Abstract
Amyotrophic lateral sclerosis (ALS) is the most common neurodegenerative disease affecting motor neurons (MN). This fatal disease is characterized by progressive muscular atrophy and unfortunately it does not have an effective treatment. Although a small proportion of ALS cases have a familiar origin, the vast majority of them are thought to have a sporadic origin. Although the pathogenesis of ALS has not been fully elucidated, various disorders in different cellular functions such as gene expression, protein metabolism, axonal transport and glial cell disorders have been linked to MN degeneration. Among them, proteostasis is one of the best studied. Retinoids are vitamin A-derived substances that play a crucial role in embryogenesis, development, programmed cell death and other cellular functions. Retinoid agonists behave as transcription factors throughout the activation of the nuclear retinoid receptors.
Several reports in the literature suggest that retinoids are involved in proteostasis regulation, by modulating its two major pathways, the ubiquitin-proteasome system and the autophagy-lysosome response. Additionally, there are some evidences for a role of retinoids themselves, in ALS pathogenesis. In this review, we discuss the importance of proteostasis disruption as a trigger for MN degeneration and the capability of retinoids to modulate it, as well as the potential therapeutic role of retinoids as a new therapy in ALS.
Keywords: Amyotrophic lateral sclerosis, Bexarotene, Neurodegeneration, Retinoids, SOD1
1. Introduction
Amyotrophic lateral sclerosis (ALS) is the most common neurodegenerative disease affecting motor neurons (MNs), with an annual incidence that ranges from 1 to 3 cases per 100,000 individuals [3,31]. Although a small proportion of cases have a familial origin related to mutations in specific genes (C9ORF72, SOD1, etc.), more than 90% of cases are sporadic. ALS typically involves both upper and lower MNs resulting in a well-defined clinical presentation that includes muscular cramps, fasciculations, weakness, amyotrophy and spasticity. Death usually occurs 2–3 years after diagnosis as a consequence of respiratory failure [3].
Although the basis of ALS pathogenesis has not yet been clearly elucidated, our knowledge about disease mechanisms has significantly improved in the last few years. The impairment of various cellular functions and signaling pathways has been related to MN degeneration [15,41,43]. Among them, dysfunction of gene expression, proteostasis, axonal transport and well as the involvement of glial cells surrounding MNs seem to be some of the most prominent mechanisms [43]. Despite considerable effort and investigation of numerous drugs, riluzole, a glutamate antagonist with a very modest effect in survival, is the only drug currently approved for ALS treatment [36]. Therefore, there is a pressing need in the search of new therapeutic approaches. In this context, retinoids appear as potential promising therapeutics for the treatment of ALS [13].
2. Retinoid metabolism
Vitamin A (retinol) and its derivatives (retinoids) play an important role in embryonic development, cellular differentiation, programmed cell death as well as in other vital cellular functions [4,30]. Vitamin A cannot be synthesized endogenously, and is obtained through the diet as retinol, retinil esters, or β-carotenes. The vitamin A metabolites can be stored in the liver and subsequently converted into various retinoid species. Thus, retinol can be irreversible transformed into all-trans retinoid acid (ATRA or RA; commonly known as retinoic acid), principally by retinal dehydrogenase. Retinol has 6 active isoforms including all-trans, 11-cis, 13-cis, 9,13-di-cis, 9-cis and 11, and 13-di-cis retinol. Remarkably ATRA is the most abundant form in the organism [37]. There are different binding proteins involved in the transport of retinoids in serum and other body fluids. The retinol binding protein (RBP) mediates the transport of retinoids from the liver to the target tissues. In these tissues there are a set of tissue-specific proteins, the cellular retinol binding proteins (CRBPs), which facilitate the incorporation of retinoids into the cells [26,48]. Retinoids are able to enter the cell as RA or as retinol molecules. Once within the cell, RA binds to another family of retinol-specific binding proteins, the cellular acid retinoid binding proteins (CRABPs), which are involved in the metabolism and nuclear import of RA. Thus, whereas CRABP-I promotes RA catabolism, CRABP-II facilitates nuclear translocation of RA to interact with retinoid nuclear receptors [6,11].
There are two main families of retinoid receptors, with three different receptor subtypes each one: the RA receptors (RARα RARβ and RAR ) and the retinoid X receptors (RXRα, RXRβ and RXR ). Typically RARs can be activated by both ATRA and 9-cis retinoic acid, while RXR will only be activated by the latter [21]. Nuclear retinoid receptors are ligand activated transcription factors that regulate transcription by binding to DNA at RA response elements (RAREs) located in the promoters and enhancers of their target genes. RXRs can act as self-sufficient homodimers, but primary form functional heterodimers with other type II nuclear receptors. Thus, RXRs can form heterodimers with RARs and other nuclear receptors whose ligands are dietary lipids and their catabolites, including fatty acids (“proliferation peroxisome activated receptor”, PPAR), biliary acids (“farnesoid X receptor”, FXR), and vitamin D (“vitamin D receptor”, VDR) [13]. Importantly, some heterodimeric receptor complexes can be activated by either the RXR ligand or the partner receptor ligand. Based on this dual-ligand regulation two categories of permissive and non-permissive heterodimers have been established. Whereas the former can be activated by ligands of either RXR or its partner, the latter can be only activated by the partner's ligand while the RXR remains inactive [13,50,51]. In this context, permissive heterodimers simultaneously binding ligands to both subunits will elicit a synergic response, exerting a more intense effect than non-permissive heterodimers.
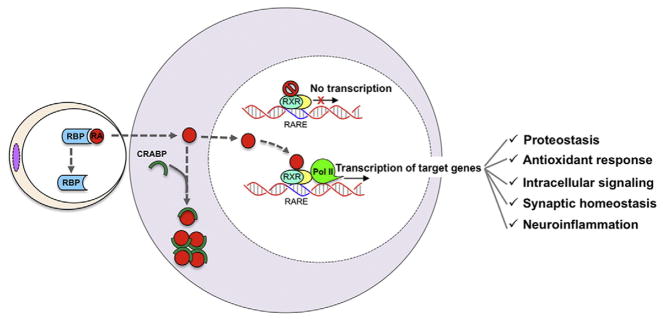
Retinoid agonists bound to RAR or RXR regulate gene transcription by binding to RAREs of target genes positioned in both the promoter and enhancers [19] leading to chromatin remodeling and ultimately to regulation of transcription of their target genes. The interaction of the receptor with regulatory regions of the genes can act to either promote gene transcription upon ligand binding or repressing transcription in the absence of ligand [13] (Fig. 1). Interestingly, these processes are highly dynamic and they must revert once the ligand-induced activation of the receptor has disappeared. Retinoid receptors interact only with those genes that have a RARE. It has been well documented that retinoids act broadly to regulate gene expression since each RXR receptor may comprise 10,000–25,000 interaction sites (cistromes), corresponding to more than 500 different genes [13,45]. Importantly, these receptors have the ability to transrepress gene expression, acting principally on genes involved in the inflammatory response [18].
Fig. 1.
Retinoid metabolism. Retinoid receptors are specifically associated to those genes which have a retinoid acid response element (RARE). This element is usually located at the promoter region. Normally retinoid receptors are inactivated, since they are associated to a group of molecules which act as transcription repressors. Once in the cell, retinoids through the action of different cellular retinoid acid binding proteins (CRABPs) can be stored or delivered to the nucleus activating the retinoid pathways. In the presence of ligand the dimeric nuclear receptor activates and changes its structural conformation, thus liberating from the inhibitor complex and promoting the transcription of its target genes. Many genes are modulated by retinoids. Among them there are genes involved in proteostasis, the antioxidant response, the intracellular signaling, the synaptic homeostasis and the inflammation.
3. The role of retinoids in proteostasis
Alterations in proteostasis, associated with protein aggregation and a dysregulation of lysosomal actions, seem to play a central role in the pathogenesis of ALS [43]. The ubiquitin proteasome system (UPS) and the lysosome-autophagy response constitute the two major cellular pathways for protein degradation. Recent investigations suggest that retinoids could contribute to modulate protein metabolism. In this context, Cheng et al. [8] showed that ATRA/RA treatment had a neuroprotective effect in cultured neuroblastoma cells under conditions of proteasome inhibition. These authors demonstrated that ATRAs allowed cells to escape from programmed cell death induced by UPS inhibition. In that study, the investigators speculated that retinoids may increase cellular tolerance to situations characterized by proteasome inhibition, thus resulting in a delay in the onset of apoptotic mechanisms [8]. Other studies suggest that retinoids also regulate the lysosome-autophagy system. Thus, Anguiano et al. [52] showed that RARα activation inhibited the chaperone-mediated autophagy. In this vein, these authors proposed that synthetic ATRAs could be useful as modulators of autophagic response. Other experimental data indicate that RA induces acidification and maturation of autophagosome structures identified as amphisomes, an essential process in the autophagy [40]. Interestingly, it seems that this mechanism does not involve the nuclear receptor activation, thus acting primarily on the autophagosomes without induce changes in gene transcription [39,40]. This effect agrees with the concept that in addition to the classic genome theory of action, RA also has extranuclear and non-genomic effects. In line with this concept, it has been reported that RA induces the rapid and transient activation of kinase cascades involved in several essential intracellular pathways [2,44].
Regarding the participation of retinoids in the cellular stress response, it is widely accepted that failure of proteostasis leads to increased levels of oxidative stress. Interestingly, in the late 90s, several studies using different experimental models demonstrated that retinoids are implicated in the response to cellular stress by modulating the gene expression of superoxide dismutase 1 (SOD1) [1,47]. Thus, retinoids would also contribute to increase cellular resistance to a variety of cellular stresses, allowing cells to escape from the final common apoptotic response.
4. Retinoids and ALS
As mentioned above, retinoids play a crucial role in cellular differentiation, programmed cell death and other vital cellular functions [4,30]. Regarding nervous system, retinoids seem to be essential in the induction of neural differentiation, motor axon outgrowth and neural patterning. In line with this, an elevated RA signaling in the adult correlates with axon outgrowth and nerve regeneration. RA is also involved in the maintenance of the differentiated state of adult neurons, and it has been reported that disruption of RA signaling in the adult leads to the degeneration of motor MNs [22,32,33]. Retinoids modulate the expression of hundreds of genes, including a large number of neuronal genes [13,29]. Apart from their effects in cellular proteostasis, in the case of ALS, retinoid-regulated neuronal genes might impact on other important cellular processes, such as the antioxidant response (SOD1), neuroinflammation and immune modulation (VEGF, IL2, subunit of NFkβ), cytoskeletal organization (neurofilament L, M, H proteins), ion transport (K+ channel, Ca++ channels), intracellular signaling (phospholipase A2, CREB) and synaptic homeostasis (ChAT, ACh and GABA transporters, NMDA receptor NR1 and kainate receptor GluR6) (Fig. 1) [27,29].
Several recent investigations (Table 1), including genetic, histopathological and experimental studies, support the concept that retinoids play an important role in ALS pathogenesis. Genetic studies have been performed in both ALS patients and animal models of the disease. Among different animal models of ALS, the murine SOD1G93A transgenic mouse model is the most commonly used in basic research. This model, which was created in 1996, is characterized by the overexpression of the human mutated transgene SOD1 [20]. The mutant transgene encodes an aberrant protein that is abnormally folded, which renders it difficult to be cleared by common proteolytic pathways. Consequently, the abnormal protein will tend to aggregate, exerting a cytotoxic effect that may initiate the pathogenetic cascade. Although it has a number of drawbacks and limitations, the SOD1 model is considered a good disease model, because it recapitulates much of the ALS pathology [43]. Thus, mice are healthy at birth, but exhibit histological abnormalities around days 45–50 of life, and neuromuscular manifestations around days 80–85 and finally die by days 125–135 of life.
Table 1.
Studies supporting the role of retinoids in ALS.
| Author | Model | Tissue studied | Main findings |
|---|---|---|---|
| Malaspina [35] | sALS-patients | Spinal cord |
|
| Corcoran [9] | Wild type rats sALS patients |
Spinal cord |
|
| Jiang [23] | sALS-patients | Microdissected MNs/spinal cord |
|
| Jokic [24] | SOD1G93A rats | Spinal cord |
|
| Crochemore [49] | SOD1G93A mice | Spinal cord |
|
| Kolarcik [27] | sALS/fALS-patients | Spinal cord/MN cultures |
|
| Riancho [42] | SOD1G93A mice | Spinal cord |
|
ATRAs (all trans retinoid acid), CRABP1 (cellular retinoid acid binding protein 1), fALS (familiar amyotrophic lateral sclerosis), MN (motor neuron), RAR (retinoic acid receptor), RBP1 (retinol binding protein 1), RXR (retinoid X receptor), and sALS (sporadic amyotrophic lateral sclerosis).
A number of recent genetic studies in ALS patients have documented alterations in some proteins related to the retinoid signaling pathways [23,34,35]. First, Malaspina and co-workers found 14 genes with significant differential expression in the spinal cord of ALS patients and controls. Remarkably, retinol binding protein 1 appeared to be overexpressed almost 3-fold in ALS patients [35]. More recently, Jiang et al. [23] performed a gene expression study in both laser-captured microdissected MNs and the whole spinal cord. Interestingly, gene expression profiles in microdissected MNs demonstrated low expression of both cellular retinoid acid binding protein 1 (CRABP1) and RAR1-γ [23]. On the other hand, gene expression combined with histological studies in the rodent model SOD1G93A have also identified changes in retinoid receptor expression at presymptomatic stages and in more advanced disease [24].
There are few experimental studies with retinoids investigating MN disease. Although one study did not demonstrate a beneficial effect of retinoids, most investigations have reported positive results. Thus, Crochemore et al. [49] performed an experimental study in the SOD1G93A transgenic murine model to evaluate if oral treatment with ATRA/RA (dose of 20–30 mg/kg) had a beneficial effect in the SOD1G93A mice. The authors reported that treatment with retinoids had a negative effect on animal survival without any particular effect on MNs in the spinal cord. By contrast, some years before, Corcoran and his colleagues [9] speculated that a defect in retinoid metabolism could predispose to MN degeneration. On this basis the investigators fed a group of wild type healthy rats with a retinoid free diet. Interestingly, they observed that rats deprived of retinoids developed a similar phenotype to that observed in ALS animal models, consisting of atrophy and muscular weakness mainly in the hind limbs. The histological analysis of the lumbar enlargement of spinal cord revealed that animals fed with a retinoid-free diet had a remarkable neuron loss, with up to 40% loss of anterior horn MNs. These authors then studied a set of patients with sporadic forms of ALS. They found that MNs of ALS patients had a defect in the retinoid receptor subunit RARα and lower levels of the enzyme retinaldehyde dehydrogenase, supporting the idea that disorders in retinoid metabolism could facilitate MN degeneration [9].
More recently, Kolarcik and Bowser [27] have also found marked alterations in retinoid pathways in patients with familiar and sporadic forms of ALS. Interestingly they observed that variations in expression and distribution of several proteins implicated in retinoid metabolism, such as retinoid-binding proteins and retinoid receptor subunits, correlated with changes in MN survival. The authors also described that those MNs overexpressing RARβ were more resistant to cellular apoptosis, suggesting that RARβ activation could mediate a neuroprotective response. To confirm these findings, the authors treated primary MNs cultures with the RARβ agonist adapalene, and demonstrated that cells treated with the drug were more resistant to oxidative-stress induced death. Consistent with this effect, when neuron cultures were pre-treated with the RARβ antagonist LE-135, cell death significantly accelerated [27].
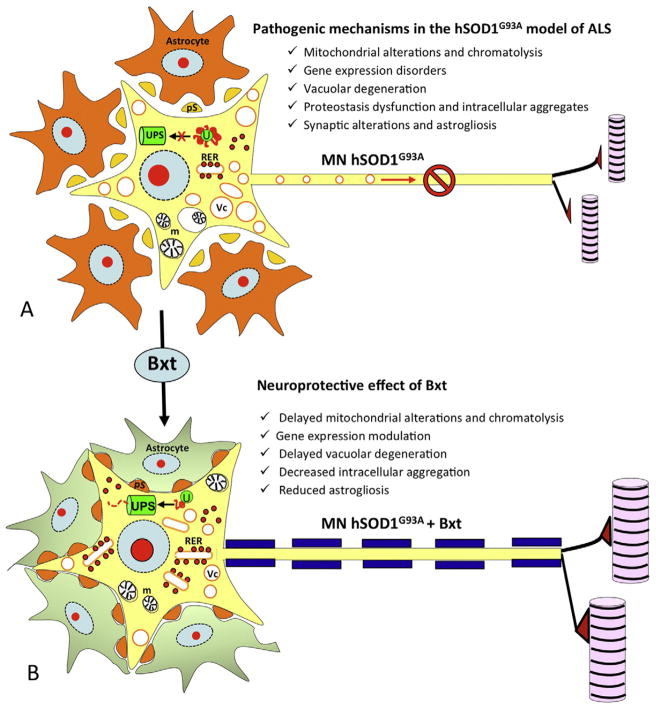
Based upon those studies, we decided to test the potential neuroprotective effect of Bexarotene (Bxt) in the transgenic murine model SOD1G93A. Bxt is a highly selective RXR agonist with a favorable safety profile. Similar to other retinoids, dyslipidemia, hypothyroidism, and cutaneous disorders are the most common adverse reactions in clinical practice [12]. Bxt has been approved by the FDA for the treatment of cutaneous T-cell lymphoma and is currently used as a long-term therapy [14]. This drug had already been tested in a murine model of Alzheimer disease demonstrating a marked reduction in amyloid deposits together significant improvements in functional capabilities. These results have been partially replicated by other groups. [5,10,16,28,46]. In our study, we found that Bxt significantly extended lifespan and delayed neuro-muscular deterioration for more than 10 and 15 days respectively, which represents almost one third of the symptomatic period. Histological studies showed that Bxt acted to preserve MN homeostasis, and ameliorated MN loss at both pre-symptomatic and early symptomatic stages of the disease. Specifically, it reduced the neuronal loss and the chromatolytic response, decreased the formation of ubiquitylated cytoplasmic inclusions, and modulated the lysosomal response. As an RXR agonist, Bxt notably induced the nuclear expression of the RXRα. Bxt also contributed to MN preservation by reducing reactive astrogliosis and preserving perisomatic synapsis [42] (Fig. 2).
Fig. 2.
Neuroprotective effect of retinoids in ALS. This figure illustrates the main mechanisms involved in the pathogenesis of the SOD1G93A murine model of ALS (A). Among them, disorders in gene expression, chromatolysis and mitochondrial alterations (m), vacuolar degeneration (Vc), ubiquitin-proteasome (UPS) system collapse with appearance of intracellular aggregates (U) and astrogliosis with perisomatic synaptic (pS) alterations seem to be some of the most important ones. Our investigations showed that treatment with the retinoid agonist bexarotene, had a beneficial effect at multiple levels (B). This retinoid helped to modulate gene expression, and delayed the protein synthesis machinery disruption and consequently decreased mitochondrial alterations and vacuolar degeneration. Additionally it reduced the intracellular aggregates. Bxt also preserved MN environment until more advanced stages.
Overall, those studies suggest that the activation of RAR and RXR may have a neuroprotective effect in ALS and other neurodegenerative disorders. The relative importance of RARα and RARβ, and RXR remain to be established. Nevertheless, it is worth mentioning that not only RAR–RXR, but also direct and indirect RARα–RARβ interactions may be involved in the regulation of target genes [7,17,25,38].
In short, there is a pressing need for the search of new therapies for ALS patients. Retinoids act as transcription factors modulating the expression of their target genes. To date, both basic and experimental studies in ALS patients and animal models of disease support the concept that these drugs could be useful in ALS treatment. Thus, they provide a rationale for future clinical trials with retinoids in ALS.
Footnotes
Conflict of interest
All authors declare they have no conflict of interest.
References
- 1.Ahlemeyer B, Bauerbach E, Plath M, Steuber M, Heers C, Tegtmeier F, Krieglstein J. Retinoic acid reduces apoptosis and oxidative stress by preservation of SOD protein level. Free Radic Biol Med. 2001;30:1067–1077. doi: 10.1016/s0891-5849(01)00495-6. [DOI] [PubMed] [Google Scholar]
- 2.Al Tanoury Z, Piskunov A, Rochette-Egly C. Vitamin A and retinoid signaling: genomic and nongenomic effects. J Lipid Res. 2013;54:1761–1775. doi: 10.1194/jlr.R030833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amato A, Russell J. Neuromuscular Disorders. McGraw Hill; China: 2008. [Google Scholar]
- 4.Barnard JH, Collings JC, Whiting A, Przyborski SA, Marder TB. Synthetic retinoids: structure–activity relationships. Chemistry. 2009;15:11430–11442. doi: 10.1002/chem.200901952. [DOI] [PubMed] [Google Scholar]
- 5.Boehm-Cagan A, Michaelson DM. Reversal of apoE4-driven brain pathology and behavioral deficits by bexarotene. J Neurosci. 2014;34:7293–7301. doi: 10.1523/JNEUROSCI.5198-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boylan JF, Gudas LJ. The level of CRABP-I expression influences the amounts and types of all-trans-retinoic acid metabolites in F9 teratocarcinoma stem cells. J Biol Chem. 1992;267:21486–21491. [PubMed] [Google Scholar]
- 7.Chen Y, Takeshita A, Ozaki K, Kitano S, Hanazawa S. Transcriptional regulation by transforming growth factor beta of the expression of retinoic acid and retinoid X receptor genes in osteoblastic cells is mediated through AP-1. J Biol Chem. 1996;271:31602–31606. doi: 10.1074/jbc.271.49.31602. [DOI] [PubMed] [Google Scholar]
- 8.Cheng B, Martinez AA, Morado J, Scofield V, Roberts JL, Maffi SK. Retinoic acid protects against proteasome inhibition associated cell death in SH-SY5Y cells via the AKT pathway. Neurochem Int. 2013;62:31–42. doi: 10.1016/j.neuint.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 9.Corcoran J, So PL, Maden M. Absence of retinoids can induce motoneuron disease in the adult rat and a retinoid defect is present in motoneuron disease patients. J Cell Sci. 2002;115:4735–4741. doi: 10.1242/jcs.00169. [DOI] [PubMed] [Google Scholar]
- 10.Cramer PE, Cirrito JR, Wesson DW, Lee CY, Karlo JC, Zinn AE, Casali BT, Restivo JL, Goebel WD, James MJ, Brunden KR, Wilson DA, Landreth GE. ApoE-directed therapeutics rapidly clear beta-amyloid and reverse deficits in AD mouse models. Science. 2012;335:1503–1506. doi: 10.1126/science.1217697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delva L, Bastie JN, Rochette-Egly C, Kraiba R, Balitrand N, Despouy G, Chambon P, Chomienne C. Physical and functional interactions between cellular retinoic acid binding protein II and the retinoic acid-dependent nuclear complex. Mol Cell Biol. 1999;19:7158–7167. doi: 10.1128/mcb.19.10.7158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duvic M, Hymes K, Heald P, Breneman D, Martin AG, Myskowski P, Crowley C, Yocum RC. Bexarotene is effective and safe for treatment of refractory advanced-stage cutaneous T-cell lymphoma: multinational phase II–III trial results. J Clin Oncol. 2001;19:2456–2471. doi: 10.1200/JCO.2001.19.9.2456. [DOI] [PubMed] [Google Scholar]
- 13.Evans RM, Mangelsdorf DJ. Nuclear receptors, RXR, and the big bang. Cell. 2014;157:255–266. doi: 10.1016/j.cell.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farol LT, Hymes KB. Bexarotene: a clinical review. Expert Rev Anticancer Ther. 2004;4:180–188. doi: 10.1586/14737140.4.2.180. [DOI] [PubMed] [Google Scholar]
- 15.Ferraiuolo L, Kirby J, Grierson AJ, Sendtner M, Shaw PJ. Molecular pathways of motor neuron injury in amyotrophic lateral sclerosis. Nat Rev Neurol. 2011;7:616–630. doi: 10.1038/nrneurol.2011.152. [DOI] [PubMed] [Google Scholar]
- 16.Fitz NF, Cronican AA, Lefterov I, Koldamova R. Comment on “ApoE-directed therapeutics rapidly clear beta-amyloid and reverse deficits in AD mouse models”. Science. 2013;340:924. doi: 10.1126/science.1235809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geisen C, Denk C, Gremm B, Baust C, Karger A, Bollag W, Schwarz E. High-level expression of the retinoic acid receptor beta gene in normal cells of the uterine cervix is regulated by the retinoic acid receptor alpha and is abnormally down-regulated in cervical carcinoma cells. Cancer Res. 1997;57:1460–1467. [PubMed] [Google Scholar]
- 18.Glass CK, Saijo K. Nuclear receptor transrepression pathways that regulate inflammation in macrophages and T cells. Nat Rev Immunol. 2010;10:365–376. doi: 10.1038/nri2748. [DOI] [PubMed] [Google Scholar]
- 19.Gosselin D, Glass CK. Epigenomics of macrophages. Immunol Rev. 2014;262:96–112. doi: 10.1111/imr.12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, Caliendo J, Hentati A, Kwon YW, Deng HX. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science. 1994;264:1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- 21.Heyman RA, Mangelsdorf DJ, Dyck JA, Stein RB, Eichele G, Evans RM, Thaller C. 9-cis retinoic acid is a high affinity ligand for the retinoid X receptor. Cell. 1992;68:397–406. doi: 10.1016/0092-8674(92)90479-v. [DOI] [PubMed] [Google Scholar]
- 22.Ji SJ, Zhuang B, Falco C, Schneider A, Schuster-Gossler K, Gossler A, Sockanathan S. Mesodermal and neuronal retinoids regulate the induction and maintenance of limb innervating spinal motor neurons. Dev Biol. 2006;297:249–261. doi: 10.1016/j.ydbio.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 23.Jiang YM, Yamamoto M, Kobayashi Y, Yoshihara T, Liang Y, Terao S, Takeuchi H, Ishigaki S, Katsuno M, Adachi H, Niwa J, Tanaka F, Doyu M, Yoshida M, Hashizume Y, Sobue G. Gene expression profile of spinal motor neurons in sporadic amyotrophic lateral sclerosis. Ann Neurol. 2005;57:236–251. doi: 10.1002/ana.20379. [DOI] [PubMed] [Google Scholar]
- 24.Jokic N, Ling YY, Ward RE, Michael-Titus AT, Priestley JV, Malaspina A. Retinoid receptors in chronic degeneration of the spinal cord: observations in a rat model of amyotrophic lateral sclerosis. J Neurochem. 2007;103:1821–1833. doi: 10.1111/j.1471-4159.2007.04893.x. [DOI] [PubMed] [Google Scholar]
- 25.Kam RK, Deng Y, Chen Y, Zhao H. Retinoic acid synthesis and functions in early embryonic development. Cell Biosci. 2012;2:11. doi: 10.1186/2045-3701-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawaguchi R, Yu J, Honda J, Hu J, Whitelegge J, Ping P, Wiita P, Bok D, Sun H. A membrane receptor for retinol binding protein mediates cellular uptake of vitamin A. Science. 2007;315:820–825. doi: 10.1126/science.1136244. [DOI] [PubMed] [Google Scholar]
- 27.Kolarcik CL, Bowser R. Retinoid signaling alterations in amyotrophic lateral sclerosis. Am J Neurodegener Dis. 2012;1:130–145. [PMC free article] [PubMed] [Google Scholar]
- 28.Landreth GE, Cramer PE, Lakner MM, Cirrito JR, Wesson DW, Brunden KR, Wilson DA. Response to comments on “ApoE-directed therapeutics rapidly clear beta-amyloid and reverse deficits in AD mouse models”. Science. 2013;340:924. doi: 10.1126/science.1234114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lane MA, Bailey SJ. Role of retinoid signalling in the adult brain. Prog Neurobiol. 2005;75:275–293. doi: 10.1016/j.pneurobio.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 30.Lanska DJ. Chapter 29: historical aspects of the major neurological vitamin deficiency disorders: overview and fat-soluble vitamin A. Handb Clin Neurol. 2010;95:435–444. doi: 10.1016/S0072-9752(08)02129-5. [DOI] [PubMed] [Google Scholar]
- 31.Lopez-Vega JM, Calleja J, Combarros O, Polo JM, Berciano J. Motor neuron disease in Cantabria. Acta Neurol Scand. 1988;77:1–5. doi: 10.1111/j.1600-0404.1988.tb06965.x. [DOI] [PubMed] [Google Scholar]
- 32.Maden M. Retinoids and spinal cord development. J Neurobiol. 2006;66:726–738. doi: 10.1002/neu.20248. [DOI] [PubMed] [Google Scholar]
- 33.Maden M. Retinoic acid in the development, regeneration and maintenance of the nervous system. Nat Rev Neurosci. 2007;8:755–765. doi: 10.1038/nrn2212. [DOI] [PubMed] [Google Scholar]
- 34.Malaspina A, de Belleroche J. Spinal cord molecular profiling provides a better understanding of amyotrophic lateral sclerosis pathogenesis. Brain Res Brain Res Rev. 2004;45:213–229. doi: 10.1016/j.brainresrev.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 35.Malaspina A, Kaushik N, de Belleroche J. Differential expression of 14 genes in amyotrophic lateral sclerosis spinal cord detected using gridded cDNA arrays. J Neurochem. 2001;77:132–145. doi: 10.1046/j.1471-4159.2001.t01-1-00231.x. [DOI] [PubMed] [Google Scholar]
- 36.Miller RG, Mitchell JD, Moore DH. Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND) Cochrane Database Syst Rev. 2012;3:CD001447. [Google Scholar]
- 37.Napoli JL. Biochemical pathways of retinoid transport, metabolism and signal transduction. Clin Immunol Immunopathol. 1996;80:S52–S62. doi: 10.1006/clin.1996.0142. [DOI] [PubMed] [Google Scholar]
- 38.Passeri D, Marcucci A, Rizzo G, Billi M, Panigada M, Leonardi L, Tirone F, Grignani F. Btg2 enhances retinoic acid-induced differentiation by modulating histone H4 methylation and acetylation. Mol Cell Biol. 2006;26:5023–5032. doi: 10.1128/MCB.01360-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rajawat Y, Hilioti Z, Bossis I. Autophagy: a target for retinoic acids. Autophagy. 2010;6:1224–1226. doi: 10.4161/auto.6.8.13793. [DOI] [PubMed] [Google Scholar]
- 40.Rajawat Y, Hilioti Z, Bossis I. Retinoic acid induces autophagosome maturation through redistribution of the cation-independent mannose-6-phosphate receptor. Antioxid Redox Signal. 2011;14:2165–2177. doi: 10.1089/ars.2010.3491. [DOI] [PubMed] [Google Scholar]
- 41.Riancho J, Ruiz-Soto M, Villagrá NT, Berciano J, Berciano MT, Lafarga M. Compensatory motor neuron response to chromatolysis in the murine hSOD1 G93A model of amyotrophic lateral sclerosis. Front Cell Neurosci. 2014;8 doi: 10.3389/fncel.2014.00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Riancho J, Ruiz-Soto M, Berciano MT, Berciano J, Lafarga M. Neuroprotective effect of bexarotene in the SOD1G93A mouse model of amyotrophic lateral sclerosis. Front Cell Neurosci. 2015;9:250. doi: 10.3389/fncel.2015.00250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Robberecht W, Philips T. The changing scene of amyotrophic lateral sclerosis. Nat Rev Neurosci. 2013;14:248–264. doi: 10.1038/nrn3430. [DOI] [PubMed] [Google Scholar]
- 44.Rochette-Egly C. Retinoic acid signaling and mouse embryonic stem cell differentiation: cross talk between genomic and non-genomic effects of RA. Biochim Biophys Acta. 2015;1851:66–75. doi: 10.1016/j.bbalip.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 45.Tang Q, Chen Y, Meyer C, Geistlinger T, Lupien M, Wang Q, Liu T, Zhang Y, Brown M, Liu XS. A comprehensive view of nuclear receptor cancer cistromes. Cancer Res. 2011;71:6940–6947. doi: 10.1158/0008-5472.CAN-11-2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tesseur I, Lo AC, Roberfroid A, Dietvorst S, Van Broeck B, Borgers M, Gijsen H, Moechars D, Mercken M, Kemp J, D'Hooge R, De Strooper B. Comment on “ApoE-directed therapeutics rapidly clear beta-amyloid and reverse deficits in AD mouse models”. Science. 2013;340:924. doi: 10.1126/science.1233937. [DOI] [PubMed] [Google Scholar]
- 47.Yoo HY, Chang MS, Rho HM. Induction of the rat Cu/Zn superoxide dismutase gene through the peroxisome proliferator-responsive element by arachidonic acid. Gene. 1999;234:87–91. doi: 10.1016/s0378-1119(99)00176-6. [DOI] [PubMed] [Google Scholar]
- 48.Zanotti G, Berni R. Plasma retinol-binding protein: structure and interactions with retinol, retinoids and transthyretin. Vitam Horm. 2004;69:271–295. doi: 10.1016/S0083-6729(04)69010-8. [DOI] [PubMed] [Google Scholar]
- 49.Crochemore C, Virgili M, Bonamassa B, Canistro D, Pena-Altamira E, Paolini M, Contestabile A. Long-term dietary administration of valproic acid does not affect, while retinoic acid decreases the lifespan of G93A mice a model for amyotrophic lateral sclerosis. Muscle Nerve. 2009;39:548–552. doi: 10.1002/mus.21260. [DOI] [PubMed] [Google Scholar]
- 50.Kurokawa R, Yu VC, Näär A, Kyakumoto S, Han Z, Silverman S, Rosenfeld MG, Glass CK. Differential orientations of the DNA-binding domain and carboxy-terminal dimerization interface regulate binding site selection by nuclear receptor heterodimers. Genes Dev. 1993;7:1423–1435. doi: 10.1101/gad.7.7b.1423. [DOI] [PubMed] [Google Scholar]
- 51.Forman BM, Umesono K, Chen J, Evans RM. Unique response pathways are established by allosteric interactions among nuclear hormone receptors. Cell. 1995;81:541–550. doi: 10.1016/0092-8674(95)90075-6. [DOI] [PubMed] [Google Scholar]
- 52.Anguiano J, Garner TP, Mahalingam M, Das BC, Gavathiotis E, Cuervo AM. Chemical modulation of chaperone-mediated autophagy by retinoic acid derivatives. Nat Chem Biol. 2013;9:374–382. doi: 10.1038/nchembio.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]