Abstract
Previous work has suggested that individual differences in infant functional neuroconnectivity are a potential biomarker for later cognitive and social outcomes, but the mechanisms are unclear. This study investigated a longitudinal model of infant frontotemporal electroencephalogram (EEG) coherence predicting toddler inhibition, which then predicted childhood social responsiveness. A structural equation model showed good fit, with increased right hemisphere frontotemporal EEG coherence predicting less inhibition at age two, which in turn predicted less social responsiveness at age four. These findings support the hypothesis that infant frontotemporal connectivity is indirectly associated with later social behavior, with toddler inhibition as a potential mechanism.
Keywords: inhibition, EEG coherence, social responsiveness, neuroconnectivity, infants, children
Neurodevelopment in infancy involves increases in white matter connections between brain areas, with individual differences in the pattern and strength of these connections (Paus et al., 2001). There is neuroimaging evidence that the pattern of developmental differences in brain connectivity in the first postnatal year can predict outcomes in social and cognitive functioning (Can, Richards, & Kuhl, 2013; Short et al., 2013). The potential of physiological markers to indicate infants who could benefit from early intervention to improve social or cognitive outcomes is an exciting possibility, and neuroconnectivity is a possible biomarker. Our goal was to evaluate the longitudinal effects of early neuroconnectivity on social development and determine if this association is dependent upon toddler cognition.
Connectivity between brain regions is related to measures of typical cognitive and social functioning during infancy. Short distance neuroconnectivity between frontal and central EEG electrodes is positively correlated with language ability at 24 months (Mundy, Fox & Card, 2003) and neuroconnectivity across the brain, including frontal and temporal regions, is associated with joint attention performance at 12 and 24 months, although significant electrode pairs were different between the two ages (Eggebrecht et al., 2017). Additionally, working memory at 8 months is associated with increased frontoparietal connectivity (Bell, 2012), whereas inhibitory control task demands are associated with increased widespread connectivity at 10 months (i.e., frontofrontal, frontoparietal, frontotemporal, frontooccipital; Cuevas, Swingler, Bell, Marcovitch, & Calkins, 2012).
Electroencephalogram (EEG) coherence is a non-invasive methodology for evaluating neuroconnectivity between brain regions and is calculated as the squared cross-correlation of EEG power at two scalp electrodes (Thatcher, Krause, & Hrybyk, 1986). Higher coherence between two electrodes represents coordinated activity of the neurons around those sites, while lower coherence indicates less coordination, thus less connectivity (Thatcher, 1994). Importantly, EEG coherence cannot provide information about white matter integrity or structural connectivity, but rather how electrical activity is coordinated between areas of cortex, representing functional connectivity. This conceptual model has been validated in adults by comparing simultaneously collected EEG coherence with structural MRI measures of connectivity (Srinivasan, Winter, Ding, & Nunez, 2007). Although similar studies have not been done in children, EEG coherence is considered a valid measure of cortical connectivity in infancy as in adulthood. We know a great deal about EEG coherence and early development (e.g. Barry et al., 2004; Thatcher, North, & Biver, 2008). Developmentally, EEG coherence between frontal and non-frontal areas follows a cyclical pattern. Specifically, connectivity decreases in the right hemisphere and increases in the left hemisphere from infancy through early childhood (Thatcher, Walker, & Giudice, 1987).
We focused on EEG coherence between frontal and temporal locations. The frontal brain regions are associated with higher-order thinking, including executive functions (EF; Diamond, 2013; Stuss & Alexander, 2000), whereas temporal regions are important for social behavior and memory (e.g., Squire & Zola-Morgan, 1991; Zahn et al., 2007). Medial prefrontal and superior temporal regions together are considered the “mentalizing” network involved in attributing mental states and advanced social cognition and this network is often altered in neurodevelopmental disorders (Happé & Frith, 2014). For example, quantifiable differences in frontotemporal EEG coherence have been reported in children and adults who have diagnoses that involve atypical social interactions (Duffy & Als, 2012; Murias, Webb, Greenson, & Dawson, 2007). As such, frontotemporal EEG coherence may an indicator of early infant neurological function that sets the stage for later typical cognitive and social development, such as inhibition and social responsiveness.
Inhibition is top-down cognitive processing to control dominant responses (Miyake et al., 2000). Inhibition is closely related to self-regulation and the association is so robust that inhibition and self-regulation are often considered as overlapping constructs (Lehto, Juujärvi, Kooistra, & Pulkkinen, 2003), with inhibition likely being a prerequisite for developing self-regulation (Hofmann, Schmeichel, & Baddeley, 2012). Inhibition, along with other EFs, undergoes rapid development beginning at about 24 months, making this an important age to investigate for early intervention (Carlson, 2005; Petersen, Hoyniak, McQuillan, Bates, & Staples, 2016).
Social responsiveness (SR) is a measure of ability to engage in appropriate social interactions and reciprocity (Gutstein & Whitney, 2002). SR is measurable in infancy, with infants preferring faces that maintain eye contact over faces that look away (Farroni, Csibra, Simion, & Johnson, 2002). SR develops into more complex forms by preschool (Peterson, Slaughter, & Paynter, 2007) and by age four can be measured using parent or teacher report of observed behaviors that disrupt social functioning (Constantino et al., 2003).
Early inhibition is associated with a myriad of concurrent and later social outcomes, including aspects of SR (Diamond, 2013). As children learn to interact with social partners they must use inhibitory control to engage appropriately, for example inhibiting the prepotent response of taking a desirable toy from another child and instead waiting their turn. Preschoolers with better inhibitory control demonstrate better socio-emotional competence (Rhoades, Greenberg, & Domitrovich, 2009) and preschool self-regulation is associated with social competence in first grade (Russell, Lee, Spieker, & Oxford, 2016). Additionally, researchers studying EF, which typically includes aspects of inhibition, report that EF is closely associated with social functioning. For example, Rinsky and Hinshaw (2011) compared childhood EF using a composite including inhibition and adolescent social functioning in girls with and without an attention-deficit/hyperactivity disorder diagnosis. Regardless of psychopathology, childhood EF was associated with adolescent social functioning. In young children with developmental delay, EF deficits are associated with reduced social communication (McEvoy, Rogers, & Pennington, 1993).
Examining the development of inhibition, and EFs in general, has shown that toddler cognition is important for developing social skills. Longitudinally, in a typically developing sample, EF at age two predicted theory of mind performance at three years, despite the two measures being uncorrelated at two years, suggesting a developmental mechanism of early EF affecting social cognition (Carlson, Mandell, & Williams, 2004). Further supporting this finding, Müller and colleagues (2012) reported that EF at two years predicted theory of mind at three years, after accounting for verbal intelligence and age. An EF intervention increased SR in children with autism spectrum disorder more than a comparable social skills training, further elucidating the link between EF and SR (Cannon, Kenworthy, Alexander, Werner, & Anthony, 2011). Diamond and Lee (2011) have argued that early improvement of EF fosters social bonds among children.
Despite relations between toddler inhibition and SR, the neural development underlying the association of these behaviors is not well understood. There is evidence, however, that infant neuroconnectivity is related to later EF. For example, Whedon, Perry, Calkins, and Bell (2016) reported that increases in left frontofrontal EEG coherence from five to ten months was associated with better performance on attentional control task at two years, as well as EF and language tasks at three years. The link between early neuroconnectivity and later social development is less well studied and the patterns appear to vary with electrode pair, hemisphere, and development. For example, cortical connectivity measured by right hemisphere frontocentral EEG coherence at 18 months was positively correlated with vocabulary size at 24, whereas left hemisphere EEG coherence at 14 months was negatively correlated with whether a child was in a high or low vocabulary group (Mundy, Fox, & Card, 2003). Language development is associated with joint attention, is important to the communication aspect of SR, and recruits temporal cortex (Tomasello, 1988). Using similar EEG methodology, Kühn-Popp, Kristen, Paulus, Meinhardt, and Sodian (2016) showed that a higher ratio of short to long left hemisphere EEG coherence at 14 months in typically developing children positively predicted social pointing at 15 months and epistemic language at 48 months, above and beyond child intelligence, suggesting that early neuroconnectivity scaffolds the development of social responsiveness. Additionally, research using fMRI has demonstrated that better white matter integrity of the uncinate fasciculus, a white matter tract which connects frontal and temporal cortices, at six months predicts responding to joint attention at nine months (Elison et al., 2013).
The association between frontotemporal connectivity and social behavior (e.g., Kühn-Popp et al., 2016) and the relation between inhibition and SR (e.g., Cannon et al., 2011; Carlson et al., 2004; Carlson & Moses, 2001) led to our hypothesis that infant frontotemporal EEG coherence would predict toddler inhibition and in turn, higher levels of toddler inhibition would predict greater SR in typically developing children. Given the various findings in the literature showing that the direction of the association between EEG coherence and cognition depends on age, electrode selection, and hemisphere, we did not predict whether this link would be positive or negative or whether it would be specific to one hemisphere. To the best of our knowledge there is no research using neural connectivity to predict both inhibition and social behavior from infancy through early childhood.
Method
This study followed ethical principles of research with human participants, with written informed consent from a parent and assent from the child before data collection began. All procedures in this study were approved by the Institutional Review Board at Virginia Tech.
Participants
One hundred six infants (56 girls; ethnicity: 4.7% Hispanic, 95.3% non-Hispanic; race: 87.7% Caucasian, 1.9% African American, 0.9% Asian, 8.5% multi-racial, 0.9% other) and their mothers were recruited for a longitudinal study investigating cognition-emotion integration in early development. The children in this analysis represent the only cohort of children in the larger longitudinal study (25% of the larger study) for which SR data are available at age four.
The sample was recruited from a rural college town in the Southeast U.S. All children were born at over 36 weeks gestation and weighed more than 6 pounds, with the exception of two infants born at 33 and 35 weeks. Both of these infants only contributed EEG data to the analysis and had baseline coherence values within two standard deviations of the group mean. All parents were high school graduates, with 73% of mothers and 66% of fathers having a college degree. Average maternal and paternal age at birth was 29.62 and 32.95 years (SD = 4.32 and 6.00), respectively, although two parents did not report age. The demographics of the 62 children who returned at 48 months were similar, with 77% of mothers and 69% of fathers having college degrees and an average maternal age of 30.03 and paternal age of 33.34. Additionally, 8% of the final sample identified as Hispanic, 87.1% as White, 1.6% as Black or African-American, 9.7% as multiracial, and 1.6% as other. Families were paid for participation and children were given a small gift at each visit.
Procedures
Infant and mother dyads first came into the laboratory at 5 months. EEG data were not available for five infants, three because of equipment failure, one refused to tolerate the cap, and one who had high levels of artifact. At the 24-month visit, 81 families returned and 13 toddlers refused to complete one of the inhibition tasks, leaving 68 who completed both measures. At the 48-month visit, 62 parents returned the SR questionnaire. There is no visible pattern of missingness in the data and Little’s test on gender, race, and maternal education failed to reject the null, χ2 (6) = 4.732, p =.579, providing evidence that the data may be missing completely at random. This, and the simplicity of the model, warranted the use of full information maximum likelihood estimation (FIML) to use all available data.
5-month EEG coherence.
When parents and infants arrived at the laboratory, procedures were explained, and consent was obtained from the parents. Infants were fitted with a 16 electrode stretch cap (Electro-Cap International, Inc.; Eaton, OH; E1-series cap) and baseline EEG was collected for 1 minute while the infant sat in the parent’s lap and watched the experimenter manipulate a multicolored ball toy approximately 1.1 meters in front of them.
EEG was recorded from 16 electrodes: frontal pole (Fp1, Fp2), medial frontal (F3, F4), lateral frontal (F7, F8), central (C3, C4), temporal (T7, T8), medial parietal (P3, P4), lateral parietal (P7, P8), and occipital (O1, O2), referenced to Cz. After the EEG cap was placed on the head, a small amount of conductive gel was placed into each recording site and the scalp rubbed. Electrode impedances were measured and accepted if they were below 20k. The electrical activity from each lead was amplified using separate Bioamps (James Long Company; Caroga Lake, NY). During data collection, the high-pass filter was a single pole RC filter with a 0.1-Hz cut-off (3 dB or half-power point) and 6 dB per octave roll-off. The low-pass filter was a two-pole Butterworth type with a 100 Hz cut-off (3 dB or half-power point) and 12-dB octave roll-off. Activity for each lead was displayed on the monitor of the acquisition computer. The EEG was digitized online at 512 samples per second for each channel to eliminate the effects of aliasing. The acquisition software was Snapshot-Snapstream (HEM Data Corp.; Southfield, MI) and the raw data were stored for later analyses. Prior to each EEG recording, a 10-Hz 50-uV peak-to-peak sine wave was input through each amplifier. This calibration signal was digitized for 30 s and stored for subsequent analysis. To ensure that EEG data being collected was as clean as possible, a visual inspection of the incoming data from each electrode was performed by a trained experimenter viewing the data on a computer in a control room adjacent to the testing room. This experimenter also viewed the testing session via camera and inserted event marks in the EEG data at the start and finish of the baseline task. These event marks were later used to segment the baseline portion from the ongoing EEG.
EEG data were examined and analyzed using EEG Analysis software developed by James Long Company. Data were first re-referenced via software to an average reference configuration (Lehmann, 1987). The average reference EEG data were artifact scored for eye movements using a peak-to-peak criterion of 100 uV or greater, and for gross motor movements using a peak-to-peak criterion of 200 uV or greater. Segments of EEG data that were scored as containing artifact were eliminated from all subsequent analyses. No artifact correction procedures were used. The data were then analyzed with a discrete Fourier transform (DFT) using a Hanning window of 1-s width and 50% overlap. Across infants, the mean number of DFT windows during the baseline was 84.00 (SD = 22.97), which is approximately 42 seconds of artifact-free EEG data. Power was computed for the 6-9 Hz infant alpha frequency band, which is the dominant frequency for infants and young children (Bell & Fox, 1992, 1994; Marshall, Bar-Haim, & Fox, 2002). Coherence was calculated as the squared cross correlation of power between electrode pairs using an algorithm by Saltzberg, Burton, Burch, Fletcher, and Michaels (1986; equation 9).
Coherence calculations require reliable EEG power values. With children between the ages of 10 and 13 years, 20 seconds of artifact-free resting EEG is sufficient for reliable calculation of EEG power (Gasser, Bacher, & Steinberg, 1985) and EEG coherence (Gasser, Jennen-Steinmetz, & Verleger, 1987). When examining 60 seconds of resting EEG from children between the ages of 8 and 13, Xu and colleagues (Xu et al., under review) report that Cronbach’s alpha for EEG power at various electrodes ranges between .95 and .98. We know of no reports of reliability assessments in infant resting EEG, although it is a common practice for infant EEG researchers to obtain approximately 1 min of baseline recording (e.g., Bell, 2012; Marshall et al., 2002; Orekhova, Stroganova, & Posikera, 2001; Thatcher, Walker, & Giudice, 1987). Although EEG data were collected from other electrode pairs, we chose to only examine F3-T7 and F4-T8, because of our a priori hypothesis based on the existing literature (e.g., Elison et al., 2013). Testing other electrode pairs would have increased the possibility of type I error and resulted in less sound scientific conclusions.
24-month inhibition.
At the 24-month visit toddlers completed two tasks to measure cognitive and behavioral inhibition: tongue task and mommy/me. The tongue task (Cuevas, Hubble, & Bell, 2012; Kochanska, Murray, & Harlan, 2000) is an inhibition task that required the child to hold a goldfish cracker on their tongue for 10, 20, and finally 30 seconds without eating it. Trials were presented with increasing duration to tax inhibitory control and challenge the children if they had been successful during previous trials. Each child received 3 trials. Six children received 4 trials because one of the delays was repeated. The proportion of trials the child managed to not eat the goldfish before time ended was the variable of interest. Intraclass Correlation Coefficient (ICC) was calculated for the coding of 21% of the sample and was .97.
The mommy/me task is a Stroop like cognitive inhibition task that requires young children to maintain two rules in working memory and inhibit a dominant response. It is conceptually similar to the day/night task, the grass/snow task, and other Stroop like tasks used with young children (Carlson, 2005; Cuevas et al., 2012; Gerstadt, Hong, & Diamond, 1994). The experimenter took pictures of the child and mother at the beginning of the appointment. This task asked the children the say “me” when shown a picture of their mother and “mommy” when shown a picture of themselves. The child was given two practice trials and ten task trials in pseudorandom order. Proportion of correct of task trials was the variable of interest. The ICC was calculated for the coding of 21% of the sample and was .99.
48-month social responsiveness.
At the 48-month visit the mother was asked to complete the Social Responsiveness Scale (SRS; Constantino, 2002). The SRS is a 65-item questionnaire made up of five subdomains: Awareness, Social Cognition, Communication, Motivation, and Restrictive and Repetitive Behaviors. Questions focus on how the child engages in social interaction and behaves in social situations. Mothers rated specific and observable aspects of social behavior on a scale from one (not true) to four (almost always true). Thus, a higher score indicates less social responsiveness. SRS scores are continuously distributed in the general population, with an overall mean of 44.35 (SD = 31.5) reported for children 4-7 years old, with an overall score of 101 considered the cutoff for Pervasive Developmental Disorder- Not Otherwise Specified (Constantino, Przybeck, Friesen, & Todd, 2000). The standardized t-scores for each of the subdomains were the variable of interest. For our sample, the SRS total score had an internal consistency of α = .87.
Results
Descriptive statistics
Means, standard deviations, and normality statistics are in Table 1. The right hemisphere EEG coherence values exhibited kurtosis and were slightly positively skewed. Infants with particularly high right hemisphere values were investigated to ensure that their parents did not report left-hand dominance, which none did; therefore no corrective action was taken. Outliers on all variables were defined as those with scores more than three standard deviations away from the mean. All outliers were investigated to ensure that the scores were not data entry errors and then were included in all further analyses. Independent sample t-tests for variable of interest showed no differences between boys and girls, except for the communication subscale of the SRS, with mothers of boys reporting lower communication; t (60) = 2.081, p = .043 (boys mean = 47.515, SD = 5.032; girls mean = 44.479, SD = 5.396). The SRS subscale t-scores of our sample ranged from 36 to 70, meaning that none of the children in the study reached criteria for diagnoses, but showed substantial variability in score.
Table 1.
Correlations and Descriptive Statistics for all Study Variables
| F3-T7 | F4-T8 | MM | TT | SRS-A | SRS-SC | SRS-C | SRS-M | SRS-RRB | |
|---|---|---|---|---|---|---|---|---|---|
| 5 m F3-T7 | - | ||||||||
| 5 m F4-T8 | .081 | - | |||||||
| 24 m MM | −.153 | −.222* | - | ||||||
| 24 m TT | −.062 | −.142 | .328* | - | |||||
| 48 m SRS-A | −.078 | .213 | −.317* | −.278 | - | ||||
| 48 m SRS-SC | .056 | .203 | −.237 | −.515** | .373** | - | |||
| 48 m SRS-C | −.004 | .309* | −.179 | −.327* | .429** | .667** | - | ||
| 48 m SRS-M | .105 | .216 | −.164 | −.161 | .305* | .389** | .602** | - | |
| 48 m SRS-RRB | −.044 | .236 | −1.30 | −.358* | .399** | .570** | .665** | .490** | - |
| Means (SD) | 0.122 (0.076) | 0.118 (0.071) | 2.425 (0.848) | 0.559 (0.403) | 49.839 (6.778) | 46.274 (5.579) | 46.226 (5.345) | 47.694 (7.224) | 48.742 (6.213) |
| Skewness | 0.915 | 1.368 | −0.928 | −0.215 | −0.169 | 0.260 | 0.522 | 0.894 | 0.544 |
| Kurtosis | 0.970 | 3.766 | 0.112 | −1.505 | −0.581 | −0.606 | 0.375 | 0.677 | −0.402 |
Note.
p<.05,
p<.01
F3-T7: EEG coherence between F3 and T7; F4-T8: EEG coherence between F7 and T8; TT: Tongue Task; MM: Mommy/me; SRS-A: Social Responsiveness Scale - Awareness; SRS-SC: Social Cognition; SRS-C: Communication; SRS-M: Motivation; SRS-RRB: Restrictive and Repetitive Behaviors.
Model
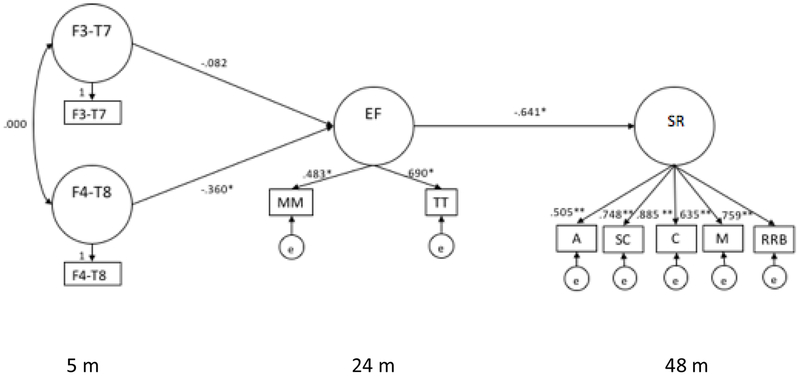
Structural equation modeling (SEM) was used to test our hypothesized longitudinal model. IBM SPSS 23.0.0 was used to calculate correlations and outliers and Amos 23.0.0 was used for the SEM analysis (see Table 2 and Figure 1). The measurement model, χ2 (13, N=106) = 13.046, p =.444; RMSEA = 0.006; ECVI = 0.543; and CFI = 1.000, indicated that both inhibition tasks loaded onto the inhibition construct: mommy/me (β = .483) and tongue task (β = .690). The five subscales of the SRS also loaded onto the SR (i.e., social behavior) construct: awareness (β = .505); social cognition (β = .748); communication (β = .885); motivation (β = 635); and restrictive and repetitive behaviors (β = .759).
Table 2.
Standardized and Unstandardized Path Coefficients for Full Structural Equation Model
| Path | β | B | SE | R2 |
|---|---|---|---|---|
| 5 m F3-T7 → 24 m Inhibition | −.082 | −0.445 | 0.770 | |
| 5 m F4-T8 → 24 m Inhibition | −.360 | −2.083* | 0.998 | |
| Inhibition | 0.142 | |||
| 24 m Inhibition → 48 m Social Responsiveness | −.641 | −7.182* | 3.088 | |
| Social Responsiveness | 0.411 |
Note.
p < .05.
Social Responsiveness = SRS score, with lower scores indicating higher levels of social responsiveness
Figure 1. Full Structural Model with Standardized Values.
χ2 (25, N=106) = 20.316, p =.730; RMSEA = 0.000; ECVI = 0.746; and CFI = 0.996 F3-T7: EEG coherence between F3 and T7; F4-T8: EEG coherence between F7 and T8; TT: Tongue Task; MM: Mommy/me; A: Awareness; SC: Social Cognition; C: Communication; M: Motivation; RRB: Restrictive and Repetitive Behaviors; I: Inhibition; SR: Social Responsiveness (i.e., SRS score). For SRS, lower scores indicate greater social responsiveness
*p<.05, **p<.001
The full structural model fit well, χ2 (25, N=106) = 20.316, p =.730; RMSEA = 0.000; ECVI = 0.746; and CFI = 0.996. The structural path from right frontotemporal coherence at 5-months (β = −.360) to inhibition at 24 months was significant at p < .05. The path from inhibition at 24 months to SR at 48 months (β = −.641) was significant at p < .05. The path between left frontotemporal connectivity (β = −.082) to inhibition was not significant. There was an indirect effect from right frontotemporal EEG coherence to 48 month SR (β = .231, p < .05). Overall, the model explained 14% of the variance in inhibition at 24 months and 41% of the variance in SR at 48 months.
Discussion
Our findings support a model of infant neuroconnectivity predicting SR in early childhood, through toddler inhibition. The pattern of significant beta weights shows that lower levels of right hemisphere infant neuroconnectivity between frontal and temporal regions is associated with higher toddler inhibition, which in turn predicts better SR (i.e., lower SRS scores and thus a negative beta weight) at age four.
Both the mommy/me and tongue tasks require the child to inhibit a prepotent response. This inhibition of stereotypical and dominant responses may be a prerequisite for the development of social responsiveness (Diamond, 2013). This supports previous research that inhibition relates to the development of social behavior (Alduncin, Huffman, Feldman, & Loe, 2014; Rints, McAuley, & Nilsen, 2015; Wolfe, Vannatta, Nelin, & Yeates, 2015), but to our knowledge is the first to specifically show that toddler inhibition predicts preschool social responsiveness. Our finding suggests that inhibitory control in toddlerhood acts as a foundation for later social responsiveness. If children do not develop the necessary inhibitory abilities as toddlers, their ability to develop and maintain social relationships in early childhood is negatively impacted. In the future, inhibitory control could act as a marker for children who might benefit from interventions to improve social skills (Kenworthy et al., 2014).
It may be, however, that children who had poorer inhibition at 24 months also had poorer social skills at that same age, meaning that inhibitory control is not a prerequisite but perhaps a comorbid symptom of children whose parents report less SR. Because the SRS questionnaire can only be administered beginning at 48 months, it is difficult to test this possibility. Future research could use an alternative measure of SR, perhaps a joint attention scale that is appropriate for use with toddlers (e.g., Mundy et al., 2007). The use of the SRS is a strength of our model, however, due to the relative ease of administration and the validity of the instrument.
We did not hypothesize a direct path from infant EEG frontotemporal coherence to SR, but we hypothesized and found indirect effects through inhibition. The indirect effect from infant neuroconnectivity to SR demonstrates that infant neurobiology impacts SR through inhibition, providing a mechanism for the development of this important skill. Our findings may inform the research literature showing infant functional hyperconnectivity in children who later develop autism spectrum disorder (ASD; Kana, Uddin, Kenet, Chugani, & Müller, 2014; Orekhova et al., 2014; Wolff et al., 2012), as well as higher functional connectivity correlating with increased social impairment in children with ASD (Supekar et al., 2013). Most importantly, our findings suggest that even in a typically developing sample, the level of right frontotemporal connectivity is related to social functioning through the development of inhibition. This indicates not only that early neural differences have an effect on the development of social functioning, but that these differences are evident in toddlerhood inhibition, providing a mechanism and timeframe for intervention.
The laterality of this neuroconnectivity effect was not hypothesized, but may be explained by the maturation patterns of the cortex. Research on cortical maturation has shown that, in general, the right cortex matures at an accelerated pace compared to homologous structures on the left (Chiron et al., 1997; Thatcher et al., 1987). Thus, it may be that children with more right frontotemporal connectivity in infancy have a disruption in their cortical maturational pattern which affects the development of inhibition. There is no consensus regarding hemisphere effects of white matter connectivity in patterns of atypical development, such as ASD. For example, in an MRI study investigating structural connectivity in toddlers, Bashat and colleagues (2007) reported more left hemisphere white matter in toddlers with ASD compared to controls. Using similar methods, Wolff and colleagues (2012) reported bilateral differences in association tracts between the group that developed ASD and controls. This example of lack of consistency in these studies may be due to grouping together participants of different ages and comparing different developmental stages, as well as different methodologies and conceptualizations of neuroconnectivity. Thus, the association between infant right frontotemporal functional connectivity and toddler inhibition we have described may only apply to the EEG coherence patterns of typically developing children within our relatively tight age range of 5 months.
The mechanism by which variability in infant neuroconnectivity translates into toddler inhibition and then preschool SR is unclear. These differences in brain function may be the result of genetic variation predisposing certain children to frontotemporal neuroconnectivity patterns and later outcomes. Alternatively, the variability could be the result of early environmental exposure, which could affect the infant’s processing of social information and brain function. Future work will be able to parse out these possibilities, but speculatively, connectivity between frontal and temporal regions allows for the prefrontal cortex to exert top-down control over regions of cortex associated with various social behaviors. Perhaps, if this functional connectivity is appropriately pruned in early infancy, children are able to develop suitable inhibition as toddlers and greater SR as preschoolers. Alternatively, if this functional connectivity is overactive in early infancy, then children develop less inhibition as toddlers and less SR as preschoolers. Further work on the genetics and environmental influences that affect neuroconnectivity is warranted to investigate this potential mechanism.
Although our findings support a model of infant neuroconnectivity predicting toddler inhibition, which in turn predicts SR in early childhood, our work is not without shortcomings. A limitation of our study is the amount of missing data, as 62 children had complete data. However, using FIML allowed for us to use all of the available data in order to obtain parameter estimates, compared to using list or pairwise deletion which can lead to biased estimates (McDonald & Ho, 2002). Despite the unavoidable attrition of a longitudinal study, our findings are robust and the simplicity of the model allows us to make some generalizations. As discussed above, an additional limitation of our findings is the lack of a measure of SR at 24 months, which would have allowed for a crosslagged design. Thus, we cannot be certain that the effect of inhibition on SR is unidirectional, although we do show temporal precedence of inhibition.
It remains to be tested if our model can be replicated with other typically developing samples. Other future directions include investigation of other ages at which EEG coherence as neuroconnectivity predicts later inhibition and SR. We included inhibition in toddlerhood as part of our model because it is associated with social behavior and at age two is about to undergo a dramatic shift in development, making it an excellent opportunity for intervention (Petersen et al., 2016). Inhibition at age three should likewise be examined, as this would capture inhibition in the midst of developmental changes. In sum, these direct and indirect effects of neuroconnectivity provide evidence that infant frontotemporal neural activity is associated with a cascade of effects that predict both cognitive and social outcomes in early childhood.
Acknowledgments
This research was supported by grants R01 HD049878 and R03 HD043057 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development awarded to the third author. The authors thank the children and their families who participated in this study.
Footnotes
The authors declare no conflicts of interest.
Contributor Information
Alleyne P.R. Broomell, Department of Psychology, Virginia Tech
Jyoti Savla, Center for Gerontology; Department of Human Development and Family Science, Virginia Tech.
Martha Ann Bell, Department of Psychology, Virginia Tech.
References
- Alduncin N, Huffman LC, Feldman HM, & Loe IM (2014). Executive function is associated with social competence in preschool-aged children born preterm or full term. Early Human Development, 90(6), 299–306. 10.1016/j.earlhumdev.2014.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry RJ, Clarke AR, McCarthy R, Selikowitz M, Johnstone SJ, & Rushby JA (2004). Age and gender effects in EEG coherence: I. Developmental trends in normal children. Clinical Neurophysiology, 115(10), 2252–2258. [DOI] [PubMed] [Google Scholar]
- Bell MA, & Fox NA (1992). The relations between frontal brain electrical activity and cognitive development during infancy. Child Development, 63(5), 1142–1163. [PubMed] [Google Scholar]
- Bell MA, & Fox NA (1994). Brain development over the first year of life: Relations between electroencephalographic frequency and coherence and cognitive and affective behaviors In Dawson G & Fischer KW (Eds.), Human behavior and the developing brain (pp. 314–345). New York, NY, US: Guilford Press. [Google Scholar]
- Ben Bashat D, Kronfeld-Duenias V, Zachor DA, Ekstein PM, Hendler T, Tarrasch R… Ben Sira L (2007). Accelerated maturation of white matter in young children with autism: A high b value DWI study. NeuroImage, 37(1), 40–47. 10.1016/j.neuroimage.2007.04.060 [DOI] [PubMed] [Google Scholar]
- Can Dilara D., Richards T, & Kuhl PK (2013). Early gray-matter and white-matter concentration in infancy predict later language skills: A whole brain voxel-based morphometry study. Brain and Language, 124(1), 34–44. 10.1016/j.bandl.2012.10.00 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon L, Kenworthy L, Alexander KC, Werner MA, & Anthony L (2011). Unstuck and on Target! An Executive Function Curriculum to Improve Flexibility for Children with Autism Spectrum Disorders. Research Edition. Brookes Publishing Company. [Google Scholar]
- Carlson SM (2005). Developmentally sensitive measures of executive function in preschool children. Developmental Neuropsychology, 28(2), 595–616. 10.1207/s15326942dn2802_3 [DOI] [PubMed] [Google Scholar]
- Carlson SM, Mandell DJ, & Williams L (2004). Executive Function and Theory of Mind: Stability and Prediction From Ages 2 to 3. Developmental Psychology, 40(6), 1105 10.1037/0012-1649.40.6.1105 [DOI] [PubMed] [Google Scholar]
- Carlson SM, & Moses LJ (2001). Individual Differences in Inhibitory Control and Children’s Theory of Mind. Child Development, 72(4), 1032–1053. 10.1111/1467-8624.00333 [DOI] [PubMed] [Google Scholar]
- Chiron C, Jambaque I, Nabbout R, Lounes R, Syrota A, & Dulac O (1997). The right brain hemisphere is dominant in human infants. Brain, 120(6), 1057–1065. 10.1093/brain/120.6.1057 [DOI] [PubMed] [Google Scholar]
- Constantino JN, Przybeck T, Friesen D, & Todd RD (2000). Reciprocal social behavior in children with and without pervasive developmental disorders. Journal of Developmental and Behavioral Pediatrics: JDBP, 21(1), 2–11. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Davis SA, Todd RD, Schindler MK, Gross MM, Brophy SL, … Reich W (2003). Validation of a Brief Quantitative Measure of Autistic Traits: Comparison of the Social Responsiveness Scale with the Autism Diagnostic Interview-Revised. Journal of Autism and Developmental Disorders, 33(4), 427–433. 10.1023/A:1025014929212 [DOI] [PubMed] [Google Scholar]
- Cuevas K, Hubble M, & Bell MA (2012). Early Childhood Predictors of Post-Kindergarten Executive Function: Behavior, Parent Report, and Psychophysiology. Early Education and Development, 23(1), 59–73. 10.1080/10409289.2011.611441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A (2013). Executive functions. Annual Review of Psychology, 64, 135–168. 10.1146/annurev-psych-113011-143750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A, & Lee K (2011). Interventions shown to Aid Executive Function Development in Children 4-12 Years Old. Science (New York, N.Y.), 333(6045), 959–964. 10.1126/science.1204529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy FH, & Als H (2012). A stable pattern of EEG spectral coherence distinguishes children with autism from neuro-typical controls - a large case control study. BMC Medicine, 10, 64 10.1186/1741-7015-10-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggebrecht AT, Elison JT, Feczko E, Todorov A, Wolff JJ, Kandala S, … Pruett JR (2017). Joint Attention and Brain Functional Connectivity in Infants and Toddlers. Cerebral Cortex, 27(3), 1709–1720. 10.1093/cercor/bhw403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elison JT, Wolff JJ, Heimer DC, Paterson SJ, Gu H, Hazlett HC, … for the IBIS Network. (2013). Frontolimbic neural circuitry at 6 months predicts individual differences in joint attention at 9 months. Developmental Science, 16(2), 186–197. 10.1111/desc.12015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farroni T, Csibra G, Simion F, & Johnson MH (2002). Eye contact detection in humans from birth. Proceedings of the National Academy of Sciences, 99(14), 9602–9605. 10.1073/pnas.152159999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstadt CL, Hong YJ, & Diamond A (1994). The relationship between cognition and action: performance of children 312-7 years old on a stroop- like day-night test. Cognition, 53(2), 129–153. 10.1016/0010-0277(94)90068-X [DOI] [PubMed] [Google Scholar]
- Gutstein SE, & Whitney T (2002). Asperger Syndrome and the Development of Social Competence. Focus on Autism and Other Developmental Disabilities, 17(3), 161–171. 10.1177/10883576020170030601 [DOI] [Google Scholar]
- Happe F, & Frith U (2014). Annual research review: Towards a developmental neuroscience of atypical social cognition. Journal of Child Psychology and Psychiatry, 55(6), 553–577. [DOI] [PubMed] [Google Scholar]
- Hofmann W, Schmeichel BJ, & Baddeley AD (2012). Executive functions and self-regulation. Trends in Cognitive Sciences, 16(3), 174–180. 10.1016/j.tics.2012.01.006 [DOI] [PubMed] [Google Scholar]
- Kenworthy L, Anthony LG, Naiman DQ, Cannon L, Wills MC, Luong-Tran C, … Wallace GL (2014). Randomized controlled effectiveness trial of executive function intervention for children on the autism spectrum. Journal of Child Psychology and Psychiatry, 55(4), 374–383. 10.1111/jcpp.12161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochanska G, Murray KT, & Harlan ET (2000). Effortful control in early childhood: Continuity and change, antecedents, and implications for social development. Developmental Psychology, 36(2), 220–232. 10.1037/0012-1649.36.2.220 [DOI] [PubMed] [Google Scholar]
- Kuhn-Popp N, Kristen S, Paulus M, Meinhardt J, & Sodian B (2016). Left hemisphere EEG coherence in infancy predicts infant declarative pointing and preschool epistemic language. Social Neuroscience, 11(1), 49–59. 10.1080/17470919.2015.1024887 [DOI] [PubMed] [Google Scholar]
- Lehto JE, Juujärvi P, Kooistra L, & Pulkkinen L (2003). Dimensions of executive functioning: Evidence from children. British Journal of Developmental Psychology, 21(1), 59–80. 10.1348/026151003321164627 [DOI] [Google Scholar]
- Marshall PJ, Bar-Haim Y, & Fox NA (2002). Development of the EEG from 5 months to 4 years of age. Clinical Neurophysiology, 113(8), 1199–1208. 10.1016/S1388-2457(02)00163-3 [DOI] [PubMed] [Google Scholar]
- McDonald RP, & Ho M-HR (2002). Principles and practice in reporting structural equation analyses. Psychological Methods, 7(1), 64–82. [DOI] [PubMed] [Google Scholar]
- McEvoy RE, Rogers SJ, & Pennington BF (1993). Executive Function and Social Communication Deficits in Young Autistic Children. Journal of Child Psychology and Psychiatry, 34(4), 563–578. 10.1111/j.1469-7610.1993.tb01036.x [DOI] [PubMed] [Google Scholar]
- Müller U, Liebermann-Finestone DP, Carpendale JIM, Hammond SI, & Bibok MB (2012). Knowing minds, controlling actions: The developmental relations between theory of mind and executive function from 2 to 4 years of age. Journal of Experimental Child Psychology, 111(2), 331–348. 10.1016/j.jecp.2011.08.014 [DOI] [PubMed] [Google Scholar]
- Mundy P, Block J, Delgado C, Pomares Y, Van Hecke AV, & Parlade MV (2007). Individual Differences and the Development of Joint Attention in Infancy. Child Development, 78(3), 938–954. 10.1111/j.1467-8624.2007.01042.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy P, Fox N, & Card J (2003). EEG coherence, joint attention and language development in the second year. Developmental Science, 6(1), 48–54. 10.1111/1467-7687.00253 [DOI] [Google Scholar]
- Murias M, Webb SJ, Greenson J, & Dawson G (2007). Resting State Cortical Connectivity Reflected in EEG Coherence in Individuals With Autism. Biological Psychiatry, 62(3), 270–273. 10.1016/j.biopsych.2006.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T, Collins DL, Evans AC, Leonard G, Pike B, & Zijdenbos A (2001). Maturation of white matter in the human brain: a review of magnetic resonance studies. Brain Research Bulletin, 54(3), 255–266. 10.1016/S0361-9230(00)00434-2 [DOI] [PubMed] [Google Scholar]
- Petersen IT, Hoyniak CP, McQuillan ME, Bates JE, & Staples AD (2016). Measuring the development of inhibitory control: The challenge of heterotypic continuity. Developmental Review, 40, 25–71. 10.1016/j.dr.2016.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson CC, Slaughter VP, & Paynter J (2007). Social maturity and theory of mind in typically developing children and those on the autism spectrum. Journal of Child Psychology and Psychiatry, 48(12), 1243–1250. 10.1111/j.1469-7610.2007.01810.x [DOI] [PubMed] [Google Scholar]
- Rinsky JR, & Hinshaw SP (2011). Linkages Between Childhood Executive Functioning and Adolescent Social Functioning and Psychopathology in Girls with ADHD. Child Neuropsychology : A Journal on Normal and Abnormal Development in Childhood and Adolescence, 17(4), 368–390. 10.1080/09297049.2010.544649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rints A, McAuley T, & Nilsen ES (2015). Social Communication Is Predicted by Inhibitory Ability and ADHD Traits in Preschool-Aged Children: A Mediation Model. Journal of Attention Disorders, 19(10), 901–911. 10.1177/1087054714558873 [DOI] [PubMed] [Google Scholar]
- Ross P, & Segalowitz SJ (2000). An EEG coherence test of the frontal dorsal versus ventral hypothesis in N-back working memory. Brain and Cognition, 43(1-3), 375–379. [PubMed] [Google Scholar]
- Russell BS, Lee JO, Spieker S, & Oxford ML (2016). Parenting and Preschool Self-Regulation as Predictors of Social Emotional Competence in 1st Grade. Journal of Research in Childhood Education, 30(2), 153–169. 10.1080/02568543.2016.1143414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltzbertg B, Burton WD, Burch NR, Fletcher J, & Michaels R (1986). Electrophysiological measures of regional neural interactive coupling. Linear and non-linear dependence relationships among multiple channel electroencephalographic recordings. International Journal of Bio-Medical Computing, 18(2), 77–87. 10.1016/0020-7101(86)90050-4 [DOI] [PubMed] [Google Scholar]
- Short SJ, Elison JT, Goldman BD, Styner M, Gu H, Connelly M, … Gilmore JH (2013). Associations between white matter microstructure and infants’ working memory. NeuroImage, 64, 156–166. 10.1016/j.neuroimage.2012.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, & Zola-Morgan S (1991). The Medial Temporal Lobe Memory System. Science; Washington, 253(5026), 1380. [DOI] [PubMed] [Google Scholar]
- Srinivasan R, Winter WR, Ding J, & Nunez PL (2007). EEG and MEG coherence: Measures of functional connectivity at distinct spatial scales of neocortical dynamics. Journal of Neuroscience Methods, 166(1), 41–52. 10.1016/j.jneumeth.2007.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuss DT, & Alexander MP (2000). Executive functions and the frontal lobes: a conceptual view. Psychological Research, 63(3-4), 289–298. 10.1007/s004269900007 [DOI] [PubMed] [Google Scholar]
- Thatcher RW, Krause PJ, & Hrybyk M (1986). Cortico-cortical associations and EEG coherence: A two-compartmental model. Electroencephalography and Clinical Neurophysiology, 64(2), 123–143. 10.1016/0013-4694(86)90107-0 [DOI] [PubMed] [Google Scholar]
- Thatcher RW, Walker RA, & Giudice S (1987). Human Cerebral Hemispheres Develop at Different Rates and Ages. Science, 236(4805), 1110–1113. [DOI] [PubMed] [Google Scholar]
- Thatcher Robert W. (1994). Cyclic cortical reorganization: Origins of human cognitive development In Dawson G & Fischer KW (Eds.), Human behavior and the developing brain (pp. 232–266). New York, NY, US: Guilford Press. [Google Scholar]
- Thatcher RW, North DM, & Biver CJ (2008). Development of cortical connections as measured by EEG coherence and phase delays. Human brain mapping, 29(12), 1400–1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasello M (1988). The role of joint attentional processes in early language development. Language Sciences, 10(1), 69–88. 10.1016/0388-0001(88)90006-X [DOI] [Google Scholar]
- Whedon M, Perry NB, Calkins SD, & Bell MA (2016). Changes in frontal EEG coherence across infancy predict cognitive abilities at age 3: The mediating role of attentional control. Developmental Psychology, 52(9), 1341–1352. 10.1037/dev0000149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe KR, Vannatta K, Nelin MA, & Yeates KO (2015). Executive functions, social information processing, and social adjustment in young children born with very low birth weight. Child Neuropsychology, 21(1), 41–54. 10.1080/09297049.2013.866217 [DOI] [PubMed] [Google Scholar]
- Wolff JJ, Gu H, Gerig G, Elison JT, Styner M, Gouttard S, … Piven J (2012). Differences in White Matter Fiber Tract Development Present From 6 to 24 Months in Infants With Autism. American Journal of Psychiatry, 169(6), 589–600. 10.1176/appi.ajp.2011.11091447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn R, Moll J, Krueger F, Huey ED, Garrido G, & Grafman J (2007). Social concepts are represented in the superior anterior temporal cortex. Proceedings of the National Academy of Sciences, 104(15), 6430–6435. 10.1073/pnas.0607061104 [DOI] [PMC free article] [PubMed] [Google Scholar]