Abstract
Objective
Increase in the evidence of global occurrence of Zika viral infection suggests that in Africa the circulation of the virus which causes 80% of asymptomatic infection could be undetected and/or overlooked. We sought to serologically detect Zika virus infection in febrile patients at Greater Accra Regional Hospital, Ghana.
Results
Of the 160 patient serum samples analyzed, 33 were found to have antibodies against Zika virus infection. Among the sero-positives 30 (91%) of the cases were anti-Zika virus IgM with the 21–30-year age group recording the highest number of 8 (26%) and 2 (7%) cases being the least for the 61 years and above age group. All sero-positive febrile patients developed at least one symptom consistent with Zika virus infection: 33 (100%) fever, 25 (76%) muscle pain, 24 (73%) joint pain, and conjunctivitis 2 (6%). Digestive symptoms recorded include 16 (49%) nausea, 12 (36%) vomiting and diarrhea 18 (55%). In addition, 28 (85%) loss of appetite, 14 (75%) rapid respiration and chest pain 15 (42%) were reported by seropositive febrile patients. Our data indicates exposure to Zika virus which suggests the possible circulation of the virus among febrile patients in Ghana with a sero-prevalence rate of 20.6%.
Keywords: Zika virus, Seroprevalence, Anti-Zika virus immunoglobulins M and G (IgM and IgG) antibodies, Enzyme linked immunosorbent assay (ELISA)
Introduction
Febrile illnesses account for about 30% and 75% of healthcare visit by children [1] and adults [2] respectively in Ghana. However, these febrile illnesses are mostly misdiagnosed for endemic diseases, malaria and typhoid fever. Zika virus infection shares overlapping signs and symptoms with most endemic diseases including malaria and typhoid fever. The etiologic agent, Zika virus was first isolated in Uganda in 1947 from a rhesus macaque monkey and Aedes africanus mosquito in 1948 [3]. First human cases of Zika virus infection were reported in Africa and Asia. In Africa, Zika virus infection was documented in Uganda and Tanzania in 1952 [4] and in Asia, notably Indonesia, Malaysia and Thailand [5]. Afterwards serological studies detected Zika virus infection in humans in Nigeria, Egypt, India, Pakistan, North Vietnam and Philippines, spreading from Africa to Southeast Asia [6, 7]. Zika virus infection in Africa and Asia never got the deserved attention due to the sporadic nature of the infection coupled with mild short-term and low-grade fever (37.8–38.5 °C) and mostly self-limiting symptoms including maculopapular and pruritic rashes, conjunctivitis (non-purulent) and arthralgia [8]. As a result, Zika virus infection appeared to have been neglected in tropical medicine and no efforts were made to develop vaccine and drugs for treatment [7]. Although Zika virus infection has not been reported in Ghana, evidence of several outbreaks and exposure to the virus have been reported in various African countries such as Gabon [9] Cape Verde, Senegal [10, 11] and Ivory Coast which share a common border with Ghana. Thus, the evidence of exposure to the virus in our neighboring countries and the presence of active vector population of Aedes spp. of mosquitoes in Ghana have necessitated the need to document the exposure levels in febrile patients. The mild or asymptomatic nature and the overlapping clinical feature with other endemic disease conditions make it probable for typical Zika virus infection to be missed out in diagnosis. Therefore, we set out to augment and improve scientific data in the sub-region and create awareness to pre-empt possible outbreaks with the detection of antibodies against Zika virus circulating among febrile patients at Greater Accra Regional Hospital, Accra Ghana.
Main text
Methods
Study design
This was a cross sectional study that involved 160 archived serum samples from febrile patients at the Greater Accra Regional Hospital between December 2016 and November 2017. These clinical specimens were obtained as part of an ongoing project with the aim of using serological and molecular tools to detect Dengue, Chikungunya and other Arboviruses in febrile patients within selected health facilities in Ghana. A structured case investigation form was used to collect information about demographic features, and clinical signs and symptoms of the febrile patients.
Eligibility criteria
Inclusion criteria for enrollment included a person with fever (body temperature above 38 °C) and at least one of the following signs or symptoms: arthralgia; or arthritis; or conjunctivitis (non-purulent/hyperemic). Patients positive for malaria by blood film diagnosis and patients who refuse to submit samples after consenting were excluded from the study. Venous blood was collected into heparinized tubes and centrifuged, and serum was harvested, aliquoted into two separate cryogenic 1.8 ml vials for each patient sample and stored at − 80 °C.
Laboratory testing
The archived sera from febrile patients were tested by enzyme linked immunosorbent assay (ELISA) for anti-Zika virus IgM and IgG antibodies with a commercial ELISA kit, Zika virus IgM and IgG capture ELISA (Abcam, Cambridge, UK). The sensitivity of this ELISA kit used (Abcam anti-Zika virus IgM and IgG) was reportedly assessed by evaluating the performances of 5 commonly used Zika virus immunoassays against an ELISA (MAC ELISA) developed by the US Centers for Disease Control and Prevention and cross-Plaque reduction neutralization test (PRNT) was reported as 57% and a specificity of 100% [12]. Manufacturer’s instructions or recommendations were followed in all tests performed.
Zika virus IgM and IgG negative control, Zika virus IgM and IgG positive control and the test serum samples were first diluted with sample dilution buffer for Zika virus IgM and IgG. Thereafter, incubated at 37 °C for an hour in micro-titre plates pre-coated with monoclonal antibody bound to recombinant Zika virus antigen. After subsequent washing, wells were treated with horseradish peroxidase (HRP) Zika virus conjugate (peroxidase labeled Zika virus antigen) except for the substrate blank well and incubated for 30 min at 37 °C. Washing was repeated after which substrate solution 3,3′,5,5′-tetramethylbenzidine (TBM) hydrogen peroxide was added and incubated in the dark at room temperature 20–25 °C for 15 min. A stop solution was then added, and the absorbance measured at 450 nm using ELISA microtiter plate reader (Human Diagnostic Worldwide, Germany) within 30 min after terminating the reaction using a reference wavelength of 620 nm. The ELISA tests results were classified according to the interpretation provided by the supplier, Abcam, Cambridge, UK.
Statistical analysis
One Way-Anova was used to test the association within groups at significance level of P-value < 0.05 using SPSS version 20.
Results
Characteristics of Zika virus sero-positives
Of the 160 samples tested, 33 (20.6%) had positive serological evidence for Zika virus. Out of the 33 Zika virus sero-positives, 30 (90.9%) were IgM positive whereas 3 (9.1%) were IgG positive. Of all the patients enrolled, 119 (74.4%) were females while 41 (25.6%) were males. Number of anti-Zika virus IgM males was 11 (36.7%) and that of females was 19 (63.3%) (Table 1). Anti-Zika virus IgG antibodies were detected only in females, however Zika virus infection was observed to affect males and females in a ratio of 1:2. Among the age groups identified, 8 (26.7%) of the anti-Zika virus IgM were recorded in age group 21–30 with 2 (6.7%) for the age group 61 years and above (Table 1). Anti-Zika virus IgG antibodies were detected only in age groups 21–30 2 (66.7%) and 41–50 1 (33.4%) (Table 1). There was significant difference between anti-Zika virus IgM among the age groups (P = 0.004).
Table 1.
Demographic distribution of seropositive febrile patients
| Variables | Zika virus antibodies, n = 33 | |||
|---|---|---|---|---|
| IgM (30) | P-value | IgG (3) | P-value | |
| Age groups | ||||
| 3–20 | 4 (13%) | 0 | ||
| 21–30 | 8 (26.7%) | 2 (66.7%) | ||
| 31–40 | 6 (20%) | 0 | ||
| 41–50 | 7 (23.3%) | 0.004* | 1 (33.3) | 0.203 |
| 51–60 | 3 (10%) | 0 | ||
| 61 and above | 2 (6.7%) | 0 | ||
| Gender | ||||
| Male | 11 (36.7%) | 0 | ||
| Female | 19 (63.3%) | 0.166 | 3 (100%) | 0.500 |
n number of Zika virus seropositive
* Statistically significant difference between age groups (P = 0.004). P is significant at 0.05
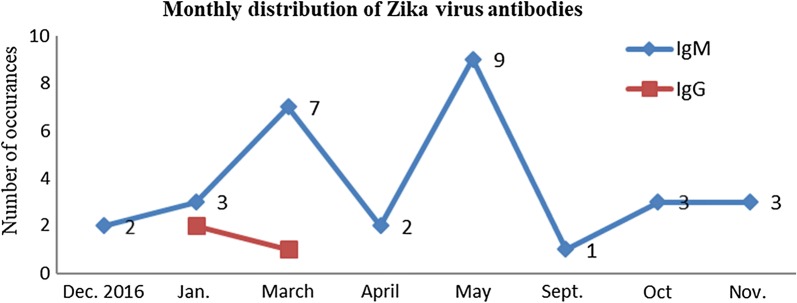
Monthly distribution of Zika virus antibodies
Anti-Zika virus IgM detection peaked in March 7 (23.3%) and May 9 (30%) and lowed in September 1 (3.3%). However, anti-Zika virus IgG antibodies were detected only in January and March 2 (66.7%) and 1 (33.3%) respectively (Fig. 1).
Fig. 1.
Monthly distribution of Zika virus antibodies
Symptoms presented by seropositive febrile patients
Thirty-three (100%) of the seropositive febrile patients were presented with fever. The least 1 (0.8%) symptom reported was conjunctivitis which was also not reported by any seropositive Zika virus female. All other symptoms were reported in both males and females (Table 2).
Table 2.
Symptoms and complications of Zika virus seropositives (n = 33)
| Symptoms | Zika positive cases | Percentage |
|---|---|---|
| Fever | 33 | 100 |
| Diarrhea | 18 | 55 |
| Nausea | 16 | 49 |
| Vomiting | 12 | 36 |
| Loss of appetite | 28 | 85 |
| Muscle pain | 25 | 76 |
| Joint pain | 24 | 73 |
| Conjunctivitis | 2 | 6 |
| Chest pain | 15 | 46 |
| Rapid respiration | 14 | 42 |
| Jaundice | 1 | 3 |
| Recent hearing loss | 1 | 3 |
Discussion
The results from the study indicate exposure levels to Zika virus which suggest the possible circulation of the virus among febrile patients in Ghana with a sero-prevalence of 20.6%. Anti-Zika virus IgM prevalence observed is likely due to the recent (in the year 2016) wave of Zika virus infection dominated headlines in certain parts of the world, especially those with tropical climate. Data presented by this study again suggests noticeable levels of susceptibility to Zika virus infections. This could mean many infected women subjects being at risk of giving birth to babies with defects. Thus, an extended scope to cover infected expectant mothers and diagnose birth defects would have been interesting and will be considered in further studies.
The sero-prevalence of Zika virus antibodies of 20.6% in this study was consistent with study findings from other African countries with a rate from as low as 0.1% in Gabon and Senegal and as high as 38% in Cameroon [13]. These differences could be due to inconsistency in the study participants’ inclusion criteria or diagnostic test used. Zika virus infection was estimated to affect females and males in a ratio of 2:1 which correlate well with several studies [14]. Considering the findings of [14] and that of this study, there may be gender-related differences in Zika virus infection incidence, which might be due to exposure differences [15]. Activeness of females during the early hours of the day exposes greater proportion of females to Zika virus-carrying Aedes spp. of mosquitoes either at work or while travelling to and from work. This may also be attributed to possible differences in who sought medical care following symptomatic Zika virus infection. High prevalence of IgM or IgG in age group 21–30 compared to 61 and above support data from earlier studies by [14] and [16]. Possible explanation to this observation could be the activeness of age group 21–30 during the early hours of the day which exposes them to the bites of Zika virus-carrying Aedes spp. of mosquitoes more frequently than age group 61 years and above. This could also suggest adults manifest with disease less, as they become immune to Zika virus. The proportions of the IgG antibody titers recorded in age group 21–30 and 41–50 may suggest that, Zika virus infection is not endemic in Ghana, rather the virus had been introduced to a non-exposed population as endemicity is attained when the adult infection decreases and only the new entrants into the population are affected more [16]. The significant difference between IgM among the age groups may be due to the pattern of erotic activities and exposure among the age groups. Active sexual activity and exposure begins early, increases simultaneously and declines as one ages. Monthly distribution of Zika virus infection with higher prevalence in the raining season as seen in this study is in harmony with reported pattern of Zika virus transmission [17]. The high frequency of IgM in the month of May and March could be due to the high amount of rainfall which provided temperatures suitable for virus survival, incubation, development and biting of Aedes aegypti and Aedes albopictus [18]. The compact clusters of cases in March and May, and the high prevalence of IgM against Zika virus are consistent with an acute outbreak of Zika virus infection in the population without previous immunity to Zika virus [19]. In this study, commonly reported symptoms of Zika virus infection documented were fever, muscle pain, joint pain and conjunctivitis. Strikingly, conjunctivitis often observed in Zika virus infection was not reported by any seropositive female. Digestive symptoms noted in Zika virus infected patients but rarely observed [20], however, found in this study were nausea, vomiting and diarrhea.
Limitation
The limitation of this study was the absence of plaque neutralization reduction test and PCR for confirmation.
Acknowledgements
We are grateful to the clinicians and paramedical staff of the Public Health Division of Greater Accra Regional Hospital, Ridge for their technical assistance, the administrative support from the head and staff of the Department of Microbiology, University of Ghana, Korle Bu and Department of Virology at Noguchi Memorial Institute for Medical Research.
Abbreviations
- AMED
Japan Agency for Medical Research and Development
- ELISA
enzyme-linked immunosorbent assay
- HRP
horseradish peroxidase
- IgG/M
immunoglobulin G/M
- J-GRID
Japan Initiative for Global Research Network on Infectious Diseases
- NMIMR
Noguchi Memorial Institute for Medical Research
- TBM
3,3′,5,5′-tetramethylbenzidine
- US CDC
United States of America’s Centers for Disease Control and Prevention
Authors’ contributions
Conceived and designed the experiments: TKA, JHKB, GAA; Performed the experiments: GAA, JHKB, DP EEA; Analyzed the data: GAA, JHKB, DP, EEA; Contributed reagents/materials: JHKB; Wrote the paper: GAA, JHKB; Revised and edited manuscript: GAA, JHKB, TKA, DP, EEA. All authors read and approved the final manuscript.
Funding
This work was supported partly by funds from the Japan Initiative for Global Research Network on Infectious Diseases (J-GRID) from the Japan Agency for Medical Research and Development (AMED) and by the Government of Ghana through financial support from the University of Ghana Research Fund. The funders did not contribute to the design of the study and collection, analysis, and interpretation of data and in writing the manuscript. This study was funded by Japan Agency for Medical Research and Development (AMED) and by the Government of Ghana through financial support from the University of Ghana Research Fund (JP18fm0108010).
Availability of data and materials
In this manuscript, the availability of data and material was not applicable.
Ethics approval and consent to participate
The study was approved by both Institutional review Committees of the College of health Sciences, University of Ghana and Noguchi Memorial Institute for Medical Research. A written consent was obtained from all eligible adult patients or guardians of patients less than 18 years old before inclusion. All study participants were assigned with unique identification number throughout the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Godson Aryee Ankrah, Email: godank1@gmail.com.
Joseph Humphrey Kofi Bonney, Phone: +233243568041, Email: Kbonney@noguchi.ug.edu.gh.
Esinam Eudosia Agbosu, Email: EAgbosu@noguchi.ug.edu.gh.
Deborah Pratt, Email: DPratt@noguchi.ug.edu.gh.
Theophilus Korku Adiku, Email: tadiku@uhas.edu.gh.
References
- 1.Section on Clinical Pharmacology and, Therapeutics; Committee on, Drugs. Farrar HC, Sullivan JE. Fever and antipyretic use in children. Pediatrics. 2011;127(3):580–587. doi: 10.1542/peds.2010-3852. [DOI] [PubMed] [Google Scholar]
- 2.Kiekkas P, Aretha D, Bakalis N, Karpouhtsi I, Marneras C, Baltopoulos GI. Fever effects and treatment in critical care: literature review. Aust Crit Care. 2012;26(3):130–135. doi: 10.1016/j.aucc.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Duffy, Chen TH, Hancock WT, Powers AM, Kool JL, Lanciotti RS, Pretrick M, Marfel M, Holzbauer S, Dubray C, Guillaumot L, Griggs A, Bel MA, Lambert AJ, Laven J, Kosoy O, Panella A, Biggersta BJ, Fischer M, Hayes EB. Zika virus outbreak on Yap Island, federated states of Micronesia. N Engl J Med. 2009;360:2536–2543. doi: 10.1056/NEJMoa0805715. [DOI] [PubMed] [Google Scholar]
- 4.Haddow AD, Schuh AJ, Yasuda CY, Kasper MR, Heang V, Huy R. Genetic characterization of Zika virus strains: geographic expansion of the Asian lineage. PLoS Negl Trop Dis. 2012;6:e1477. doi: 10.1371/journal.pntd.0001477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hayes EB. Zika virus outside Africa. Emerg Infect Dis. 2009;15(9):1347–1350. doi: 10.3201/eid1509.090442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calveta GA, Filippis AM, Mendonça MC, Sequeira PC, Siqueira AM, Veloso VG, et al. First detection of autochthonous Zika virus transmission in a HIV-infected patient in Rio de Janeiro, Brazil. J Clin Virol. 2016;74:1–3. doi: 10.1016/j.jcv.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 7.Lanciotti RS, Kosoy OL, Laven JJ, Velez JO, Lambert AJ, Johnson AJ, Stanfield SM, Duffy MR. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis. 2008;14(8):1232–1239. doi: 10.3201/eid1408.080287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brasil Patrícia, Pereira José P., Moreira M. Elisabeth, Ribeiro Nogueira Rita M., Damasceno Luana, Wakimoto Mayumi, Rabello Renata S., Valderramos Stephanie G., Halai Umme-Aiman, Salles Tania S., Zin Andrea A., Horovitz Dafne, Daltro Pedro, Boechat Marcia, Raja Gabaglia Claudia, Carvalho de Sequeira Patrícia, Pilotto José H., Medialdea-Carrera Raquel, Cotrim da Cunha Denise, Abreu de Carvalho Liege M., Pone Marcos, Machado Siqueira André, Calvet Guilherme A., Rodrigues Baião Ana E., Neves Elizabeth S., Nassar de Carvalho Paulo R., Hasue Renata H., Marschik Peter B., Einspieler Christa, Janzen Carla, Cherry James D., Bispo de Filippis Ana M., Nielsen-Saines Karin. Zika Virus Infection in Pregnant Women in Rio de Janeiro. New England Journal of Medicine. 2016;375(24):2321–2334. doi: 10.1056/NEJMoa1602412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grard G, Caron M, Mombo IM, Nkoghe D, MbouiOndo S, Jiolle D, et al. Zika virus in Gabon (Central Africa)—2007: a new threat from Aedes albopictus? PLoS Negl Trop Dis. 2014;8:e2681. doi: 10.1371/journal.pntd.0002681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heang V, Yasuda CY, Sovann L, Haddow AD, Travassosda-Rosa AP, Tesh RB, et al. Zika virus infection, Cambodia, 2010. Emerg Infect Dis. 2012;18:349–351. doi: 10.3201/eid1802.111224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heang V, Yasuda C, Ngan C, et al. Zika virus from fever syndromic surveillance in Cambodia. Am J Trop Med Hyg. 2011;85(6 suppl 1):183. [Google Scholar]
- 12.Safronetz D, Sloan A, Stein DR, Mendoza E, Barairo N, Ranadheera C, Scharikow L, Holloway K, Robinson A, Traykova-Andonova M, Makowski K, Dimitrova K, Giles E, Hiebert J, Mogk R, Beddome S, Drebot M. Evaluation of 5 commercially available Zika virus immunoassays. Emerg Infect Dis. 2017;23(9):1577–1580. doi: 10.3201/eid2309.162043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saiz J-C, Vázquez-Calvo Á, Blázquez AB, Merino-Ramos T, Escribano-Romero E, Martín-Acebes MA. Zika virus: the latest newcomer. Front Microbiol. 2016;7:496. doi: 10.3389/fmicb.2016.00496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lozier Matthew J, Burke Rachel M, Lopez Juan, Acevedo Veronica, Amador Manuel, Read Jennifer S, Jara Amanda, Waterman Stephen H, Barrera Roberto, Muñoz-Jordan Jorge, Rivera-Garcia Brenda, Sharp Tyler M. Differences in Prevalence of Symptomatic Zika Virus Infection, by Age and Sex—Puerto Rico, 2016. The Journal of Infectious Diseases. 2017;217(11):1678–1689. doi: 10.1093/infdis/jix630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaplan JE, et al. Epidemiologic investigations of dengue infection in Mexico. Am J Epidemiol. 1983;117:335–343. doi: 10.1093/oxfordjournals.aje.a113546. [DOI] [PubMed] [Google Scholar]
- 16.Kumar M, Sharma R, Parihar G, Sharma M. Seroprevalence of dengue in central Rajasthan: a study at a tertiary care hospital. Int J Curr Microbiol Appl Sci. ISSN: 2319-7706. 2015;4(9): 933–40. http://www.ijcmas.com.
- 17.Gupta E, Dar L, Kapoor G, Broor S. The changing epidemiology of dengue in Delhi, India. Virol J. 2006;3:92. doi: 10.1186/1743-422X-3-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mordecai E, Cohen J, Evans MV, Gudapati P, Johnson LR, Miazgowicz K. et al. Temperature determines Zika, dengue and chikungunya transmission potential in the Americas. bioRxiv. 2016;63735.
- 19.Mark RD, Tai-Ho C, Thane HW, Ann MP, Jacob LK, Robert SL, Moses P, Maria M, Stacey H, Christine D, Laurent G, Anne G, Martin B, Amy JL, Janeen L, Olga K, Amanda P, Brad JB, Marc F, Edward B. Zika virus outbreak on Yap Island, federated states of Micronesia. N Engl J Med. 2009;360:2536–2543. doi: 10.1056/NEJMoa0805151. [DOI] [PubMed] [Google Scholar]
- 20.Oehler E, Watrin L, Larre P, Leparc-Goffart I, Lastere S, Valour F. Zika virus infection complicated by Guillain–Barre syndrome—case report, French Polynesia. Euro Surveill. 2014;19:20720. doi: 10.2807/1560-7917.ES2014.19.9.20720. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
In this manuscript, the availability of data and material was not applicable.