Abstract
OBJECTIVE
To determine the effects of orthostatic hypotension (OH) measurement timing on its associations with dizziness, falls, fractures, cardiovascular disease (CVD), and mortality.
METHODS
We analyzed OH measurements from the Action to Control Cardiovascular Risk in Diabetes BP trial, which evaluated two blood pressure (BP) goals (systolic BP [SBP] < 120 mm Hg vs. SBP < 140 mm Hg) and incident CVD among adults with diabetes and hypertension. Seated BP was measured after 5 minutes of rest at baseline and follow-up visits (12 months, 48 months, and exit). Standing BP was measured 3 consecutive times (M1–M3) after standing, starting at 1 minute with each measurement separated by 1 minute. Consensus OH was defined as a drop in SBP ≥ 20 mm Hg or diastolic BP (DBP) ≥ 10 mm Hg. Participants were asked about orthostatic dizziness, recent falls, and recent fractures, and underwent surveillance for CVD events and all-cause mortality.
RESULTS
There were 4,268 participants with OH assessments over 8,450 visits (mean age 62.6 years [SD = 6.6]; 46.6% female; 22.3% black). Although all measures of consensus OH were significantly associated with dizziness, none were associated with falls, and only M2 (~3 minutes) was significantly associated with fractures. No measurements were associated with CVD events, but later measurements were significantly associated with mortality. BP treatment goal did not increase risk of OH regardless of timing. Associations were not consistently improved by the mean or minimum of M1–M3.
CONCLUSION
In this population of adults with hypertension and diabetes, neither single time nor set of measurements were clearly superior with regard to outcomes. These findings support the use of a flexibly timed, single measurement to assess OH in clinical practice.
CLINICAL TRIALS REGISTRATION
Trial Number NCT00000620
Keywords: blood pressure, cardiovascular disease, diabetes, dizziness, fall, fracture, hypertension, lightheadedness, mortality, orthostatic hypotension, trial
Orthostatic hypotension (OH) is a readily measured and frequently encountered physical sign in clinical practice with a prevalence of over 20% in older populations.1 However, substantial debate remains regarding the timing of its measurement and interpretation. Although some observational studies have identified OH as a risk factor for dizziness, falls, fractures, cardiovascular disease (CVD), and mortality,1–5 others have not found it to be informative for these outcomes.1,6–8 One explanation may relate to when OH is assessed. Recent studies of continuous blood pressure (BP) measurements after standing suggest that early drops in BP are associated with dizziness,9 whereas others report that delayed recovery in orthostatic BP beyond 1 minute (i.e., sustained OH) is more strongly associated with falls.10 In contrast, we demonstrated in a predominantly healthy, middle-aged population that measurements within 1 minute of standing were more informative for dizziness, falls, and fractures than later measurements between 1 and 2 minutes.3 However, our study did not examine measures within 2–3 minutes or beyond 3 minutes, time intervals commonly recommended in clinical practice guidelines.11–16
The Action to Control Cardiovascular Risk in Diabetes (ACCORD) BP trial examined the effects of systolic BP (SBP) goal (<120 mm Hg vs. <140 mm Hg) on CVD events and mortality in adults with diabetes and hypertension.17 In addition, participants were asked about prior orthostatic dizziness, falls, and fractures.6,18 An assessment of OH was introduced later as part of the trial protocol with 3 standardized measurements occurring between 1 and 5 minutes after standing. Ultimately, this trial did not show a relationship between BP treatment and OH. Furthermore, although OH was associated with death, it was not associated with major CVD events.6 However, whether these associations differed by time of assessment was not reported. Given the higher prevalence of diabetic autonomic neuropathy with potentially more severe or delayed recovery of BP, ACCORD BP offers a unique opportunity to explore the implications of timing in a highly prevalent and potentially vulnerable, yet understudied population.
We revisited the ACCORD study to examine the effects of timing on the association of OH with (i) postural dizziness, (ii) clinical events related to OH, namely falls and fractures, and (iii) long-term major cardiovascular events or all-cause mortality. Our last objective was (iv) to examine the effects of timing on BP treatment goal and OH. We hypothesized that earlier measurements of OH would be more important for dizziness, falls, and fractures than later OH measurements or OH based on the mean or minimum of all 3 measurements. We also hypothesized that timing would be less informative for CVD, mortality, or the effects of BP treatment on OH.
METHODS
Study participants
As reported previously, ACCORD was a 2 × 2 factorial trial that included 10,251 participants from 77 clinical sites in the United States and Canada.17,19 All participants were randomized to intensive vs. standard glycated hemoglobin targets (<6% vs. 7%–7.9%), whereas half were included in the ACCORD BP trial, a sub-study examining BP treatment goal (described later). All participants were required to have a diagnosis of type 2 diabetes mellitus with a hemoglobin A1c of at least 7.5%. In addition, participants were required to have an elevated risk of CVD, namely, a prior history of CVD for adults aged 40–79 years or in absence of prior CVD for adults aged 55–79 years, albuminuria, left ventricular hypertrophy, anatomic evidence of subclinical atherosclerosis, or 2 or more risk factors for CVD: obesity, smoking, hypertension, or hyperlipidemia. Participants with a body mass index greater than 45 kg/m2 or a serum creatinine above 1.5 mg/dl were excluded.19
The ACCORD BP trial was a sub-study of ACCORD, which included 4,733 participants of whom 4,329 had OH measurements (see Supplementary Material SM1).6,17 Eligibility for ACCORD BP required an SBP between 130 and 180 mm Hg, a regimen of no more than 3 antihypertensive medications, and a 24-hour urinary protein <1 g. Our study was further limited to participants who successfully completed all three standing BP assessments intended by the OH protocol. As a result, we excluded 9 participants who were missing 1 or 2 standing measurements (N = 4,320). We further excluded 52 participants missing covariate information (N = 4,268). This secondary analysis was performed using a de-identified, public dataset maintained by the National Institutes of Health/National Heart, Lung, and Blood Institute BioLINCC. The Beth Israel Deaconess Institutional Review Board determined that this secondary analysis was not considered human subjects research.
Study design
Recruitment into the ACCORD BP trial was performed in two phases, one between January and June 2001 and the other between February 2003 and October 2005.17,19 There were 2,362 participants randomized to an intensive treatment goal (SBP < 120 mm Hg), and 2,371 randomized to a standard treatment goal (SBP < 140 mm Hg). Participants assigned to the intensive goal attended visits monthly for the first 4 months followed by visits every 2 months. Participants in the standard goal treatment group attended visits at month 1, 4, and then every 4 months. Additional visits were scheduled as needed to monitor treatment response and trial implementation. In the original BP trial, the mean SBP at 4 months in the intensive treatment goal was 119.3 mm Hg vs. 133.5 mm Hg in the standard treatment goal. At 1 year, the intensive group was on 3.4 antihypertensive medications vs. 2.1 in the standard group. Follow-up for the trial ended in June 2009.
OH and postural dizziness
The OH protocol was initiated in October 2004 in all participants in the ACCORD BP sub-study for baseline visits (among newly enrolled participants), 12-month, 48-month, and exit visits (in participants with no study events). Participants were asked to sit for at least 5 minutes with minimal activity. Then, using an Omron HEM-907 with a programmed 1-minute interval, participants underwent a series of 3 seated measurements. BP measurements were typically performed over the upper right arm using a cuff size selected based on measured arm circumference. They were then asked to stand (see Supplementary Material SM2 for the original manual of procedures and Supplementary Material SM3 for a schematic of the protocol). One minute after standing, another series of three measurements (separated by 1-minute intervals) was initiated. The same arm and cuff were used for both seated and standing assessments. Participants were also asked whether they experienced dizziness or lightheadedness during the standing process.
Postural change in SBP or diastolic BP (DBP) from seated to standing (reported per −10 mm Hg) was determined by taking the standing measure and subtracting the mean seated measure for SBP or DBP and then multiplying by −1/10. OH was defined in the following way: systolic OH (a decrease in SBP of at least 20 mm Hg), diastolic OH (a decrease in DBP of at least 10 mm Hg), or consensus OH (a decrease in SBP of at least 20 mm Hg or a decrease in DBP of at least 10 mm Hg), based on thresholds used in the original consensus definition.12,20 OH was determined using each of the three standing measurements individually, an average of the three standing measurements (a more precise and specific definition), or the minimum of the three measurements (a more sensitive definition).
Self-reported events: fall and fracture
Beginning in January 2006, participants were asked about falls or fractures as part of an ancillary study called ACCORD-BONE.18,21 This ancillary study included 5 of the 7 clinical center networks, comprising 54 of the original 77 sites and 3,099 ACCORD-BP trial participants (1,534 in the intensive arm and 1,565 in the standard arm). At every annual visit (earliest being the first 12-month follow-up visit), participants were asked about falls and fractures (see Supplementary Material SM4). For falls, participants were asked: “In the last 12 months have you fallen and landed on the floor or ground, or fallen and hit an object like a table or stair?” For fractures, participants were asked: “Has a doctor or other health care provider told you that you have broken or fractured any bones since your last annual ACCORD visit?” For 2006 (the year these questionnaires were initiated), the time interval of the fracture question was altered to include any fractures since randomization (as opposed to last annual visit).
Prospective clinical events: major CVD events, mortality, and secondary outcomes
Participants were followed for CVD events throughout the study. The primary outcome in the original ACCORD trial was the first occurrence of a major CVD event defined as nonfatal myocardial infarction, nonfatal stroke, or death from CVD. The secondary analysis was death from any cause. All events were adjudicated by a central committee, who were blinded to the study group assignments. Other outcomes in the ACCORD trial were examined in supplemental analyses, including death from cardiovascular causes, nonfatal stroke, total stroke, hospitalization or death due to heart failure, and a composite outcome composed of the primary outcome plus revascularization or hospitalization for congestive heart failure (CHF; called the “expanded macrovascular outcomes”).
Other covariates
Demographic characteristics included age, sex, and race (white, black, Hispanic, other). History of CVD, history of heart failure, history of stroke, history of myocardial infarction, smoking status, diuretic use, central alpha-adrenergic agonist use, and beta blockers use were self-reported. History of CVD and heart failure was asked as part of a participant’s medical history during the baseline visit, whereas history of myocardial infarction and stroke was asked during the screening visit while assessing eligibility. Body mass index was determined from standardized measurements of height and weight. Fasting glucose concentrations were measured in plasma collected prior to randomization using standard assays.22 Resting baseline SBP, DBP, or heart rate was determined during the baseline visit.
Statistical analysis
Study population characteristics were described using means (SD) and proportions, overall and by BP goal. We examined the association of postural change in SBP or DBP or 3 OH metrics (systolic OH, diastolic OH, or consensus OH) with self-reported dizziness, falls, or fractures at any point during follow-up using generalized estimating equations (binomial family, logit link, exchangeable correlation structure, robust variance estimator) adjusted for baseline age, sex, race, trial network, treatment arm (both BP goal and glycemic goal assignments), baseline SBP, baseline DBP, baseline heart rate, baseline body mass index, baseline fasting plasma glucose, history of CVD, history of myocardial infarction, history of stroke, history of heart failure, and smoking status. Postural change in BP and OH definitions were examined by measurement (1, 2, or 3), as an average value, or based on the minimum of the three measurements. BP assessments contributing to these analyses were performed at baseline, 12-month, 48-month, and exit visits.
We also examined the association of postural change in SBP or DBP or the 3 OH metrics with major CVD events or mortality from any cause, using Cox proportional hazards models adjusted for the aforementioned covariates. For survival models, postural change assessments were treated as time-varying covariates based on measurements from baseline, 12-month, 48-month, and exit visits. Models were left-censored to begin with the first visit during which an assessment of postural change occurred with values updated at subsequent visits (for most participants, the first visit was at baseline or the 12-month visit). Follow-up ended if a participant experienced an event of interest or when the study ended (administrative censoring). We included OH assessments during the “Exit” visit in this analysis. Because the date of the “Exit” visit was not made publicly available, this value was estimated using the administrative censoring date among living participants at the end of the study with the assumption that all participants survived at least 1 hour after their postural change BP assessment. As a result, there was minimal contribution of the exit visit to follow-up. These models were conducted for each measurement time as well as the average and minimum of all three standing measurements.
All the aforementioned Cox models, using postural change in BP or the 3 OH definitions, were repeated for our secondary outcomes: cardiovascular mortality, nonfatal stroke, total stroke, or CHF death or hospitalization. We also examined postural change in BP or the 3 OH definitions in relation to 1 composite secondary outcome: expanded macrovascular outcomes (primary outcome plus revascularization or hospitalization for CHF). We confirmed the Cox proportional hazards assumption of all models for both primary and secondary outcomes using Schoenfeld residuals.
Finally, we looked at the effect of BP goal (intensive vs. standard) on postural change in SBP or DBP from measurement 1–3, the average, and the minimum, determined during post-randomization visits. First, we plotted the distribution of standing SBP and DBP obtained via measurements 1–3 by treatment goal. Then we performed a repeat measures analysis of all available follow-up assessments, examining postural change in SBP or DBP (measurements 1–3, the average, and the minimum) via generalized estimating equations (normal family, identity link, exchangeable correlation structure, robust variance estimator). We repeated these analyses examining the effect of BP goal on odds of systolic OH, diastolic OH, or consensus OH (binomial family, logit link, exchangeable correlation structure, robust variance estimator). These models were performed for OH determined based on each individual measurement as well as for OH based on the average and the minimum of all three standing measurements. All treatment effect models were adjusted for glycemia goal assignment as well, accounting for the ACCORD factorial design.
Analyses were conducted with Stata version 14.0 (Stata Corporation, College Station, TX). P values less than 0.05 were considered significant without adjustment for multiple comparisons.
RESULTS
Population characteristics
Of the 4,268 participants in this study, the mean age was 62.6 years (SD = 6.6), 46.6% were women, and 22.3% were black (Table 1). The mean baseline SBP was 139.0 (15.7) mm Hg, DBP was 75.9 (10.3) mm Hg, and heart rate was 72.9 (11.6) bpm. Mean body mass index was 32.1 (5.5) kg/m2 and mean fasting plasma glucose was 174.7 (57.5) mg/dl. Population characteristics were similar across randomized groups. There were 1,313 participants with standing BP measured at baseline, 2,630 with standing BP measured at 12 months, 3,061 with standing BP measured at 48 months, and 1,456 with standing BP measured at study exit for a total of 8,450 visits with an OH assessment. OH was more prevalent with measurement 1 vs. measurement 3 (Supplementary Table ST1). The average of the 3 measurements was the most specific, whereas the minimum standing BP was the most sensitive definition for OH.
Table 1.
Baseline characteristics overall and by treatment group, Mean (SD) or N (%)
| Overall, N = 4,268 | Standard BP Goal, N = 2,151 | Intensive BP Goal, N = 2,117 | |
|---|---|---|---|
| Age, yr | 62.6 (6.6) | 62.6 (6.7) | 62.6 (6.5) |
| Female, N (%) | 1,987 (46.6) | 999 (46.4) | 988 (46.7) |
| Race, N (%) | |||
| White | 2,577 (60.4) | 1,273 (59.2) | 1,304 (61.6) |
| Black | 953 (22.3) | 495 (23.0) | 458 (21.6) |
| Hispanic | 279 (6.5) | 147 (6.8) | 132 (6.2) |
| Other | 459 (10.8) | 236 (11.0) | 223 (10.5) |
| Mean SBP, mm Hg | 139.0 (15.7) | 139.2 (15.4) | 138.9 (16.0) |
| Mean DBP, mm Hg | 75.9 (10.3) | 75.9 (10.2) | 75.9 (10.5) |
| Mean heart rate, mm Hg | 72.9 (11.6) | 72.8 (11.4) | 73.0 (11.8) |
| Body mass index, kg/m2 | 32.1 (5.5) | 32.1 (5.4) | 32.2 (5.6) |
| Fasting plasma glucose, mg/dl | 174.7 (57.5) | 173.5 (57.7) | 176.0 (57.3) |
| History of cardiovascular disease, N (%) | 1,409 (33.0) | 715 (33.2) | 694 (32.8) |
| History of myocardial infarction, N (%) | 581 (13.6) | 309 (14.4) | 272 (12.8) |
| History of stroke, N (%) | 255 (6.0) | 121 (5.6) | 134 (6.3) |
| History of heart failure, N (%) | 168 (3.9) | 78 (3.6) | 90 (4.3) |
| Smoking status, N (%) | |||
| Never | 1,901 (44.5) | 965 (44.9) | 936 (44.2) |
| Former | 1,821 (42.7) | 909 (42.3) | 912 (43.1) |
| Current | 546 (12.8) | 277 (12.9) | 269 (12.7) |
| Diuretic use, N (%) | 1,564 (36.6) | 790 (36.7) | 774 (36.6) |
| Central alpha antagonist, N (%) | 69 (1.6) | 25 (1.2) | 44 (2.1) |
| Beta blocker, N (%) | 1,170 (27.4) | 578 (26.9) | 592 (28.0) |
Abbreviations: BP, blood pressure; DBP, diastolic blood pressure; SBP, systolic blood pressure.
Dizziness, falls, and fractures
Negative postural changes in SBP based on each of the three individual measurements, the average measurement, and the minimum SBP measurement were associated with dizziness (odds ratios [ORs] of 1.12–1.21 per −10 mm Hg; Table 2). Similarly, all measures of systolic OH were significantly associated with dizziness (ORs of 1.90–2.82). Meanwhile, the first measurements of negative postural change in DBP (OR 1.16 per −10 mm Hg; 95% CI: 1.01, 1.32) or diastolic OH (OR 1.78; 95% CI: 1.22, 2.58) were most strongly associated with dizziness. The consensus definition was associated with dizziness with all 3 individual measurements, the average, and the minimum measurement (ORs of 1.53–2.01) although the first two measurements demonstrated a slightly greater magnitude and were more statistically significant than the third measurement.
Table 2.
The association of orthostatic hypotension at 3 different times, the average, or a minimum of 3 standing measurements with dizziness upon standing, fall in the previous year, or fracture in the previous year
| Dizziness, N = 422/8,450 visits | Falls, N = 986/5,070 visits | Fracture, N = 151/5,071 visits | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| SBP per −10 mm Hg | ||||||
| M1 | 1.16 (1.07, 1.26) | 0.0002 | 1.05 (0.99, 1.11) | 0.12 | 1.03 (0.89, 1.20) | 0.66 |
| M2 | 1.16 (1.07, 1.26) | 0.0004 | 1.03 (0.97, 1.10) | 0.28 | 1.13 (0.97, 1.31) | 0.12 |
| M3 | 1.12 (1.03, 1.22) | 0.01 | 1.03 (0.97, 1.09) | 0.39 | 1.01 (0.88, 1.16) | 0.85 |
| Ave | 1.18 (1.08, 1.29) | 0.0002 | 1.04 (0.98, 1.11) | 0.20 | 1.07 (0.91, 1.25) | 0.41 |
| Min | 1.21 (1.12, 1.31) | <0.0001 | 1.08 (1.01, 1.14) | 0.02 | 1.09 (0.94, 1.28) | 0.26 |
| Systolic orthostatic hypotension (drop in SBP ≥ 20 mm Hg) | ||||||
| M1 | 1.90 (1.33, 2.72) | 0.0004 | 1.36 (0.99, 1.87) | 0.06 | 1.66 (0.86, 3.20) | 0.13 |
| M2 | 2.49 (1.71, 3.61) | <0.0001 | 1.49 (1.06, 2.11) | 0.02 | 2.86 (1.61, 5.08) | 0.0003 |
| M3 | 2.10 (1.43, 3.09) | 0.0002 | 1.35 (0.94, 1.92) | 0.10 | 1.15 (0.51, 2.58) | 0.74 |
| Ave | 2.82 (1.91, 4.18) | <0.0001 | 1.64 (1.11, 2.42) | 0.01 | 2.08 (1.00, 4.31) | 0.0501 |
| Min | 2.09 (1.57, 2.79) | <0.0001 | 1.29 (1.00, 1.67) | 0.0504 | 1.80 (1.07, 3.03) | 0.03 |
| DBP per −10 mm Hg | ||||||
| M1 | 1.16 (1.01, 1.32) | 0.04 | 1.02 (0.93, 1.13) | 0.62 | 1.01 (0.80, 1.28) | 0.93 |
| M2 | 1.05 (0.92, 1.20) | 0.46 | 0.95 (0.87, 1.05) | 0.33 | 0.99 (0.79, 1.25) | 0.96 |
| M3 | 1.07 (0.93, 1.23) | 0.32 | 0.95 (0.86, 1.04) | 0.28 | 0.93 (0.74, 1.16) | 0.51 |
| Ave | 1.11 (0.96, 1.29) | 0.16 | 0.97 (0.87, 1.08) | 0.58 | 0.97 (0.75, 1.26) | 0.82 |
| Min | 1.14 (0.99, 1.30) | 0.06 | 0.99 (0.89, 1.09) | 0.78 | 0.98 (0.77, 1.26) | 0.90 |
| Diastolic orthostatic hypotension (drop in DBP ≥ 10 mm Hg) | ||||||
| M1 | 1.78 (1.22, 2.58) | 0.003 | 1.14 (0.82, 1.59) | 0.44 | 1.16 (0.55, 2.46) | 0.70 |
| M2 | 1.27 (0.79, 2.02) | 0.32 | 1.19 (0.81, 1.74) | 0.38 | 2.07 (1.06, 4.07) | 0.03 |
| M3 | 1.21 (0.75, 1.97) | 0.43 | 0.76 (0.49, 1.16) | 0.20 | 1.59 (0.74, 3.41) | 0.24 |
| Ave | 1.33 (0.77, 2.28) | 0.30 | 1.05 (0.65, 1.68) | 0.85 | 1.13 (0.39, 3.27) | 0.82 |
| Min | 1.38 (0.99, 1.92) | 0.06 | 1.07 (0.82, 1.39) | 0.63 | 1.37 (0.79, 2.36) | 0.26 |
| Consensus orthostatic hypotension (drop in SBP ≥ 20 mm Hg or DBP ≥ 10 mm Hg) | ||||||
| M1 | 1.71 (1.27, 2.30) | 0.0004 | 1.22 (0.94, 1.58) | 0.14 | 1.16 (0.64, 2.12) | 0.63 |
| M2 | 1.73 (1.24, 2.41) | 0.001 | 1.30 (0.98, 1.73) | 0.07 | 2.52 (1.56, 4.08) | 0.0002 |
| M3 | 1.53 (1.09, 2.15) | 0.01 | 0.99 (0.74, 1.34) | 0.96 | 1.23 (0.65, 2.31) | 0.53 |
| Ave | 2.01 (1.41, 2.84) | 0.0001 | 1.22 (0.88, 1.70) | 0.24 | 1.62 (0.84, 3.10) | 0.15 |
| Min | 1.61 (1.25, 2.08) | 0.0003 | 1.16 (0.94, 1.43) | 0.18 | 1.50 (0.96, 2.35) | 0.07 |
Models were adjusted for baseline age, female, race, trial network, treatment arm, baseline SPB, baseline DBP, baseline heart rate, baseline body mass index, baseline fasting plasma glucose, history of cardiovascular disease, history of myocardial infarction, history of stroke, history of heart failure, and smoking status. All participants represented in this table had all 3 standing blood pressure measurements. These analyses included 4,267 participants for dizziness; 3,658 participants for fall; and 3,659 participants for fracture. Abbreviations: Ave, average; CI, confidence interval; DBP, diastolic blood pressure; Min, minimum; OR, odds ratio; SBP, systolic blood pressure.
The minimum measurement for postural change in SBP was most strongly associated with falls (OR 1.08; 95% CI: 1.01, 1.14). In contrast, the 2nd measurement and the average measurement of systolic OH were more strongly associated with falls (OR 1.49 [95% CI: 1.06, 2.11] and OR 1.64 [95% CI: 1.11, 2.42], respectively), whereas the 2nd measurement of systolic OH (2.86; 95% CI: 1.61, 5.08), the minimum of systolic OH (OR 1.80; 95% CI: 1.07, 3.03), the 2nd measurement of diastolic OH (OR 2.07; 95% CI: 1.06, 4.07), and consensus OH (OR 2.52; 95% CI: 1.56, 4.08) were more strongly associated with fractures than the other measurements.
Incident CVD events and mortality
None of the measurements of postural change in SBP, postural change in DBP, systolic OH, or consensus OH were significantly associated with major CVD events, regardless of measurement timing (Table 3). The measures most associated with major CVD events were the 3rd and minimum standing measurement for diastolic OH (HR 1.95 [95% CI: 1.25, 3.04] and 1.50 [95% CI: 1.03, 2.18]), respectively.
Table 3.
The association of orthostatic hypotension at 3 different times, the average, or a minimum of 3 standing measurements with cardiovascular events or mortality (HR, 95% CI)
| Primary outcome (fatal cardiovascular disease, myocardial infarction, or stroke), N = 270 events/4,152 participants | Mortality from any cause, N = 173 events/4,259 participants |
|||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| SBP per −10 mm Hg | ||||
| M1 | 1.07 (0.98, 1.17) | 0.13 | 1.15 (1.03, 1.28) | 0.01 |
| M2 | 1.00 (0.91, 1.10) | 0.98 | 1.10 (0.98, 1.23) | 0.10 |
| M3 | 0.99 (0.90, 1.08) | 0.84 | 1.09 (0.98, 1.22) | 0.11 |
| Average | 1.02 (0.93, 1.13) | 0.64 | 1.14 (1.01, 1.28) | 0.04 |
| Minimum | 1.02 (0.93, 1.12) | 0.73 | 1.14 (1.02, 1.27) | 0.02 |
| Systolic orthostatic hypotension (drop in SBP ≥ 20 mm Hg) | ||||
| M1 | 1.08 (0.65, 1.80) | 0.77 | 1.71 (1.00, 2.93) | 0.051 |
| M2 | 1.22 (0.71, 2.11) | 0.47 | 2.18 (1.27, 3.75) | 0.005 |
| M3 | 1.10 (0.63, 1.94) | 0.73 | 1.39 (0.73, 2.66) | 0.32 |
| Average | 1.28 (0.71, 2.30) | 0.41 | 1.81 (0.94, 3.48) | 0.07 |
| Minimum | 1.05 (0.68, 1.63) | 0.81 | 1.79 (1.14, 2.80) | 0.01 |
| DBP per −10 mm Hg | ||||
| M1 | 1.15 (0.99, 1.34) | 0.07 | 1.08 (0.90, 1.30) | 0.40 |
| M2 | 1.06 (0.91, 1.24) | 0.42 | 1.02 (0.85, 1.23) | 0.83 |
| M3 | 1.08 (0.93, 1.26) | 0.32 | 1.02 (0.85, 1.23) | 0.80 |
| Average | 1.12 (0.95, 1.32) | 0.18 | 1.05 (0.86, 1.29) | 0.63 |
| Minimum | 1.14 (0.97, 1.33) | 0.10 | 1.06 (0.88, 1.29) | 0.53 |
| Diastolic orthostatic hypotension (drop in DBP ≥ 10 mm Hg) | ||||
| M1 | 1.36 (0.85, 2.19) | 0.20 | 0.88 (0.43, 1.81) | 0.74 |
| M2 | 1.40 (0.84, 2.34) | 0.20 | 1.78 (0.98, 3.22) | 0.06 |
| M3 | 1.95 (1.25, 3.04) | 0.003 | 2.14 (1.25, 3.67) | 0.006 |
| Average | 1.60 (0.95, 2.72) | 0.08 | 1.39 (0.68, 2.85) | 0.37 |
| Minimum | 1.50 (1.03, 2.18) | 0.03 | 1.69 (1.07, 2.66) | 0.02 |
| Consensus orthostatic hypotension (drop in SBP ≥ 20 mm Hg or DBP ≥ 10 mm Hg) | ||||
| M1 | 1.21 (0.81, 1.80) | 0.35 | 1.24 (0.75, 2.04) | 0.40 |
| M2 | 1.21 (0.78, 1.86) | 0.39 | 1.88 (1.18, 2.99) | 0.008 |
| M3 | 1.38 (0.92, 2.07) | 0.12 | 1.77 (1.11, 2.81) | 0.02 |
| Average | 1.20 (0.75, 1.92) | 0.45 | 1.48 (0.85, 2.58) | 0.17 |
| Minimum | 1.20 (0.86, 1.69) | 0.28 | 1.68 (1.14, 2.46) | 0.008 |
Models adjusted for baseline age, female, race, trial network, treatment arm, baseline SBP, baseline DBP, baseline heart rate, baseline body mass index, baseline fasting plasma glucose, history of cardiovascular disease, history of myocardial infarction, history of stroke, history of heart failure, and smoking status. All participants represented in this table had all 3 standing blood pressure measurements. Abbreviations: CI, confidence interval; DBP, diastolic blood pressure; SBP, systolic blood pressure.
Measurement time was inconsistently associated with mortality from any cause. For SBP, mortality from any cause was most strongly associated with postural change in SBP measurement 1 (HR 1.15; 95% CI: 1.03, 1.28), and systolic OH measurement 2 (HR 2.18; 95% CI: 1.27, 3.75). The average postural change in SBP was also significantly associated with mortality (HR 1.14; 95% CI: 1.01, 1.28). Furthermore, the minimum standing SBP for postural change (HR 1.14; 95% CI: 1.02, 1.27) and systolic OH were associated with mortality (HR 1.79; 95% CI: 1.14, 2.80). In contrast, later measurements of DBP were significantly associated with mortality from any cause, specifically, diastolic OH measurement 3 (HR 2.14; 95% CI: 1.25, 3.67). The minimum standing DBP for diastolic OH was also associated with mortality (HR 1.69; 95% CI: 1.07, 2.66). Consensus OH was significantly associated with mortality from any cause when assessed via measurement 2 (HR 1.88; 95% CI: 1.18, 2.99) and 3 (HR 1.77; 95% CI: 1.11, 2.81) as well as the minimum (HR 1.68; 95% CI: 1.14, 2.46).
Other CVD outcomes
Neither postural change in SBP nor systolic OH was significantly associated with cardiovascular mortality, nonfatal stroke, total stroke, or CHF death or hospitalization (Supplementary Table ST1). Similarly, postural change in DBP was not significantly associated with these outcomes with the exception of CHF death or hospitalization. Greater drops in DBP upon standing were significantly associated with higher odds of CHF death or hospitalization when assessed at measurement 2 (HR 1.31; 95% CI: 1.04, 1.65), measurement 3 (HR 1.34; 95% CI: 1.06, 1.70), the average of all 3 standing measurements (HR 1.38; 95% CI: 1.07, 1.78), or the minimum of the 3 standing measurements (HR 1.35; 95% CI: 1.07, 1.70). Diastolic OH and consensus OH were also not significantly associated with any of the supplemental outcomes with a few notable exceptions. Diastolic OH measured during measurement 3 or as the minimum of the 3 standing BP measurements were significantly associated with cardiovascular mortality (HR 4.00 [95% CI: 2.02, 7.93] and 2.25 [95% CI: 1.17, 4.34], respectively), while consensus OH measured during measurement 3 was significantly associated with cardiovascular mortality (HR 2.20; 95% CI: 1.11, 4.37) and consensus OH based on the minimum standing BP measurement was significantly associated with CHF death or hospitalization (HR 1.79; 95% CI: 1.10, 2.92).
Treatment effects on postural change and OH
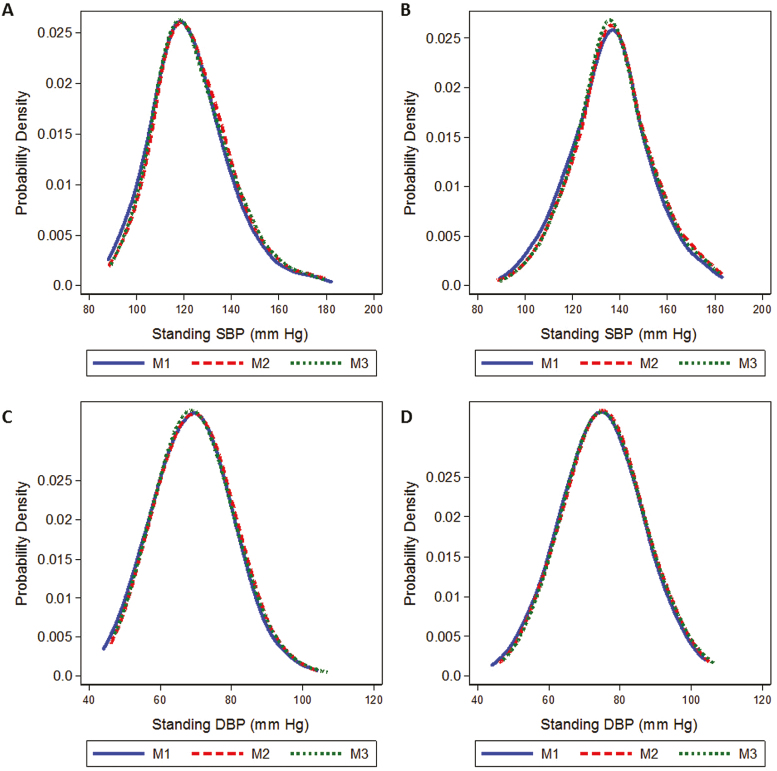
A more intensive BP goal caused a leftward shift in the distribution of standing SBP and DBP (Figure 1a–d). However, this effect varied little by measurement time. This was reflected by the virtual absence of an effect of treatment goal on postural change in SBP, systolic OH, and postural change in DBP (Table 4). There was evidence of a lower odds of having diastolic OH (OR 0.74; 95% CI: 0.57, 0.97) or consensus OH (OR 0.80; 95% CI: 0.65, 0.98) as assessed during measurement 3 alone. Also, intensive treatment was significantly associated with lower odds of diastolic OH when based on the minimum standing DBP (OR 0.78; 95% CI: 0.65, 0.94) and consensus OH when based on the minimum standing BP (OR 0.80; 95% CI: 0.69, 0.94).
Figure 1.
Kernel density plots of the distribution of standing systolic blood pressure (SBP) for intensive (a) and standard (b) blood pressure treatment goals, and diastolic blood pressure (DBP) for intensive (c) and standard (d) blood pressure treatment goals. Each line represents measurement 1–3 (M1 solid, M2 dash, M3 dot). There were 2,110 participants assigned the intensive BP goal, and 2,145 participants assigned the standard goal, contributing 7,153 follow-up visits. M1, standing measurement 1; M2, standing measurement 2; M3, standing measurement 3.
Table 4.
Effects of blood pressure treatment goal on orthostatic hypotension
| Intensive vs. moderate BP goal, N = 7,147 | ||
|---|---|---|
| β or odds ratio (95% CI) | P | |
| Postural change in SPB per 1 mm Hg | ||
| M1 | −0.08 (−0.72, 0.56) | 0.81 |
| M2 | 0.14 (−0.51, 0.79) | 0.67 |
| M3 | −0.01 (−0.67, 0.65) | 0.98 |
| Average | 0.01 (−0.58, 0.61) | 0.96 |
| Minimum | 0.29 (−0.33, 0.91) | 0.36 |
| Systolic orthostatic hypotension (drop in SBP ≥ 20 mm Hg) | ||
| M1 | 1.02 (0.80, 1.29) | 0.88 |
| M2 | 1.00 (0.76, 1.30) | 0.99 |
| M3 | 0.91 (0.70, 1.18) | 0.48 |
| Average | 1.14 (0.84, 1.54) | 0.40 |
| Minimum | 0.89 (0.74, 1.08) | 0.24 |
| Postural change in DBP per 1 mm Hg | ||
| M1 | 0.07 (−0.32, 0.47) | 0.71 |
| M2 | 0.15 (−0.24, 0.55) | 0.44 |
| M3 | 0.02 (−0.38, 0.41) | 0.94 |
| Average | 0.08 (−0.28, 0.44) | 0.67 |
| Minimum | 0.25 (−0.13, 0.62) | 0.20 |
| Diastolic orthostatic hypotension (drop in DBP ≥ 10 mm Hg) | ||
| M1 | 0.96 (0.76, 1.21) | 0.71 |
| M2 | 0.86 (0.66, 1.11) | 0.24 |
| M3 | 0.74 (0.57, 0.97) | 0.03 |
| Average | 0.96 (0.71, 1.29) | 0.78 |
| Minimum | 0.78 (0.65, 0.94) | 0.01 |
| Consensus orthostatic hypotension (drop in SBP ≥ 20 mm Hg or DBP ≥ 10 mm Hg) | ||
| M1 | 0.92 (0.76, 1.11) | 0.39 |
| M2 | 0.89 (0.72, 1.09) | 0.24 |
| M3 | 0.80 (0.65, 0.98) | 0.03 |
| Average | 1.01 (0.80, 1.28) | 0.93 |
| Minimum | 0.80 (0.69, 0.94) | 0.006 |
This analysis was limited to participants with orthostatic hypotension assessments after baseline. Abbreviations: BP, blood pressure; CI, confidence interval; DBP, diastolic blood pressure; M1, standing measurement 1; M2, standing measurement 2; M3, standing measurement 3; SBP, systolic blood pressure.
DISCUSSION
In this secondary analysis of the ACCORD trial, we examined timing of OH assessments in adults with diabetes and hypertension, and we found that earlier OH assessments were more significantly associated with dizziness upon standing, whereas middle measurements (closest to 3 minutes) were more significantly associated with falls and fracture in the previous year. The clinical significance of the timing of OH assessment was not as consistent for CVD or mortality from any cause. Intriguingly, a more intensive BP treatment goal was associated with lower odds of OH as measured by the last measurement. Neither the average nor minimum of the 3 measurements were more consistently associated with outcomes than the individual timed measurements.
Postural dizziness is an important manifestation of OH23 and a likely mediator of clinical events like falls.24 Drops in BP upon standing that do not maintain adequate perfusion pressure are thought to contribute to cerebral hypoperfusion, manifesting as dizziness.25,26 However, there is substantial debate about which pattern of postural change in BP contributes most to the clinical symptom of dizziness. Although a number of reports have shown that OH identified within 1 minute of standing is associated with dizziness,3,27 others have shown that adults with rapid recovery of BP within 30 seconds of standing were less likely to report OH symptoms.28 This is thought to be related to the ability to autoregulate cerebral blood flow. Previous studies have also emphasized the importance of SBP rather than DBP in relation to dizziness.28 In our study, although virtually any drop in SBP was associated with dizziness, only the earliest drops in DBP were associated with dizziness. Our data are consistent with other studies, in that one need not wait for a longer time to capture systolic OH, while later assessments of diastolic OH could result in misclassification and null association.29 Notably, the earliest measurement was more consistently associated with dizziness than the average or minimum of three measurements. This suggests that timing may be particularly important for identifying OH associated with dizziness.
Falls and fracture are variably associated with OH in the literature.3,7,30 In the Atherosclerosis Risk in Communities study, OH measures within 1 minute of standing in a middle-aged, community-based population were most associated with risk of falls or fracture.3 Similarly, in a population of older adults with hypertension, OH detected at 1 minute was more strongly associated with falls than detection at 3 minutes.29 In ACCORD, OH was not consistently associated with falls, but the second measurement (which on average would be closest in time to 3 minutes) was associated with a higher odds of fracture. This differed from our hypothesis that dizziness would track with falls and fracture. Whether this discrepancy is related to population differences (e.g., OH in a diabetic population), outcome reporting (recalled vs. incident claims), or an absence of measures within 1 minute of standing cannot be determined in our study. However, future study protocols should evaluate OH measurements within 1 minute and at 3 minutes with fall and fracture risk to better answer this question.
In ACCORD, OH assessment was not consistently associated with CVD or its subtypes regardless of timing. This contrasts with numerous other studies, which have shown OH to be associated with CVD.1,5,31–36 We also did not observe an association between systolic OH and stroke or diastolic OH and myocardial disease as reported by others.37 We speculate that this discordance may be secondary to ACCORD’s study population of adults with diabetes. Although OH may predominantly reflect subclinical CVD in ambulatory populations,36 OH in adults with diabetes may disproportionately reflect comorbid autonomic dysfunction or hypovolemia.38,39
With regard to mortality from any cause, both earlier systolic OH and later diastolic OH measurements were more strongly associated with mortality from any cause compared to OH detected at other time points. In contrast, prior studies of OH assessment timing have found OH to be associated with mortality regardless of measurement time.3 Some hypothesize that sustained OH contributes to greater risk of death.40 However, this may also reflect increased variability in BP regulation or underlying cardiovascular pathology, which are important risk factors for mortality.41 Notably, systematic reviews of cohort studies examining OH and mortality show associations between OH and mortality across variably timed collection protocols,42,43 which supports the perspective that timing is less consistently important for predicting mortality. However, if timing was truly not a factor, then one might expect that the mean measurement would provide the most precise assessment of standing BP and thus the strongest association with mortality, and yet this was not observed in our study.
Current OH guidelines vary with regard to the timing of standing BP assessments. Some recommend measurements close to 1 minute,44 2 minutes,13,14 within 3 minutes,12,20 or at 1 and 3 minutes.15 Although the 2nd measurement, closest to 3 minutes, was the most reliably associated with outcomes, our study does not provide robust evidence for any of the aforementioned timing recommendations. However, the fact that single measurements were as reliable as the mean or minimum supports the use of single measurement in clinical practice, which is less time intensive than either a mean or minimum of 3 measurements.
Our study has several limitations. First, OH measures began at 1 minute and were separated by 1-minute intervals, likely extending between 1.5 minutes to 4.5 minutes after standing. However, the actual time of the OH result was not reported. Furthermore, we are unable to compare these later measurements with measurements within 1 minute. It is possible that associations may differ with postural change within 1 minute, as we have shown previously.3 Second, OH was largely not associated with CVD in this study. It is unclear why this was the case, perhaps due to the fact that OH among adults with diabetes reflects pathways that are not specific to subclinical CVD. Third, power was limited by the late implementation of the OH protocol, variable assessments of self-reported outcomes (falls and fracture), and lack of post-exit follow-up (cardiovascular events and mortality), minimizing the contribution of exit visit assessments. This could account for some of the nonsignificant associations, particularly between OH with falls and fractures. Fourth, additional outcomes of interest were uncommon in this population (i.e., syncope). As a result, we were unable to include this in our study. Fifth, the OH protocol was from seated to standing, which may result in a less sensitive OH assessment. Sixth, ACCORD was not designed to examine the effect of antihypertensive class on OH. Seventh, the many comparisons in this study create an opportunity for chance associations. As a result, isolated measurements should be interpreted with caution. Finally, analyses of OH as a risk factor are inherently observational and hence subject to residual confounding from unmeasured characteristics of study participants.
Our study has numerous strengths. OH assessments were rigorously performed by trained personnel, using a standardized protocol. Furthermore, repeat assessments increased power and afforded an opportunity to model OH as a time-varying covariate. Our study encompassed a large, relevant population, known to have a higher prevalence of OH. Furthermore, CVD and mortality events were adjudicated by a trained, expert committee. Finally, the randomized BP goal design allowed for a causal evaluation of the effects of BP treatment goal on OH at various time points.
These findings have several potential implications. Earlier assessments of OH are more strongly related to dizziness, but assessments closer to 3 minutes are more strongly associated with falls and fracture. There is less consistency in the relationship between the timing of OH and CVD and mortality from any cause. These findings suggest that timing of OH may associate differently with different outcomes, potentially reflecting unique patterns of BP stabilization after standing. For example, it is possible that initial OH causes transient cerebral hypoperfusion, while delayed OH represents autonomic dysfunction or BP dysregulation due to underlying diseases (e.g., diabetes).45 These findings highlight a need for better subtyping of OH at the time of detection. Importantly, regardless of timing of OH measurement, the more intensive target BP goal did not increase risk of OH, confirming findings of several large randomized trials of BP goal.6,46,47 This observation does not support prior reports suggesting that BP treatment is a cause of OH.48,49
In conclusion, in a population of diabetic and hypertensive adults, a single OH assessment within 1–5 minutes after standing was as consistently associated with outcomes as the average or the minimum of 3 standing measurements and may represent a more simple way to assess OH. However, given the number of comparisons in our study, these findings require confirmation in other cohorts.
DISCLOSURE
The authors declared no conflict of interest.
Supplementary Material
ACKNOWLEDGMENTS
S.P.J. is supported by a NIH/NHLBI K23HL135273-03 and R21HL144876-01. This data were organized and provided to investigators by the NIH/NHLBI BioLincc. We thank the staff and participants of the ACCORD study for their important contributions. This research was also supported by grants R01 AG041785 and R01 AG025037 to L.A.L from the National Institute on Aging. L.A.L. holds the Irving and Edyth S. Usen and Family Chair in Geriatric Medicine at Hebrew SeniorLife, Boston, Massachusetts.
REFERENCES
- 1. Rutan GH, Hermanson B, Bild DE, Kittner SJ, LaBaw F, Tell GS. Orthostatic hypotension in older adults. The cardiovascular health study. CHS Collaborative Research Group. Hypertension 1992; 19:508–519. [DOI] [PubMed] [Google Scholar]
- 2. Juraschek SP, Daya N, Appel LJ, Miller ER 3rd, Windham BG, Pompeii L, Griswold ME, Kucharska-Newton A, Selvin E. Orthostatic hypotension in middle-age and risk of falls. Am J Hypertens 2017; 30:188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Juraschek SP, Daya N, Rawlings AM, Appel LJ, Miller ER 3rd, Windham BG, Griswold ME, Heiss G, Selvin E. Association of history of dizziness and long-term adverse outcomes with early vs later orthostatic hypotension assessment times in middle-aged adults. JAMA Intern Med 2017; 177:1316–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yatsuya H, Folsom AR, Alonso A, Gottesman RF, Rose KM; ARIC Study Investigators Postural changes in blood pressure and incidence of ischemic stroke subtypes: the ARIC study. Hypertension 2011; 57:167–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fedorowski A, Stavenow L, Hedblad B, Berglund G, Nilsson PM, Melander O. Orthostatic hypotension predicts all-cause mortality and coronary events in middle-aged individuals (The Malmo Preventive Project). Eur Heart J 2010; 31:85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fleg JL, Evans GW, Margolis KL, Barzilay J, Basile JN, Bigger JT, Cutler JA, Grimm R, Pedley C, Peterson K, Pop-Busui R, Sperl-Hillen J, Cushman WC. Orthostatic hypotension in the ACCORD (Action to Control Cardiovascular Risk in Diabetes) blood pressure trial: prevalence, incidence, and prognostic significance. Hypertension 2016; 68:888–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chu LW, Chi I, Chiu AY. Incidence and predictors of falls in the Chinese elderly. Ann Acad Med Singapore 2005; 34:60–72. [PubMed] [Google Scholar]
- 8. Ensrud KE, Nevitt MC, Yunis C, Hulley SB, Grimm RH, Cummings SR. Postural hypotension and postural dizziness in elderly women. The study of osteoporotic fractures. The Study of Osteoporotic Fractures Research Group. Arch Intern Med 1992; 152:1058–1064. [PubMed] [Google Scholar]
- 9. Stewart JM, Clarke D. “He’s dizzy when he stands up”: an introduction to initial orthostatic hypotension. J Pediatr 2011; 158:499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Finucane C, O’Connell MD, Donoghue O, Richardson K, Savva GM, Kenny RA. Impaired orthostatic blood pressure recovery is associated with unexplained and injurious falls. J Am Geriatr Soc 2017; 65:474–482. [DOI] [PubMed] [Google Scholar]
- 11. Kaufmann H. Consensus statement on the definition of orthostatic hypotension, pure autonomic failure and multiple system atrophy. Clin Auton Res 1996; 6:125–126. [DOI] [PubMed] [Google Scholar]
- 12. Freeman R, Wieling W, Axelrod FB, Benditt DG, Benarroch E, Biaggioni I, Cheshire WP, Chelimsky T, Cortelli P, Gibbons CH, Goldstein DS, Hainsworth R, Hilz MJ, Jacob G, Kaufmann H, Jordan J, Lipsitz LA, Levine BD, Low PA, Mathias C, Raj SR, Robertson D, Sandroni P, Schatz I, Schondorff R, Stewart JM, van Dijk JG. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res 2011; 21:69–72. [DOI] [PubMed] [Google Scholar]
- 13. National Heart Foundation of Australia. Guideline for the diagnosis and management of hypertension in adults – 2016. Melbourne: National Heart Foundation of Australia, 2016.” https://www.heartfoundation.org.au/images/uploads/publications/PRO-167_Hypertension-guideline-2016_WEB.pdf. [Google Scholar]
- 14. Hypertension Canada. 2017 Hypertension Canada Guidelines for the Management of Hypertension (FULL VERSION). Can J Cardiol 2017; 33:557–576. [DOI] [PubMed] [Google Scholar]
- 15. Mancia G, Fagard R, Narkiewicz K, Redón J, Zanchetti A, Böhm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchhof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B, Zannad F; Task Force Members 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 2013; 31:1281–1357. [DOI] [PubMed] [Google Scholar]
- 16. Lahrmann H, Cortelli P, Hilz M, Mathias CJ, Struhal W, Tassinari M. EFNS guidelines on the diagnosis and management of orthostatic hypotension. Eur J Neurol 2006; 13:930–936. [DOI] [PubMed] [Google Scholar]
- 17. Cushman WC, Evans GW, Byington RP, Goff DC Jr, Grimm RH Jr, Cutler JA, Simons-Morton DG, Basile JN, Corson MA, Probstfield JL, Katz L, Peterson KA, Friedewald WT, Buse JB, Bigger JT, Gerstein HC, Ismail-Beigi F; ACCORD Study Group Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362:1575–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Margolis KL, Palermo L, Vittinghoff E, Evans GW, Atkinson HH, Hamilton BP, Josse RG, O’Connor PJ, Simmons DL, Tiktin M, Schwartz AV. Intensive blood pressure control, falls, and fractures in patients with type 2 diabetes: the ACCORD trial. J Gen Intern Med 2014; 29:1599–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gerstein HC, Miller ME, Genuth S, Ismail-Beigi F, Buse JB, Goff DC Jr, Probstfield JL, Cushman WC, Ginsberg HN, Bigger JT, Grimm RH Jr, Byington RP, Rosenberg YD, Friedewald WT; ACCORD Study Group Long-term effects of intensive glucose lowering on cardiovascular outcomes. N Engl J Med 2011; 364:818–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Consensus statement on the definition of orthostatic hypotension, pure autonomic failure, and multiple system atrophy. The Consensus Committee of the American Autonomic Society and the American Academy of Neurology. Neurology. 1996; 46:1470. [DOI] [PubMed] [Google Scholar]
- 21. Schwartz AV, Margolis KL, Sellmeyer DE, Vittinghoff E, Ambrosius WT, Bonds DE, Josse RG, Schnall AM, Simmons DL, Hue TF, Palermo L, Hamilton BP, Green JB, Atkinson HH, O’Connor PJ, Force RW, Bauer DC. Intensive glycemic control is not associated with fractures or falls in the ACCORD randomized trial. Diabetes Care 2012; 35:1525–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Appel LJ, Wright JT Jr, Greene T, Agodoa LY, Astor BC, Bakris GL, Cleveland WH, Charleston J, Contreras G, Faulkner ML, Gabbai FB, Gassman JJ, Hebert LA, Jamerson KA, Kopple JD, Kusek JW, Lash JP, Lea JP, Lewis JB, Lipkowitz MS, Massry SG, Miller ER, Norris K, Phillips RA, Pogue VA, Randall OS, Rostand SG, Smogorzewski MJ, Toto RD, Wang X; AASK Collaborative Research Group Intensive blood-pressure control in hypertensive chronic kidney disease. N Engl J Med 2010; 363:918–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim HA, Yi HA, Lee H. Recent advances in orthostatic hypotension presenting orthostatic dizziness or vertigo. Neurol Sci 2015; 36:1995–2002. [DOI] [PubMed] [Google Scholar]
- 24. Graafmans WC, Ooms ME, Hofstee HM, Bezemer PD, Bouter LM, Lips P. Falls in the elderly: a prospective study of risk factors and risk profiles. Am J Epidemiol 1996; 143:1129–1136. [DOI] [PubMed] [Google Scholar]
- 25. Novak P. Cerebral blood flow, heart rate, and blood pressure patterns during the tilt test in common orthostatic syndromes. Neurosci J 2016; 2016:6127340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Novak V, Spies JM, Novak P, McPhee BR, Rummans TA, Low PA. Hypocapnia and cerebral hypoperfusion in orthostatic intolerance. Stroke 1998; 29:1876–1881. [DOI] [PubMed] [Google Scholar]
- 27. Wieling W, Krediet CT, van Dijk N, Linzer M, Tschakovsky ME. Initial orthostatic hypotension: review of a forgotten condition. Clin Sci (Lond) 2007; 112:157–165. [DOI] [PubMed] [Google Scholar]
- 28. Romero-Ortuno R, Cogan L, Foran T, Kenny RA, Fan CW. Continuous noninvasive orthostatic blood pressure measurements and their relationship with orthostatic intolerance, falls, and frailty in older people. J Am Geriatr Soc 2011; 59:655–665. [DOI] [PubMed] [Google Scholar]
- 29. Gangavati A, Hajjar I, Quach L, Jones RN, Kiely DK, Gagnon P, Lipsitz LA. Hypertension, orthostatic hypotension, and the risk of falls in a community-dwelling elderly population: the maintenance of balance, independent living, intellect, and zest in the elderly of Boston study. J Am Geriatr Soc 2011; 59:383–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ooi WL, Hossain M, Lipsitz LA. The association between orthostatic hypotension and recurrent falls in nursing home residents. Am J Med 2000; 108:106–111. [DOI] [PubMed] [Google Scholar]
- 31. Fan XH, Wang Y, Sun K, Zhang W, Wang H, Wu H, Zhang H, Zhou X, Hui R. Disorders of orthostatic blood pressure response are associated with cardiovascular disease and target organ damage in hypertensive patients. Am J Hypertens 2010; 23:829–837. [DOI] [PubMed] [Google Scholar]
- 32. Verwoert GC, Mattace-Raso FU, Hofman A, Heeringa J, Stricker BH, Breteler MM, Witteman JC. Orthostatic hypotension and risk of cardiovascular disease in elderly people: the Rotterdam study. J Am Geriatr Soc 2008; 56:1816–1820. [DOI] [PubMed] [Google Scholar]
- 33. Fedorowski A, Hedblad B, Melander O. Early postural blood pressure response and cause-specific mortality among middle-aged adults. Eur J Epidemiol 2011; 26:537–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rose KM, Tyroler HA, Nardo CJ, Arnett DK, Light KC, Rosamond W, Sharrett AR, Szklo M. Orthostatic hypotension and the incidence of coronary heart disease: the Atherosclerosis Risk in Communities study. Am J Hypertens 2000; 13:571–578. [DOI] [PubMed] [Google Scholar]
- 35. Rose KM, Eigenbrodt ML, Biga RL, Couper DJ, Light KC, Sharrett AR, Heiss G. Orthostatic hypotension predicts mortality in middle-aged adults: the Atherosclerosis Risk In Communities (ARIC) Study. Circulation 2006; 114:630–636. [DOI] [PubMed] [Google Scholar]
- 36. Juraschek SP, Daya N, Appel LJ, Miller ER, McEvoy JW, Matsushita K, Ballantyne CM, Selvin E. Orthostatic hypotension and risk of clinical and subclinical cardiovascular disease in middle-aged adults. J Am Heart Assoc 2018; 7:e008884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fedorowski A, Wahlstrand B, Hedner T, Melander O. Systolic and diastolic component of orthostatic hypotension and cardiovascular events in hypertensive patients: the Captopril Prevention Project. J Hypertens 2014; 32:75–81. [DOI] [PubMed] [Google Scholar]
- 38. Zhou Y, Ke SJ, Qiu XP, Liu LB. Prevalence, risk factors, and prognosis of orthostatic hypotension in diabetic patients: a systematic review and meta-analysis. Medicine (Baltimore) 2017; 96:e8004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Laederach-Hofmann K, Weidmann P, Ferrari P. Hypovolemia contributes to the pathogenesis of orthostatic hypotension in patients with diabetes mellitus. Am J Med 1999; 106:50–58. [DOI] [PubMed] [Google Scholar]
- 40. Frith J, Bashir AS, Newton JL. The duration of the orthostatic blood pressure drop is predictive of death. QJM 2016; 109:231–235. [DOI] [PubMed] [Google Scholar]
- 41. Gavish B, Bursztyn M. Blood pressure and heart period variability ratios derived from 24-h ambulatory measurements are predictors of all-cause mortality. J Hypertens 2015; 33:491–8; discussion 498. [DOI] [PubMed] [Google Scholar]
- 42. Angelousi A, Girerd N, Benetos A, Frimat L, Gautier S, Weryha G, Boivin JM. Association between orthostatic hypotension and cardiovascular risk, cerebrovascular risk, cognitive decline and falls as well as overall mortality: a systematic review and meta-analysis. J Hypertens 2014; 32:1562–1571; discussion 1571. [DOI] [PubMed] [Google Scholar]
- 43. Ricci F, Fedorowski A, Radico F, Romanello M, Tatasciore A, Di Nicola M, Zimarino M, De Caterina R. Cardiovascular morbidity and mortality related to orthostatic hypotension: a meta-analysis of prospective observational studies. Eur Heart J 2015; 36:1609–1617. [DOI] [PubMed] [Google Scholar]
- 44. National Institute for Health and Care Excellence. Hypertension in Adults: Diagnosis and Management | Guidance and guidelines. <https://www.nice.org.uk/guidance/cg127/chapter/1-guidance> Accessed 30 October 2017.
- 45. van Wijnen VK, Harms MPM, Wieling W. Orthostatic hypotension in the first minute after standing Up: what is the clinical relevance and do symptoms matter? Hypertension 2018; 71:816–818. [DOI] [PubMed] [Google Scholar]
- 46. SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373:2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Juraschek SP, Appel LJ, Miller ER 3rd, Mukamal KJ, Lipsitz LA. Hypertension treatment effects on orthostatic hypotension and its relationship with cardiovascular disease. Hypertension 2018; 72:986–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kamaruzzaman S, Watt H, Carson C, Ebrahim S. The association between orthostatic hypotension and medication use in the British Women’s Heart and Health Study. Age Ageing 2010; 39:51–56. [DOI] [PubMed] [Google Scholar]
- 49. Fotherby MD, Potter JF. Orthostatic hypotension and anti-hypertensive therapy in the elderly. Postgrad Med J 1994; 70:878–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.