Abstract
Background
Although oncogenic driver mutations were thought to be mutually exclusive in non‐small cell lung cancer (NSCLC), certain tumors harbor co‐occurring mutations and represent a rare molecular subtype. The evaluation of the clinical features and therapeutic response associated with this NSCLC subtype will be vital for understanding the heterogeneity of treatment response and improving the management of these patients.
Methods
This retrospective study included 3774 samples from patients diagnosed with NSCLC. All samples were screened for EGFR, ALK, ROS1, KRAS, and BRAF mutation using the amplification‐refractory mutation system. The relationship between concomitant driver mutations and clinicopathologic characteristics, and patient clinical outcomes were evaluated.
Results
Sixty‐three (1.7%) samples had more than one driver gene mutation. Among these, 43 were coalterations with an EGFR mutation, 20 with an ALK rearrangement, and eight with an ROS1 rearrangement. Except for ROS1 concomitant mutations that were more frequent in male patients (87.5%, P = 0.020), the clinicopathological features of the concomitant mutation patients were not significantly different from those harboring a single EGFR, ALK, or ROS1 mutation. Furthermore, first‐line EGFR‐TKI treatment did not significantly improve the progression‐free survival (PFS) of patients harboring EGFR concomitant mutation, compared to patients harboring a single EGFR mutation. However, for EGFR concomitant mutation patients, TKI therapy was more effective than chemotherapy (median PFS of 10.8 vs 5.2 months, P = 0.023). Lastly, KRAS mutations did not influence the EGFR‐TKI therapy treatment effect.
Conclusion
In this study, concomitant mutations were found in 1.7% of the NSCLC. EGFR‐TKI therapy was more effective than chemotherapy for patients harboring EGFR concomitant mutation, and ROS1 concomitant mutations were more frequent in male patients. For patients harboring coalterations with an ALK or ROS1 rearrangement, we should be cautious when considering the therapeutic options.
Keywords: ALK, concomitant mutations, EGFR, NSCLC, ROS1
1. INTRODUCTION
Tyrosine kinase inhibitors (TKIs) targeting epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase (ALK), and c‐ros oncogene 1 (ROS1) have been established as efficient cancer treatments.1, 2, 3, 4, 5 However, concomitant mutations in these driver genes, as well as in the KRAS and BRAF oncogenes, have been reported frequently in recent years.6, 7 Moreover, case series reports presented various treatment procedures and different results.
Concomitant driver gene mutations in EGFR and ALK have been detected in 1.3%‐15.4% of the patients with non‐small cell lung cancer (NSCLC), depending on the method used.8, 9 Among the patients harboring EGFR/ALK coalterations, some responded to treatment with an EGFR‐TKI (ie, gefitinib, erlotinib, icotinib, or afatinib),8, 10, 11, 12, 13 while others responded to treatment with an ALK‐TKI (crizotinib) 9, 13 or both.14, 15 To the best of our knowledge, there is currently no consensus for the optimal management for these patients. Although several studies provided recommendations on how to treat these patients,8, 9, 15 we still need further evidence to improve the efficacy of the therapy. While the co‐occurrence of driver gene mutations in patients harboring a ROS1 rearrangement has been described as rare,16, 17, 18 Wiesweg et al recently reported that the rate of concomitant mutations could be as high as 36%.19 They detected ROS1/ALK, ROS1/EGFR, and ROS1/KRAS coalterations, and accordingly, new studies are required to establish how to treat these patients.
KRAS proto‐oncogene (KRAS) and B‐Raf proto‐oncogene (BRAF) have been frequently studied in NSCLC, and mutation testing for these genes is recommended in the National Comprehensive Cancer Network guidelines for NSCLC KRAS mutations have been found more frequently in non‐Asian patients and former or current smokers and have been associated with mucinous adenocarcinoma.20 Furthermore, evidence suggested that KRAS mutations combined with EGFR mutations or ALK rearrangements could negatively impact the effects of TKI therapy.6, 13, 21, 22 Meanwhile, the BRAF V600E mutation has been reported to be mutually exclusive with EGFR and KRAS mutations 23 but has been found to co‐occur with other driver gene mutations.19
Rare driver gene mutations, such as the erb‐b2 receptor tyrosine kinase 2 (HER2) mutation or MET proto‐oncogene (MET) exon 14 skipping, have also been found to co‐occur with other oncogenic driver mutations and were reported to respond well to TKI therapy.12, 24 Meanwhile, other concomitant mutations, such as TP53 or PIK3CA mutations combined with EGFR mutations or ALK rearrangements, have been found to influence the treatment effect of TKI therapy.25, 26, 27, 28 However, mutation testing for these genes is not routinely performed in most clinical practices. Since there is no consensus for treating patients with concomitant driver gene mutations, studying coalterations in EGFR, ALK, ROS1, KRAS, and BRAF remains vital to improve treatments for these patients. Therefore, in this center, alterations in these five driver genes were regularly tested at diagnosis, and the clinical features and outcomes were analyzed for the patients with concomitant mutations.
2. PATIENTS AND METHODS
2.1. Patients
Between June 2014 and June 2017, 3774 samples from stage IIIB or IV Chinese patients diagnosed with NSCLC were screened for the five driver gene mutations. All patients provided written informed consent before molecular detection. All patients with concomitant mutations who received therapy at the center were included in the study. The EGFR‐TKIs were gefitinib, erlotinib, icotinib, or afatinib, the ALK‐TKIs were crizotinib or alectinib, and the ROS1‐TKI was crizotinib. All the patients received TKI doses consistent with the package insert recommendations. Follow‐up data were collected until July 2018 to assess progression‐free survival (PFS) and overall survival (OS). This study was approved by the Ethics Committee of Shanghai Pulmonary Hospital (2014‐FK02).
2.2. ARMS for the detection of EGFR, ALK, ROS1, KRAS, and BRAF mutations
Formalin‐fixed paraffin‐embedded (FFPE), fine/core needle aspiration, biopsy, or pleural effusion samples were used for the detection of alterations in the EGFR, KRAS, BRAF, ALK, and ROS1 genes. Genomic DNA and total RNA were extracted from FFPE samples using the AmoyDx FFPE DNA/RNA extraction kit (ADx‐FF03, Amoy Diagnostics, Xiamen, China) according to the manufacturer's recommendations, and from all other samples using the AmoyDx Tissue DNA/RNA extraction kit (ADx‐TI03, Amoy Diagnostics). EGFR, KRAS, and BRAF mutations were detected using 80 ng, 120 ng, and 10 ng of DNA, respectively. ALK and ROS1 rearrangements were detected using 100‐1000 ng of total RNA. The EGFR 29 Mutations Detection Kit (ADx‐EG01), KRAS Mutation Detection Kit (ADx‐KR01), BRAF V600 Mutations Detection Kit (ADx‐BR01), EML4‐ALK Fusion Gene Detection Kit (ADx‐AE01), and ROS1 Gene Fusions Detection Kit (ADx‐RO02) from Amoy Diagnostics were used to detect alterations. For ALK and ROS1 reverse transcription, RNA and 0.5 μL of EA Reverse Transcriptase were added to an EA RT and ROS1 RT Reaction Mix tube, respectively. The samples were then incubated for 1 hour at 42℃ followed by 5 minutes at 95℃. All real‐time qPCRs were performed on a Stratagene Mx3000P™ cycler (Agilent, Santa Clara, CA, USA) using the following program: 5 minutes at 95℃ (1 cycle); 25 seconds at 95℃, 20 seconds at 64℃, and 20 seconds at 72℃ (15 cycles); 25 seconds at 93℃, 35 seconds at 60℃, and 20 seconds at 72℃ (31 cycles). Ultrapure water was used as negative control, different commercial products were used as positive control for different gene detection, and the conservative sequences of the corresponding gene were used as quality control. Alterations in EGFR, ALK/ROS1, KRAS, and BRAF were defined as Ct values <26, <30, <26, and <28, respectively. For samples positive for ALK and ROS1 rearrangements, DNA sequencing was performed to distinguish the variants as previously described.29, 30
2.3. Statistical analysis
All statistical analyses were performed using the SPSS v.20 software (SPSS Inc, Chicago, IL, USA). Comparisons of clinicopathological features in Table 1 and 2 were all evaluated using Pearson Chi‐square test, except that the analysis of pathology was evaluated using Fisher's exact test in Table 2. PFS and OS were analyzed using the Kaplan‐Meier method. The two‐sided significance level was set at P < 0.05.
Table 1.
Clinicopathological features of EGFR, ALK, and ROS1 single mutations with their concomitant mutations
| EGFR | ALK | ROS1 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Concomitant n(%) | Single n(%) | P | Concomitant n(%) | Single n(%) | P | Concomitant n(%) | Single n(%) | P | |
| Sex | |||||||||
| Female | 25 (58.1) | 61 (61.0) | 0.853 | 11 (55.0) | 51 (51.0) | 0.810 | 1 (12.5) | 30 (60.0) | 0.020 |
| Male | 18 (41.9) | 39 (39.0) | 9 (45.0) | 49 (49.0) | 7 (87.5) | 20 (40.0) | |||
| Age (y) | |||||||||
| ˂65 | 29 (67.4) | 59 (59.0) | 0.357 | 15 (75.0) | 84 (84.0) | 0.521 | 6 (75.0) | 40 (80.0) | >0.9999 |
| ≥65 | 14 (32.6) | 41 (41.0) | 5 (25.0) | 16 (16.0) | 2 (25.0) | 10 (20.0) | |||
| Smoking status | |||||||||
| Never/light | 34 (79.1) | 77 (77.0) | 0.831 | 14 (70.0) | 73 (73.0) | >0.9999 | 5 (62.5) | 38 (76.0) | 0.666 |
| Smoking | 9 (20.9) | 23 (23.0) | 6 (30.0) | 27 (27.0) | 3 (37.5) | 12 (24.0) | |||
| Pathology | |||||||||
| Adenocarcinoma | 39 (90.7) | 91 (91.0) | >0.9999 | 20 (100.0) | 90 (90.0) | 0.210 | 7 (87.5) | 42 (84.0) | >0.9999 |
| Others | 4 (9.3) | 9 (9.0) | 0(0) | 10 (10.0) | 1 (12.5) | 8 (16.0) | |||
Table 2.
Clinicopathological features of EGFR concomitant or single mutation patients treated with first‐line EGFR‐TKI
| Concomitant n(%) | Single n(%) | P | |
|---|---|---|---|
| Sex | |||
| Female | 9 (81.8) | 61 (61.0) | 0.208 |
| Male | 2 (18.2) | 39 (39.0) | |
| Age | |||
| ˂65 | 7 (63.6) | 59 (59.0) | >0.9999 |
| ≥65 | 4 (36.4) | 41 (41.0) | |
| Smoking status | |||
| Never/light | 10 (90.9) | 77 (77.0) | 0.451 |
| Smoking | 1 (9.1) | 23 (23.0) | |
| Pathology | |||
| Adenocarcinoma | 11 (100.0) | 91 (91.0) | 0.595 |
| Others | 0 | 9 (9.0) | |
| Treatment effect | |||
| PR | 7 (63.6) | 66 | 0.613 |
| SD | 2 (18.2) | 26 | |
| PD | 2 (18.2) | 8 | |
3. RESULTS
3.1. Frequency and outcomes of patients harboring concomitant mutations
Among the 3774 patients tested, the single EGFR, ALK, ROS1, KRAS, and BRAF mutation rates were 39.0% (1470), 5.5% (207), 2.1% (80), 8.0% (303), and 0.6% (23), respectively. Sixty‐three patients (1.7%) harbored mutations in two or three of these genes (Figure 1), and among these patients, EGFR/KRAS was the most frequent coalteration (31.7%), followed by ALK/KRAS (17.5%). Out of 3774 patients, only two harbored a triple EGFR/ROS1/KRAS coalteration. Within the patients harboring coalterations with an EGFR, ALK, or ROS1 alteration, 21 received TKI therapy as first‐line treatment, with an objective response rate (ORR) of 61.9% (13/21) and a median PFS of 11.8 months.
Figure 1.
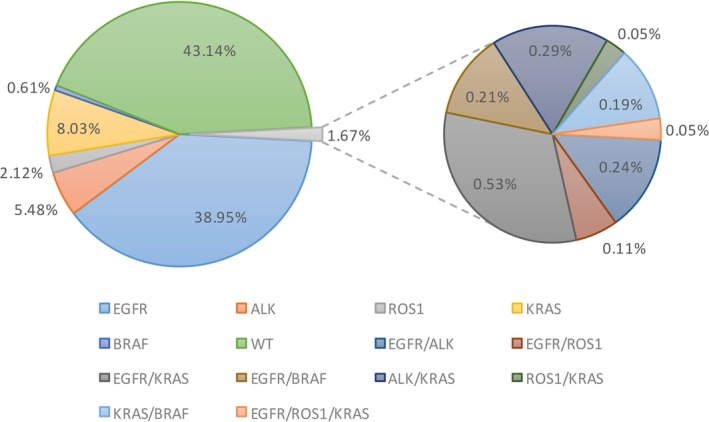
Frequency of EGFR, ALK, ROS1, KRAS and BRAF mutations in NSCLC patients. Wild‐type of the five genes was 43.14%, concomitant mutations was 1.67% and EGFR/KRAS was the most frequent mutation type (0.53%). Other mutation types are also listed in the figure
3.2. Patients harboring a coalteration with an EGFR mutation
Out of 1513 patients harboring an EGFR mutation, 43 (2.8%) carried an additional alteration. Notably, among the patients harboring an EGFR 19DEL or L858R mutation, 15 received EGFR‐TKI therapy as first‐line treatment and had a tumor response assessment (10 partial responses [PR], three stable diseases [SD], and two progressive diseases [PD]) with 11 patients achieving PFS, while seven received chemotherapy and had a tumor response assessment (two PR, three SD, and two PD) with six patients achieving PFS. We compared the clinical features of 100 randomly selected patients harboring a single EGFR mutation and receiving EGFR‐TKI therapy as first‐line treatment with those of the 43 patients harboring coalterations with an EGFR mutation but found no significant differences (Table 1). Furthermore, when we compared the clinical features and treatment effect of the 11 patients of the PFS group to those of the 100 randomly selected patients, we found no statistically significant differences (Table 2). Moreover, the PFS between the patients harboring a single EGFR mutation and those harboring coalterations with an EGFR mutation were not significantly different (10.8 vs 9.6 months, P = 0.747, Figure 2A). We also explored whether the patients harboring co‐alterations with an EGFR mutation benefited from TKI therapy or chemotherapy. The results showed that patients receiving TKI therapy as first‐line treatment (11 patients with coalterations: three EGFR/ALK, five EGFR/KRAS, two EGFR/BRAF, and one EGFR/ROS1/KRAS) had improved PFS compared to those receiving chemotherapy (six patients with coalterations: two EGFR/ALK, three EGFR/KRAS, and one EGFR/ROS1/KRAS) (10.8 vs 5.2 months, P = 0.023, Figure 2B). In contrast, sex, age, and smoking status were not associated with PFS of first‐line treatment (Table 3). For the patients harboring EGFR/KRAS and EGFR/non‐KRAS coalterations, and receiving EGFR‐TKI therapy as first‐line treatment, the ORR was 62.5% (5/8) and 71.4% (5/7), respectively. Finally, the PFS comparisons between patients harboring an EGFR/KRAS coalteration and those harboring a single EGFR mutation (Figure 2C), or between patients harboring an EGFR/KRAS coalteration and those harboring an EGFR/non‐KRAS coalteration (Figure 2D) showed no significant differences (P = 0.392 and P = 0.159, respectively).
Figure 2.
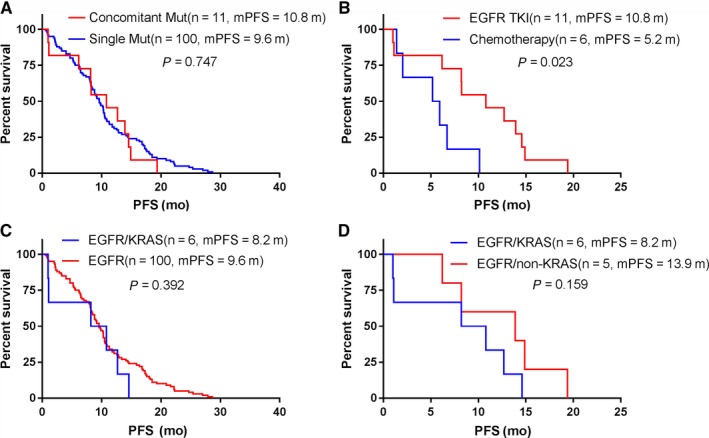
Clinical outcomes of EGFR concomitant mutation patients. Survival data were analyzed using Kaplan‐Meier method. A, PFS between EGFR concomitant mutation and single EGFR mutation patients treated with first‐line TKIs. B, PFS of EGFR concomitant mutation patients treated with first‐line TKI or chemotherapy. C, PFS between EGFR/KRAS concomitant and EGFR single mutation patients treated with first‐line TKIs. D, PFS of EGFR/KRAS and EGFR/non‐KRAS concomitant mutation patients treated with first‐line TKIs
Table 3.
Survival analysis of EGFR concomitant mutation patients treated with first‐line EGFR‐TKI or chemotherapy
| No.(N = 17) | P | |
|---|---|---|
| Sex | ||
| Female | 12 | 0.456 |
| Male | 5 | |
| Age (y) | ||
| ˂65 | 12 | 0.814 |
| ≥65 | 5 | |
| Smoking status | ||
| Never/light | 15 | 0.799 |
| Smoking | 2 | |
| Treatment | ||
| TKIs | 11 | 0.023 |
| Chemotherapy | 6 | |
3.3. Patients harboring a coalteration with an ALK rearrangement
Out of 227 patients harboring an ALK rearrangement, 20 (8.8%) carried an additional alteration. Eight of these patients received crizotinib, with an ORR of 37.5% (3/8) across all treatment lines and 60.0% (3/5) for first‐line treatment (Table 4). We compared the clinical features of 100 randomly selected patients harboring a single ALK rearrangement with those of the 20 patients harboring a coalteration with an ALK rearrangement but found no significant differences (Table 1). At the time of the study, only three of the patients who received crizotinib as first‐line treatment achieved PFS. Therefore, we did not compare the outcomes of these patients with those of the patients harboring a single ALK rearrangement.
Table 4.
Characteristics of ALK and ROS1 concomitant mutation patients
| Patient No. | Sex | Age | Smoking history (pack year) | Pathologya | Stage | Mutation | ALK/ROS1 variant | First‐line treatment | Effect | PFS (mo) | ALK(ROS1)‐TKI treatment line | TKI Effect | TKI PFS (mo) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 48 | 0 | A | IV | EGFR/ALK | / | Chemotherapy | SD | 10.1 | / | ||
| 2 | F | 69 | 0 | A | IIIA | EGFR/ALK | V1 | Chemotherapy | PR | 5.9 | / | ||
| 3 | F | 46 | 0 | A | IIIB | ALK/KRAS | V3 | Crizotinib | PR | / | 1 | PR | |
| 4 | M | 58 | 45 | A | IV | ALK/KRAS | / | Crizotinib | PD | 1.1 | 1 | PD | 1.1 |
| 5 | M | 60 | 10 | A | IV | ALK/KRAS | V3 | Crizotinib | PR | 28.0 | 1 | PR | 28.0 |
| 6 | M | 36 | 5 | A | IV | ALK/KRAS | V5 | Crizotinib | PR | 14.8 | 1 | PR | 14.8 |
| 7 | M | 55 | 25 | A | IV | ALK/KRAS | V1 | Chemotherapy | SD | / | / | ||
| 8 | F | 51 | 0 | A | IV | ALK/KRAS | V3 | Crizotinib | SD | / | 1 | SD | |
| 9 | F | 54 | 0 | A | IV | ALK/KRAS | / | Chemotherapy | SD | 9.3 | 2 | SD | |
| 10 | M | 73 | 30 | A | IV | ALK/KRAS | / | Chemotherapy | SD | 83.2 | 3 | PD | 1.2 |
| 11 | F | 49 | 0 | A | IV | ALK/KRAS | V2 | Chemotherapy | PD | 1.2 | 3 | SD | 2.9 |
| 12 | M | 59 | 80 | A | IV | EGFR/ROS1 | EZR‐E10;ROS1‐E34 | Chemotherapy | SD | / | / | ||
| 13 | M | 54 | 0 | A | IIIB | EGFR/ROS1 | CD74‐E6;ROS1‐E34 | Crizotinib | SD | / | 1 | SD | |
| 14 | M | 53 | 0 | A | IV | EGFR/ROS1/KRAS | EZR‐E10;ROS1‐E34 | Chemotherapy | PD | 2.0 | 2 | PD | 1.0 |
| 15 | M | 52 | 20 | A | IV | EGFR/ROS1/KRAS | / | Gefitinib | PR | 12.7 | / | ||
| 16 | M | 77 | 0 | A | IIIA | ROS1/KRAS | EZR‐E10;ROS1‐E34 | Chemotherapy | PD | 0.9 | / |
A, adenocarcinoma
3.4. Patients harboring a coalteration with a ROS1 rearrangement
Out of 88 patients harboring an ROS1 rearrangement, eight (9.1%) had coalterations. We compared the clinical features of 50 randomly selected patients harboring a single ROS1 rearrangement with those of the eight patients harboring a coalteration with an ROS1 rearrangement and found that coalterations with an ROS1 rearrangement occurred more frequently in male patients (P = 0.020) (Table 1). Concerning the outcomes, among the two patients treated with crizotinib, one had SD after receiving crizotinib as first‐line treatment, and the other had PD after receiving crizotinib as second‐line treatment (Table 4). Furthermore, two patients harbored EGFR/ROS1/KRAS triple co‐alterations (patient 14 and 15). Patient 14 had PD after receiving crizotinib as second‐line treatment and PR after receiving icotinib as third‐line treatment (PFS of 27.5 months), whereas patient 15 had PR after receiving gefitinib as first‐line treatment (PFS of 12.7 months).
3.5. Patients harboring a coalteration with a KRAS or BRAF mutation
Out of the 42 patients harboring a coalteration with a KRAS mutation, 13 (31.0%) had a G12C substitution, 10 (23.8%) G12D, six (14.3%) G12S, five (11.9%) G12V, and eight (19.0%) a different mutation. Furthermore, three patients harbored two types of KRAS mutation with one each carrying G12R/C, G12D/V, and G12S/C mutations. Concerning the 22 patients harboring EGFR/KRAS coalterations, the most frequent types of KRAS mutation were G12C (31.8%) and G12D (31.8%). Meanwhile, G12C was the most frequent KRAS mutation (54.5%) among the 11 patients harboring ALK/KRAS coalterations. Seventeen of the 42 patients harboring a coalteration with a KRAS mutation received first‐line chemotherapy, with an ORR of 26.7% (four PR, five SD, and six PD) and a median PFS of 4 months. Moreover, 15 patients harbored a coalteration with a BRAF mutation, with eight and seven patients carrying EGFR/BRAF and KRAS/BRAF coalterations, respectively. Seven of these patients received EGFR‐TKI therapy as first‐line treatment (two PR and one SD), and two of them achieved PFS (19.4 and 14.9 months, respectively). In comparison, five patients received chemotherapy (four SD and one PD), and three of them achieved PFS (8.1, 2.8, and 17.4 months, respectively).
4. DISCUSSION
Concomitant driver gene mutations in NSCLC patients have been reported in previous case series studies. However, the standard treatment of these patients was still ongoing. Therefore, it is of great importance to determine the clinical features and outcomes of these patients to provide the most effective treatments. To the best of our knowledge, this report is the first comprehensive study of concomitant driver gene mutations in Chinese patients harboring EGFR, ALK, ROS1, KRAS, and BRAF alterations. We identified 63 patients who harbored concomitant mutations. Our data showed that patients harboring coalterations with an EGFR mutation had better PFS with TKI therapy than with chemotherapy, while coalterations with an ROS1 rearrangement occurred more frequently in male patients.
A previous study reported that concomitant mutations were found in approximately 5% of the patients with lung adenocarcinoma.31 However, using more precise detection methods, the same patient cohort could exhibit different results.9 Indeed, EGFR/ALK coalterations have been reported to occur in 3.9%‐13.6% and 15.4%‐18.8% of the patients harboring EGFR mutations and ALK rearrangements, respectively.8, 9, 32 Furthermore, EGFR/KRAS coalterations have been reported to occur in 5.8%‐35.8% of the patients harboring EGFR mutations.16, 32 In the present study, EGFR/ALK coalterations were detected in 0.6% and 4.0% of the patients harboring EGFR and ALK alterations, respectively, which was lower than in other available studies. Most of the samples we used had been prepared for cytological analyses, which could explain why certain mutation‐positive tumor cells were not detected by comparison with detection performed on resected tumor tissues or biopsy tissue samples. Besides, the use of more precise methods to detect the alterations, such as next‐generation sequencing (NGS), would most likely result in a higher ratio of concomitant mutations.
The choice between EGFR‐TKI and ALK‐TKI therapy as first‐line treatment for patients harboring EGFR/ALK concomitant mutations has been debated since their discovery. In certain studies, EGFR‐TKI therapy gave better results, while in others it was the other way around.8, 9, 10, 11, 14, 15 Apart from the influence of the level of protein phosphorylation on treatment effect, we think that there may be at least three other possible explanations for these contradicting observations. First, we must consider tumor heterogeneity. Different mutations may coexist in the same tumor cells 15 or may be present in different areas (ie, different cells) of the tumor.33 If tumor cells carry both EGFR and ALK alterations, the two types of inhibitors may be able to kill tumor cells and could have a positive treatment effect. However, in cases where the mutations affect different tumor cells, a given type of TKI can only target corresponding tumor cells (eg, EGFR‐TKI can only target tumor cells with EGFR alterations), and other tumor cells (ie, not carrying the targeted mutation) may be able to proliferate rapidly. Second, the existence of gene mutation subtypes may impact the treatment effect. For example, various ALK rearrangement variants result in different responses to ALK‐TKI therapy and resistance patterns.34, 35 Furthermore, a similar phenomenon has also been reported for classical EGFR mutations.36, 37 In this situation, the types of mutation should be considered carefully before making any decision regarding the treatment. Finally, we cannot exclude the possibility of unknown mechanisms impacting the outcomes. The pathological subtype, passenger mutations, and mutations in other genes,27, 28 as well as smoking‐related genomic patterns,38 might influence the treatment effect. In this study, since only three of the patients harboring EGFR/ALK coalterations and receiving TKI therapy achieved PFS, we did not compare the clinical outcomes of these patients with those of the patients harboring a single EGFR or ALK alteration. Regarding the whole group of patients harboring coalterations with an EGFR mutation, we did not record significant differences with the group of patients harboring a single EGFR mutation following TKI therapy. However, they benefited more from TKI therapy than from chemotherapy. In previous studies, KRAS mutations have been associated with primary resistance to EGFR‐TKI therapy.6, 21, 22 In this study, we did not record significant differences of PFS between the patients harboring EGFR/KRAS coalterations and those harboring a single EGFR mutation, or between the patients harboring EGFR/KRAS coalterations and those harboring EGFR/non‐KRAS coalterations. However, the study included only a small number of patients with such coalterations, and future studies would greatly benefit from larger patient cohorts.
In this study, the cohort of patients harboring coalterations with an ALK or ROS1 rearrangement who received TKI therapy as first‐line treatment was too small to compare the clinical outcomes with those of patients harboring a single ALK or ROS1 rearrangement. Nevertheless, we were able to determine that ALK‐TKI therapy for the treatment of patients harboring a coalteration with an ALK rearrangement was more efficient in first‐line treatment than in later lines of treatment. Moreover, for patients harboring EGFR/ROS1/KRAS triple coalterations, EGFR‐TKI therapy may have been more efficient than ROS1‐TKI therapy. However, this result must be supported by additional evidence.
KRAS mutations were reported to be negatively correlated with the treatment effect of TKI therapy, which highlighted the lack of effective targeted drugs for these patients. Therefore, chemotherapy remains the primary therapy for patients harboring KRAS mutations. However, we found that the PFS for chemotherapy as first‐line treatment for patients harboring coalterations with a KRAS mutation was only four months. For patients with single KRAS mutation, G12C, G12D, and G12V have been reported as the most frequent mutation subtypes.39 For patients harboring concomitant mutations, G12C/D and G12C KRAS mutation subtypes were the most frequent in KRAS/EGFR and KRAS/ALK coalterations, respectively. The development of targeted therapy for the treatment of patients harboring the BRAF V600E mutation has also been relatively slow until the combination of dabrafenib plus trametinib was approved for the treatment of these patients.40, 41 However, no patient included in this study received this combination therapy. Moreover, our data suggested that the PFS of patients harboring EGFR/BRAF coalterations could likely be improved with the use of TKI therapy.
Rare mutations, such as the HER2 mutation and MET exon 14 skipping, can co‐occur with EGFR mutations, and patients with these types of coalteration have been reported to respond well to EGFR‐TKI or combined therapy.12, 24 In our center, patients first diagnosed with NSCLC were routinely screened for EGFR, ALK, ROS1, KRAS, and BRAF alterations. Notably, HER2 mutations, MET exon 14 skipping, and RET rearrangements were only detected in patients who carried wild‐type alleles of EGFR, ALK, ROS1, KRAS, and BRAF. As mentioned before, NGS can be used to detect mutations that would otherwise be missed by regular screening methods. Besides coalterations among the five driver genes studied here, mutations in TP53, PIK3CA, and other genes can also co‐occur with EGFR or ALK alterations, in which case they have been shown to decrease the treatment effect of TKI therapy.25, 26, 27, 28 For patients affected by this type of coalteration, combination therapy such as TKI plus chemotherapy should be considered. However, to date, these mutations have not been routinely tested in most clinical practices. Collectively, these data highlight the importance and necessity of studying coalterations involving one or more of the EGFR, ALK, ROS1, KRAS, and BRAF driver genes.
In conclusion, concomitant driver gene mutations define a small group of NSCLC patients. Patients harboring coalterations with an EGFR mutation tend to benefit more from TKI therapy than from chemotherapy. However, further studies are needed to evaluate the treatment outcomes of patients harboring an ALK or ROS1 rearrangement combined with an EGFR mutation.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
DATA AVAILABILITY STATEMENT
Cancer Medicine encourages authors to share the data and other artefacts supporting the results in the paper by archiving it in an appropriate public repository. Authors may provide a data availability statement, including a link to the repository they have used, in order that this statement can be published in their paper. Shared data should be cited.
ACKNOWLEDGMENTS
Xibin Zhuang and Xuefei Li designed this study; Chao Zhao and Jiayu Li performed the procedure for detection; Chunxia Su, Xiaoxia Chen and Shengxiang Ren collected the clinical data; Chao Zhao and Xibin Zhuang performed statistical analyses; Xuefei Li and Caicun Zhou gave critical comments and suggestions; Chao Zhao and Xuefei Li drafted the manuscript.
Zhuang X, Zhao C, Li J, et al. Clinical features and therapeutic options in non‐small cell lung cancer patients with concomitant mutations of EGFR, ALK, ROS1, KRAS or BRAF . Cancer Med. 2019;8:2858–2866. 10.1002/cam4.2183
Xibin Zhuang, Chao Zhao and Jiayu Li contributed equally to this work.
Data Availability Statement: Cancer Medicine encourages authors to share the data and other artefacts supporting the results in the paper by archiving it in an appropriate public repository. Authors may provide a data availability statement, including a link to the repository they have used, in order that this statement can be published in their paper. Shared data should be cited.
Contributor Information
Xuefei Li, Email: bug_lily2003@163.com.
Caicun Zhou, Email: caicunzhoudr@163.com.
REFERENCES
- 1. Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin‐paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947‐957. [DOI] [PubMed] [Google Scholar]
- 2. Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first‐line treatment for patients with advanced EGFR mutation‐positive non‐small‐cell lung cancer (OPTIMAL, CTONG‐0802): a multicentre, open‐label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735‐742. [DOI] [PubMed] [Google Scholar]
- 3. Solomon BJ, Mok T, Kim DW, et al. PROFILE 1014 investigators. First‐line crizotinib versus chemotherapy in ALK‐positive lung cancer. N Engl J Med. 2014;371(23):2167‐2177. [DOI] [PubMed] [Google Scholar]
- 4. Shaw AT, Ou SH, Bang YJ, et al. Crizotinib in ROS1‐rearranged non‐small‐cell lung cancer. N Engl J Med. 2014;371(21):1963‐1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Soria JC, Ohe Y, Vansteenkiste J, et al. FLAURA investigators. Osimertinib in untreated EGFR‐Mutated advanced non‐small‐cell lung cancer. N Engl J Med. 2018;378(2):113‐125. [DOI] [PubMed] [Google Scholar]
- 6. Shigematsu H, Gazdar AF. Somatic mutations of epidermal growth factor receptor signaling pathway in lung cancers. Int J Cancer. 2006;118(2):257‐262. [DOI] [PubMed] [Google Scholar]
- 7. Gainor JF, Varghese AM, Ou SH, et al. ALK rearrangements are mutually exclusive with mutations in EGFR or KRAS: an analysis of 1,683 patients with non‐small cell lung cancer. Clin Cancer Res. 2013;19(15):4273‐4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yang JJ, Zhang XC, Su J, et al. Lung cancers with concomitant EGFR mutations and ALK rearrangements: diverse responses to EGFR‐TKI and crizotinib in relation to diverse receptors phosphorylation. Clin Cancer Res. 2014;20(5):1383‐1392. [DOI] [PubMed] [Google Scholar]
- 9. Won JK, Keam B, Koh J, et al. Concomitant ALK translocation and EGFR mutation in lung cancer: a comparison of direct sequencing and sensitive assays and the impact on responsiveness to tyrosine kinase inhibitor. Ann Oncol. 2015;26(2):348‐354. [DOI] [PubMed] [Google Scholar]
- 10. Lou NN, Zhang XC, Chen HJ, et al. Clinical outcomes of advanced non‐small‐cell lung cancer patients with EGFR mutation, ALK rearrangement and EGFR/ALK co‐alterations. Oncotarget. 2016;7(40):65185‐65195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ye C, Wang J, Zheng S, Chai Y. Effective treatment with icotinib in lung adenocarcinoma with EGFR and ALK co‐alterations and brain metastasis. Onco Targets Ther. 2016;26(9):6605‐6608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hu W, Liu Y, Chen J. Concurrent gene alterations with EGFR mutation and treatment efficacy of EGFR‐TKIs in Chinese patients with non‐small cell lung cancer. Oncotarget. 2017;8(15):25046‐25054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schmid S, Gautschi O, Rothschild S, et al. Clinical outcome of ALK‐Positive Non‐Small Cell Lung Cancer (NSCLC) patients with De Novo EGFR or KRAS Co‐Mutations receiving Tyrosine Kinase Inhibitors (TKIs). J Thorac Oncol. 2017;12(4):681‐688. [DOI] [PubMed] [Google Scholar]
- 14. Lee T, Lee B, Choi YL, Han J, Ahn MJ, Um SW. Non‐small cell lung cancer with concomitant EGFR, KRAS, and ALK mutation: clinicopathologic features of 12 cases. J Pathol Transl Med. 2016;50(3):197‐203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Baldi L, Mengoli MC, Bisagni A, Banzi MC, Boni C, Rossi G. Concomitant EGFR mutation and ALK rearrangement in lung adenocarcinoma is more frequent than expected: report of a case and review of the literature with demonstration of genes alteration into the same tumor cells. Lung Cancer. 2014;86(2):291‐295. [DOI] [PubMed] [Google Scholar]
- 16. Zhu YC, Liao XH, Wang WX, et al. Dual drive coexistence of EML4‐ALK and TPM3‐ROS1 fusion in advanced lung adenocarcinoma. Thorac Cancer. 2018;9(2):324‐327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Deng H, Liu C, Zhang G, Wang X, Liu Y. Lung adenocarcinoma with concurrent ALK and ROS1 rearrangement: a case report and review of the literatures. Pathol Res Pract. 2018;214(12):2103‐2105. [DOI] [PubMed] [Google Scholar]
- 18. Zhu YC, Lin XP, Li XF, et al. Concurrent ROS1 gene rearrangement and KRAS mutation in lung adenocarcinoma: a case report and literature review. Thorac Cancer. 2018;9(1):159‐163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wiesweg M, Eberhardt W, Reis H, et al. High prevalence of concomitant oncogene mutations in prospectively identified patients with ROS1‐positive metastatic lung cancer. J Thorac Oncol. 2017;12(1):54‐64. [DOI] [PubMed] [Google Scholar]
- 20. Finberg KE, Sequist LV, Joshi VA, et al. Mucinous differentiation correlates with absence of EGFR mutation and presence of KRAS mutation in lung adenocarcinomas with bronchioloalveolar features. J Mol Diagn. 2007;9(3):320‐326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sahnane N, Frattini M, Bernasconi B, et al. EGFR and KRAS mutations in ALK‐positive lung adenocarcinomas: biological and clinical effect. Clin Lung Cancer. 2016;17(1):56‐61. [DOI] [PubMed] [Google Scholar]
- 22. Guibert N, Barlesi F, Descourt R, et al. Characteristics and outcomes of patients with lung cancer harboring multiple molecular alterations: results from the IFCT study biomarkers France. J Thorac Oncol. 2017;12(6):963‐973. [DOI] [PubMed] [Google Scholar]
- 23. Kinno T, Tsuta K, Shiraishi K, et al. Clinicopathological features of nonsmall cell lung carcinomas with BRAF mutations. Ann Oncol. 2014;25(1):138‐142. [DOI] [PubMed] [Google Scholar]
- 24. Kauffmann‐Guerrero D, Kahnert K, Kumbrink J, Syunyaeva Z, Tufman A, Huber RM. Successful treatment of a patient with NSCLC Harboring an EGFR mutation and a concomitant met exon 14 skipping mutation combining afatinib and crizotinib. Clin Lung Cancer. 2019;20(1):59‐62. [DOI] [PubMed] [Google Scholar]
- 25. Eng J, Woo KM, Sima CS, et al. Impact of concurrent PIK3CA mutations on response to EGFR tyrosine kinase inhibition in EGFR‐mutant lung cancers and on prognosis in oncogene‐driven lung adenocarcinomas. J Thorac Oncol. 2015;10(12):1713‐1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim Y, Lee B, Shim JH, et al. Concurrent genetic alterations predict the progression to target therapy in EGFR‐mutated advanced non‐small cell lung cancer. J Thorac Oncol. 2019;14(2):193–202. [DOI] [PubMed] [Google Scholar]
- 27. Hong S, Gao F, Fu S, et al. Concomitant genetic alterations with response to treatment and epidermal growth factor receptor tyrosine kinase inhibitors in patients With EGFR‐mutant advanced non‐small cell lung cancer. JAMA Oncol. 2018;4(5):739‐742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kron A, Alidousty C, Scheffler M, et al. Impact of TP53 mutation status on systemic treatment outcome in ALK‐rearranged non‐small‐cell lung cancer. Ann Oncol. 2018;29(10):2068‐2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li X, Zhao C, Su C, Ren S, Chen X, Zhou C. Epidemiological study of HER‐2 mutations among EGFR wild‐type lung adenocarcinoma patients in China. BMC Cancer. 2016;16(1):828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang Y, Zhang S, Wu F, et al. Outcomes of Pemetrexed‐based chemotherapies in HER2‐mutant lung cancers. BMC Cancer. 2018;18(1):326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kris MG, Johnson BE, Kwiatkowski DJ, et al. Identification of driver mutations in tumor specimens from 1,000 patients with lung adenocarcinoma: the NCI's Lung Cancer Mutation Consortium (LCMC). J Clin Oncol. 2011;29(suppl): CRA7506. [Google Scholar]
- 32. Ulivi P, Chiadini E, Dazzi C, et al. Nonsquamous, non‐small‐cell lung cancer patients who carry a double mutation of EGFR, EML4‐ALK or KRAS: frequency, clinical‐pathological characteristics, and response to therapy. Clin Lung Cancer. 2016;17(5):384‐390. [DOI] [PubMed] [Google Scholar]
- 33. Cai W, Lin D, Wu C, et al. Intratumoral heterogeneity of ALK‐Rearranged and ALK/EGFR coaltered lung adenocarcinoma. J Clin Oncol. 2015;33(32):3701‐3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yoshida T, Oya Y, Tanaka K, et al. Differential crizotinib response duration among ALK fusion variants in ALK‐positive non‐small‐cell lung cancer. J Clin Oncol. 2016;34(28):3383‐3389. [DOI] [PubMed] [Google Scholar]
- 35. Lin JJ, Zhu VW, Yoda S, et al. Impact of EML4‐ALK variant on resistance mechanisms and clinical outcomes in ALK‐positive lung cancer. J Clin Oncol. 2018;36(12):1199‐1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yang JC, Wu YL, Schuler M, et al. Afatinib versus cisplatin‐based chemotherapy for EGFR mutation‐positive lung adenocarcinoma (LUX‐Lung 3 and LUX‐Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol. 2015;16(2):141‐151. [DOI] [PubMed] [Google Scholar]
- 37. Ke EE, Zhou Q, Zhang QY, et al. A higher proportion of the EGFR T790M mutation may contribute to the better survival of patients with exon 19 deletions compared with those with L858R. J Thorac Oncol. 2017;12(9):1368‐1375. [DOI] [PubMed] [Google Scholar]
- 38. Karlsson A, Ringnér M, Lauss M, et al. Genomic and transcriptional alterations in lung adenocarcinoma in relation to smoking history. Clin Cancer Res. 2014;20(18):4912‐4924. [DOI] [PubMed] [Google Scholar]
- 39. Lindsay CR, Jamal‐Hanjani M, Forster M, Blackhall F. KRAS: reasons for optimism in lung cancer. Eur J Cancer. 2018;99:20‐27. [DOI] [PubMed] [Google Scholar]
- 40. Planchard D, Besse B, Groen H, et al. Dabrafenib plus trametinib in patients with previously treated BRAF(V600E)‐mutant metastatic non‐small cell lung cancer: an open‐label, multicentre phase 2 trial. Lancet Oncol. 2016;17(7):984‐993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Planchard D, Smit EF, Groen H, et al. Dabrafenib plus trametinib in patients with previously untreated BRAFV600E‐mutant metastatic non‐small‐cell lung cancer: an open‐label, phase 2 trial. Lancet Oncol. 2017;18(10):1307‐1316. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Cancer Medicine encourages authors to share the data and other artefacts supporting the results in the paper by archiving it in an appropriate public repository. Authors may provide a data availability statement, including a link to the repository they have used, in order that this statement can be published in their paper. Shared data should be cited.


