Abstract
Decreases in cognitive function related to increases in oxidative stress and inflammation occur with ageing. Acknowledging the free radical-quenching activity and anti-inflammatory action of the carotenoid lycopene, the aim of the present review was to assess if there is evidence for a protective relationship between lycopene and maintained cognitive function or between lycopene and development or progression of dementia. A systematic literature search identified five cross-sectional and five longitudinal studies examining these outcomes in relation to circulating or dietary lycopene. Among four studies evaluating relationships between lycopene and maintained cognition, three reported significant positive relationships. Neither of the two studies reporting on relationship between lycopene and development of dementia reported significant results. Of four studies investigating circulating lycopene and pre-existing dementia, only one reported significant associations between lower circulating lycopene and higher rates of Alzheimer's disease mortality. Acknowledging heterogeneity among studies, there is insufficient evidence and a paucity of data to draw firm conclusions or tease apart direct effects of lycopene. Nevertheless, as low circulating lycopene is a predictor of all-cause mortality, further investigation into its relationship with cognitive longevity and dementia-related mortality is warranted.
Key words: Lycopene, Cognitive function, Cognition, Dementia, Alzheimer's disease
Abbreviations: AD, Alzheimer's disease; ICD, International Statistical Classification of Diseases and Related Health Problems; MMSE, Mini-Mental State Examination; ROS, reactive oxygen species
As life expectancy increases, so too does the number of individuals with dementia and cognitive decline. In 2015, there were an estimated 46·8 million adults living with dementia worldwide, with a new case of dementia diagnosed every 3·2 s(1). Unfortunately, there are currently few effective treatments once a dementia diagnosis is made. Thus, nutrition interventions to reduce the prevalence of cognitive decline are of great interest to public health.
Decreases in cognitive function related to increases in oxidative stress commonly occur with ageing. High rates of energy production required to maintain the central nervous system subject the brain to risk of oxidative imbalance, leading to neuro-inflammation(2). Although reactive oxygen species (ROS) serve as signalling molecules at physiological levels, oxidative stress ensues when ROS production exceeds homoeostatic mechanisms to detoxify. The accumulation of ROS along with oxidised protein, lipid and nucleic acids from prolonged oxidative imbalances damages neurons and myelin sheaths. Furthermore, chronic oxidative stress in the brain leads to neuro-inflammation, a hallmark feature of cognitive ageing and dysfunction along with neurodegenerative diseases(2,3). In contrast to the deleterious effects of oxidative imbalance on cognition, systemic oxidative balance may positively influence cognitive longevity or maintained cognitive performance.
Fruit and vegetables contain numerous bioactive compounds exhibiting antioxidant and anti-inflammatory functionality, such that their intake may have protective effects against cognitive decline by maintaining and restoring oxidative and inflammatory balance. According to a recent meta-analysis of nine studies (n 31 104), increased consumption of fruit and vegetables was associated with significant reduction in the risk of cognitive impairment and dementia (OR = 0·87, 95 % CI 0·77, 0·99)(4); however, equivocal findings were reported in a recent systematic review of the literature reporting on associations between cognitive function or dementia and intake of specific antioxidants in fruit and vegetables including β-carotene, vitamins C and E and flavonoids(5). In contrast, higher intake of the bioactive carotenoid lutein has been linked to better cognitive performance(6). Although the exact mechanisms of the neuroprotective activity of lutein are unknown, the beneficial effects of this carotenoid compound are thought to be attributable to its ability to decrease oxidative stress. Divergent associations between carotenoids, specifically β-carotene and lutein, and cognitive function or dementia may be the result of differences in tissue distribution of these carotenoids as well as differing antioxidant functionality. For example, lycopene, a carotenoid present in several fruits including tomatoes and watermelon, has demonstrated greater singlet oxygen quenching abilities compared with the other carotenoids, such as β-carotene, lutein and zeaxanthin(7). In recent studies using animal models, lycopene has been shown to exhibit neuroprotective effects by mitigating oxidative stress, suppressing production of inflammatory cytokines, and attenuating amyloidosis or accumulation of amyloid plaques(8,9); furthermore, lycopene has been shown to attenuate cognitive deficits by improving inflammation in the gut–liver–brain axis as well improving glycolipid metabolism(10). Additional mechanisms by which lycopene confers neurocognitive protection include the inhibition of neuronal apoptosis and restoration of mitochondrial function(11). To date, the current evidence on the relationship between lycopene and cognition in humans has yet to be synthesised.
As there are several stages of cognitive decline, so, too, are there several methods for assessing and subsequently diagnosing loss of cognitive function. For example, the term ‘dementia’ describes a very broad range of symptoms that may include loss of memory, language, focus, problem-solving and visual perception(12). Currently, Alzheimer's disease (AD) is the most common form of dementia worldwide(13). As such, the aim of the present review is to assess if there is evidence for a protective relationship between lycopene and maintained cognitive function as well as assess its relationship with the development and progression of dementia.
Methods
Literature search
A systematic literature search was conducted according to published guidelines for systematic reviews. Searches were conducted using PubMed and EBSCOhost. The selected databases offer different filters for search results. Filters for ‘peer reviewed’, ‘scholarly journals’, and ‘ages 65+ years’, ‘middle aged: 45–64 years’, ‘aged (65 years & older)’, ‘aged 80 & over’, ‘very old (85 years & older)’ and ‘middle aged (40–64 years)’ were included in the EBSCOhost search. Filters for human studies were placed on the PubMed search. Articles were included if published after 2005, with titles and abstracts in English, and in full text rather than as an abstract or presentation format only.
Application of inclusion/exclusion criteria
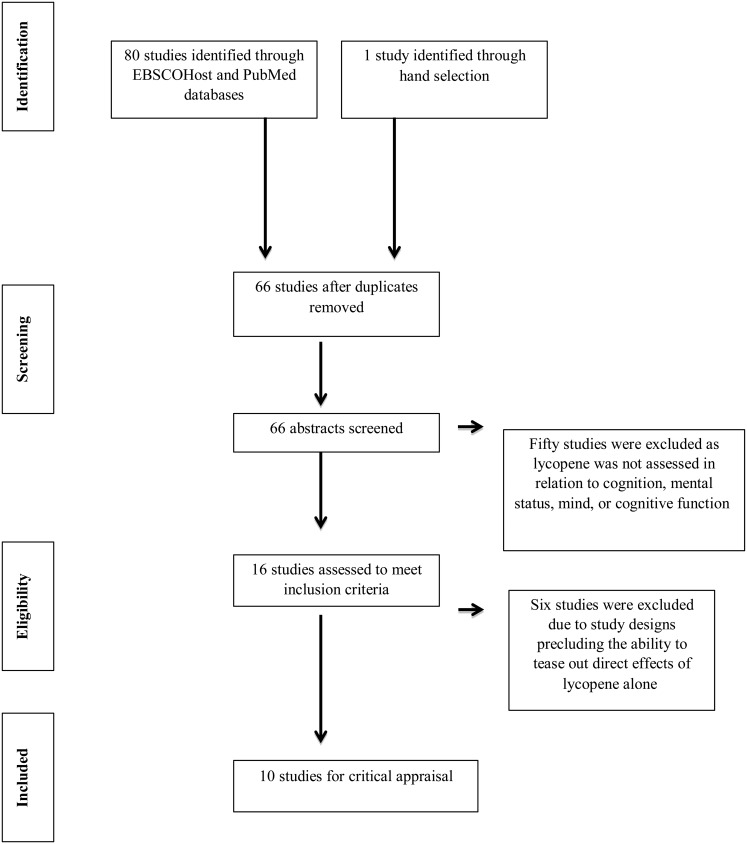
Boolean search terms were used to identify the primary articles to be considered for the review. Search terms for ‘lycopene’ and ‘cognition or mental status or brain function or cognitive function or memory or brain’ and ‘elderly or older adults or geriatric’ were applied. Identical searches were conducted using EBSCOhost and PubMed databases. Additionally, searches were also conducted using lycopene-rich foods such as tomatoes as search terms; however, no additional studies meeting the inclusion criteria were identified. One publication did not appear in search results or in the article references but was hand-selected due to its topical relevance to the objective of this study(14). After duplicate articles were removed, sixty-six publications remained. The first author (K. M. C.-W.) screened articles identified in the searches by abstract and a keyword search of the full paper. Fifty articles were excluded as they did not assess lycopene in relation to ‘cognition’, ‘mental status’, ‘mind’ or ‘cognitive function’. The references of identified articles were also reviewed to check for any articles not identified by the Boolean searches; however, no new articles were identified for inclusion following abstract review by the first author. The review process is reported in Fig. 1.
Fig. 1.
Search strategy flow diagram for research evaluating the relationship between lycopene and cognition.
Data extraction and analysis
To prevent risk of bias in reporting, the remaining sixteen articles were independently analysed by all authors for quality according to the Academy of Nutrition and Dietetics Evidence Analysis Library Worksheet and Quality Criteria Checklist(15). Following article review and data abstraction, six articles were excluded. Although the abstracts mentioned lycopene, the papers did not meet the inclusion criteria(16–21). One study only considered incidence of depression in participants rather than specific areas of cognitive functioning and was thus excluded for unrelated outcome measures(16). Another article assessed mental health status, not cognition(18). The third reported on cognition and the Mediterranean diet as a whole rather than lycopene specifically(17). The remaining three papers describe studies providing a combination supplement or a beverage that included lycopene together with other antioxidants(19–21). These were not included due to the inability to tease out any direct effects from lycopene alone.
Results
Study characteristics of research identified
Of the eighty-one articles that were identified and considered for the present review, ten articles fit the inclusion criteria (Table 1)(13,14,22–29). The articles were published between 2007 and 2015 from the USA, France, Germany, Scotland and Brazil. Of the ten studies, five had a cross-sectional study design(13,22,23,27,28) and five assessed longitudinal data with study duration lasting from 1·8 years to an average of 17 years(14,24–26,29). These ten publications assessed lycopene and cognition in both men and women between the ages of 45 and 102 years.
Table 1.
Results from studies assessing the relationship between lycopene and cognitive function
| Study population | Study design | Carotenoid measurement | Cognitive measures | Reference |
|---|---|---|---|---|
| France n 589 (61·3 %F) Age: 68–79 years Race: NR |
Cross-sectional | Plasma | Mini-Mental State Examination, Trail Making Test Part A, Trail Making Test Part B, Digit–Symbol Substitution, Finger Tapping Test, Word Fluency Test | Akbaraly et al.(22) |
| Germany n 193 (51·8 %F) Age: 45–102 years Race: NR |
Cross-sectional | Plasma FFQ |
Mini-Mental State Examination, Clock Drawing Test, Dem-Tect Scale | Polidori et al.(23) |
| USA n 16 010 (100 %F) Age: ≥ 70 years Race: NR |
Longitudinal: 17 years | 61-item FFQ 130-item FFQ |
TICS, delayed recall of the TICS, East Boston Memory Test – immediate and delayed recalls, Backward Digit Span, Category Fluency Test | Devore et al.(24) |
| France n 2983 (46·3 %F) Age: 45–60 years Race: NR |
Longitudinal: 13–15 years | Plasma Bimonthly 24 h recall |
RI-48 Cued Recall Task, Semantic Fluency Task, Phonemic Fluency Task, Forward and Backward Digit Span, Delis–Kaplan Trail Making Test | Kesse-Guyot et al.(25) |
| Scotland n 201 (44·2 %F) Age: 77 years Race: NR |
Longitudinal: 11 years | FFQ Plasma |
Mini-Mental State Examination | Whalley et al.(26) |
| France n 1092 (%F NR) Age: mean 74·4 years Race: NR |
Longitudinal: 1·8–10·8 years | Plasma | Mini-Mental State Examination, Isaac's Set Test, Benton Visual Retention Test | Feart et al.(14) |
| Germany AD n 27 (63 %F) AD Plus n 16 (56·3 %F) Control n 33 (66·6 %F) Age: AD 80·5 (sd 5·7) years AD Plus 79·4 (sd 4·7) years Control 73·2 (sd 8·3) years Race: NR |
Cross-sectional | Plasma | Mini-Mental State Examination | Dias et al.(13) |
| Germany Mild dementia n 74 (33·3 %F) Control n 148 (33·3 %F) Age: 65–90 years Race: NR |
Cross-sectional | Plasma | Mini-Mental State Examination, Semantic Fluency Task, CERAD word list | von Arnim et al.(27) |
| Brazil Treatment n 23 (%F NR) Control n 42 (%F NR) Age: 82 years (mean) Race: NR |
Cross-sectional | Plasma | Mini-Mental State Examination | Giavarotti et al.(28) |
| USA n 6958 (52·1 %F) Age: > 50 years Race: white, black, Hispanic, other |
Longitudinal: up to 12 years | Serum | Alzheimer's disease-associated mortality | Min & Min(29) |
%F, percentage female; NR, not reported; TICS, Telephone Interview for Cognitive Status; AD, Alzheimer's disease; CERAD, Consortium to Establish a Registry for Alzheimer's Disease.
Discussion of the ten articles is divided into sections on the relationship between lycopene intake or circulating levels of lycopene and (1) cognitive performance, (2) development of dementia and (3) influence on pre-existing dementia.
Lycopene and cognitive performance
Of the ten articles included in this review, four focused on lycopene and maintenance of normal cognitive function at a single time point or the relationship between lycopene intake at one point and cognitive outcomes years later(22–25).
In a cross-sectional study by Akbaraly et al.(22), low plasma lycopene was correlated with low cognitive performance in a cohort of 589 community-dwelling men and women aged 68–79 years. After adjustment for possible confounders, participants in the lowest percentile of cognitive function were more likely to have the lowest levels of circulating lycopene. When cognitive function tests were administered, low levels of lycopene were significantly associated with the Trail Making Test B (P = 0·008) and Digit–Symbol Substitution test (P = 0·01) (OR = 1·76 and 2·02, respectively).
In another cross-sectional analysis, Polidori et al.(23) examined plasma lycopene relative to cognition in a German cohort of 193 men and women aged 45–102 years. FFQ were used to group participants into ‘high’ or ‘low’ intake groups for fruit and vegetables. Participants in the ‘high intake’ group for fruit and vegetables had significantly higher plasma lycopene compared with those in the ‘low intake’ group (P < 0·0001). Specifically, higher plasma lycopene levels were associated with higher test scores on the Mini-Mental State Examination (MMSE) (P = 0·005), the Dem-Tect Scale (P = 0·02) and the Clock Drawing Test (P < 0·0001).
In the Nurses' Health Study, cognition was assessed in 16 010 female nurses aged 70 years and older using a battery of six cognitive tests that were repeated three times at 2-year intervals(24). The cognitive tests included the Telephone Interview for Cognitive Status (TICS) test, East Boston Memory Test – immediate and delayed recalls, a category fluency test, a delayed recall of the TICS ten-word list, and backward digit span test. The global score consisted of the average z scores of all six tests, and the verbal score included the immediate and delayed recalls of the East Boston Memory Test and the TICS ten-word list. Additionally, an average of five FFQ was given over approximately 17 years. The dietary assessments were collected in the years prior to cognitive testing and were assumed to depict the participants' usual intake before and during the cognitive assessment period. Lycopene intake was associated with a slower decline in both the global and verbal composite scores (P = 0·05, P = 0·009, respectively) over the course of the 6 years that the cognitive tests were administered. Results suggest a potential relationship between long-term consumption of foods high in lycopene and preserved cognitive function.
Another longitudinal study reported an association between carotenoid-rich dietary patterns and cognition later in life(25). In this group of 2983 men and women aged 45–60 years, dietary data were obtained from twelve individual 24-h dietary recalls administered over 2 years. Plasma concentrations of lutein, zeaxanthin, β-cryptoxanthin, lycopene, α-carotene, trans-β-carotene and cis-β-carotene were measured at baseline for a subset of the participants (n 381). Cognitive function was assessed 13 years later by the RI-48 cued recall task, several verbal fluency tasks, the forward and backward digit span tests and the Trail Making Test. Carotenoid-rich diet patterns ascertained from 24-h recalls at baseline were correlated with total plasma carotenoid concentrations collected at the same time, although a statistically significant association with plasma lycopene specifically was not observed. Dietary patterns rich in total carotenoids over the first 2 years of data collection were associated with a higher composite score of cognitive function at the 13-year follow-up (P < 0·05); however, correlations between plasma lycopene at baseline and cognitive scores 13 years later were not reported. Thus, although this study does provide evidence for a relationship between higher intakes of carotenoid-rich foods and preserved cognitive function with advancing age, it does not provide evidence for the effects of lycopene specifically.
Lycopene and the development of dementia
Only two studies considered lycopene status and risk of dementia diagnosis later in life(14,26), and both reported circulating lycopene levels rather than a self-reported measure of intake. Based on the relationship between high homocysteine and increased risk of AD, Whalley et al.(26) investigated circulating homocysteine, folate and vitamin B12 among a sample of 201 men and women born in 1921. Plasma lycopene was analysed among a subsample of 173 participants, and associations were examined between baseline blood levels of these circulating nutrients and development of dementia across 11 years. Dementia diagnosis was based on International Statistical Classification of Diseases and Related Health Problems (ICD)-10 criteria. Plasma lycopene, among several other antioxidant compounds, showed a significant inverse relationship with plasma homocysteine levels (P = 0·017); furthermore, it was reported that low plasma lycopene concentrations were associated with increased risk of dementia (P value not reported). Based on the analyses, it is unclear whether this was an independent risk or if it was associated with the risk of high homocysteine, given the inverse association between lycopene and homocysteine.
Feart et al.(14) did not find significant inverse associations between plasma lycopene and dementia diagnosis in a longitudinal study of 1092 French men and women with an average age of 74·4 years. In this study, researchers assessed the relationship between plasma carotenoids (β-carotene, α-carotene, lycopene, lutein, zeaxanthin and β-cryptoxanthin) measured at baseline and the subsequent risk of developing dementia or AD. All participants were free of dementia at baseline, and cognitive status was assessed by a team of neurologists over the 10-year average follow-up period. It was determined that decreased risk of dementia and AD was associated only with higher lutein concentrations in the older adult participants (P < 0·05). Although circulating levels of lycopene were lower in participants later diagnosed with dementia compared with those without dementia, differences did not reach statistical significance.
Lycopene and pre-existing dementia
Four studies assessed lycopene among participants with pre-existing dementia(13,27–29). Each of the studies utilised a comparison group and reported circulating lycopene levels rather than a self-reported intake. Dias et al.(13) analysed blood samples from twenty-seven AD participants without cardiovascular co-morbidities (AD only), sixteen AD participants with concomitant cardiovascular co-morbidities (AD plus), and a control group of thirty-three healthy older adults. Among the primary outcome measures of this study were HDL-cholesterol concentrations, MMSE scores and plasma carotenoid concentrations (lycopene, β-carotene, α-carotene, lutein, zeaxanthin and cryptoxanthin). Participants with ‘AD plus’ had lower plasma concentrations of HDL-cholesterol (P < 0·05) and lycopene (P < 0·05) compared with the control and ‘AD only’ groups. Lycopene was not significantly lower in the ‘AD only’ group when compared with the control group. Based on the results, it is unclear if there is an inter-relationship between circulating lycopene, CVD and AD.
In a cross-sectional study of 222 German men and women aged 65–90 years, von Arnim et al.(27) investigated the association between mild dementia and serum antioxidants (vitamin C, vitamin E, β-carotene, lycopene, coenzyme Q10). Mild dementia was defined by an MMSE score < 24, and diagnosis was confirmed by a battery of neurological tests. According to these criteria, there were seventy-four men and women in the group designated as having mild dementia. The study enrolled 148 men and women who scored over 24 on the MMSE for an age- and sex-matched control group. Though descriptive findings suggest that individuals consuming more lycopene are at lower odds of having dementia compared with those that consume lesser amounts of lycopene, statistical findings from the adjusted models were not significant. Of concern, the authors do note, however, that ‘measurement error in the outcome variable’ of serum lycopene could have occurred.
In a cross-sectional study by Giavarotti et al.(28), plasma lycopene levels in twenty-three elderly men and women with probable AD were compared with forty-two controls (mean age of all participants = 82 years). Cognitive status was assessed by MMSE and Clinical Dementia Rating scores. Although mean plasma levels of lycopene were lower in participants with probable AD compared with those with normal cognitive function, the difference was not significant.
Min & Min(29) evaluated existing data from the Third National Health and Nutrition Examination Survey (NHANES III) and the NHANES III Linked Mortality File to assess the relationship between serum carotenoids and risk of death from AD. The study population which included 6968 individuals over the age of 50 years was assessed at two cross-sectional time points. Serum carotenoids (α-carotene, β-carotene, β-cryptoxanthin, lycopene, lutein plus zeaxanthin) were measured at baseline (between 1988 and 1994), and deaths attributable to AD were quantified from ICD-9 and ICD-10 codes through the year 2000. Mortality data showed seventy-five deaths from AD over the follow-up period, and participants with AD mortality had significantly lower serum levels of lycopene compared with those without AD mortality (P < 0·0001). Results suggest a potential association between decreases in serum lycopene and death attributable to AD.
Discussion
The present review is the first of its kind to assess available research on the relationship between lycopene and cognition. A key discovery was the paucity of research in this area, especially of intervention studies. Among the four studies evaluating the relationship between circulating or dietary lycopene and maintained cognition, three reported significant positive relationships(22–24). In contrast, neither of the two studies reporting on the relationship between lycopene and future dementia development reported significant results(14,26). However, in both studies, plasma lycopene was lower in those with future dementia diagnosis albeit non-significantly. Lastly, among the four studies investigating circulating lycopene in older adults with pre-existing dementia, only one reported significantly lower circulating levels of lycopene among those with AD mortality matched to a comparison group(29). Taken collectively, four of the ten articles identified for inclusion in the present review reported statistically significant protective associations(22–24,29). Nevertheless, the study designs preclude the ability to ascribe causality both due to potential confounding and a lack of knowledge about the temporal relationship between variables of interest.
Although functional MRI studies have associated plasma lycopene with enhanced cognitive performance, findings reported herein warrant additional discussion as several factors probably mediate the relationship between lycopene and cognition(30). It must be acknowledged that lycopene accumulates differently in tissues, and this accumulation is influenced by both genetic factors and phenotypic influences(31,32). Thus, inter-personal variability in the bioavailability and distribution of lycopene would be expected to influence its relationship with cognition. Furthermore, dietary factors such as the food matrix may also have a significant impact on the bioavailability of lycopene(33). For this reason, circulating levels of lycopene are superior to self-reported measures of dietary intake in assessing the relationship between lycopene and cognition. All but one study included in the present review included a circulating measure of lycopene, and more specifically, three of the four studies reporting significant protective associations utilised a circulating measure of lycopene(22,23,29). Given the dietary factors influencing carotenoid bioavailability, it has been proposed that a relative carotenoid bioavailability measure be used to compare circulating levels of single carotenoids induced by fruit and vegetable intake with that of purified carotenoid supplementation(33). Such a measure would strengthen experimental research aimed at understanding the matrix-mediated effect on lycopene bioavailability and its subsequent relationship with cognition. Nevertheless, elucidating the health benefit of individual carotenoids is fraught with challenges given the common dietary sources and highly correlated nature of their presence in food. Thus, it cannot be ruled out that carotenoids may act synergistically as evidenced by the significant positive association reported by Kesse-Guyot et al.(25) between total plasma carotenoids rather than lycopene alone and cognitive function. Such synergy between lycopene and other compounds in the food matrix has been highlighted by Burton-Freeman & Sesso(34) in a review evaluating the evidence on tomato intake v. lycopene supplementation on cardiovascular risk factors. In short, tomato supplementation elicited more favourable results on oxidative stress, inflammation, endothelial function and lipid metabolism whereas lycopene supplementation was more effective in blood pressure management. Taken collectively, traditional reductive approaches may not fully capture the synergy between foods and nutrients, specifically lycopene and other carotenoid compounds(35).
Age-related changes in metabolic processes as well as accumulation of ROS-induced damaged biomolecules with advancing age may influence overall lycopene status. For example, results from the Women's Health and Aging Study reveal that older adults have significantly lower circulating lycopene levels compared with younger individuals of matched ethnic and dietary history(36). Factors influencing these findings include decreases in intestinal absorption of carotenoids with ageing and lifespan accumulation of health conditions associated with systemic oxidative stress(37,38). Additional determinants of circulating lycopene include age, lipid levels and smoking status(39,40). Among studies included in the present review, six of the ten studies reported adjusting statistical models for at least one of these potential covariates(14,22–25,29).
Furthermore, it must be acknowledged that decreases in both cognitive function and overall intake of carotenoids occur with advancing age(41). More specifically, diet quality, whether it be due to physiological, psychological or socio-economic reasons, often declines with age(42). For example, a recent study examining dietary patterns among a diverse group of 416 men and women aged 65 years and older reported that fewer than 50 % of participants were meeting nutrient recommendations, with many particularly lacking optimal intake of fruits and vegetables(43). In collective assessment of these potential mediating variables, age-related oxidative imbalances and low lycopene bioavailability along with diminished carotenoid intake represent the perfect storm for precipitating cognitive decline.
The present review reveals several important gaps in the literature related to lycopene intake and cognition. Divergent findings are probably attributed to the various study designs and cognitive measures employed; furthermore, the studies controlled for different covariates, examined different populations and utilised different outcome measures to evaluate cognitive performance (n 4), development of dementia (n 2) and influence on pre-existing dementia (n 4). Implications of the present review support the International Academy on Nutrition and Aging call for prospective studies of durational periods to sufficiently monitor diet and disease or cognitive decline(44). Future research would also be strengthened by utilising a select battery of tests to allow for cross-study comparisons; additionally, more sensitive tests that tease apart different domains of cognition such as executive functioning, reasoning and short-term and working memory should be used rather than tests assessing global cognition only. Likewise, all future studies must control for known confounders in order to tease apart direct effects for guiding evidence-based practice. While this study provides implications for future research, it is not exempt from some limitations, namely the lack of meta-analytic assessment. Such analysis was not conducted due to incongruences in outcome measures coupled with the small number of articles in each outcome grouping which reduces statistical power for analysis. Also, database search filters used in identifying potential articles for inclusion in the present review were limited to articles with titles and abstracts available in English.
As low circulating lycopene is a predictor of all-cause mortality(45), this carotenoid compound warrants further investigation into its relationship with disease-specific mortality including dementia. At this time, there is insufficient evidence and a paucity of data to draw firm conclusions and to tease apart direct effects of lycopene alone, thus warranting additional research. Until such research is conducted, adopting a Mediterranean-type diet pattern may benefit cognitive longevity as greater adherence results in higher circulating concentrations of carotenoids including lycopene(46).
Acknowledgements
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
K. M. C.-W., T. A. P. and A. C. E. developed the research question. All authors independently analysed and critically appraised articles included in the review. K. M. C.-W. and T. A. P. conducted the literature search and wrote the manuscript. A. C. E. edited the manuscript.
There were no conflicts of interest.
References
- 1.Alzheimer's Disease International (2015) Dementia statistics https://www.alz.co.uk/research/statistics (accessed April 2017).
- 2.Glade MJ (2010) Oxidative stress and cognitive longevity. Nutrition 26, 595–603. [DOI] [PubMed] [Google Scholar]
- 3.Mittal K & Katare DP (2016) Shared links between type 2 diabetes mellitus and Alzheimer's disease: a review. Diabetes Metab Syndr 10, Suppl. 1, S144–S149. [DOI] [PubMed] [Google Scholar]
- 4.Jiang X, Huang J, Song D, et al. (2017) Increased consumption of fruit and vegetable is related to a reduced risk of cognitive impairment and dementia: meta-analysis. Front Aging Neurosci 9, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crichton GE, Bryan J & Murphy KJ (2013) Dietary antioxidants, cognitive function and dementia – a systematic review. Plant Foods Hum Nutr 68, 279–292. [DOI] [PubMed] [Google Scholar]
- 6.Johnson EJ (2014) Role of lutein and zeaxanthin in visual and cognitive function throughout the lifespan. Nutr Rev 72, 605–612. [DOI] [PubMed] [Google Scholar]
- 7.Di Mascio P, Kaiser S & Sies H (1989) Lycopene as the most efficient biological carotenoid singlet oxygen quencher. Arch Biochem Biophys 274, 532–538. [DOI] [PubMed] [Google Scholar]
- 8.Wang J, Li L, Wang Z, et al. (2018) Supplementation of lycopene attenuates lipopolysaccharide-induced amyloidogenesis and cognitive impairments via mediating neuroinflammation and oxidative stress. J Nutr Biochem 56, 16–25. [DOI] [PubMed] [Google Scholar]
- 9.Liu CB, Wang R, Yi YF, et al. (2018) Lycopene mitigates β-amyloid induced inflammatory response and inhibits NF-κB signaling at the choroid plexus in early stages of Alzheimer's disease rats. J Nutr Biochem 53, 66–71. [DOI] [PubMed] [Google Scholar]
- 10.Wang J, Wang Z, Li B, et al. (2018) Lycopene attenuates Western-diet-induced cognitive deficits via improving glycolipid metabolism dysfunction and inflammatory responses in gut–liver–brain axis. Int J Obes (Lond) (epublication ahead of print version 11 December 2018). [DOI] [PubMed] [Google Scholar]
- 11.Chen D, Huang C & Chen Z (2019) A review for the pharmacological effect of lycopene in central nervous system disorders. Biomed Pharmacother 111, 791–801. [DOI] [PubMed] [Google Scholar]
- 12.Mayo Clinic (2017) Dementia – diagnosis and treatment https://www.mayoclinic.org/diseases-conditions/dementia/diagnosis-treatment/drc-20352019 (accessed February 2018).
- 13.Dias IH, Polidori MC, Li L, et al. (2014) Plasma levels of HDL and carotenoids are lower in dementia patients with vascular comorbidities. J Alzheimers Dis 40, 399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feart C, Letenneur L, Helmer C, et al. (2016) Plasma carotenoids are inversely associated with dementia risk in an elderly French cohort. J Gerontol A Biol Sci Med Sci 71, 683–688. [DOI] [PubMed] [Google Scholar]
- 15.Academy of Nutrition and Dietetics Evidence Analysis Library (2016) Evidence analysis manual https://www.andeal.org/evidence-analysis-manual (accessed September 2017).
- 16.Niu K, Guo H, Kakizaki M, et al. (2013) A tomato-rich diet is related to depressive symptoms among an elderly population aged 70 years and over: a population-based, cross-sectional analysis. J Affect Disord 144, 165–170. [DOI] [PubMed] [Google Scholar]
- 17.Shahar DR, Houston DK, Hue TF, et al. (2012) Adherence to Mediterranean diet and decline in walking speed over 8 years among community-dwelling older adults. J Am Geriatr Soc 60, 1881–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilkinson S, Farrelly S, Low J, et al. (2008) The use of complementary therapy by men with prostate cancer in the UK. Eur J Cancer Care (Engl) 17, 492–499. [DOI] [PubMed] [Google Scholar]
- 19.Yasuno F, Tanimukai S, Sasaki M, et al. (2012) Combination of antioxidant supplements improved cognitive function in the elderly. J Alzheimers Dis 32, 895–903. [DOI] [PubMed] [Google Scholar]
- 20.Bun S, Ikejima C, Kida J, et al. (2015) A combination of supplements may reduce the risk of Alzheimer's disease in elderly Japanese with normal cognition. J Alzheimers Dis 45, 15–25. [DOI] [PubMed] [Google Scholar]
- 21.Nilsson A, Salo I, Plaza M, et al. (2017) Effects of a mixed berry beverage on cognitive functions and cardiometabolic risk markers; a randomized cross-over study in healthy older adults. PLOS ONE 12, e0188173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akbaraly NT, Faure H, Gourlet V, et al. (2007) Plasma carotenoid levels and cognitive performance in an elderly population: results of the EVA study. J Gerontol A Biol Sci Med Sci 62, 308–316. [DOI] [PubMed] [Google Scholar]
- 23.Polidori MC, Praticόc D, Mangialasche F, et al. (2009) High fruit and vegetable intake is positively correlated with antioxidant status and cognitive performance in healthy subjects. J Alzheimers Dis 17, 921–927. [DOI] [PubMed] [Google Scholar]
- 24.Devore EE, Kang JH, Stampfer MJ, et al. (2013) The association of antioxidants and cognition in the Nurses’ Health Study. Am J Epidemiol 177, 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kesse-Guyot E, Andreeva VA, Ducros V, et al. (2013) Carotenoid-rich dietary patterns during midlife and subsequent cognitive function. Br J Nutr 111, 915–923. [DOI] [PubMed] [Google Scholar]
- 26.Whalley LJ, Duthie SJ, Collins AR, et al. (2014) Homocysteine, antioxidant micronutrients and late onset dementia. Eur J Nutr 53, 277–285. [DOI] [PubMed] [Google Scholar]
- 27.von Arnim CA, Herbolsheimer F, Nikolaus T, et al. (2012) Dietary antioxidants and dementia in a population-based case–control study among older people in South Germany. J Alzheimers Dis 31, 712–724. [DOI] [PubMed] [Google Scholar]
- 28.Giavarotti L, Simon KA, Azzalis LA, et al. (2013) Mild systemic oxidative stress in the subclinical stage of Alzheimer's disease. Oxid Med Cell Longev 2013, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Min J & Min K (2014) Serum lycopene, lutein and zeaxanthin, and the risk of Alzheimer's disease mortality in older adults. Dement Geriatr Cogn Disord 37, 246–256. [DOI] [PubMed] [Google Scholar]
- 30.Zwilling CE, Talukdar T, Zamroziewicz MK, et al. (2019) Nutrient biomarker patterns, cognitive function, and fMRI measures of network efficiency in the aging brain. NeuroImage 188, 239–251. [DOI] [PubMed] [Google Scholar]
- 31.Moran NE, Erdman JW & Clinton SK (2013) Complex interactions between dietary and genetic factors impact lycopene metabolism and distribution. Arch Biochem Biophys 539, 171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yeum KJ & Russell RM (2002) Carotenoid bioavailability and bioconversion. Annu Rev Nutr 22, 483–504. [DOI] [PubMed] [Google Scholar]
- 33.van het Hof KH, West CE, Weststrate JA, et al. (2000) Dietary factors that affect the bioavailability of carotenoids. J Nutr 130, 503–506. [DOI] [PubMed] [Google Scholar]
- 34.Burton-Freeman BM & Sesso HD (2014) Whole food versus supplement: comparing the clinical evidence of tomato intake and lycopene supplementation on cardiovascular risk factors. Adv Nutr 5, 457–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoffman K, Schulze MB, Schienkiewitz A, et al. (2004) Application of a new statistical method to derive dietary patterns in nutritional epidemiology. Am J Epidemiol 159, 935–944. [DOI] [PubMed] [Google Scholar]
- 36.Semba RD, Patel KV, Ferrucci L, et al. (2010) Serum antioxidants and inflammation predict red cell distribution width in older women: the Women's Health and Aging Study I. Clin Nutr 29, 600–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akhtar S, Ahmed A, Randhawa MA, et al. (2013) Prevalence of vitamin A deficiency in South Asia: causes, outcomes, and possible remedies. J Health Popul Nutr 31, 413–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petyaev IM (2016) Lycopene deficiency in ageing and cardiovascular disease. Oxid Med Cell Longev 2016, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Re R, Mishra GD, Thane CW, et al. (2003) Tomato consumption and plasma lycopene concentration in people aged 65 y and over in a British national survey. Eur J Clin Nutr 57, 1545. [DOI] [PubMed] [Google Scholar]
- 40.Mayne ST, Cartmel B, Silva F, et al. (1999) Plasma lycopene concentrations in humans are determined by lycopene intake, plasma cholesterol concentrations and selected demographic factors. J Nutr 129, 849–854. [DOI] [PubMed] [Google Scholar]
- 41.Lauretani F, Semba RD, Dayhoff-Brannigan M, et al. (2008) Low total plasma carotenoids are independent predictors of mortality among older persons: the InCHIANTI Study. Eur J Nutr 47, 335–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Porter Starr KN, McDonald SR & Bales CW (2015) Nutritional vulnerability in older adults: a continuum of concerns. Curr Nutr Rep 4, 176–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hsiao PY, Mitchell DC, Coffman DL, et al. (2013) Dietary patterns and diet quality among diverse older adults: the University of Alabama at Birmingham Study of Aging. J Nutr Health Aging 17, 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gillette Guyonnet S, Abellan Van Kan G, Andrieu S, et al. (2007) IANA task force on nutrition and cognitive decline with aging. J Nutr Health Aging 11, 132–152. [PubMed] [Google Scholar]
- 45.Shardell MD, Alley DE, Hicks GE, et al. (2011) Low-serum carotenoid concentrations and carotenoid interactions predict mortality in US adults: the Third National Health and Nutrition Examination Survey. Nutr Res 31, 178–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Daviglus ML, Plassman BL, Pirzada A, et al. (2011) Risk factors and preventive interventions for Alzheimer disease: state of the science. Arch Neurol 68, 1185–1190. [DOI] [PubMed] [Google Scholar]