2.
Characteristics of studies included in the meta-analysis of ONJ.
| Study first author | Year | Country | Sample size | Number of ONJ | Percentage of ONJ | Population | Study design |
|---|---|---|---|---|---|---|---|
| Aguiar Bujanda41 | 2007 | Spain | 35 | 4 | 0.114 | Patients receiving zoledronic acid for bone metastasis | Retrospective study |
| Bamias42 | 2005 | Greece | 70 | 2 | 0.029 | Cancer patients started treatment with bisphosphonate since January 1997 until December 31, 2003 and received at least six infusions. | Retrospective study |
| Boonyapakorn43 | 2008 | Germany | 10 | 5 | 0.500 | Multiple myeloma and other malignancies treated with bisphosphonate | Prospective study |
| Brufsky44 | 2013 | United States | 159 | 6 | 0.038 | Women with bone metastases from BC treated with intravenous bisphosphonates from January 1999 to June 2008. | Retrospective cohort study |
| Christodoulou45 | 2009 | Greece | 75 | 2 | 0.027 | Osseous metastases from various tumors from June 2007 to June 2008 | Retrospective cohort study |
| Ding46 | 2012 | China | 181 | 1 | 0.006 | Breast cancer patients with BM | Retrospective study |
| Fehm47 | 2009 | Germany | 345 | 10 | 0.029 | Breast cancer or gynecological malignancies receiving bisphosphonates | Retrospective study |
| Fusco48 | 2013 | Italy | 78 | 27 | 0.346 | Cancer and myeloma patients treated with bisphosphonates | Retrospective study |
| Guarneri49 | 2010 | Italy | 425 | 10 | 0.024 | Cancer patients receiving i.v. bisphosphonate s for ⩾24 months | Retrospective study |
| Guarneri50 | 2005 | Italy | 48 | 3 | 0.063 | Patients with HER2-negative LR/MBC | Prospective cohort study |
| Hoff51 | 2008 | Brazil | 1338 | 16 | 0.012 | Patients treated with intravenous bisphosphonates between 1996 and 2004 | Retrospective study |
| Ibrahim52 | 2008 | Italy | 220 | 5 | 0.023 | Patients with bone metastases treated from June 2002 to December 2006 with i.v. bisphosphonates | Retrospective study |
| Loyson53 | 2018 | Belgium | 192 | 13 | 0.068 | Patients with solid tumors and bone metastases treated with denosumab after prior treatment with bisphosphonates | Retrospective study |
| Manfredi54 | 2017 | Italy | 111 | 12 | 0.108 | Patients treated with zoledronic acid for bone metastases from solid tumors | Retrospective study |
| Pilanci55 | 2015 | Turkey | 97 | 13 | 0.134 | Patients with metastatic breast cancer who had bone metastases and underwent treatment with ZA between March 2006 and December 2013 | Retrospective study |
| Rathbone56 | 2013 | England | 1678 | 26 | 0.015 | Women with stage II or III breast cancer | Randomized Controlled Trial |
| Ripamonti57 | 2009 | Italy | 966 | 26 | 0.027 | Patients with bone metastases (PRE-Group) and treated for the first time with bisphosphonates from January 1999 to April 2005 | Retrospective study |
| Rugani58 | 2014 | Austria | 48 | 10 | 0.208 | From 2000 to 2008, 63 hormone receptor-positive, premenopausal breast cancer patients who were free of metastases | Retrospective study |
| Sanna59 | 2006 | Italy | 81 | 5 | 0.062 | Advanced breast cancer patients with bone metastases under bisphosphonate treatment | Observational study |
| Thumbigere-Math60 | 2012 | United States | 576 | 18 | 0.031 | Patients with cancer treated with intravenous pamidronate and/or zoledronate between January, 2003 and December, 2007 | Retrospective study |
| Vahtsevanos61 | 2009 | Greece | 1621 | 80 | 0.049 | Women with Stage IV breast cancer and osteolytic metastases | Retrospective chart review |
| Vidal-Real62 | 2015 | Spain | 15 | 4 | 0.267 | Cancer patients treated with IV bisphosponates, | Retrospective study |
| Walter63 | 2009 | Germany | 75 | 4 | 0.053 | Breast cancer patients treated in the breast unit from January 2000 to March 2006 | Retrospective study |
| Wang64 | 2007 | United States | 81 | 2 | 0.025 | Patients evaluated and/or treated between January 1, 2000, and December 31, 2005, and had received zoledronic acid and/or pamidronate | Retrospective chart review |
| 24 studies | 8525 |
ONJ, osteonecrosis of the jaw; LR/MBC, locally recurrent or metastatic breast cancer; BM, bone metastasis.
Assessment of adverse events
Here, a total of 23 RCT studies was assessed for the adverse events of breast cancer patients. This meta-analysis included a pooled study population that consisted of 12,073 individuals, with 6484 being patients with bisphosphonates and 5598 being patients without bisphosphonates.
In the included studies, we observed a total of 105 adverse events. Those adverse events mentioned in fewer than three studies were excluded as rare or individual variations rather than truly common adverse events. The descriptions of same adverse events might vary from study to study. We combined the studies, resulting in 28 adverse events being analyzed in this study. These 28 adverse events were classified according to organ system (Table 3). Each adverse event was described in a different number of included studies. We combined and calculated the incidence separately for all 28 adverse events, noting that the numbers of studies mentioning each adverse event were inconsistent. We combined the RR of the bisphosphonates group with that of the control group for the incidence of adverse events. When compared with the control group, the most substantial increase in adverse events was noted for influenza-like illness. The RR of influenza-like illness was estimated at 4.52 (95% CI 2.22–9.23; p < 0.0001; I2 = 33%). Increased fatigue rates were reported by 21 studies, and the relative risk of fatigue was estimated at 1.08 (95% CI 1.01–1.16; p = 0.02; I2 = 7%). Bisphosphonates were also associated with a significantly higher RR of fever (RR 1.82, 95% CI 1.28–2.59; I2 = 59%), dyspepsia (RR 1.25, 95% CI 1.1–1.42; I2 = 48%), anorexia (RR 1.29, 95% CI 1.13–1.46; I2 = 48%), and urinary tract infection (RR 1.32, 95% CI 1.02–1.72). We did not observe significantly higher RR for the other 22 adverse events. Interestingly, the incidence of peripheral edema was lower in treatment with bisphosphonates compared with control groups (RR 0.85, 95% CI 0.73–0.99; p < 0.05; I2 = 19%). The results of the meta-analysis are summarized in Figure 3 and Table 3.
Table 3.
Summary of the absolute event rates for adverse events with and without bisphosphonate.
| Adverse Events | Classification | Number of studies included | Events with bisphosphonate | Events without bisphosphonate | Overall effect RR | Overall effect p value |
|---|---|---|---|---|---|---|
| Abdominal pain | Gastrointestinal Disorders | 5 | 219/1379 | 189/1387 | 1.16 | 0.15 |
| Anorexia | 7 | 452/2227 | 325/2024 | 1.29 | 0.0001 | |
| Constipation | 10 | 648/2819 | 651/2829 | 0.98 | 0.61 | |
| Diarrhea | 12 | 602/2841 | 486/2890 | 1.27 | 0.07 | |
| Dyspepsia | 11 | 471/3720 | 341/2879 | 1.25 | 0.0008 | |
| Nausea | 27 | 1893/6925 | 1870/6963 | 1.01 | 0.63 | |
| Alopecia | General disorders and administration site conditions | 5 | 229/1554 | 202/1562 | 1.14 | 0.15 |
| Back pain | 8 | 358/1993 | 320/1982 | 1.1 | 0.27 | |
| Dizziness | 3 | 138/875 | 117/682 | 0.98 | 0.87 | |
| Fatigue | 21 | 1295/5195 | 1196/5375 | 1.08 | 0.02 | |
| Fever | 8 | 449/1419 | 352/1597 | 1.82 | 0.0009 | |
| Headache | 11 | 390/2179 | 417/2182 | 0.92 | 0.21 | |
| Hot flashes | 7 | 214/1009 | 229/1013 | 0.94 | 0.47 | |
| Influenza-like illness | 3 | 38/115 | 8/111 | 4.52 | < 0.0001 | |
| Metabolic and nutritional disorders | 3 | 43/2254 | 32/1354 | 1.31 | 0.62 | |
| Peripheral edema | 10 | 263/1816 | 303/1860 | 0.85 | 0.03 | |
| Anemia | Hematologic disorders | 8 | 583/2175 | 544/2177 | 1.06 | 0.23 |
| Granulocytopenia | 9 | 179/1835 | 178/1847 | 1.01 | 0.94 | |
| Hepatic dysfunction | Hepatobiliary disorders | 3 | 74/2705 | 57/1857 | 1.33 | 0.45 |
| Urinary tract infection | Infections: urinary tract | 6 | 147/2611 | 86/1583 | 1.32 | 0.04 |
| Arthralgia | Musculoskeletal and connective tissue disorders | 14 | 622/2477 | 539/2473 | 1.09 | 0.08 |
| Myalgia | 31 | 1608/8124 | 1354/7120 | 1.09 | 0.21 | |
| Depression | Psychiatric disorders | 7 | 190/3158 | 175/2321 | 1 | 1 |
| Insomnia | 8 | 184/1302 | 215/1342 | 0.87 | 0.12 | |
| Coughing | Respiratory disorders | 7 | 285/1405 | 250/1438 | 1.15 | 0.06 |
| Dyspnea | 10 | 519/2411 | 513/2414 | 0.97 | 0.61 | |
| Dermatologic | Skin/rash | 7 | 210/3089 | 194/2241 | 1.04 | 0.82 |
| Renal AEs | Urinary disorders | 5 | 129/3529 | 98/2649 | 1.24 | 0.1 |
RR, Risk ratio
Figure 3.
Summary figure demonstrating the risk ratio (RR) for adverse events for patients with and without exposure to bisphosphonates. CI, Confidence interval.
Assessment incidence of ONJ
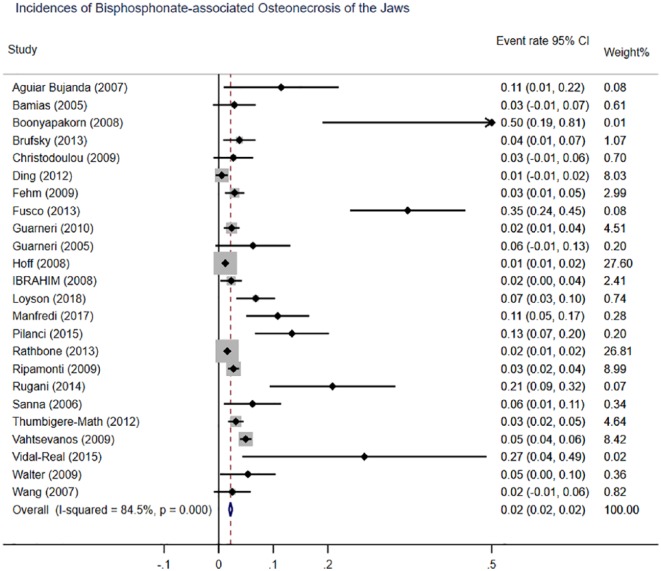
We next screened the database for our systematic review of the incidence of ONJ. Here, 24 retrospective studies with 8525 patients were pooled in the meta-analysis. Meta-analysis of the incidence of ONJ among patients with bisphosphonates revealed significant between-study heterogeneity (I2 = 84.5%). Therefore, we used a random effects model for meta-analysis. Of the patients with bisphosphonates, 304 had a diagnosis of ONJ. We found that the overall incidence of ONJ in patients exposed to bisphosphonates therapies is 2% (95% CI 0.02–0.02; p < 0.001) (Figure 4).
Figure 4.
Meta-analysis of incidence of bisphosphonates-associated osteonecrosis of the jaw. CI, Confidence interval.
Assessment of publication bias
We conducted six analyses out of seven comparisons with dominant pooled RR (fatigue, fever, dyspepsia, anorexia, urinary tract infection, peripheral edema) to assessment of publication bias. We did not conduct the Egger’s side effect test because of paucity of included studies (as few as three studies) for pooled RR of influenza-like illness. We produced a p value for Egger’s test as shown in supplemental Figures S1 and S2. As can be seen, all six p values were >0.1, suggesting no presence of significant publication bias (Table 4).
Table 4.
Egger’s and Begg’s test p value.
| Adverse events | Egger’s p value | Begg’s p value |
|---|---|---|
| Anorexia | 0.703 | 0.688 |
| Fatigue | 0.213 | 0.212 |
| Peripheral edema | 0.486 | 0.477 |
| Fever | 0.151 | 0.153 |
| Dyspepsia | 0.946 | 0.954 |
| Urinary tract infection | 0.872 | 0.878 |
We also applied Egger’s test to the ONJ studies to produce an incidence of ONJ on the basis of pooled comparison; this resulted in a p-value of <0.001, suggesting the presence of publication bias (see Egger’s regression chart in Figure 5a and Begg’s funnel plot in Figure 5b). We adopted the trim-fill method to further analyze the bias, with the resulting plot (Figure 5c) suggesting that an unbiased state could be achieved through filling with an additional nine studies .
Figure 5.

Results of Egger’s test (a), Begg’s test (b), and the fill method (c) for bisphosphonates-associated osteonecrosis of the jaw (ONJ).
Discussion
Our study enrolled 47 clinical trials involving 20,607 participants in a network meta-analysis. Six bisphosphonates regimens were included: alendronate, clodronate, ibandronate, pamidronate, risedronate, and zoledronic. Our data suggest that patients with breast cancer treated by bisphosphonates are at higher risk of fatigue, anorexia, peripheral edema, dyspepsia, fever, influenza-like illness, and urinary tract infection relative to controls, and that 2% of breast cancer patients treated with bisphosphonates develop ONJ.
In recent years, bisphosphonates have emerged as a highly effective therapeutic option for prevention of SREs, especially in patients who have breast cancer and metastatic bone disease.16,65 Epidemiological studies have suggested that bisphosphonates may increase bone mineral density in lumbar and hip joints in breast cancer patients, including premenopausal and postmenopausal women.15,39,66 Several experimental studies have also proposed that bisphosphonates might have antitumor effects, including inducing apoptosis, reducing proliferation, and inhibiting tumor cell migration and invasion.40 Since bisphosphonates are considered as an effective adjuvant drug for prevention of SREs, these adverse effects should be clarified and weighted. Our present systematic review and meta-analysis confirms and quantifies the adverse effects associated with bisphosphonates as an adjuvant treatment. Accordingly, we anticipate that our findings may help physicians and their patients gauge the risk-benefit of adding bisphosphonates to a patient with advanced malignancy.
Studies have reported that nephrotoxicity, including toxic acute tubular necrosis and focal segmental glomerulosclerosis, is a potential limiting factor for intravenous bisphosphonates.67,68 Adverse events such as influenza-like illness and chills were more common in the zoledronate group compared with the placebo group.15 In addition, the following adverse events were also more frequently observed in the zoledronate group as compared with the observation group: sensory neuropathy and other nervous system disorders, gastrointestinal, skin, myalgia, pain, fatigue, fever, and other general condition disorders.40 When compared with patients in the placebo group, patients in the ibandronate group had an excess of adverse events in specific system organ classes: infection, cardiac, gastrointestinal, hepatobiliary, and general condition.13 Our meta-analysis included 23 studies, all of which were RCTs. The results demonstrated that only seven adverse events (i.e. influenza-like illness, fatigue, anorexia, dyspepsia, fever, urinary tract infection, and peripheral edema) are related to the use of bisphosphonates. Influenza-like illness is the most common adverse event. This may indicate that these adverse events are mediated through bisphosphonates. However, no significantly higher risk was shown in the incidence of myalgia and nausea, which were the most common adverse events of bisphosphonates. Interestingly, we observed a decreased risk of peripheral edema in patients treatment with bisphosphonates compared with control groups. Thus, clinicians should be aware of these potential adverse effects in clinical use.
ONJ is a destructive bone process in patients undergoing bisphosphonate therapy who show bone exposure of over 8 weeks of development and who did not undergo radiotherapy of the head and neck.53,54,62 Some ONJ patients may show no symptoms at all, but in others ONJ may cause severe pain, swelling and bleeding of oral cavity tissue, continuous purulent secretion accompanied with or without fistula in the oral cavity, severe bad breath and an abnormal feeling of the lower lip related to loosened teeth. Predominantly, ONJ leads to a severe deterioration of the patient’s quality of life. However, some researchers have shown a very variable prevalence of ONJ. A retrospective study of 194 Spanish patients who had undergone intravenous bisphosphonate therapy showed that the prevalence of ONJ was 12.9%.62 Another retrospective analysis showed that 8 of 190 patients (4.2%) with breast cancer developed ONJ.60 Ding and colleagues retrospectively analyzed the safety data of bisphosphonates in 181 breast cancer patients with bone metastasis who received intravenous bisphosphonates for more than 2 years; only 1 of these patients was diagnosed with ONJ, giving an incidence rate of 0.6%.46 The purpose of the study reported in this article was to determine the prevalence of ONJ in breast cancer patients who have undergone intravenous bisphosphonate therapy, and relate the risk factors described to establish a protocol to reduce the risk of developing ONJ. In the current analysis, we observed that 2.0% of breast cancer patients treated with bisphosphonates developed ONJ. It is worth noting that Boonyapakorn and colleagues reported a relevantly greater number (50%) of ONJ in patients treated with bisphosphonates.43 The smaller sample in the latter study (n = 10) might explain the high incidence of ONJ. The different prevalence of ONJ is not well understood but may possibly be related to each patient’s systemic factors, such as diabetes, osteoporosis (in oncology patients), and medication related to steroids, or immunosuppressive and antiangiogenic drugs.62,69 In addition, local factors are also related to the appearance of ONJ, such as oral hygiene and overall periodontal state.
The present study has certain limitations. First, we analyzed the data on adverse events provided in published clinical trials. These adverse events were chosen by the authors according to varied criteria. Therefore, many related adverse events may have be considered irrelevant and therefore have not been reported. Second, 23 studies of ONJ are single-group trials, rather than RCTs. Nevertheless, major cases are enrolled in the study, and are consistent with the research progress; hence, the conclusions have a certain value and significance. Third, as with any meta-analysis, the results described here are affected by the limitations of the individual clinical trials that were selected for this meta-analysis. Last, the pooled between-studies of ONJ suggested significant publication bias. According to the trim-fill method, publication bias might be corrected to an unbiased state by adding nine studies. At present we lack the relevant studies from among the articles searched.
Conclusion
In summary, the results of this meta-analysis suggest that the use of bisphosphonates is associated with seven adverse events: fatigue, anorexia, peripheral edema, dyspepsia, fever, influenza-like illness, and urinary tract infection. During bisphosphonate therapy, 2% of patients might develop ONJ. This study should help to convey information to clinicians and patients on the correct and rational use of bisphosphonates in the treatment of breast cancer, avoiding unnecessary dose reduction and treatment interruptions, and thus minimizing the impact on patient quality of life.
Supplemental Material
Supplemental material, Supplement_Figure_1_ for The incidence and relative risk of adverse events in patients treated with bisphosphonate therapy for breast cancer: a systematic review and meta-analysis by Yan-Li Yang, Zi-Jian Xiang, Jing-Hua Yang, Wen-Jie Wang and Ruo-Lan Xiang in Therapeutic Advances in Medical Oncology
Supplemental Material
Supplemental material, Supplement_Figure_2 for The incidence and relative risk of adverse events in patients treated with bisphosphonate therapy for breast cancer: a systematic review and meta-analysis by Yan-Li Yang, Zi-Jian Xiang, Jing-Hua Yang, Wen-Jie Wang and Ruo-Lan Xiang in Therapeutic Advances in Medical Oncology
Acknowledgments
We thank Beijing Zhiyun Data Technology Co. LTD, for data analysis service.
Footnotes
Funding: This work was funded by the National Natural Science Foundation of China (grant number 81570993) and Beijing Natural Science Foundation (grant number 7162100).
Conflict of interest statement: The authors declare that there is no conflict of interest.
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Yan-Li Yang, Department of Respiratory and Critical Care Medicine, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China.
Zi-Jian Xiang, Beijing Zhiyun Data Technology Co. LTD, China.
Jing-Hua Yang, Beijing Zhiyun Data Technology Co. LTD, China.
Wen-Jie Wang, Beijing Zhiyun Data Technology Co. LTD, China.
Ruo-Lan Xiang, Department of Physiology and Pathophysiology, Peking University School of Basic Medical Sciences, Key Laboratory of Molecular Cardiovascular Sciences, Ministry of Education, and Beijing Key Laboratory of Cardiovascular Receptors Research, China.
References
- 1. Palmieri C, Fullarton JR, Brown J. Comparative efficacy of bisphosphonates in metastatic breast and prostate cancer and multiple myeloma: a mixed-treatment meta-analysis. Clin Cancer Res 2013; 19: 6863–6872. [DOI] [PubMed] [Google Scholar]
- 2. Clement-Demange L, Clezardin P. Emerging therapies in bone metastasis. Curr Opin Pharmacol 2015; 22: 79–86. [DOI] [PubMed] [Google Scholar]
- 3. Saad F, Lipton A, Cook R, et al. Pathologic fractures correlate with reduced survival in patients with malignant bone disease. Cancer 2007; 110: 1860–1867. [DOI] [PubMed] [Google Scholar]
- 4. Van Poznak CH, Temin S, Yee GC, et al. American society of clinical oncology executive summary of the clinical practice guideline update on the role of bone-modifying agents in metastatic breast cancer. J Clin Oncol 2011; 29: 1221–1227. [DOI] [PubMed] [Google Scholar]
- 5. Landesberg R, Eisig S, Fennoy I, et al. Alternative indications for bisphosphonate therapy. J Oral Maxillofac Surg 2009; 67: 27–34. [DOI] [PubMed] [Google Scholar]
- 6. Bock O, Felsenberg D. Bisphosphonates in the management of postmenopausal osteoporosis–optimizing efficacy in clinical practice. Clin Interv Aging 2008; 3: 279–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Su G, Xiang Y, He G, et al. Bisphosphonates may protect against bone loss in postmenopausal women with early breast cancer receiving adjuvant aromatase inhibitor therapy: results from a meta-analysis. Arch Med Res 2014; 45: 570–579. [DOI] [PubMed] [Google Scholar]
- 8. Kimmel DB. Mechanism of action, pharmacokinetic and pharmacodynamic profile, and clinical applications of nitrogen-containing bisphosphonates. J Dent Res 2007; 86: 1022–1033. [DOI] [PubMed] [Google Scholar]
- 9. Anagha PP, Sen S. The efficacy of bisphosphonates in preventing aromatase inhibitor induced bone loss for postmenopausal women with early breast cancer: a systematic review and meta-analysis. J Oncol 2014; 2014: 625060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature 2003; 423: 337–342. [DOI] [PubMed] [Google Scholar]
- 11. Costa L. Bisphosphonates in adjuvant setting for breast cancer: a review of the meta-analysis of bisphosphonates’ effects on breast cancer recurrence presented in December 2013 at San Antonio Breast Conference. Curr Opin Support Palliat Care 2014; 8: 414–419. [DOI] [PubMed] [Google Scholar]
- 12. Hortobagyi G, Poznak C, Harker W, et al. Continued treatment effect of zoledronic acid dosing every 12 vs 4 weeks in women with breast cancer metastatic to bone: the OPTIMIZE-2 randomized clinical trial. JAMA Oncol 2017; 3: 906–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Von Minckwitz G, Möbus V, Schneeweiss A, et al. German adjuvant intergroup node-positive study: a phase III trial to compare oral ibandronate versus observation in patients with high-risk early breast cancer. J Clin Oncol 2013; 31: 3531–3539. [DOI] [PubMed] [Google Scholar]
- 14. Powles T, Paterson A, McCloskey E, et al. Reduction in bone relapse and improved survival with oral clodronate for adjuvant treatment of operable breast cancer [ISRCTN83688026]. Breast Cancer Res 2006; 8: R13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kyvernitakis I, Kann PH, Thomasius F, et al. Prevention of breast cancer treatment-induced bone loss in premenopausal women treated with zoledronic acid: final 5-year results from the randomized, double-blind, placebo-controlled ProBONE II trial. Bone 2018; 114: 109–115. [DOI] [PubMed] [Google Scholar]
- 16. Hadji P, Coleman RE, Wilson C, et al. Adjuvant bisphosphonates in early breast cancer: consensus guidance for clinical practice from a European panel. Ann Oncol 2016; 27: 379–390. [DOI] [PubMed] [Google Scholar]
- 17. Markowitz GS, Fine PL, Stack JI, et al. Toxic acute tubular necrosis following treatment with zoledronate (Zometa). Kidney Int 2003; 64: 281–289. [DOI] [PubMed] [Google Scholar]
- 18. Perazella MA, Markowitz GS. Bisphosphonate nephrotoxicity. Kidney Int 2008; 74: 1385–1393. [DOI] [PubMed] [Google Scholar]
- 19. Wang X, Yang KH, Wanyan P, et al. Comparison of the efficacy and safety of denosumab versus bisphosphonates in breast cancer and bone metastases treatment: a meta-analysis of randomized controlled trials. Oncol Lett 2014; 7: 1997–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health are interventions: explanation and elaboration. PLoS Med 2009; 6: e1000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lipton A, Steger GG, Figueroa J, et al. Extended efficacy and safety of denosumab in breast cancer patients with bone metastases not receiving prior bisphosphonate therapy. Clin Cancer Res 2008; 14: 6690–6696. [DOI] [PubMed] [Google Scholar]
- 23. Fizazi K, Lipton A, Mariette X, et al. Randomized phase II trial of denosumab in patients with bone metastases from prostate cancer, breast cancer, or other neoplasms after intravenous bisphosphonates. J Clin Oncol 2009; 27: 1564–1571. [DOI] [PubMed] [Google Scholar]
- 24. Powles TJ, McCloskey E, Paterson AHG, et al. Oral clodronate and reduction in loss of bone mineral density in women with operable primary breast cancer. J Natl Cancer Inst 1998; 90: 704–708. [DOI] [PubMed] [Google Scholar]
- 25. Powles T, Paterson S, Kanis J, et al. Randomized, placebo-controlled trial of clodronate in patients with primary operable breast cancer. J Clin Oncol 2002; 20: 3219–3224. [DOI] [PubMed] [Google Scholar]
- 26. McLachlan SA, Cameron D, Murray R, et al. Safety of oral ibandronate in the treatment of bone metastases from breast cancer: long-term follow-up experience. Clin Drug Invest 2006; 26: 43–48. [DOI] [PubMed] [Google Scholar]
- 27. Rosen L, Gordon D, Kaminski M, et al. Long-term efficacy and safety of zoledronic acid compared with pamidronate disodium in the treatment of skeletal complications in patients with advanced multiple myeloma or breast carcinoma: a randomized, double-blind, multicenter, comparative trial. Cancer 2003; 98: 1735–1744. [DOI] [PubMed] [Google Scholar]
- 28. Rosen LS, Gordon DH, Dugan W, Jr, et al. Zoledronic acid is superior to pamidronate for the treatment of bone metastases in breast carcinoma patients with at least one osteolytic lesion. Cancer 2004; 100: 36–43. [DOI] [PubMed] [Google Scholar]
- 29. Hines SL, Mincey BA, Sloan JA, et al. Phase III randomized, placebo-controlled, double-blind trial of risedronate for the prevention of bone loss in premenopausal women undergoing chemotherapy for primary breast cancer. J Clin Oncol 2009; 27: 1047–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Greenspan SL, Vujevich KT, Brufsky A, et al. Prevention of bone loss with risedronate in breast cancer survivors: a randomized, controlled clinical trial. Osteoporosis Int 2015; 26: 1857–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Berenson JR, Rosen LS, Howell A, et al. Zoledronic acid reduces skeletal-related events in patients with osteolytic metastases. Cancer 2001; 91: 1191–1200. [DOI] [PubMed] [Google Scholar]
- 32. Kohno N, Aogi K, Minami H, et al. Zoledronic acid significantly reduces skeletal complications compared with placebo in Japanese women with bone metastases from breast cancer: a randomized, placebo-controlled trial. J Clin Oncol 2005; 23: 3314–3321. [DOI] [PubMed] [Google Scholar]
- 33. Brufsky A, Harker WG, Beck JT, et al. Zoledronic acid inhibits adjuvant letrozole-induced bone loss in postmenopausal women with early breast cancer. J Clin Oncol 2007; 25: 829–836. [DOI] [PubMed] [Google Scholar]
- 34. Safra T, Bernstein-Molho R, Greenberg J, et al. The protective effect of zoledronic acid on bone loss in postmenopausal women with early breast cancer treated with sequential tamoxifen and letrozole: a prospective, randomized, phase II trial. Oncology 2011; 81: 298–305. [DOI] [PubMed] [Google Scholar]
- 35. Takahashi S, Iwase T, Kohno N, et al. Efficacy of zoledronic acid in postmenopausal Japanese women with early breast cancer receiving adjuvant letrozole: 12-month results. Breast Cancer Res Treat 2012; 133: 685–693. [DOI] [PubMed] [Google Scholar]
- 36. Charehbili A, Ven S, Smit V, et al. Addition of zoledronic acid to neoadjuvant chemotherapy does not enhance tumor response in patients with HER2-negative stage II/III breast cancer: the NEOZOTAC trial (BOOG 2010–01). Ann Oncol 2014; 25: 998–1004. [DOI] [PubMed] [Google Scholar]
- 37. Hadji P, Kauka A, Ziller M, et al. Effects of zoledronic acid on bone mineral density in premenopausal women receiving neoadjuvant or adjuvant therapies for HR+ breast cancer: the ProBONE II study. Osteoporos Int 2014; 25: 1369–1378. [DOI] [PubMed] [Google Scholar]
- 38. Henry D, Vadhan-Raj S, Hirsh V, et al. Delaying skeletal-related events in a randomized phase 3 study of denosumab versus zoledronic acid in patients with advanced cancer: an analysis of data from patients with solid tumors. Support Care Cancer 2014; 22: 679–687. [DOI] [PubMed] [Google Scholar]
- 39. Jacobs C, Kuchuk I, Bouganim N, et al. A randomized, double-blind, phase II, exploratory trial evaluating the palliative benefit of either continuing pamidronate or switching to zoledronic acid in patients with high-risk bone metastases from breast cancer. Breast Cancer Res Tr 2016; 155: 77–84. [DOI] [PubMed] [Google Scholar]
- 40. Von Minckwitz G, Rezai M, Tesch H, et al. Zoledronate for patients with invasive residual disease after anthracyclines-taxane-based chemotherapy for early breast cancer: the Phase III NeoAdjuvant Trial Add-oN (NaTaN) study (GBG 36/ABCSG 29). Eur J Cancer 2016; 64: 12–21. [DOI] [PubMed] [Google Scholar]
- 41. Aguiar Bujanda D, Bohn Sarmiento U, Cabrera Suárez MÁ, et al. Assessment of renal toxicity and osteonecrosis of the jaws in patients receiving zoledronic acid for bone metastasis. Ann Oncol 2007; 18: 556–560. [DOI] [PubMed] [Google Scholar]
- 42. Bamias A, Kastritis E, Bamia C, et al. Osteonecrosis of the jaw in cancer after treatment with bisphosphonates: incidence and risk factors. J Clin Oncol 2005; 23: 8580–8587. [DOI] [PubMed] [Google Scholar]
- 43. Boonyapakorn T, Schirmer I, Reichart PA, et al. Bisphosphonate-induced osteonecrosis of the jaws: prospective study of 80 patients with multiple myeloma and other malignancies. Oral Oncology 2008; 44: 857–869. [DOI] [PubMed] [Google Scholar]
- 44. Brufsky AM, Sereika SM, Mathew A, et al. Long-term treatment with intravenous bisphosphonates in metastatic breast cancer: a retrospective study. Breast J 2013; 19: 504–511. [DOI] [PubMed] [Google Scholar]
- 45. Christodoulou C, Pervena A, Klouvas G, et al. Combination of bisphosphonates and antiangiogenic factors induces osteonecrosis of the jaw more frequently than bisphosphonates alone. Oncology 2009; 76: 209–211. [DOI] [PubMed] [Google Scholar]
- 46. Ding X, Fan Y, Ma F, et al. Prolonged administration of bisphosphonates is well-tolerated and effective for skeletal-related events in Chinese breast cancer patients with bone metastasis. Breast 2012; 21: 544–549. [DOI] [PubMed] [Google Scholar]
- 47. Fehm T, Beck V, Banys M, et al. Bisphosphonate-induced osteonecrosis of the jaw (ONJ): incidence and risk factors in patients with breast cancer and gynecological malignancies. Gynecol Oncol 2009; 112: 605–609. [DOI] [PubMed] [Google Scholar]
- 48. Fusco V, Galassi C, Berruti A, et al. Decreasing frequency of osteonecrosis of the jaw in cancer and myeloma patients treated with bisphosphonates: the experience of the oncology network of piedmont and aosta valley (North-Western Italy). ISRN Oncol 2013; 2013: 672027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Guarneri V, Donati S, Nicolini M, et al. Renal safety and efficacy of i.v. bisphosphonates in patients with skeletal metastases treated for up to 10 Years. Oncologist 2005; 10: 842–848. [DOI] [PubMed] [Google Scholar]
- 50. Guarneri V, Miles D, Robert N, et al. Bevacizumab and osteonecrosis of the jaw: incidence and association with bisphosphonate therapy in three large prospective trials in advanced breast cancer. Breast Cancer Res Tr 2010; 122: 181–188. [DOI] [PubMed] [Google Scholar]
- 51. Hoff AO, Toth BB, Altundag K, et al. Frequency and risk factors associated with osteonecrosis of the jaw in cancer patients treated with intravenous bisphosphonates. J Bone and Miner Res 2008; 23: 826–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ibrahim T, Barbanti F, Giorgio-Marrano G, et al. Osteonecrosis of the jaw in patients with bone metastases treated with bisphosphonates: a retrospective study. Oncologist 2008; 13: 330–336. [DOI] [PubMed] [Google Scholar]
- 53. Loyson T, Van Cann T, Schöffski P, et al. Incidence of osteonecrosis of the jaw in patients with bone metastases treated sequentially with bisphosphonates and denosumab. Acta Clin Belg 2018; 73: 100–109. [DOI] [PubMed] [Google Scholar]
- 54. Manfredi M, Mergoni G, Goldoni M, et al. A 5-year retrospective longitudinal study on the incidence and the risk factors of osteonecrosis of the jaws in patients treated with zoledronic acid for bone metastases from solid tumors. Med Oral Patol Oral 2017; 22: e342–e348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pilanci KN, Alco G, Ordu C, et al. Is administration of trastuzumab an independent risk factor for developing osteonecrosis of the jaw among metastatic breast cancer patients under zoledronic acid treatment? Medicine (Baltimore) 2015; 94: e671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Rathbone EJ, Brown JE, Marshall HC, et al. Osteonecrosis of the jaw and oral health-related quality of life after adjuvant zoledronic acid: an adjuvant zoledronic acid to reduce recurrence trial subprotocol (BIG01/04). J Clin Oncol 2013; 31: 2685–2691. [DOI] [PubMed] [Google Scholar]
- 57. Ripamonti CI, Maniezzo M, Campa T, et al. Decreased occurrence of osteonecrosis of the jaw after implementation of dental preventive measures in solid tumour patients with bone metastases treated with bisphosphonates. The experience of the National Cancer Institute of Milan. Ann Oncol 2009; 20: 137–145. [DOI] [PubMed] [Google Scholar]
- 58. Rugani P, Luschin G, Jakse N, et al. Prevalence of bisphosphonate-associated osteonecrosis of the jaw after intravenous zoledronate infusions in patients with early breast cancer. Clin Oral Invest 2014; 18: 401–407. [DOI] [PubMed] [Google Scholar]
- 59. Sanna G, Preda L, Bruschini R, et al. Bisphosphonates and jaw osteonecrosis in patients with advanced breast cancer. Ann Oncol 2006; 17: 1512–1516. [DOI] [PubMed] [Google Scholar]
- 60. Thumbigere-Math V, Tu L, Huckabay S, et al. A retrospective study evaluating frequency and risk factors of osteonecrosis of the jaw in 576 cancer patients receiving intravenous bisphosphonates. Am J Clin Oncol 2012; 35: 386–392. [DOI] [PubMed] [Google Scholar]
- 61. Vahtsevanos K, Kyrgidis A, Verrou E, et al. Longitudinal cohort study of risk factors in cancer patients of bisphosphonate-related osteonecrosis of the jaw. J Clin Oncol 2009; 27: 5356–5362. [DOI] [PubMed] [Google Scholar]
- 62. Vidal-Real C, Pérez-Sayáns M, Suárez-Peñaranda JM, et al. Osteonecrosis of the jaws in 194 patients who have undergone intravenous bisphosphonate therapy in Spain. Med Oral Patol Oral 2015; 20: e267–e272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Walter C, Al-Nawas B, Bois AD, et al. Incidence of bisphosphonate-associated osteonecrosis of the jaws in breast cancer patients. Cancer 2009; 115: 1631–1637. [DOI] [PubMed] [Google Scholar]
- 64. Wang EP, Kaban LB, Strewler GJ, et al. Incidence of osteonecrosis of the jaw in patients with multiple myeloma and breast or prostate cancer on intravenous bisphosphonate therapy. J Oral Maxil Surg 2007; 65: 1328–1331. [DOI] [PubMed] [Google Scholar]
- 65. Baba K, Kaida H, Hattori C, et al. Tumoricidal effect and pain relief after concurrent therapy by strontium-89 chloride and zoledronic acid for bone metastases. Hell J Nucl Med 2018; 21: 15–23. [DOI] [PubMed] [Google Scholar]
- 66. Sun S, Wang F, Dou H, et al. Preventive effect of zoledronic acid on aromatase inhibitor-associated bone loss for postmenopausal breast cancer patients receiving adjuvant letrozole. OncoTargets Ther 2016; 9: 6029–6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Luedders DW, Steinhoff J, Thill M, et al. Lack of difference in acute nephrotoxicity of intravenous bisphosphonates zoledronic acid and ibandronate in women with breast cancer and bone metastases. Anticancer Res 2015; 35: 1797–1802. [PubMed] [Google Scholar]
- 68. Macpherson IR, Bray C, Hopkins C, et al. Loading dose ibandronate versus standard oral ibandronate in patients with bone metastases from breast cancer. Clin Breast Cancer 2015; 15: 117–127. [DOI] [PubMed] [Google Scholar]
- 69. Hoff AO, Toth B, Hu M, et al. Epidemiology and risk factors for osteonecrosis of the jaw in cancer patients. Ann N Y Acad Sci 2011; 1218: 47–54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplement_Figure_1_ for The incidence and relative risk of adverse events in patients treated with bisphosphonate therapy for breast cancer: a systematic review and meta-analysis by Yan-Li Yang, Zi-Jian Xiang, Jing-Hua Yang, Wen-Jie Wang and Ruo-Lan Xiang in Therapeutic Advances in Medical Oncology
Supplemental material, Supplement_Figure_2 for The incidence and relative risk of adverse events in patients treated with bisphosphonate therapy for breast cancer: a systematic review and meta-analysis by Yan-Li Yang, Zi-Jian Xiang, Jing-Hua Yang, Wen-Jie Wang and Ruo-Lan Xiang in Therapeutic Advances in Medical Oncology