Abstract
Background
Afatinib is an irreversible ErbB family blocker that improves progression‐free survival (PFS) of advanced EGFR‐mutant lung adenocarcinoma compared to chemotherapy. However, afatinib leads to more adverse events than first‐generation EGFR inhibitors. Hence, exploration of the optimal afatinib initial dose and its efficacy and safety in Asian patients has drawn extensive attention.
Methods
We retrospectively evaluated demographic and clinical information, survival data, and adverse events in advanced non‐small cell lung cancer patients treated with afatinib from 27 February 2017 to 30 October 2018.
Results
A total of 60 patients were included in the study. Thirty‐nine (65%) patients received afatinib as first‐line treatment. The median PFS was 12.3 months (95% confidence internal 7.6–17.0). Multivariate Cox regression analysis revealed that age, gender, smoking history, baseline brain metastasis status, afatinib starting dose, and mutation type did not significantly influence PFS. No significant difference in median PFS between patients treated with an initial dose of afatinib of 40 mg or 30 mg, either in the first‐line (14.5 vs. 5.2 months; P = 0.101) or in a second or later‐line setting (3.0 vs. 5.0 months; P = 0.375) was observed. The incidence of all grades of rash/acne (92.5% vs. 61.1%; P = 0.011) and paronychia (82.5% vs. 50.0%; P = 0.010) in the 40 mg group was significantly higher than in the 30 mg group.
Conclusion
First‐line afatinib treatment is beneficial for advanced lung adenocarcinoma patients with sensitive EGFR mutations. Initial dose and baseline brain metastasis status do not significantly impact PFS.
Keywords: Afatinib, lung adenocarcinoma, non‐small‐cell lung, ErbB receptors, molecular targeted therapy
Introduction
Non‐small cell lung cancer (NSCLC) accounts for more than 80% of all lung cancer cases, with adenocarcinoma and squamous cell carcinoma the dominant subtypes.1 Approximately 50% of Chinese adenocarcinoma patients harbor EGFR mutations.2, 3 The most common sensitive (classic) EGFR mutations are in‐frame deletions in exon 19 (19del) and exon 21 substitution of leucine for arginine (L858R).4, 5, 6, 7 Other uncommon sensitive (non‐classical) mutations have also been detected, including G719X, L861Q, 19 insertions, A763_Y764 insFQEA, and S768I mutations.8, 9, 10, 11, 12
Afatinib is an oral irreversibly‐binding ErbB family blocker that can effectively block signaling from EGFR (ErbB1), HER2/ErbB2, ErbB4, and all relevant ErbB family members.13, 14 The LUX‐Lung 3 and 6 trials revealed that first‐line treatment with afatinib significantly prolongs the progression‐free survival (PFS) of patients with common or uncommon sensitive EGFR mutations compared to chemotherapy.6, 12, 15 In the LUX‐Lung 7 trial, first‐line afatinib treatment even generated longer PFS than gefitinib for advanced lung adenocarcinoma patients with sensitive EGFR mutations.16
Although Asian patients were enrolled in the LUX‐lung 6 trial, the efficacy and safety outcomes were obtained from a controlled environment and patient population. More real‐world data of Chinese patients treated with afatinib are required, as confounding factors during clinical practice may influence efficacy and toxicity.
Herein, we conducted a retrospective real‐world study to explore the efficacy and toxicity of afatinib in a Chinese population of advanced lung adenocarcinoma patients with sensitive EGFR mutations.
Methods
Patients
We retrospectively screened advanced NSCLC patients treated with afatinib at the National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences (Beijing, China) from 27 February 2017 to 30 October 2018. The ethics committee of the National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College (Approval No. 18‐016/1618) approved the study.
Patients that met the following criteria were included: (i) a histologically or cytologically‐verified diagnosis of locally advanced, recurrent, or metastatic NSCLC; (ii) sensitive EGFR mutations; (iii) aged ≥ 18 years; and (iv) administration of at least one month of afatinib. The exclusion criteria were: (i) combination with other anticancer drugs; (ii) lack of necessary survival data; (iii) irregular administration of afatinib; and (iv) accompanying with other malignant tumors. PCR or next generation sequencing were used to determine EGFR mutations. Patients received 30 or 40 mg afatinib daily as a starting dose, with proper adjustments as necessary. The starting dose was determined by clinicians’ judgment according to patient age, body surface area, Eastern Cooperative Oncology Group performance status (ECOG PS), and the severity of adverse events from previous target therapy.
Data collection and evaluation
Clinical data were extracted from patients’ medical history and supplemented by follow‐up if needed. Follow‐up was conducted through regular patient visits or telephone calls. Demographic and clinical data were collected. Patient PS was assessed according to ECOG score. Response to afatinib was evaluated by regular imaging examinations, in accordance with Response Evaluation Criteria in Solid Tumors, version 1.1 (RECIST 1.1). Survival outcomes were collected from the initiation of afatinib treatment to the patient's death or the end of the study at March 31, 2019. Adverse events were assessed using the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0.
Statistical analysis
The Kaplan–Meier method was applied to estimate progression‐free survival (PFS) and overall survival (OS). Predictive factors for survival outcomes were analyzed with proportional hazard models (multivariate Cox regression). Comparison of demographic characteristics and the incidence of adverse events between 40 mg and 30 mg afatinib groups were evaluated with χ2 or Fisher's exact tests. All analyses were performed using SPSS version 25.0.
Results
Demographic and clinical characteristics of patients
A total of 60 patients were included in the study. The median age of all patients was 58.1 (range: 36.3–82.7) years and most patients were non‐smokers (Table 1). All patients had an ECOG PS score of 0–1. Twenty‐six (43.3%) patients harbored exon 19 del, 16 (26.7%) patients harbored exon 21 L858R, and 18 (30.0%) patients harbored uncommon sensitive EGFR mutations, among whom five patients had both common and uncommon mutations.
Table 1.
Demographic and clinical characteristics of patients
| Characteristics | All patients | First‐line afatinib | ≥ Second‐line afatinib |
|---|---|---|---|
| N | 60 | 39 | 21 |
| Age | |||
| Median (years) | 58.1 | 57.2 | 59.9 |
| Range | 36.3–82.7 | 36.3–82.7 | 39.7–75.5 |
| Age distribution, N (%) | |||
| ≥ 65 | 13 (21.7%) | 8 (20.5%) | 5 (23.8%) |
| < 65 | 47 (78.3%) | 31 (79.5%) | 16 (76.2%) |
| Gender | |||
| Male | 30 (50.0%) | 16 (41.0%) | 14 (66.7%) |
| Female | 30 (50.0%) | 23 (59.0%) | 7 (33.3%) |
| Smoking history | |||
| Yes | 18 (30.0%) | 10 (25.6%) | 8 (38.1%) |
| No | 42 (70.0%) | 29 (74.4%) | 13 (61.9%) |
| ECOG PS score | |||
| 0–1 | 60 (100.0%) | 39 (100.0%) | 21 (100%) |
| 2–4 | 0 | 0 | 0 |
| EGFR mutation | |||
| Exon 19 deletion | 26 (43.3%) | 19 (48.7%) | 7 (33.3%) |
| Exon 21 L858R | 16 (26.7%) | 7 (17.9%) | 9 (42.9%) |
| Uncommon mutations† | 18 (30.0%) | 13 (33.3%) | 5 (23.8%) |
| Baseline brain metastasis | |||
| Yes | 24 (40.0%) | 14 (35.9%) | 10 (47.6%) |
| No | 36 (60.0%) | 25 (64.1%) | 11 (52.4%) |
| Starting dose of afatinib | |||
| 40 mg | 41 (68.3%) | 29 (74.4%) | 12 (57.1%) |
| 30 mg | 19 (31.7%) | 10 (25.6%) | 9 (42.9%) |
Four patients had both exon 21 L858R and uncommon mutations and one patient had both exon 19 deletion and uncommon mutations. ECOG PS, Eastern Cooperative Oncology Group performance status.
Efficacy of afatinib in the first‐line setting
Thirty‐nine (65%) patients received afatinib as first‐line treatment, with a median follow‐up duration of 15.3 months. The objective response rate (ORR) was 56.4% and the disease control rate (DCR) was 97.4%. Median PFS was 12.3 months (95% confidence internal [CI], 7.6–17.0) (Fig 1a), while the median OS has not yet been reached. Multivariate Cox regression analysis indicated that age (< 65 vs. ≥ 65 years), gender, smoking history, baseline brain metastasis status, initial afatinib dose (30 mg vs. 40 mg), and mutation type (common only vs. uncommon) did not significantly influence PFS. The median PFS of patients with common sensitive EGFR mutations (L858R or 19del) was 15.6 months (95% CI 9.5–21.8), and the median PFS of patients with uncommon sensitive mutations was 5.2 months (95% CI 3.6–6.9; P = 0.099) (Fig 1b).
Figure 1.
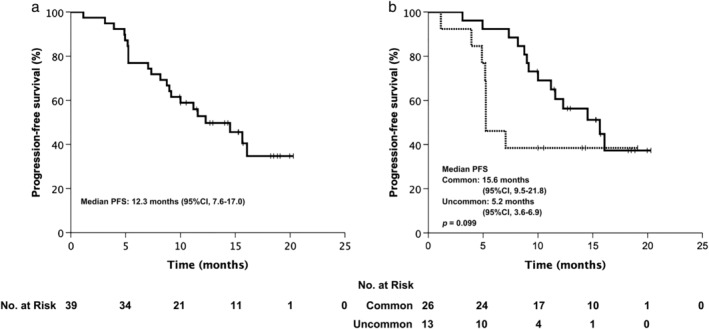
Progression‐free survival (PFS) of (a) first‐line treatment,  censored; and (b) first‐line treatment stratified by mutation type.
censored; and (b) first‐line treatment stratified by mutation type.  Common sensitive mutations,
Common sensitive mutations,  uncommon sensitive mutations,
uncommon sensitive mutations,  censored. CI, confidence interval.
censored. CI, confidence interval.
Efficacy of afatinib in the second or later‐line setting
Twenty‐one patients received afatinib as second or later‐line treatment and the median follow‐up duration was 12.0 months. The ORR was 33.3% and the DCR was 85.7%. Median PFS was 4.1 months (95% CI 1.3–7.0) (Fig 2a), while the median OS has not yet been reached. Multivariate Cox regression analysis indicated that age (< 65 vs. ≥ 65 years), gender, smoking history, baseline brain metastasis status, initial afatinib dose (30 mg vs. 40 mg), and mutation type (common only vs. uncommon) did not significantly influence PFS. The median PFS of patients with common sensitive EGFR mutations (L858R or 19del) was 3.0 months (95% CI 0.4–5.5), while the median PFS of patients with uncommon sensitive mutations was 6.6 months (95% CI 5.0–8.2; P = 0.119) (Fig 2b).
Figure 2.
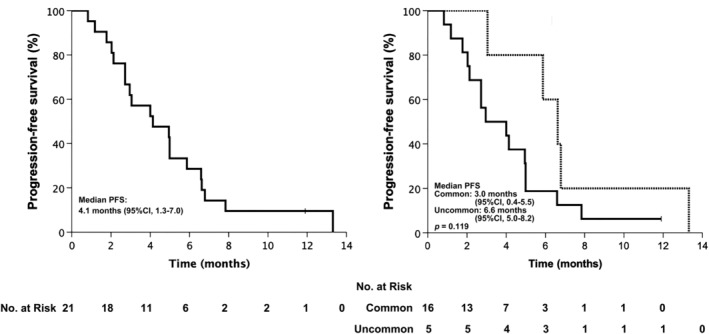
Progression‐free survival (PFS) of (a) second or later‐line treatment  Censored; and (b) second or later‐line treatment stratified by mutation type.
Censored; and (b) second or later‐line treatment stratified by mutation type.  Common sensitive mutations,
Common sensitive mutations,  uncommon sensitive mutations,
uncommon sensitive mutations,  Censored. CI, confidence interval.
Censored. CI, confidence interval.
Efficacy of afatinib for patients with baseline brain metastasis
In this study, a total of 24 patients had baseline brain metastasis. Nine patients received whole brain radiation therapy or stereotactic radiosurgery before or during afatinib treatment. The intracranial ORR of patients that did not receive local treatment for brain metastasis was 33.3%. Thirteen (54.2%) patients experienced intracranial progression. Multivariate Cox regression analysis of age (< 65 vs. ≥ 65 years), gender, smoking history, initial afatinib dose (30 mg vs. 40 mg), mutation type (common only vs. uncommon), line of afatinib (first‐line vs. ≥ second‐line), local brain metastasis treatment status, and intracranial progression status indicated that the line of afatinib was the only variable that influenced PFS (first‐line vs. ≥ second‐line hazard ratio [HR] 0.066, 95% CI 0.010–0.448; P = 0.005). Fourteen patients with baseline brain metastasis received afatinib as first‐line treatment and the median PFS of these patients was 15.6 months (95% CI 10.8–20.5). Ten patients received afatinib in second or later‐lines and the median PFS of these patients was 5.0 months (95% CI 4.9–5.0; P < 0.001) (Fig 3a). Eighteen patients with baseline brain metastasis received 40 mg afatinib daily as a starting dose and the median PFS of these patients was 10.0 months (95% CI 0.0–22.6). Six patients with baseline brain metastasis received 30 mg afatinib daily as a starting dose and the median PFS of these patients was 6.6 months (95% CI 4.5–8.8; P = 0.776) (Fig 3b).
Figure 3.
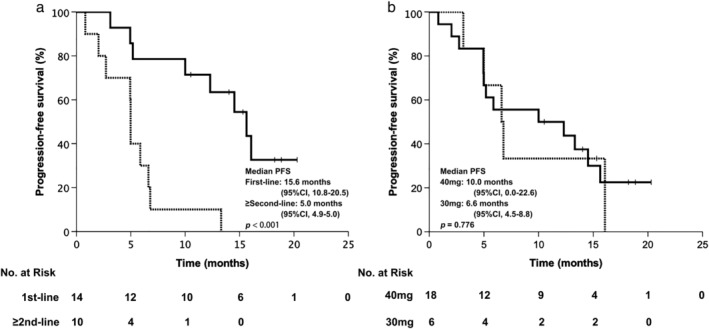
Progression‐free survival (PFS) of patients with baseline brain metastasis stratified by (a) line of afatinib,  First‐line,
First‐line,  ≥ second‐line,
≥ second‐line,  censored; and (b) initial dose of afatinib.
censored; and (b) initial dose of afatinib.  40 mg,
40 mg,  30 mg,
30 mg,  censored. CI, confidence interval.
censored. CI, confidence interval.
Efficacy of afatinib at an initial dose of 40 or 30 mg
Forty‐one patients received 40 mg afatinib daily as a starting dose and the remaining 19 patients received 30 mg daily. The characteristics of these two subgroups are summarized in Table 2. There were no significant differences in various characteristics between these two dose groups, including age distribution, gender, smoking status, ECOG PS score, EGFR mutation type, baseline brain metastasis status, or line of afatinib. No significant differences were observed in median PFS between patients treated with an initial dose of 40 mg and 30 mg either in first‐line (14.5 vs. 5.2 months; P = 0.101) (Fig 4a) or in second or later‐line settings (3.0 vs. 5.0 months; P = 0.375) (Fig 4b).
Table 2.
Comparison of characteristics between 40 mg and 30 mg afatinib groups
| Characteristics | All patients | 40 mg | 30 mg | P |
|---|---|---|---|---|
| N | 60 | 41 | 19 | |
| Age | ||||
| Median (years) | 58.1 | 57.2 | 58.1 | |
| Range | 36.3–82.7 | 36.3–70.9 | 44.6–82.7 | |
| Age distribution, N (%) | ||||
| ≥ 65 | 13 (21.7%) | 6 (14.6%) | 7 (36.8%) | 0.108 |
| < 65 | 47 (78.3%) | 35 (85.4%) | 12 (63.2%) | |
| Gender | ||||
| Male | 30 (50.0%) | 21 (51.2%) | 9 (47.4%) | 0.781 |
| Female | 30 (50.0%) | 20 (48.8%) | 10 (52.6%) | |
| Smoking history | ||||
| Yes | 18 (30.0%) | 13 (31.7%) | 5 (26.3%) | 0.672 |
| No | 42 (70.0%) | 28 (68.3%) | 14 (73.7%) | |
| ECOG PS score | ||||
| 0–1 | 60 (100%) | 41 (100%) | 41 (100%) | — |
| 2–4 | 0 | 0 | 0 | |
| EGFR mutation | ||||
| Exon 19 deletion | 26 (43.3%) | 20 (48.8%) | 6 (31.6%) | 0.370 |
| Exon 21 L858R | 16 (26.7%) | 9 (22.0%) | 7 (36.8%) | |
| Uncommon mutations† | 18 (30.0%) | 12 (29.3%) | 6 (31.6%) | |
| Baseline brain metastasis | ||||
| Yes | 24 (40.0%) | 18 (43.9%) | 6 (31.6%) | 0.365 |
| No | 36 (60.0%) | 23 (56.1%) | 13 (68.4%) | |
| Line of afatinib | ||||
| First line | 39 (65.0%) | 29 (70.7%) | 10 (52.6%) | 0.172 |
| ≥ Second line | 21 (35.0%) | 12 (29.3%) | 9 (47.4%) | |
Four patients had both exon 21 L858R and uncommon mutations and one patient had both exon 19 deletion and uncommon mutations. ECOG PS, Eastern Cooperative Oncology Group performance status.
Figure 4.
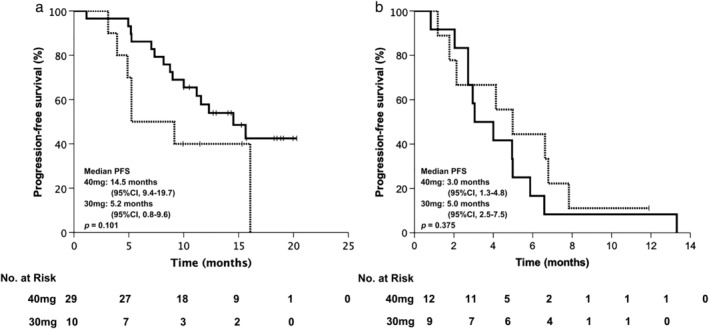
Progression‐free survival (PFS) of afatinib treatment stratified by initial dose. PFS in the (a) first‐line; and (b) second or later‐line setting.  40 mg,
40 mg,  30 mg,
30 mg,  censored. CI, confidence interval.
censored. CI, confidence interval.
Afatinib treatment‐related adverse events
A total of 58 patients were evaluable for adverse event incidence and the profiles were in line with expectations (Table 3). The most common adverse events included diarrhea (86.2%), rash/acne (82.8%), paronychia (72.4%), and stomatitis/mucositis (70.7%). The incidence of all grade rash/acne (92.5% vs. 61.1%; P = 0.011) and paronychia (82.5% vs. 50.0%; P = 0.010) was significantly higher among patients in the 40 mg group than patients in the 30 mg group. Four patients in the 40 mg group experienced a reduction in dose to 30 mg daily (2 for grade 3 diarrhea and 2 for grade 3 rash/acne). One patient experienced temporary dose modification as a result of grade 3 diarrhea.
Table 3.
Afatinib‐related adverse events
| All patients | Afatinib 40 mg | Afatinib 30 mg | P | ||||
|---|---|---|---|---|---|---|---|
| N = 58 | N = 40 | N = 18 | |||||
| Adverse events | N | % | N | % | N | % | |
| Diarrhea | 50 | 86.2 | 36 | 90.0 | 14 | 77.8 | 0.402 |
| ≥ Grade 3 | 6 | 10.3 | 5 | 12.5 | 1 | 5.6 | 0.736 |
| Rash/acne | 48 | 82.8 | 37 | 92.5 | 11 | 61.1 | 0.011 |
| ≥ Grade 3 | 2 | 3.4 | 2 | 5.0 | 0 | 0.0 | 1.000 |
| Paronychia | 42 | 72.4 | 33 | 82.5 | 9 | 50.0 | 0.010 |
| ≥ Grade 3 | 2 | 3.4 | 2 | 5.0 | 0 | 0.0 | 1.000 |
| Stomatitis/mucositis | 41 | 70.7 | 29 | 72.5 | 12 | 66.7 | 0.652 |
| ≥ Grade 3 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | — |
| Dry skin | 22 | 37.9 | 16 | 40.0 | 6 | 33.3 | 0.628 |
| ≥ Grade 3 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | — |
| Pruritus | 9 | 15.5 | 7 | 17.5 | 2 | 11.1 | 0.818 |
| ≥ Grade 3 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | — |
Discussion
This study is a large‐sample, retrospective, real‐world study of the efficacy and safety of afatinib in Chinese advanced NSCLC patients with sensitive EGFR mutations. In this study, all patients were adenocarcinoma, had a relatively good ECOG PS score of 0‐1 and the median age of all patients was 58.1, which made the results of this study comparable to the LUX‐Lung 3 and 6 trials.6, 15
Previous prospective clinical trials reported median PFS of first‐line afatinib treatment of 13.6–13.8 months in patients with common EGFR mutations (L858R and 19del).6, 15 In our study, the median PFS in common EGFR‐mutant patients was 15.6 months, which was longer than that reported in clinical trials, but may be explained by the proportion of patients with 19del mutations in our study. Twenty‐six patients with common EGFR mutations received afatinib as first‐line treatment, 19 (73.1%) of whom had 19del and 7 (26.9%) had L858R mutations, while the proportions of 19del and L858R mutations were similar in the clinical trials. Subgroup analysis of PFS in clinical trials showed that the HR of 19del was superior to that of L858R when compared to chemotherapy.6, 15 Further pooled analysis indicated a significant improvement in OS in the 19del subgroup.17, 18 These outcomes may lead to a tendency in clinical practice to prescribe afatinib to patients with 19del mutations. Several real‐world studies have also demonstrated this tendency and reported longer median PFS in common EGFR mutation groups.19, 20, 21 Kim et al. revealed that in a subgroup of patients with 19del mutations, the median PFS of afatinib was significantly superior to gefitinib or erlotinib (19.1 vs. 15.0 and 16.3 months, respectively; P = 0.01). However, there was no such significant difference in the L858R subgroup (P = 0.46).19
Combined analysis of the results of the LUX‐Lung 2, 3, and 6 trials suggests that patients with point mutations or duplications in exons 18–21 could also achieve a median PFS of 10.7 months (95% CI 5.6–14.7).12 However, in our study, the median PFS of uncommon EGFR mutant patients was only 5.2 months in the first‐line setting. There were only 13 patients with uncommon mutations receiving afatinib as first‐line treatment in our study, which may lead to bias and partly account for the outcome.
The results of multivariate Cox regression analysis in our study suggested that there was no significant difference in PFS between patients with and without brain metastasis. This result was consistent with the results of previous clinical trials. Combined analysis of the results of the LUX‐Lung 3 and 6 trials demonstrated that the PFS of patients with brain metastases was significantly prolonged in the afatinib group compared to the chemotherapy group (8.2 vs. 5.4 months, HR 0.50; P = 0.0297). The extent of improvement in PFS from afatinib treatment of these patients was similar to that of patients without brain metastases.22
One real world study indicated that a starting afatinib dose of 30mg daily had similar PFS to 40mg daily, but led to fewer serious adverse events.21 Our research also supported this conclusion. In this study, there were no significant differences in various characteristics and median PFS between these two dose groups. Multivariate COX regression analysis further confirmed that no significant differences were observed in PFS between patients with these two initial doses either in the first‐line treatment or in the second‐ or later‐line treatment.
A previous real world study indicated that a starting afatinib dose of 30 mg daily achieved similar PFS to 40 mg daily, but led to fewer serious adverse events.21 Our results support this conclusion. In this study, there were no significant differences in various characteristics and median PFS between the two dose groups. Multivariate Cox regression analysis further confirmed no significant differences in PFS between patients treated with these initial doses either in first, second or later‐lines. Meanwhile, patients treated with 40 mg afatinib were significantly more likely to experience rash/acne and paronychia. However, Tan et al. revealed that among patients with advanced EGFR mutant NSCLC with brain metastasis, the initiation of 40 mg afatinib once daily was associated with improved PFS compared to 30 mg once daily.23 We did not observe this outcome, which may result from our relatively small sample of patients with baseline brain metastasis treated with 30 mg afatinib.
There were some limitations to this study. Firstly, as a single center, retrospective study, unavoidable bias may have been introduced. Secondly, the number of patients harboring uncommon EGFR mutations in our study may not have been large enough to confirm the efficacy of afatinib in these patients. A larger sample of patients with uncommon EGFR mutations are needed for further study.
In conclusion, first‐line afatinib treatment is beneficial to advanced lung adenocarcinoma patients with sensitive EGFR mutations. Initial dose and baseline brain metastasis status do not have a significant impact on PFS in these patients.
Disclosure
No authors report any conflict of interest.
References
- 1. Noone AM, Howlader N, Krapcho M. SEER Cancer Statistics Review, 1975–2015, based on November 2017 SEER data submission. Bethesda, MD: National Cancer Institute; 2018. Available from URL: https://seer.cancer.gov/csr/1975_2015/. Accessed date: May 9, 2019.
- 2. Shi Y, Au JS, Thongprasert S et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non‐small‐cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol 2014; 9: 154–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lu RL, Hu CP, Yang HP, Li YY, Gu QH, Wu L. Biological characteristics and epidermal growth factor receptor tyrosine kinase inhibitors efficacy of EGFR mutation and its subtypes in lung adenocarcinoma. Pathol Oncol Res 2014; 20: 445–51. [DOI] [PubMed] [Google Scholar]
- 4. Mok TS, Wu YL, Thongprasert S et al. Gefitinib or carboplatin‐paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009; 361: 947–57. [DOI] [PubMed] [Google Scholar]
- 5. Rosell R, Carcereny E, Gervais R et al. Erlotinib versus standard chemotherapy as first‐line treatment for European patients with advanced EGFR mutation‐positive non‐small‐cell lung cancer (EURTAC): A multicentre, open‐label, randomised phase 3 trial. Lancet Oncol 2012; 13: 239–46. [DOI] [PubMed] [Google Scholar]
- 6. Sequist LV, Yang JC, Yamamoto N et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013; 31: 3327–34. [DOI] [PubMed] [Google Scholar]
- 7. Sharma SV, Bell DW, Settleman J, Haber DA. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer 2007; 7: 169–81. [DOI] [PubMed] [Google Scholar]
- 8. Yasuda H, Park E, Yun CH et al. Structural, biochemical, and clinical characterization of epidermal growth factor receptor (EGFR) exon 20 insertion mutations in lung cancer. Sci Transl Med 2013; 5: 216ra177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kobayashi Y, Mitsudomi T. Not all epidermal growth factor receptor mutations in lung cancer are created equal: Perspectives for individualized treatment strategy. Cancer Sci 2016; 107: 1179–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chiu CH, Yang CT, Shih JY et al. Epidermal growth factor receptor tyrosine kinase inhibitor treatment response in advanced lung adenocarcinomas with G719X/L861Q/S768I mutations. J Thorac Oncol 2015; 10: 793–9. [DOI] [PubMed] [Google Scholar]
- 11. Castellanos E, Feld E, Horn L. Driven by mutations: The predictive value of mutation subtype in EGFR‐mutated non‐small cell lung cancer. J Thorac Oncol 2017; 12: 612–23. [DOI] [PubMed] [Google Scholar]
- 12. Yang JC, Sequist LV, Geater SL et al. Clinical activity of afatinib in patients with advanced non‐small‐cell lung cancer harbouring uncommon EGFR mutations: A combined post‐hoc analysis of LUX‐Lung 2, LUX‐Lung 3, and LUX‐Lung 6. Lancet Oncol 2015; 16: 830–8. [DOI] [PubMed] [Google Scholar]
- 13. Solca F, Dahl G, Zoephel A et al. Target binding properties and cellular activity of afatinib (BIBW 2992), an irreversible ErbB family blocker. J Pharmacol Exp Ther 2012; 343: 342–50. [DOI] [PubMed] [Google Scholar]
- 14. Li D, Ambrogio L, Shimamura T et al. BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models. Oncogene 2008; 27: 4702–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wu YL, Zhou C, Hu CP et al. Afatinib versus cisplatin plus gemcitabine for first‐line treatment of Asian patients with advanced non‐small‐cell lung cancer harbouring EGFR mutations (LUX‐Lung 6): An open‐label, randomised phase 3 trial. Lancet Oncol 2014; 15: 213–22. [DOI] [PubMed] [Google Scholar]
- 16. Park K, Tan EH, O'Byrne K et al. Afatinib versus gefitinib as first‐line treatment of patients with EGFR mutation‐positive non‐small‐cell lung cancer (LUX‐Lung 7): A phase 2B, open‐label, randomised controlled trial. Lancet Oncol 2016; 17: 577–89. [DOI] [PubMed] [Google Scholar]
- 17. Greenhalgh J, Dwan K, Boland A et al. First‐line treatment of advanced epidermal growth factor receptor (EGFR) mutation positive non‐squamous non‐small cell lung cancer. Cochrane Database Syst Rev 2016; Cd010383. [DOI] [PubMed] [Google Scholar]
- 18. Yang JC, Wu YL, Schuler M et al. Afatinib versus cisplatin‐based chemotherapy for EGFR mutation‐positive lung adenocarcinoma (LUX‐Lung 3 and LUX‐Lung 6): Analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol 2015; 16: 141–51. [DOI] [PubMed] [Google Scholar]
- 19. Kim Y, Lee SH, Ahn JS, Ahn MJ, Park K, Sun JM. Efficacy and safety of afatinib for EGFR‐mutant non‐small cell lung cancer, compared with gefitinib or erlotinib. Cancer Res Treat 2019; 51: 502–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yang CJ, Tsai MJ, Hung JY et al. The clinical efficacy of afatinib 30 mg daily as starting dose may not be inferior to afatinib 40 mg daily in patients with stage IV lung adenocarcinoma harboring exon 19 or exon 21 mutations. BMC Pharmacol Toxicol 2017; 18: 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liang SK, Hsieh MS, Lee MR, Keng LT, Ko JC, Shih JY. Real‐world experience of afatinib as a first‐line therapy for advanced EGFR mutation‐positive lung adenocarcinoma. Oncotarget 2017; 8: 90430–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schuler M, Wu YL, Hirsh V et al. First‐line afatinib versus chemotherapy in patients with non‐small cell lung cancer and common epidermal growth factor receptor gene mutations and brain metastases. J Thorac Oncol 2016; 11: 380–90. [DOI] [PubMed] [Google Scholar]
- 23. Tan WL, Ng QS, Lim C et al. Influence of afatinib dose on outcomes of advanced EGFR‐mutant NSCLC patients with brain metastases. BMC Cancer 2018; 18: 1198. [DOI] [PMC free article] [PubMed] [Google Scholar]


