Abstract
Adeno-associated virus serotype 1 (AAV1) has many advantages as a gene therapy vector, but the presence of pre-existing neutralizing antibodies (NAbs) is an important limitation. This study was designed to determine: (1) characteristics of AAV NAbs in human subjects, (2) prevalence of AAV1 NAbs in heart failure patients and (3) utility of aggressive immunosuppressive therapy in reducing NAb seroconversion in an animal model. NAb titers were assessed in a cohort of heart failure patients and in patients screened for a clinical trial of gene therapy with AAV1 carrying the sarcoplasmic reticulum calcium ATPase gene (AAV1/SERCA2a). AAV1 NAbs were found in 59.5% of 1552 heart failure patients. NAb prevalence increased with age (P = 0.001) and varied geographically. The pattern of NAb titers suggested that exposure is against AAV2, with AAV1 NAb seropositivity due to crossreactivity. The effects of immunosuppression on NAb formation were tested in mini-pigs treated with immunosuppressant therapy before, during and after a single AAV1/SERCA2a infusion. Aggressive immunosuppression did not prevent formation of AAV1 NAbs. We conclude that immunosuppression is unlikely to be a viable solution for repeat AAV1 dosing. Strategies to reduce NAbs in heart failure patients are needed to increase eligibility for gene transfer using AAV vectors.
INTRODUCTION
Gene therapy is a promising approach for the treatment of heart failure. Unlike conventional pharmacologic therapy, gene therapy has the potential to correct underlying defects and mediate long-lasting improvements in cardiomyocyte function.1 A recombinant vector containing a viral genome flanked by two inverted terminal repeats of adeno-associated virus serotype 2 (AAV2) that is encapsulated in an AAV serotype 1 (AAV1) capsid transduces human cardiomyocytes with high efficiency2 and has become the vector of choice in multiple cardiac gene therapy studies, including the CUPID (Calcium Upregulation by Percutaneous Administration of Gene Therapy in Cardiac Disease) trials.3-6
Despite its many favorable features as a gene transfer vector for cardiac therapies, a notable drawback to the use of AAV1 is the presence of neutralizing antibodies (NAbs) formed during prior natural exposure. Data from clinical studies suggest that AAV1 NAbs have the ability to prevent efficient gene transduction and reduce or negate the effects of therapy.3-5,7,8
Although NAbs against AAV1 appear to be common in all populations studied, comparisons among studies are limited by the lack of a standardized definition or assay for the assessment of AAV1 NAbs.9 In general, however, studies suggest that the seroprevalence of AAV1 NAbs varies by geographic area and increases with age.10-14 AAV1 NAbs appear to prevent gene transduction by two mechanisms: inhibition of AAV1 receptor binding and interference with a key post-attachment step.15 The presence of NAbs against viral vectors has the potential to substantially reduce the eligible population for gene therapies. In addition, it is possible that patients might require more than one application of viral-based gene therapy, and hence strategies to reduce induction of NAbs are needed.
On the basis of results of the phase 1/2 CUPID study suggesting that pre-existing NAbs may limit the effect of therapy by blocking gene transduction,3-5 CUPID-2, a phase 2b randomized, placebo-controlled, multinational trial of AAV1/sarcoplasmic reticulum calcium ATPase (SERCA2a) that enrolled 250 patients6 was designed so that only patients with AAV1 NAbs at a titer < 1:2 were enrolled. Screening for this study in heart failure patients from around the United States as well as in Western and Central Europe and Israel allowed us the opportunity to collect data on the prevalence and pattern of NAbs in heart failure patients. For this report, we utilized data from a cohort of normal subjects and from heart failure patients to characterize AAV1 NAbs as well as data from the CUPID-2 trial to provide an in-depth evaluation of AAV1 NAb prevalence by geographic region, age, gender and month of testing. We also assessed the effects of aggressive immunosuppression during AAV1 exposure.
RESULTS
Patterns of AAV1 and AAV2 NAb titers
In a pilot study conducted in 2006, serum samples from 30 healthy subjects and 30 subjects with heart failure were evaluated for AAV1 and AAV2 NAbs. Titer results were reported as < 1:4 or the specific endpoint titer. For this pilot study, titers ⩾ 1:4 were considered positive on the basis of studies conducted in mini-pigs, in which a titer of 1:4 reduced vector transduction by >80% following AAV1/SERCA2a administration by intracoronary infusion (KM Zsebo, unpublished data). A total of 32 of 60 subjects (53.3%) had titers < 1:4 for both AAV1 and AAV2 (20/30 (66.7%) healthy subjects and 12/30 (40.0%) heart failure patients). For the 28 subjects (46.7%) with titers of ⩾ 1:4 (Table 1), titers were higher for AAV2 than for AAV1 in all but 3 subjects (no. 22 (healthy) and nos. 6 and 15 (heart failure)), suggesting a primary exposure to AAV2 during natural infection.
Table 1.
AAV1 and AAV2 neutralizing antibody titers in subjects with titers ⩾ 1:4a
| Sample ID | AAV1 titer | AAV2 titer |
|---|---|---|
| Healthy subjects | ||
| 24 | ⩾ 1:512 | ⩾ 1:512 |
| 7 | 1:128 | 1:256 |
| 10 | 1:64 | 1:256 |
| 3 | 1:32 | 1:128 |
| 8 | 1:32 | 1:64 |
| 28 | 1:16 | 1:64 |
| 30 | 1:16 | 1:64 |
| 9 | < 1:4 | 1:16 |
| 22 | 1:8 | 1:4 |
| 25 | < 1:4 | 1:4 |
| Subjects with heart failure | ||
| 5 | 1:64 | ⩾ 1:512 |
| 27 | 1:64 | ⩾ 1:512 |
| 8 | 1:16 | ⩾ 1:512 |
| 1 | 1:128 | 1:512 |
| 10 | 1:8 | 1:256 |
| 23 | 1:64 | 1:128 |
| 14 | 1:16 | 1:128 |
| 9 | 1:32 | 1:64 |
| 29 | 1:32 | 1:64 |
| 26 | 1:16 | 1:64 |
| 13 | 1:16 | 1:32 |
| 6 | 1:32 | 1:16 |
| 15 | 1:4 | < 1:4 |
| 7 | < 1:4 | 1:16 |
| 22 | < 1:4 | 1:8 |
| 25 | < 1:4 | 1:8 |
| 11 | < 1:4 | 1:4 |
| 21 | < 1:4 | 1:4 |
Abbreviations: AAV1, adeno-associated virus 1; AAV2, adeno-associated virus 2.
An additional 32 subjects (20 healthy, 12 with heart failure) had titers of < 1:4 for both AAV1 and AAV2.
Prevalence of AAV1 NAbs in patients with heart failure
During prescreening for the CUPID-2 study between July 2012 and February 2014, sera were collected from 1552 patients with heart failure and screened with a companion diagnostic assay that measures the inhibition of infectivity of serum-treated AAV in permissive HEK-293A cells as determined by expression of a green fluorescent protein (GFP) reporter transgene. Titer results were reported as either qualifying (< 1:2 (negative) or equivocal (approaching the cutoff for a 1:2 titer)) or nonqualifying (1:2, 1:4 and ⩾ 1:8, all reported as positive) based on the highest dilution that reduced fluorescence below a designated threshold. The meanαs.d. age of the patients was 60.5 ± 11.5 years and 1241 (80.0%) were male. The overall prevalence of subjects with AAV1 NAb-positive results (titer ⩾ 1:2) was 59.5% (923/1552) in this population. The remaining patients were negative (titer < 1:2; 544/1552 (35.1%)) or equivocal (85/1552 (5.5%). The majority of patients who were positive for AAV1 NAbs had titers ⩾ 1:8 (741/923 (80.3%)). Titers of 1:4 (111/923 (12.0%)) and 1:2 (71/923 (7.7%)) were less common.
The stability and reproducibility of titers were evaluated by comparing prescreening and baseline (day of planned infusion) titers in 242 of 250 enrolled patients. AAV1 NAb titer results for 235 of the 242 remained qualifying at baseline. Of the seven subjects with positive titers at baseline, six had a very low titer of 1:2 and one had a titer of ⩾ 1:8. To determine whether these seven results indicated a true change in AAV1 NAb titers from prescreening, the prescreening and baseline samples from these subjects were reassayed, and both titers were analyzed by cutoff value and raw inhibition data. Based on this evaluation, only one subject appeared to have a clear change in AAV1 NAb titer between prescreen (negative) and baseline (⩾ 1:8), probably because of intercurrent AAV infection. Changes in titer status in the other six subjects were likely because of slight variations in the assay.
We also reevaluated NAb titers at later time points in 20 subjects with an initial positive titer at prescreening that made them ineligible for the study at that time. Three subjects changed from a nonqualifying titer to a qualifying titer. One such subject had an initial titer of 1:4 that dropped to a qualifying titer of equivocal 1.5 months later, allowing this subject to be enrolled in the trial. Two other subjects had an initial titer of 1:2 that dropped to a qualifying titer of equivocal at 1.5 and 3 months after the prescreening test, respectively; neither subject was enrolled. Titers in the other 17 subjects either did not change or did not drop to qualifying levels.
Cellular immune responses were followed by use of an anti-AAV1 enzyme-linked immunosorbent spot (ELISpot) assay. Although a few sporadic, asymptomatic cases were observed, no significant positive cases were detected.
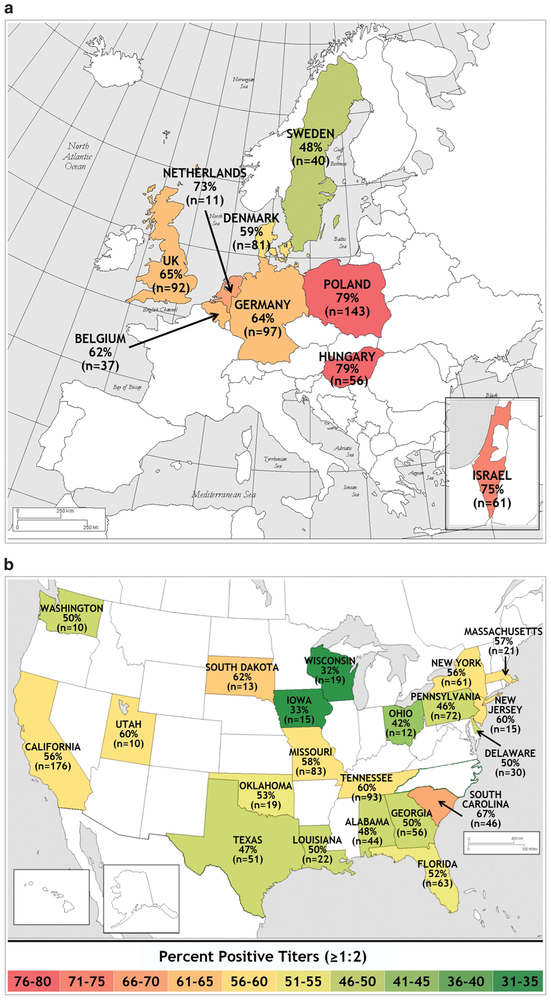
Prevalence of AAV1 NAbs by geographic region
The prevalence of AAV1 NAbs (titers ⩾ 1:2) was higher in Europe (377/557 (67.7%)) than in the United States (500/934 (53.5%); P < 0.0001; Figure 1). Israel had an AAV1 NAb prevalence of 75.4% (46/61). Within Europe, AAV1 NAb prevalence results ranged from a low of 48% in Sweden to a high of 79% in Poland and Hungary. Central European countries appeared to have a higher prevalence than more northern climes (Figure 1a), but the number and geographical locations of the countries involved limit our conclusions. Geographic variation was also observed in AAV1 NAb prevalence results in the United States, ranging from a low of 32% in Wisconsin to a high of 67% in South Carolina (Figure 1b). There was no obvious geographic pattern to the distribution of AAV1 NAbs across the United States.
Figure 1.
Prevalence of AAV1 NAbs in (a) Europe and Israel (N = 618) and (b) the United States (N = 934) as determined by titers ⩾ 1:2. One state (North Carolina) was not included because of small sample size (n = 3). AAV1, adeno-associated virus 1; NAbs, neutralizing antibodies.
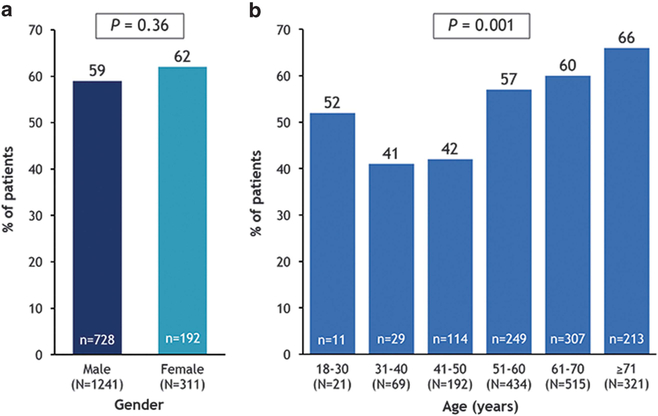
Prevalence of AAV1 NAbs by demographic characteristics and season
The prevalence of AAV1 NAbs (titers ⩾ 1:2) did not differ significantly by gender (59% in males and 62% in females (P = 0.36); Figure 2a). However, positive titers were significantly associated with increasing age (P = 0.001; Figure 2b).
Figure 2.
Prevalence of AAV1 NAbs by (a) gender and (b) age as determined by titers ⩾ 1:2 (N = 1552).
Samples for this study were collected throughout the year, ranging from a low of 108 samples collected in July to a high of 158 samples collected in December, thus providing an opportunity to investigate whether the prevalence of AAV1 NAbs varied by month or season. An analysis of positive titers by time of year of sample collection showed no significant differences by month (P = 0.63) or season (P = 0.61). By month, the prevalence of AAV1 NAbs ranged from a low of 53.3% for samples collected in September (n = 137) to a high of 66.1% for samples collected in May (n = 118).
Development of NAbs in immunosuppressed mini-pigs
We utilized an animal model (mini-pigs) to examine whether seroconversion could potentially be prevented by continuous aggressive immunosuppression (before, during and after intravenous administration of AAV1/SERCA2a). At baseline, animals had no detectable AAV1 NAbs (titer < 1:2). Infusion with AAV1/SERCA2a was well tolerated in non-immunosuppressed and immunosuppressed female Göttingen mini-pigs, but AAV1 Nabs were detected at approximately the same levels following AAV1/SERCA2a dosing regardless of the use of immunosuppressive therapy (Table 2).
Table 2.
AAV1 NAb titers in non-immunosuppressed and immunosuppressed mini-pigs
| ID | Prescreen | Day 0 | Day 30/32 | Day 56/57 | Day 84/86 | Day 105 | Day 126 |
|---|---|---|---|---|---|---|---|
| Non-immunosuppressed | |||||||
| Control | |||||||
| 1 | < 1:2 | 1:2 | 1:8 | 1:4 | 1:8 | 1:2 | < 1:2 |
| 2 | < 1:2 | < 1:2 | < 1:2 | 1:2 | 1:4 | < 1:2 | < 1:2 |
| 3 | < 1:2 | < 1:2 | < 1:2 | < 1:2 | < 1:2 | < 1:2 | 1:2 |
| 4 | NA | < 1:2 | < 1:2 | 1:2 | 1:2 | < 1:2 | 1:2 |
| 5 | NA | < 1:2 | 1:2 | < 1:2 | 1:2 | < 1:2 | < 1:2 |
| 6 | NA | < 1:2 | < 1:2 | < 1:2 | < 1:2 | < 1:2 | < 1:2 |
| AAV1/SERCA2a | |||||||
| 7 | < 1:2 | < 1:2 | 1:1024 | 1:1024 | 1:1024 | 1:1024 | 1:2048 |
| 8 | < 1:2 | < 1:2 | 1:256 | 1:64 | 1:128 | 1:512 | 1:512 |
| 9 | < 1:2 | < 1:2 | 1:256 | 1:256 | 1:256 | 1:512 | 1:512 |
| 10 | < 1:2 | < 1:2 | 1:512 | 1:256 | 1:512 | 1:256 | 1:512 |
| 11 | < 1:2 | < 1:2 | 1:128 | 1:256 | 1:256 | 1:512 | 1:512 |
| 12 | < 1:2 | 1:8 | 1:512 | 1:512 | Died (day 69) | ||
| Immunosuppressed | |||||||
| Control | |||||||
| 13 | < 1:2 | < 1:2 | < 1:2 | < 1:2 | 1:16 | 1:8 | 1:2 |
| 14 | < 1:2 | < 1:2 | < 1:2 | < 1:2 | < 1:2 | < 1:2 | < 1:2 |
| 15 | < 1:2 | < 1:2 | 1:4 | 1:2 | 1:2 | < 1:2 | 1:2 |
| 16 | < 1:2 | < 1:2 | 1:16 | 1:8 | 1:4 | Died (day 89) | |
| 17 | < 1:2 | 1:2 | 1:32 | 1:32 | 1:32 | 1:32 | 1:32 |
| 18 | NA | 1:2 | < 1:2 | < 1:2 | < 1:2 | < 1:2 | < 1:2 |
| AAV1/SERCA2a | |||||||
| 19 | < 1:2 | < 1:2 | 1:256 | Died (day 33) | |||
| 20 | 1:2 | < 1:2 | 1:256 | 1:32 | Died (day 79) | ||
| 21 | < 1:2 | < 1:2 | 1:256 | 1:64 | 1:64 | 1:64 | 1:128 |
| 22 | < 1:2 | 1:2 | 1:512 | 1:256 | 1:256 | 1:256 | 1:256 |
| 23 | < 1:2 | < 1:2 | 1:256 | 1:128 | 1:128 | 1:128 | 1:64 |
| 24 | < 1:2 | < 1:2 | 1:8 | 1:16 | 1:16 | 1:16 | 1:16 |
Abbreviations: AAV1, adeno-associated virus 1; NA, not available; Nab, neutralizing antibody; SERCA2a, sarcoplasmic reticulum calcium ATPase.
DISCUSSION
The data reported here support the high prevalence of AAV1 NAbs and extend previous observations concerning geographic variations in the prevalence of AAV1 NAbs.10-12 Our findings further suggest that AAV1 NAbs may be because of natural exposure to AAV2, as most patients with measurable titers to AAV2 also had measurable titers to AAV1, and AAV2 titers were uniformly higher than AAV1 titers in patients with positive NAb results. These data support previously published reports suggesting that AAV1-positive results are because of crossreactivity with AAV2.16 We also observed slight variations in AAV1 NAb titer over time in a few subjects, likely explained by elevations and declines in immunoglobulins because of polyclonal immunoglobulin production during a communicable infection and subsequent recovery, or other types of inflammatory episodic events.
Overall, ~40% of patients with heart failure had qualifying AAV1 NAb titers that could potentially support gene transduction. It should be noted that our study employed a more stringent cutoff for qualifying titers (1:2) than those used by most other investigators (1:2 to 1:20).9 The prevalence of AAV1 NAbs was higher in Europe and Israel than in the United States, and there appeared to be a trend toward higher prevalences in central European countries compared with more northern regions. However, more data with additional geographic locations are required to confirm these observations. There was no clear pattern of geographic distribution within the United States. Although the prevalence of AAV1 NAb did not vary significantly by gender or by time of year of sample collection, we did observe increasing NAb prevalence with increasing age. Although the youngest age group in our analysis (18 to 30 years of age) deviated somewhat from the overall statistical trend, this was likely because of random variation, as this group consisted of only 21 subjects. A study of healthy volunteers and patients with inflammatory bowel disease conducted in the Netherlands also found a significant effect of age on AAV1 NAb prevalence,11 although the subjects in that study were younger (mean of 49 years of age for healthy volunteers and 43 for inflammatory bowel disease patients) than in the study reported here.
The ability of AAV1 NAbs to impede myocardial gene transfer was supported by data from the CUPID 1 trial that utilized a recombinant AAV1 vector to deliver the gene for SERCA2a, a key regulator of calcium uptake that is deficient in patients with heart failure.17 Entry criteria for the phase 1, open-label portion of the trial included qualifying AAV1 NAb titers of 1:2 or < 1:2.3 Of the nine patients who received AAV1/SERCA2a, two had AAV1 NAb titers of 1:2 and the remaining seven patients had undetectable titers (< 1:2). Quantitative evidence of biological activity was observed except in the two NAb-positive patients who failed to improve;one received a mechanical assist device at week 6 and the other received a transplant at month 8.
On the basis of the phase 1 data, the phase 2 portion of CUPID 1, a randomized, placebo-controlled, double-blind trial, required eligible patients to have undetectable AAV1 NAb titers (<1:2) at screening.4,5 Of the 509 patients who were prescreened for AAV1 NAb in the phase 2 portion of CUPID, 265 (52.1%) were excluded because of detectable NAb. Three of the 39 patients enrolled in this trial seroconverted to detectable NAb levels between NAb screening (up to 6 months before day of dosing) and day 0, and received a single infusion of AAV1/SERCA2a (MYDICAR, Celladon Corporation, San Diego, CA, USA) or placebo. Eight of the nine high-dose AAV1/SERCA2a patients improved in their disease status relative to placebo; the sole exception was the single NAb-positive patient who had the worst individual efficacy score in that group and was discontinued from the study before month 9 for worsening heart failure and chronic inotropic support before receiving a heart transplant at month 11.4
The high prevalence of pre-existing AAV1 NAbs poses a potential dilemma for the widespread use of AAV1-based gene therapy, as more than half of gene therapy candidates may be excluded on the basis of NAb titer. In addition, patients who have received AAV-based gene therapy typically develop antibodies to the vectors,4,18,19 thus making these individuals poorly suited for retreatment with the therapeutic agent or treatment with other forms of gene therapy that utilize an AAV vector. Although cellular immunity is another potential barrier to gene transduction with AAV vectors,12 we did not detect other than sporadic T cell-mediated responses during our study.
A number of possible solutions to the problem of NAbs have been proposed, including development of NAb-resistant AAV variants, either through manipulation of capsid genes or through chemical modification of the AAV capsid, immunosuppression to modulate the immune response during transduction, and plasmapharesis to deplete AAV NAbs before introduction of the gene therapy vector.9 Our data indicate that immunosuppressive therapy is unlikely to provide a viable solution for repeat transduction with AAV1, as the administration of aggressive immunosuppressive therapy before, during and after AAV1/SERCA2a infusion did not prevent the development of NAbs in mini-pigs. Nonetheless, a study in nonhuman primates that used the anti-CD20 monoclonal antibody rituximab in combination with cyclosporine A demonstrated that NAbs against AAV6 could be suppressed in a single animal injected with an AAV8 vector encoding factor IX.20
Plasmapharesis may be a more promising strategy. In studies with nonhuman primates, plasmapharesis was more successful in improving AAV transduction efficiency in seropositive animals than pharmacological immunosuppression with prednisone alone or with a triple combination of prednisone, tacrolimus and mycophenolate mofetil.21 A pilot study of the impact of plasmapheresis in 10 subjects found that frequent (up to 5) sessions of plasmapheresis efficiently reduced AAV1 NAb titers in most patients.22 Similarly, a small retrospective study on stored plasma samples received from six patients who had undergone five sessions of plasmapheresis before kidney transplant demonstrated a 2- to 16-fold reduction in the AAV1 NAb titer of positive samples (KM Zsebo. Data on file: Celladon Corporation, 2014). These results suggest that plasmapheresis may be a viable option for circumventing the presence of low levels of pre-existing AAV1 NAbs in candidates for gene therapy, although clinical trials will be required to verify the efficacy and safety of this strategy.
In conclusion, our data indicate that the prevalence of AAV1 NAbs varies with geographic location and increases with age. Overall, ~60% of heart failure patients have positive AAV1 NAb titers and are therefore not suitable candidates for AAV-based therapy. Immunosuppression is unlikely to be a viable solution for repeat AAV1 dosing and therefore other options for reducing or circumventing the presence of AAV1 NAbs, including plasmapharesis, should be explored.
MATERIALS AND METHODS
Serum samples
Serum samples were collected from healthy adult (18 to 80 years of age) subjects (n = 30) or adult patients with chronic systolic heart failure (n = 30) in a pilot study in 2006, and between July 2012 and February 2014 as part of screening for the CUPID-2 trial ( NCT01643330) (n = 1552).6 The study protocol for this trial was approved by the institutional biosafety committees and review boards at each site, and patients signed written informed consent and release of medical information forms before screening. Samples were frozen, shipped to a central laboratory on dry ice (LabCorp Clinical Trials, Los Angeles, CA, USA) and stored at − 80 ± 20°C until analysis.
Cell culture
The immortalized human embryonic kidney cell line QBI-HEK-293A (Qbiogene, Carlsbad, CA, USA) was maintained in Dulbecco’s modification of Eagle’s medium supplemented with 10% fetal bovine serum, 25 mM HEPES and 1 × penicillin G sodium, streptomycin sulfate and l-glutamine. Cells were cultured at 37 ± 2 °C with 5 ± 0.5% CO2.
Assays for AAV1 Nabs and cellular immune responses
The assay for AAV1 NAbs used in this study is a diagnostic companion assay for MYDICAR that contains proprietary modifications to the general method described by Moskalenko et al.23 Briefly, dilutions of each subject’s serum were incubated with an AAV recombinant particle carrying the GFP reporter transgene (AAV/GFP;Virovek, Hayward, CA, USA). This mixture was then tested for vector activity/infectivity in vitro using a HEK-293A-permissive cell line. GFP-expressing cells were evaluated using SpectraMax Gemini XPS Fluorescent Microplate Reader (Molecular Devices, Sunnyvale, CA, USA). All assays were performed at one laboratory (LabCorp Clinical Trials, Los Angeles, CA, USA). Each assay included a background control (cell culture media only), vector control (AAV1/GFP in cell culture media, negative control (pooled negative human serum) and positive control (intravenous immunoglobulin in pooled negative human serum); test acceptance criteria ensured the validity of each run. The analytical procedure was validated. The intraassay coefficient of variability was 17% and the interassay coefficient of variability was 25%.
The NAb titer was calculated based on the highest dilution at which mean GFP fluorescence intensity was reduced by a defined level. All titers ⩾ 1:2 were considered positive for AAV1 NAbs and titers < 1:2 were considered negative. Equivocal samples were defined as those with a mean background adjusted fluorescence value at a 1:2 dilution that was at a predefined value near the cutoff for reporting the result as negative (< 1:2 titer) or positive (⩾ 1:2 titer).
Cellular immune responses were followed by use of interferon-γ (ELISpot, Cellular Technology Limited, Shaker Heights, OH, USA).
Immunosuppression in mini-pigs
A single intravenous infusion of AAV1/SERCA2a (dose of 5 × 1012 DNase-resistant particles AAV1/SERCA2a; ~4–5 × 1011 DNase-resistant particles per kg) was administered to normal female Gottingen mini-pigs (Marshall BioResources, North Rose, NY, USA) with (n = 12) and without (n = 12) immunosuppressive therapy. The animals were allocated to groups using simple randomization. A sample size of 12 was expected to be sufficient to assess the initial safety of AAV1/SERCA2a in immunosuppressed animals. All animals were 15 to 20 weeks old and had negative AAV1 NAb titers (< 1:2) at baseline. The animal care and use protocol was reviewed and approved by the institutional animal care and use committee of Bio-Quant (San Diego, CA, USA) and/or attending veterinarian as necessary and all animal welfare concerns were addressed and documented. All procedures were in compliance with the Animal Welfare Act, the Guide for the Care and Use of Laboratory Animals and the Office of Laboratory Animal Welfare. The day of dosing with AAV1/SERCA2a was designated day 0. Mycophenolate mofetil (CellCept tablet, Genentech, South San Francisco, CA, USA) was administered daily as an oral gavage at a dose of 250 mg per animal for at least 120 days, from at least day −14 to day 105. Sirolimus (Rapamune tablet, Pfizer, New York, NY, USA) was administered daily as an oral gavage at a dose of 2 mg per animal for at least 120 days, from at least day −14 to day 105. Methylprednisolone sodium succinate (Solu-Medrol, Pfizer, 1 ml) was administered as a daily intramuscular injection at a dose of 10 mg kg−1 for 30 days (from day 0 to day 29), followed by 5 mg kg−1 for an additional 76 days (from day 30 to day 105). Blood was drawn every 4 weeks for detection of AAV1 NAbs. Investigators were not blinded to treatment assignment.
Statistical analysis
Tests for differences by geographic region, demographics and time of year were done using X2 tests of association. A two-sided test for trend with increasing age was done using the Cochran–Armitage test. Analyses were done using SAS v9.4 (SAS Institute, Cary, NC, USA). The P-values of < 0.05 were considered statistically significant.
ACKNOWLEDGEMENTS
Sharon L Cross provided medical writing support and Julia Andres provided graphics support on behalf of Celladon Corporation. Dr Thomas Weber provided critical editing of the manuscript. RJH is supported by NIH R01 HL 117505, HL 119046, a P50 HL112324 and a Transatlantic Fondation Leducq grant.
Footnotes
CONFLICT OF INTEREST
This study was funded by Celladon Corporation (San Diego, CA, USA). Drs Greenberg, Butler, Felker, Ponikowski, Voors and Hajjar have received compensation for consultancies or board memberships from Celladon Corporation, the sponsor of this study. Drs Pogoda, Provost, Guerrero and Zsebo are former employees of Celladon.
REFERENCES
- 1.Pleger SV, Brinks H, Ritterhoff J, Raake P, Koch WJ, Katus HA et al. Heart failure gene therapy: the path to clinical practice. Circ Res 2013; 113: 792–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Du L, Kido M, Lee DV, Rabinowitz JE, Samulski RJ, Jamieson SW et al. Differential myocardial gene delivery by recombinant serotype-specific adeno-associated viral vectors. Mol Ther 2004; 10: 604–608. [DOI] [PubMed] [Google Scholar]
- 3.Jaski BE, Jessup ML, Mancini DM, Cappola TP, Pauly DF, Greenberg B et al. Calcium upregulation by percutaneous administration of gene therapy in cardiac disease (CUPID trial), a first-in-human phase 1/2 clinical trial. J Card Fail 2009; 15: 171–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jessup M, Greenberg B, Mancini D, Cappola T, Pauly DF, Jaski B et al. Calcium upregulation by percutaneous administration of gene therapy in cardiac diseases (CUPID): a phase 2 trial of intracoronary gene therapy of sarcoplasmic reticulum CA2 +-ATPase in patients with advanced heart failure. Circulation 2011; 124: 304–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zsebo K, Yaroshinsky A, Rudy JJ, Wagner K, Greenberg B, Jessup M et al. Long-term effects of AAV1/SERCA2a gene transfer in patients with severe heart failure. Analysis of recurrent cardiovascular events and mortality. Circ Res 2014; 114: 101–108. [DOI] [PubMed] [Google Scholar]
- 6.Greenberg B, Yaroshinsky A, Zsebo KM, Butler J, Felker GM, Voors AA et al. Design of a phase 2b trial of intracoronary administration of AAV1/SERCA2a in patients with advanced heart failure: the CUPID-2 trial (Calcium Up-regulation by Percutaneous Administration of Gene Therapy in Cardiac Disease Phase 2b). JACC Heart Fail 2014; 2: 84–92. [DOI] [PubMed] [Google Scholar]
- 7.Manno CS, Arruda VR, Pierce GF, Glader B, Ragni M, Rasko J et al. Successful transduction of liver in hemophilia by AAV-factor IX and limitations imposed by the host immune response. Nat Med 2006; 12: 342–347. [DOI] [PubMed] [Google Scholar]
- 8.Mendell JR, Rodino-Klapac LR, Rosales XQ, Coley BD, Galloway G, Lewis S et al. Sustained alpha-sarcoglycan gene expression following gene transfer in limb-girdle muscular dystrophy, type 2D. Ann Neurol 2010; 68: 629–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Louis Jeune V, Joergensen JA, Hajjar RJ, Weber T. Pre-existing anti-adeno-associated virus antibodies as a challenge in AAV gene therapy. Hum Gene Ther Methods 2013; 24: 59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calcedo R, Vandenberghe LH, Gao G, Lin J, Wilson JM. Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J Infect Dis 2009; 199: 381–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van der Marel S, Comijn EM, Verspaget HW, van Deventer S, van den Brink GR, Petry H et al. Neutralizing antibodies against adeno-associated viruses in inflammatory bowel disease patients: implications for gene therapy. Inflamm Bowel Dis 2011; 17: 2436–2442. [DOI] [PubMed] [Google Scholar]
- 12.Veron P, Leborgne C, Monteilhet V, Boutin S, Martin S, Moullier P et al. Humoral and cellular capsid-specific immune responses to adeno-associated virus type 1 in randomized healthy donors. J Immunol 2012; 188: 6418–6424. [DOI] [PubMed] [Google Scholar]
- 13.Liu Q, Huang W, Zhao C, Zhang L, Meng S, Gao D et al. The prevalence of neutralizing antibodies against AAV serotype 1 in healthy subjects in China: implications for gene therapy and vaccines using AAV1 vector. J Med Virol 2013; 85: 1550–1556. [DOI] [PubMed] [Google Scholar]
- 14.Calcedo R, Morizono H, Wang L, McCarter R, He J, Jones D et al. Adeno-associated virus antibody profiles in newborns, children, and adolescents. Clin Vaccine Immunol 2011; 18: 1586–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harbison CE, Weichert WS, Gurda BL, Chiorini JA, Agbandje-McKenna M, Parrish CR. Examining the cross-reactivity and neutralization mechanisms of a panel of mAbs against adeno-associated virus serotypes 1 and 5. J Gen Virol 2012; 93: 347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erles K, Seböková P, Schlehofer JR. Update on the prevalence of serum antibodies (IgG and IgM) to adeno-associated virus (AAV). J Med Virol 1999; 59: 406–411. [DOI] [PubMed] [Google Scholar]
- 17.Eisner D, Caldwell J, Trafford A. Sarcoplasmic reticulum Ca-ATPase and heart failure 20 years later. Circ Res 2013; 113: 958–961. [DOI] [PubMed] [Google Scholar]
- 18.Brantly ML, Chulay JD, Wang L, Mueller C, Humphries M, Spencer LT et al. Sustained transgene expression despite T lymphocyte responses in a clinical trial of rAAV1-AAT gene therapy. Proc Natl Acad Sci USA 2009; 106: 16363–16368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flotte TR, Trapnell BC, Humphries M, Carey B, Calcedo R, Rouhani F et al. Phase 2 clinical trial of a recombinant adeno-associated viral vector expressing α1-anti-trypsin: interim results. Hum Gene Ther 2011; 22: 1239–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mingozzi F, Chen Y, Murphy SL, Edmonson SC, Tai A, Price SD et al. Pharmacological modulation of humoral immunity in a nonhuman primate model of AAV gene transfer for hemophilia B. Mol Ther 2012; 20: 1410–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chicoine LG, Montgomery CL, Bremer WG, Shontz KM, Griffin DA, Heller KN et al. Plasmapharesis eliminates the negative impact of AAV antibodies on micro-dystrophin gene expression following vascular delivery. Mol Ther 2014; 22: 338–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Monteilhet V, Saheb S, Boutin S, Leborgne C, Veron P, Montus MF et al. A 10 patient case report on the impact of plasmapharesis upon neutralizing factors against adeno-associated virus (AAV) types 1, 2, 6, and 8. Mol Ther 2011; 19: 2084–2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moskalenko M, Chen L, van Roey M, Donahue BA, Snyder RO, McArthur JG et al. Epitope mapping of human anti-adeno-associated virus type 2 neutralizing antibodies: implications for gene therapy and virus structure. J Virol 2000; 74: 1761–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]