Abstract
Background: Adiponectin has been suggested as a marker of many cardiovascular diseases. However, the association between serum adiponectin and incidence of atrial fibrillation (AF) in general population remains unclear. A meta-analysis was performed to systematically evaluate the potential influence of serum adiponectin at baseline on the incidence of AF during follow-up in general population.
Methods: Prospective cohort studies were identified via electronic search of PubMed and Embase databases. A randomized effect model was applied to combine the results. Predefined subgroup analyses were performed to evaluate the influence of study characteristics on the association between baseline adiponectin and risk of new-onset AF.
Results: Six cohort studies with 18558 community-derived participants were included, and 3165 AF cases were developed with a mean follow-up duration of up to 22 years. Meta-analysis showed that higher baseline circulating adiponectin was significantly associated with higher risk of new-onset AF during follow-up (hazard ratio [HR]: 1.17, 95% confidence interval [CI]: 1.08–1.27, P<0.001, I2 = 52%). Subgroup analyses showed that the association between adiponectin and new-onset AF was significant in studies with mean follow-up duration over 10 years (five cohorts, HR = 1.22, P<0.001), but not in that with a follow-up duration < 10 years (one cohort, HR = 0.95, P=0.51; P for subgroup difference = 0.002).
Conclusions: Higher circulating adiponectin at baseline may be an independent risk factor for the development of new-onset AF during follow-up, particularly in cohort studies with longer follow-up durations.
Keywords: Adiponectin, Atrial fibrillation, Inflammation, Meta-analysis, Prospective cohort study
Introduction
Atrial fibrillation (AF) is one of the most common chronic arrhythmia which is associated with increased risk of stroke, heart failure (HF) and all-cause mortality [1]. The prevalence of AF was estimated at 33 million in 2015 all over the world. With the aging of global population, the prevalence of AF has been expected to increase 2.5-fold in the next 50 years [2]. Current treatments for AF mainly include rhythm control, rate control and anticoagulation [1,3]. Although catheter ablation to achieve pulmonary vein isolation may terminate AF in some patients, the recurrence remains high in long-term follow-up [3]. Therefore, identification of risk factors for the development of AF in general population is important for early prevention of AF and revealing of potential treatment targets.
Currently, established risk factors for AF include known cardiovascular diseases (CVDs), such as coronary artery disease (CAD), HF, and valvular heart disease, as well as conventional risk factors of CVDs, including aging, hypertension, diabetes mellitus (DM), and tobacco smoking [4]. Moreover, accumulating evidence suggests that insulin resistance and inflammation may also play important roles in the pathogenesis of AF [5,6]. Adiponectin, a cytokine generated by adipocytes, has been demonstrated to exert insulin-sensitizing, anti-inflammatory, and anti-atherogenic properties [7–10], which is suggested to serve as a potential biomarker for the risk of CVDs [11,12], including AF. Indeed, some cohort studies have been performed to evaluate the association between adiponectin and AF risk in community-derived general population [13–18]. However, results of these studies are inconsistent. Therefore, we aimed to perform a meta-analysis to comprehensively evaluate the association between baseline level of circulating adiponectin and incidence of AF in general population.
Methods
We followed the MOOSE (Meta-analysis of Observational Studies in Epidemiology) [19] and Cochrane’s Handbook [20] guidelines during the design, implementation, analysis, and reporting for the present study.
Database search
We searched the databases of PubMed and Embase for relevant records, using the terms ‘adiponectin’ and ‘atrial fibrillation’. We limited the search to studies in humans published in English language. The reference lists of original and review articles were also analyzed using a manual approach. The final literature search was performed on 10 July 2018.
Study selection
Articles were included in the current meta-analysis if they met all the following criteria: (i) published as full-length article in English; (ii) reported as prospective cohort studies (regardless of sample size) with the follow-up duration of at least 1 year; (iii) included community-based adult population (≥18 years of age); (iv) circulating level of adiponectin was measured and identified as exposure of interest at baseline; (v) documented the incidences of AF during follow-up; (vi) reported the hazard ratios (HRs, at least adjusted for age and gender) and their corresponding 95% confidence intervals (CIs) of the risk of new-onset AF per 1-standard deviation (SD) increase in logarithmically transformed baseline adiponectin levels, or these data could be calculated. The diagnosis of new-onset AF was based on the definitions and criteria of the original articles. Reviews, letters, editorials, and studies with designs other than prospective cohort study were excluded from the current meta-analysis.
Data extraction and quality evaluation
The processes of database searching, data extraction, and quality assessment were performed by two independent authors according to the predefined criteria. Discrepancies were resolved by discussion with the third author. Data that were extracted include: (i) study names, locations, and periods; (ii) characteristics of the participants (numbers, mean ages, and gender); (iii) forms and methods of adiponectin measurements; (iv) follow-up durations; and (v) outcomes (AF outcome assessment, numbers of cases with new-onset AF, and the variables adjusted when presenting the results); and (vi) primary data regarding the HRs and 95% CIs for the incidence of AF per 1-SD increase in logarithmically transformed adiponectin at baseline. The quality of each study was evaluated using the Newcastle–Ottawa Scale [21] which ranges from 1 to 9 stars and judges each study regarding three aspects: selection of the study groups; the comparability of the groups; and the ascertainment of the outcome of interest.
Statistical analyses
Association between baseline adiponectin and risk of new-onset AF was presented as HRs and 95% CIs for the incidence AF per 1-SD increase in logarithmically transformed adiponectin, and the most adequately adjusted data were extracted. Data of HRs and their corresponding stand errors (SEs) were calculated from 95% CIs or P values, and were logarithmically transformed to stabilize variance and normalized the distribution [20]. We used the Cochrane’s Q test and I2 test to evaluate the heterogeneity among the included cohort studies [22]. A significant heterogeneity was considered if I2 > 50%. A random-effect model was applied to synthesize the HR data because this model is expected to retrieve a more generalized result via incorporation of the potential heterogeneity [20]. Sensitivity analyses, by removing individual study one at a time, were performed to evaluate whether the results of the meta-analysis was primarily driven by one influential study [23]. Predefined subgroup analyses were performed to evaluate whether the association between baseline adiponectin and risk of new-onset AF was affected by study characteristics such as sample sizes, mean ages, proportions of males, methods of adiponectin measurement, follow-up durations, AF incidences of the cohorts, and quality scores of the studies. Medians of the continuous variables were defined as the cut-off values for the stratification of the subgroups. In addition, potential publication bias was assessed by funnel plots with the Egger regression asymmetry test [24]. RevMan (Version 5.1; Cochrane Collaboration, Oxford, U.K.) and STATA software (Version 12.0; Stata Corporation, College Station, TX) were used for the meta-analysis and statistical analyses.
Results
Results of literature search
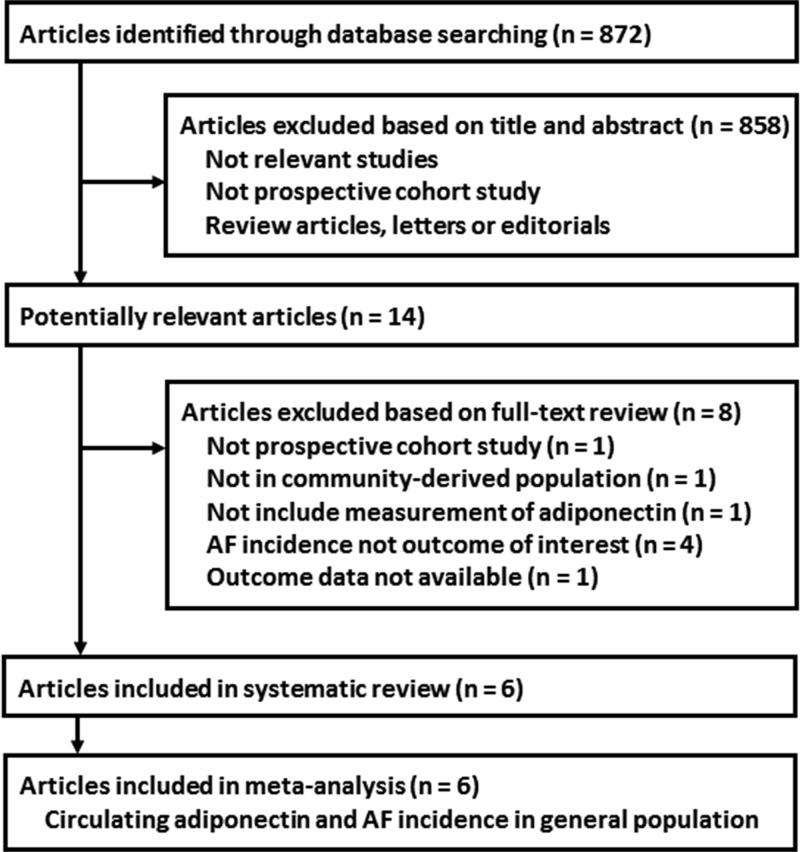
The processes of literature search and study selection were presented in Figure 1. Briefly, 872 studies were obtained via initial literature search, and 858 were excluded via title and abstract screenings because they were irrelevant to the study purpose. The remaining 14 studies underwent full-text review. Of them, eight were further excluded because one of them was not a prospective cohort study, one did not include community-derived population, one study did not provide adiponectin level at baseline, four did not report AF incidence during follow-up, and the other one was without an available outcome data. Finally, six prospective cohort studies [13–18] were included.
Figure 1. Flowchart of database search and study selection.
Study characteristics and quality evaluation
The characteristics of the included prospective cohort studies are presented in Table 1. Overall, our meta-analysis included 18558 community-derived participants from six cohort studies [13–18]. Four of them were performed in the U.S.A. [13,15–17], one in Australia [14], and the other one in Austria [18]. All the included studies reported the baseline levels of total adiponectin, while one of them also reported the level of high molecular weight (HMW) adiponectin [16]. Adiponectin was measured with enzyme-linked immunosorbent assay (ELISA) in four studies [13,14,16,18], while radioimmunoassay and multiplex assay were adopted in the other two studies respectively [15,17]. Confirmation of new-onset AF was made by electrocardiograph (ECG) and Holter in one study [13], while in the other five studies both ECG results and documented AF hospitalization were considered as new-onset AF events [14–18]. The follow-up durations varied from 7.6 to 20.0 years, and the incidences of AF in the included cohorts ranged from 8.0 to 27.8%. The Newcastle–Ottawa Scale varied from 8 to 9 in the included cohort studies.
Table 1.
Characteristics of the included prospective cohort studies
| Study | Design and location | Study periods | Number of participants | Mean age | Male | Adiponectin measurement | AF outcome assessment | Follow-up duration | AF cases | Adjusted factors | Quality score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Year | Years | % | Years | n (%) | |||||||
| 2012 Framingham Offspring Study [13] | PC, U.S.A. | 1999–2009 | 2487 | 61 | 46 | Total, ELISA | ECG or Holter | 7.6 | 206 (8.3) | Age, sex, BMI, SBP, treatment of hypertension, PR interval, clinically significant cardiac murmur, HF, and CRP | 9 |
| 2014 Busselton Health Study [14] | PC, Australia | 1995–2010 | 4267 | 52 | 47 | Total, ELISA | Hospitalization of AF | 15.0 | 343 (8.0) | Age, sex, height, hypertension treatment and BMI | 8 |
| 2015 Cardiovascular Health Study [16] | PC, U.S.A. | 1992–2009 | 3190 | 74 (>65) | 36 | Total and HMW, ELISA | ECG or hospitalization of AF | 11.4 | 886 (27.8) | Age, sex, race, educational status, height, weight, SBP, treatment of hypertension, smoking, alcohol, self-reported health status, estimated GFR, NT-proBNP, subclinical CVD, DM, LDL-C, HDL-C, TG and hsCRP | 9 |
| 2015 Health ABC Study [15] | PC, U.S.A. | 1992–2013 | 2768 | 73 (70–79) | 48 | Total, RIA | ECG or hospitalization of AF | 10.9 | 721 (26.0) | Race, age, sex, BMI, smoking, alcohol, statin treatment, hypertension, DM, CAD, HF and study site | 9 |
| 2016 Women’s Health Initiative Study [17] | PC, U.S.A. | 1993–2014 | 4937 | 66 (50–79) | 0 | Total, Multiplex assay | ECG or hospitalization of AF | 11.1 | 892 (18.1) | Age, race, education, hypertension, DM, hyperlipidemia, CAD, HF, PAD, smoking, history of cancer and BMI | 9 |
| 2017 Bruneck Study [18] | PC, Austria | 1990–2010 | 909 | 59 (40–79) | 50.7 | Total, ELISA | ECG or Holter or hospitalization of AF | 20.0 | 117 (12.9) | Age and sex | 8 |
Abbreviations: BMI, body mass index; CRP, C-reactive protein; GFR, glomerular filtrating rate; HDL-C, high-density lipoprotein cholesterol; hsCRP, highly sensitive C-reactive protein; LDL-C, low-density lipoprotein cholesterol; NT-proBNP, N-terminal pro-B type natriuretic peptide; PAD, peripheral artery disease; PC, prospective cohort; RIA, radioimmunoassay; SBP, systolic blood pressure; TG, triglyceride.
Association between adiponectin and new-onset AF
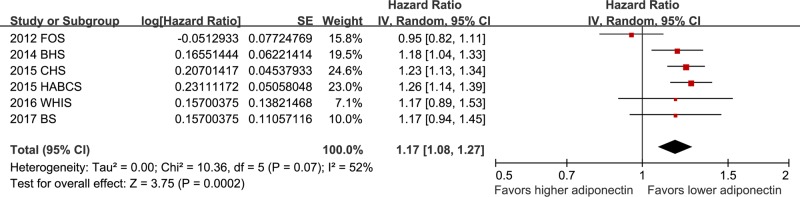
Pooled results with a random-effect model showed that higher baseline circulating adiponectin was significantly associated with higher risk of new-onset AF during follow-up (HR: 1.17, 95% CI: 1.08–1.27, P<0.001; Figure 2) with considerable heterogeneity (P for Cochrane’s Q test =0.07, I2 = 52%). Sensitivity analyses by excluding one study at a time did not change the results (HR: 1.14–1.22, P all <0.05; Table 2). Heterogeneity significantly reduced (P for Cochrane’s Q test =0.92, I2 = 0%) after excluding the Framingham Offspring Study [13], indicating that this study is the major contributor of heterogeneity of the meta-analysis.
Figure 2. Forest plots for the meta-analysis of the association between adiponectin at baseline and subsequent risk of new-onset AF in general population.
Data were presented as HRs and 95% CIs for the incidence of AF per 1-SD increase in logarithmically transformed adiponectin at baseline.
Table 2.
Sensitivity analyses
| Studies excluded | HR (95% CI) | I2 | P for heterogeneity | P for outcome effect |
|---|---|---|---|---|
| 2012 Framingham Offspring Study [13] | 1.22 (1.16, 1.29) | 0% | 0.92 | <0.001 |
| 2014 Busselton Health Study [14] | 1.16 (1.05, 1.29) | 61% | 0.03 | 0.005 |
| 2015 Cardiovascular Health Study [16] | 1.15 (1.03, 1.27) | 58% | 0.05 | 0.01 |
| 2015 Health ABC Study [15] | 1.14 (1.03, 1.26) | 53% | 0.08 | 0.009 |
| 2016 Women’s Health Initiative Study [17] | 1.17 (1.07, 1.28) | 61% | 0.03 | <0.001 |
| 2017 Bruneck Study [18] | 1.17 (1.06, 1.28) | 61% | 0.04 | 0.001 |
Subgroup analyses
Subsequent results of subgroup analyses indicated that study characteristics, such as sample sizes of the cohorts, mean ages of the participants, gender, methods of adiponectin measurements, AF incidences of the study cohorts, or the quality scores of the studies, did not significantly affect the association between adiponectin and new-onset AF (P for subgroup differences, all >0.05, Table 3). However, the association between adiponectin and new-onset AF was significant in studies with mean follow-up duration over 10 years (five cohorts, HR = 1.22, 95% CI: 1.16–1.29, P<0.001), but not in that with a follow-up duration < 10 years (one cohort, HR = 0.95, 95% CI: 0.82–1.11, P= 0.51; P for subgroup difference =0.002; Table 3).
Table 3.
Subgroup analyses
| Variables and cutoff | Number of studies | HR (95% CI) for subgroup | I2 | P for subgroup effect | P for subgroup difference |
|---|---|---|---|---|---|
| Sample sizes | |||||
| <3000 | 3 | 1.12 [0.93, 1.35] | 79% | 0.23 | |
| ≥3000 | 3 | 1.21 [1.13, 1.30] | 0% | <0.001 | 0.46 |
| Mean ages (years) | |||||
| <65 | 3 | 1.09 [0.94, 1.27] | 61% | 0.24 | |
| ≥65 | 3 | 1.24 [1.16, 1.32] | 0% | <0.001 | 0.12 |
| Male (%) | |||||
| <40 | 2 | 1.22 [1.12, 1.33] | 0% | <0.001 | |
| ≥40 | 4 | 1.14 [1.01, 1.29] | 68% | 0.04 | 0.36 |
| Adiponectin measurements | |||||
| ELISA | 4 | 1.13 [1.01, 1.27] | 64% | 0.03 | |
| Others | 2 | 1.25 [1.14, 1.37] | 0% | <0.001 | 0.20 |
| AF confirmation | |||||
| Include AF hospitalization | 5 | 1.22 [1.16, 1.29] | 0% | <0.001 | |
| Not include AF hospitalization | 1 | 0.95 [0.82, 1.11] | — | 0.51 | 0.002 |
| Follow-up duration (years) | |||||
| <10 | 1 | 0.95 [0.82, 1.11] | — | 0.51 | |
| ≥10 | 5 | 1.22 [1.16, 1.29] | 0% | <0.001 | 0.002 |
| AF incidence (%) | |||||
| <15 | 3 | 1.09 [0.94, 1.27] | 61% | 0.24 | |
| ≥15 | 3 | 1.24 [1.16, 1.32] | 0% | <0.001 | 0.12 |
| Quality scores | |||||
| =8 | 2 | 1.18 [1.06, 1.31] | 0% | 0.003 | |
| =9 | 4 | 1.16 [1.02, 1.31] | 71% | 0.02 | 0.83 |
Publication bias
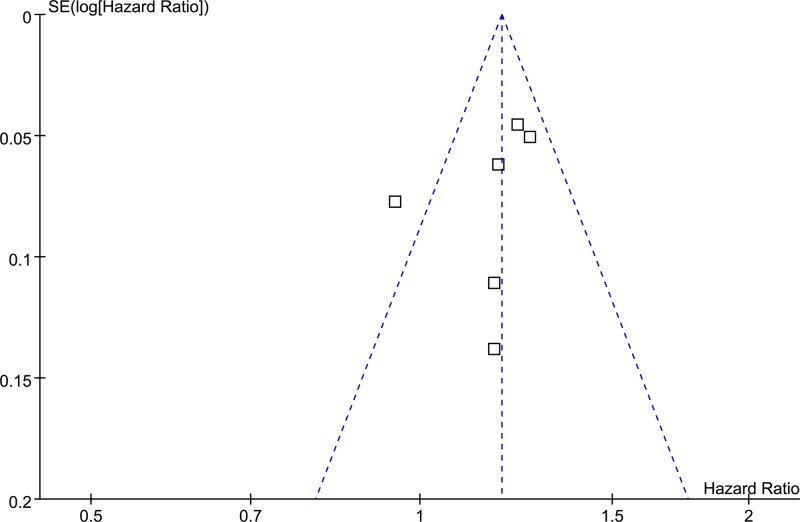
The funnel plots regarding adiponectin at baseline and the risk of AF during follow-up is shown in Figure 3. The funnel plot was symmetrical on visual inspection. Results of Egger regression test suggested that no significant publication bias was detected (P=0.33).
Figure 3. Funnel plots for the meta-analysis of the association between adiponectin and subsequent risk of new-onset AF during follow-up in general population.
Discussion
In the present study, by pooling the results of all available prospective cohort studies, results of our meta-analysis showed that higher circulating adiponectin at baseline is independently associated with increased risk of AF incidence in general population. Subgroup analyses indicated that the association between adiponectin and AF incidence is significant in cohorts with longer follow-up durations (≥10 years), but not in studies with shorter follow-up durations (<10 years). These findings are paradoxical to previous notion that adiponectin may be a protective factor against AF incidence since it is an anti-inflammatory factor.
To the best of our knowledge, our study is the first meta-analysis that evaluates the association between circulating adiponectin at baseline and the risk of AF in general population. We found that higher adiponectin at baseline appeared to be an independent risk factor for AF during follow-up. Although findings from experimental studies demonstrated that adiponectin, via exerting its insulin-sensitizing, anti-inflammatory, and anti-atherogenic properties, may be cardioprotecive, findings from epidemiological studies regarding the association between adiponectin and CVDs showed different results. In a meta-analysis of 24 prospective studies, circulating adiponectin at baseline was found to have no significant association with CAD incidence [25]. Moreover, in patients with established CVDs, higher adiponectin was independently associated with increased risk of CAD recurrence [25]. These findings were further confirmed in a subsequent meta-analysis which showed that adiponectin was associated with increased mortality in patients with already established CVDs [26]. Similarly, increased serum adiponectin was related to an elevated risk of ischemic stroke in a previous meta-analysis of 17 prospective studies with a total of 23717 participants [27]. Moreover, accumulating evidence from prospective cohort studies suggests that higher serum adiponectin may be an independent risk factor for HF incidence in community-based population [28,29]. Our results expanded these findings by showing that higher serum adiponectin was associated with increased risk of AF in general population. These findings further added the potential complexity regarding the association between adiponectin and CVDS. In fact, the potential role of adiponectin in patients with established AF was also controversial according to previous studies. In a prospective study including 874 patients with paroxysmal AF, high circulating adiponectin is independently associated with AF recurrence after catheter ablation [30]. However, another study showed that low plasma adiponectin was significantly associated with major cardiovascular events in female anticoagulated patients with nonvalvular AF [31]. Moreover, adiponectin was found to be inversely associated with the degree of platelet activation and risk of stroke in anticoagulated patients with AF, indicating a potential protective effect of higher adiponectin against stroke incidence in AF patients [32]. Taken together, although current findings suggest that increased adiponectin may be a marker of higher risk for AF development in general population, the influence of adiponectin on the pathogenesis, progression, and prognosis of AF may be much more complicated. Moreover, the potential mechanisms involved are largely unknown. Further studies are needed to clarify the exact relationship between adiponectin and AF.
Sensitivity analyses and subgroup analyses indicated that the Framingham Offspring Study [13] is the major contributor to the heterogeneity of the meta-analysis. Of note, the mean follow-up duration in the Framingham Offspring Study is shorter than others, suggesting that the association between adiponectin and AF incidence is significant in cohorts with longer follow-up durations (≥10 years), but not in studies with shorter follow-up durations (<10 years). Moreover, AF incidence was identified by the results of ECG and Holter in the Framingham Offspring Study [13], while other studies also included AF hospitalization as an indicator of AF incidence. These differences in the confirmation of AF incidence may also contribute to the heterogeneity. However, results of subgroup analyses regarding the potential influence of follow-up duration on the association between adiponectin and AF risk should be interpreted with caution since limited number of studies were included in each stratum.
Our study has limitations. First, adiponectin was measured via different methods in the included studies, and the strategies for the detection of new-onset AF varied. These may confound the result and contribute to the heterogeneity. In addition, as mentioned above, the results of subgroup analyses should be interpreted cautiously because limited studies were available and the results were based on data of study-level rather than individual patient-level. Moreover, a dose–response association between circulating adiponectin at baseline and the AF risk was unable to determine, because limited cohorts were included. Besides, as a common limitation of meta-analyses of observational studies, the association between adiponectin and AF incidence may be confounded by residual factors that were unadjusted when presenting the results. Finally, the clinical relevance of the findings of the meta-analysis should be investigated in the future. Currently, we were unable to determine whether the relationship between higher adiponectin and increased risk of AF incidence is causative. Future studies are needed to elucidate the potential mechanisms underlying the association between higher adiponectin and increased risk of AF incidence.
In conclusion, our meta-analysis indicated that higher circulating adiponectin at baseline may be an independent risk factor for the development of new-onset AF during follow-up, particularly in cohort studies with longer follow-up durations. Whether increased adiponectin is a surrogate marker or a therapeutic target during the pathogenesis of AF in general population deserves further investigation.
Abbreviations
- AF
atrial fibrillation
- CAD
coronary artery disease
- CI
confidence interval
- CVD
cardiovascular disease
- ECG
electrocardiograph
- HF
heart failure
- HR
hazard ratio
- SD
standard deviation
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
This work was supported by the Medical and Health Science and Technology Development Project of Shandong Province [grant number 2013BJYB03 (to J.W.)].
Author Contribution
Y.G. and J.W. designed the study. Y.G. and L.L. collected the data and performed statistical analyses. Y.G. and J.W. interpreted the results. Y.G. drafted the manuscript. L.L. and J.W. critically reviewed the manuscript.
References
- 1.Andrade J.G., Macle L., Nattel S., Verma A. and Cairns J. (2017) Contemporary Atrial Fibrillation Management: a comparison of the Current AHA/ACC/HRS, CCS, and ESC Guidelines. Can. J. Cardiol. 33, 965–976 10.1016/j.cjca.2017.06.002 [DOI] [PubMed] [Google Scholar]
- 2.Benjamin E.J., Virani S.S., Callaway C.W.. et al. (2018) Heart Disease and Stroke Statistics-2018 Update: a report from the American Heart Association. Circulation 137, e67–e492 10.1161/CIR.0000000000000558 [DOI] [PubMed] [Google Scholar]
- 3.Calkins H., Hindricks G., Cappato R.. et al. (2018) 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Europace 20, e1–e160 10.1093/europace/eux274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lau D.H., Nattel S., Kalman J.M. and Sanders P. (2017) Modifiable risk factors and atrial fibrillation. Circulation 136, 583–596 10.1161/CIRCULATIONAHA.116.023163 [DOI] [PubMed] [Google Scholar]
- 5.Guo Y., Lip G.Y. and Apostolakis S. (2012) Inflammation in atrial fibrillation. J. Am. Coll. Cardiol. 60, 2263–2270 10.1016/j.jacc.2012.04.063 [DOI] [PubMed] [Google Scholar]
- 6.Maria Z., Campolo A.R., Scherlag B.J., Ritchey J.W. and Lacombe V.A. (2018) Dysregulation of insulin-sensitive glucose transporters during insulin resistance-induced atrial fibrillation. Biochim. Biophys. Acta. 1864, 987–996 10.1016/j.bbadis.2017.12.038 [DOI] [PubMed] [Google Scholar]
- 7.Li F.Y., Lam K.S. and Xu A. (2012) Therapeutic perspectives for adiponectin: an update. Curr. Med. Chem. 19, 5513–5523 10.2174/092986712803833173 [DOI] [PubMed] [Google Scholar]
- 8.Ruan H. and Dong L.Q. (2016) Adiponectin signaling and function in insulin target tissues. J. Mol. Cell Biol. 8, 101–109 10.1093/jmcb/mjw014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turer A.T. and Scherer PE. (2012) Adiponectin: mechanistic insights and clinical implications. Diabetologia 55, 2319–2326 10.1007/s00125-012-2598-x [DOI] [PubMed] [Google Scholar]
- 10.Li S., Shin H.J., Ding E.L. and van Dam R.M. (2009) Adiponectin levels and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA 302, 179–188 10.1001/jama.2009.976 [DOI] [PubMed] [Google Scholar]
- 11.Ebrahimi-Mamaeghani M., Mohammadi S., Arefhosseini S.R., Fallah P. and Bazi Z. (2015) Adiponectin as a potential biomarker of vascular disease. Vasc. Health Risk Manag. 11, 55–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee S. and Kwak HB. (2014) Role of adiponectin in metabolic and cardiovascular disease. J. Exerc. Rehabil. 10, 54–59 10.12965/jer.140100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rienstra M., Sun J.X., Lubitz S.A.. et al. (2012) Plasma resistin, adiponectin, and risk of incident atrial fibrillation: the Framingham Offspring Study. Am. Heart J. 163, e111, 10.1016/j.ahj.2011.09.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knuiman M., Briffa T., Divitini M.. et al. (2014) A cohort study examination of established and emerging risk factors for atrial fibrillation: the Busselton Health Study. Eur. J. Epidemiol. 29, 181–190 10.1007/s10654-013-9875-y [DOI] [PubMed] [Google Scholar]
- 15.Dewland T.A., Vittinghoff E., Harris T.B.. et al. (2015) Inflammation as a mediator of the association between race and atrial fibrillation: results from the Health, Aging, and Body Composition Study. JACC Clin. Electrophysiol. 1, 248–255 10.1016/j.jacep.2015.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Macheret F., Bartz T.M., Djousse L.. et al. (2015) Higher circulating adiponectin levels are associated with increased risk of atrial fibrillation in older adults. Heart 101, 1368–1374 10.1136/heartjnl-2014-307015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ermakov S., Azarbal F., Stefanick M.L.. et al. (2016) The associations of leptin, adiponectin and resistin with incident atrial fibrillation in women. Heart 102, 1354–1362 10.1136/heartjnl-2015-308927 [DOI] [PubMed] [Google Scholar]
- 18.Willeit K., Pechlaner R., Willeit P.. et al. (2017) Association between vascular cell adhesion molecule 1 and atrial fibrillation. JAMA Cardiol. 2, 516–523 10.1001/jamacardio.2017.0064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stroup D.F., Berlin J.A., Morton S.C.. et al. (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283, 2008–2012 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 20.Higgins J. and Green S. (2011) Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. Cochrane Collab. www.cochranehandbook.org [Google Scholar]
- 21.Wells G.A., Shea B., O’Connell D.. et al. (2010) The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [Google Scholar]
- 22.Higgins J.P. and Thompson S.G. (2002) Quantifying heterogeneity in a meta-analysis. Stat. Med. 21, 1539–1558 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 23.Patsopoulos N.A., Evangelou E. and Ioannidis J.P. (2008) Sensitivity of between-study heterogeneity in meta-analysis: proposed metrics and empirical evaluation. Int. J. Epidemiol. 37, 1148–1157 10.1093/ije/dyn065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Egger M., Davey Smith G., Schneider M. and Minder C. (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sook Lee E., Park S.S., Kim E.. et al. (2013) Association between adiponectin levels and coronary heart disease and mortality: a systematic review and meta-analysis. Int. J. Epidemiol. 42, 1029–1039 10.1093/ije/dyt087 [DOI] [PubMed] [Google Scholar]
- 26.Wu Z.J., Cheng Y.J., Gu W.J. and Aung L.H. (2014) Adiponectin is associated with increased mortality in patients with already established cardiovascular disease: a systematic review and meta-analysis. Metabolism 63, 1157–1166 10.1016/j.metabol.2014.05.001 [DOI] [PubMed] [Google Scholar]
- 27.Hao G., Li W., Guo R.. et al. (2013) Serum total adiponectin level and the risk of cardiovascular disease in general population: a meta-analysis of 17 prospective studies. Atherosclerosis 228, 29–35 10.1016/j.atherosclerosis.2013.02.018 [DOI] [PubMed] [Google Scholar]
- 28.Witberg G., Ayers C.R., Turer A.T.. et al. (2016) Relation of adiponectin to all-cause mortality, cardiovascular mortality, and major adverse cardiovascular events (from the Dallas Heart Study). Am. J. Cardiol. 117, 574–579 10.1016/j.amjcard.2015.11.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lindberg S., Jensen J.S., Bjerre M.. et al. (2014) Cardio-adipose tissue cross-talk: relationship between adiponectin, plasma pro brain natriuretic peptide and incident heart failure. Eur. J. Heart Fail. 16, 633–638 10.1002/ejhf.82 [DOI] [PubMed] [Google Scholar]
- 30.Kim T.H., Lee J.S., Uhm J.S., Joung B., Lee M.H. and Pak H.N. (2018) High circulating adiponectin level is associated with poor clinical outcome after catheter ablation for paroxysmal atrial fibrillation. Europace 20, 1287–1293 [DOI] [PubMed] [Google Scholar]
- 31.Hernandez-Romero D., Jover E., Marin F.. et al. (2013) The prognostic role of the adiponectin levels in atrial fibrillation. Eur. J. Clin. Invest. 43, 168–173 10.1111/eci.12028 [DOI] [PubMed] [Google Scholar]
- 32.Carnevale R., Pastori D., Peruzzi M.. et al. (2014) Total adiponectin is inversely associated with platelet activation and CHA(2)DS(2)-VASc score in anticoagulated patients with atrial fibrillation. Mediators Inflamm. 2014, 908901. 10.1155/2014/908901 [DOI] [PMC free article] [PubMed] [Google Scholar]