Abstract
Background
The aim of this study was to determine whether poor collaterals contribute to the occurrence of certain types of cognitive disorders in asymptomatic middle cerebral artery stenosis (MCAS).
Material/Methods
Patients aged ≥45 years with asymptomatic MCAS confirmed by computed tomography angiography were included in a single-center retrospective study. They did not have prior stroke or dementia. Within 7 days of admission, MRI and comprehensive neuropsychological assessment were performed. Cognitive assessment was conducted after 2 years. Two independent neuroradiologists evaluated intracranial collaterals by using the Miteff scale. Demographic date and Fazekas scores were collected.
Results
A total of 173 patients with asymptomatic MCAS (66% men, mean age 59.4 years) and 42 controls (45% men, mean age 61.4 years) were enrolled. Executive function, attention, and information-processing speed in poor collateral circulation patients were more frequently and more often impaired than those in good collateral circulation patients. Throughout the study period, patients with poor collateral circulation had worse executive function, attention, and information-processing speed than those with moderate collateral circulation. Over time, MCAS patients with good collateral circulation did not show an association with cognitive function.
Conclusions
MCAS patients with moderate and poor collateral circulation have impairment of ≥1 cognitive domain over time. The affected domains are consistent with the profile of vascular cognitive impairment. Good collateral circulation is more important in patients with MCAS, and is associated with less risk of cognitive disorders.
MeSH Keywords: Cerebral Arterial Diseases, Cognitive Dissonance, Collateral Circulation
Background
Atherosclerosis is a chronic systemic inflammatory disease affecting all arteries in the body [1]. It is a leading cause of mortality world-wide [2]. Intracranial atherosclerosis is the most common vascular lesion in Asian patients [3,4], In China, intracranial atherosclerotic stenosis is not only a causal risk factor of ischemic stroke [5,6], but is also an independent risk factor for cognitive decline [7]. The middle cerebral artery (MCA) is one of the most common sites of intracranial atherosclerosis [8]. Cognitive performance has been reported to be affected in patients with severe middle cerebral artery stenosis (MCAS) and the causal link has been demonstrated [9]. However, it is unclear whether patients with asymptomatic severe MCAS suffer from persistent cognitive decline in the following years.
Leptomeningeal collaterals are a network of small blood vessels connecting distal regions of the intracerebral arterial system [10,11]. Leptomeningeal collaterals maintain the perfusion of ischemic areas after MCA stenosis or occlusions [12]. Good leptomeningeal collateral status is associated with better functional outcomes, lower risk of hemorrhagic transformation, and smaller infarct volumes after a proximal arterial stenosis or occlusion [13–18]. Nevertheless, to the best of our knowledge, the factors associated with cognitive dysfunction after severe MCAS with different degrees of collateral circulation are unclear.
We therefore performed a prospective cohort, cross-sectional, single-center study. The cognitive performances of patients after severe MCAS with different degrees of collateral circulation were evaluated. We hypothesized that severe MCAS with poor collateral circulation would be associated with poor cognitive function over time.
Material and Methods
Participants
We registered consecutive patients who had been screened for MCAS by transcranial Doppler (TCD) at the stroke screening outpatient clinic or medical examination center of Shengli Oilfield Central Hospital from January 2013 and June 2016. This was a prospective, long-term follow-up study on the cognitive function of unilateral asymptomatic severe MCAS in Chinese adults. Severe MCAS was defined as stenosis of 71% or more in MCA. The degree of stenosis was measured by computed tomography angiography (CTA). Exclusion criteria for patients and healthy volunteers were as follows: (1) History of stroke or transient ischemic attack, (2) Presence of neurologic deficits, (3) Stenosis or occlusion of 50% or more in large intracranial vessels (anterior cerebral artery, internal carotid artery, basilar artery, and vertebral artery), (4) Prior dementia, (5) Mini-Mental State Examination score less than 24, and (6) Neurological diseases affecting cognitive function (e.g., Parkinson’s disease).
This prospective cohort study was approved by the hospital ethics committee and consent was obtained from all participants.
Control group
Forty-two healthy controls were recruited from the medical examination center. All participants were free from unilateral asymptomatic MCAS based on age and number of vascular risk factors (including smoking status, history of diabetes mellitus, history of hypertension, and history of dyslipidemia). All detailed baseline data were recorded. The controls underwent the same imaging and neuropsychological assessment as did the patients with MCAS.
Imaging
All patients underwent CTA using a 64-slice multidetector CT scanner (Brilliance-64, Philips Healthcare, Best, The Netherlands) as previously described [19]. The scanning coverage was from arch to vertex with continuous axial sections parallel to the orbitomeatal line and with 0.6–1.25 mm section thickness, and 50 mL of contrast media was injected followed by 50 mL of saline with a flow of 5 mL/s. Scans were considered optimal for scoring collaterals if they included complete coverage from base of skull to vertex, It ensures adequate time for retrograde opacification of the leptomeningeal collateral-dependent slower filling MCA branches distal to the M1 occlusion in our study. Using criteria established in the Warfarin-Aspirin Symptomatic Intracranial Disease Trial, severe MCAS (i.e., no detectable stenosis, <50% stenosis, 51%–70% stenosis, 71–99% stenosis, and occlusion) was evaluated by craniocervical CTA [20].
Brain MRI was performed (3.0-T Magnetom scanner; Achieva 3.0T, Philips Medical Systems, Best, the Netherlands) within 7 days after admission according to the following parameters. Diffusion-weighted imaging used repetition time (TR)/echo time (TE)=3800/93 milliseconds, 2-mm interslice gap, field of view (FOV)=250×250 mm, slice thickness=5 mm, and b-values of 0 s/mm2 and 1000 s/mm2.
Two certified readers independently evaluated the images in a blinded manner. Each CTA study was evaluated for leptomeningeal collaterals according to Miteff system.
Imaging analysis
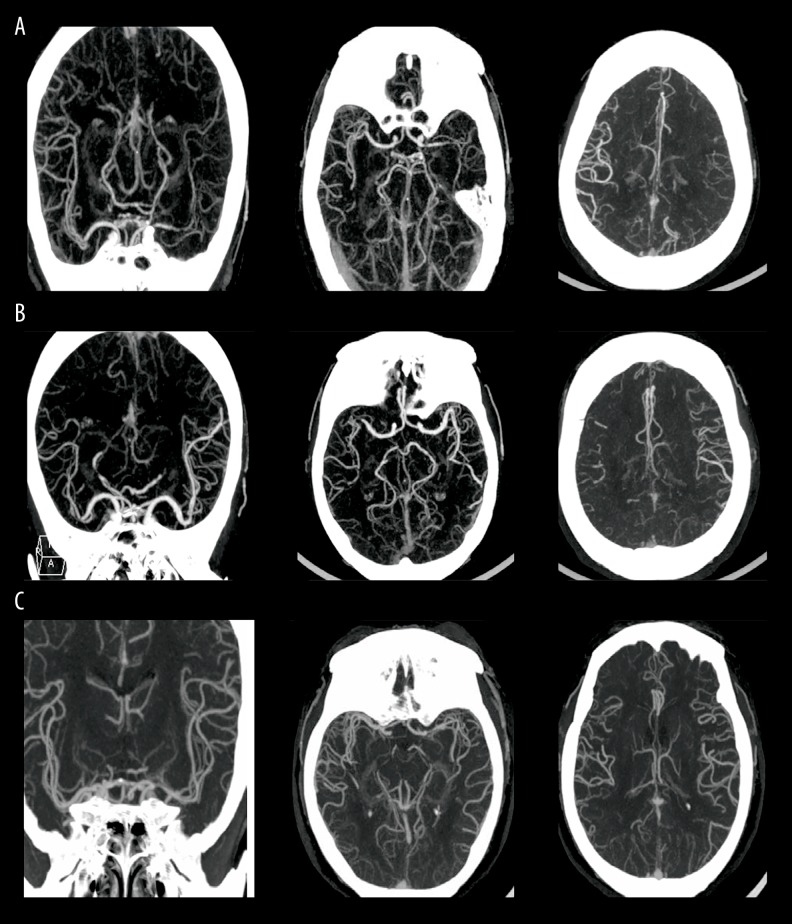
The Miteff scale is a 3-point scale that grades middle cerebral artery collateral branches with respect to the Sylvain fissure [21]. The grades assigned are as follows: a score of 1 indicated “poor” collateral status when the contrast opacification was merely observed in the distal superficial branches (Figure 1A); a score of 2 was designated “moderate” collateral status if vessels were observed at the Sylvian fissure (Figure 1B); and a score of 3 was designated “good” collateral status, which showed the entire MCA distal to the stenosis segment reconstituted with contrast (Figure 1C).
Figure 1.
The axial views of MCAS with poor, moderate, and good collateral circulation. (A) A score of 1 indicated “poor” collateral status; (B) A score of 2 indicated “moderate” collateral status; (C) A score of 3 indicated “good” collateral status.
Neuropsychological assessment
Neuropsychological assessment was performed at follow-up after 2 years. The main cognitive domains were evaluated within 7 days after the qualifying event. Executive functioning, including response generation and inhibition, was evaluated with a verbal fluency task (VFT) and the Abbreviated Stroop Color Word Test, respectively [22]. Information-processing speed was tested with cards I and II of the Abbreviated Stroop Color Word Test and the Symbol-Digit Modalities Test (SDMT) [23,24]. The Auditory Verbal Learning Test (AVLT) was used to measure working memory, including immediate recall and delayed free recall [25], and attention was evaluated by the Paced Auditory Serial Addition Test (PASAT) [26].
Other measurements
Education level, age, body mass index, and gender were recorded. Baseline vascular risk factor assessment (diabetes mellitus, hypertension, dyslipidemia, current smoking habit, and current drinking habit) was performed. The Hospital Anxiety and Depression Scale depression subscale was used for assessing depressive symptoms [27].
White matter hyperintensity (WMH) was classified into deep white matter hyperintensities (d-WMHs) and periventricular white matter hyperintensities (p-WMHs) [28]. The severity of the WMHs was rated using the Fazekas scale, ranging from 0 to 6 [29]. D-WMHs scores assigned are the following: 0: absent, 1: punctate foci, 2: beginning confluence of foci, and 3: large confluent areas. P-WMHs scores assigned are the following: 0: absent, 1: caps or pencil lining, 2: smooth halo, and 3: irregular periventricular WMH extending into deep white matter. The total WMH score was calculated by summing the scores of p-WMHs and d-WMHs.
Statistical analysis
IBM Statistical Product and Service Solutions Statistics version 20 (Armonk, NY, USA) was used for data analysis. Patients were grouped according to Miteff scale (good collateral circulation, moderate collateral circulation, and poor collateral circulation). Continuous variables were expressed as means ±SD, and categorical variables were expressed as frequencies and percentages. Baseline characteristics were analyzed with Mann-Whitney U test, Pearson χ2, and t test. Baseline domain-specific cognitive performance was analyzed with ANCOVA. The relationship of cognitive domains with collateral circulation was evaluated with multiple logistic regression analyses. The age, silent brain infarct, and leukoaraiosis were adjusted in all analyses. The critical value of α was set at 0.05.
Results
Baseline characteristics
A total of 181 patients were admitted to Shengli Oilfield Central Hospital from January 2014 to June 2016, including 58 females (33.5%) and 115 males (66.5%). During the 2-year follow-up, 8 patients (5 patients due to stroke and 3 patients due to Mini-Mental State Examination score less than 24) were excluded; thus, 173 patients were enrolled for analysis (mean age 59.4 years, 66.5% males). The baseline characteristics according to collateral circulation grades of MCAS are shown in Table 1. There were fewer women in the “moderate” and “poor” collateral status groups. Individuals with “moderate” collateral status or “poor” collateral status were more likely to have silent brain infarct (P=0.025 and P<0.001, respectively). There was a significant difference in Fazekas score between each group (P=0.001). Although the percentage of incomplete Circle of Willis (CoW) increased along with changes of collateral status, there was no significant difference among the groups. Other baseline characteristics of the MCAS patients and control group in the study are shown in Table 1.
Table 1.
Comparison of groups on the basis of baseline patient characteristics.
| Baseline characteristic | Control group (N=42) | Leptomeningeal collateral status of MCAS | F//z χ2 | P | ||
|---|---|---|---|---|---|---|
| Good (N=48) | Moderate (N=66) | Poor (N=59) | ||||
| Male sex | 19/45.23 | 25/52.08 | 48/72.73ab | 42/71.19ab | 10.941 | 0.012 |
| Smoker | 14/33.33 | 18/37.50 | 30/45.45 | 26/44.07 | 2.035 | 0.565 |
| Alcohol | 5/11.90 | 6/12.50 | 10/15.15 | 11/18.64 | 1.172 | 0.760 |
| Diabetes | 8/19.05 | 12/25.00 | 14/21.21 | 12/20.33 | 0.549 | 0.908 |
| Hypertension | 33/78.57 | 36/75.00 | 54/81.82 | 44/74.58 | 1.198 | 0.754 |
| Dyslipidemia | 25/59.52 | 26/54.17 | 40/60.61 | 38/64.41 | 1.172 | 0.760 |
| Incomplete CoW | 18/42.86 | 22/45.83 | 38/57.58 | 36/61.02 | 4.802 | 0.187 |
| Education level (years of schooling) <6 | 9/21.43 | 12/25.00 | 24/36.36 | 21/35.59 | 4.089 | 0.252 |
| Age, y | 61.41±6.89 | 61.38±6.57 | 59.21±7.34 | 58.65±7.30 | 1.197 | 0.314 |
| BMI, (kg/m2) | 25.71±3.49 | 25.14±3.49 | 25.27±3.79 | 26.94±4.49 | 1.538 | 0.208 |
| Silent brain infarct | N/A | 6/12.50a | 20/30.30ab | 27/45.76ab | 29.863 | <0.001 |
| Fazekas score (median (Q1–Q3)) | 0 (0~1) | 1(0~2)a | 2(1~2)ab | 3(2~4)abc | 29.227 | <0.001 |
BMI – body mass index; CoW – Circle of Willis.
Significantly different from control group, P<0.05;
Significantly different from good leptomeningeal collateral, P<0.05;
Significantly different from moderate leptomeningeal collateral, P<0.05.
Cognition evaluation
Baseline cognitive tests were performed at a mean of 2.6 days (interquartile range, 0–5 days; median, 2 days mean) after admission. MCAS patients had worse performance than controls in individual cognitive test and cognitive domains, but not episodic memory (Table 2). Specifically, cognitive test of VFT (P=0.001 and P<0.001, respectively), Stroop task (P<0.001 and P<0.001, respectively), Stroop interference task (P<0.001 and P<0.001, respectively), SDMT (P=0.004 and P<0.001, respectively), and PASAT (P=0.001 and P<0.001, respectively) in “moderate” collateral status and “poor” collateral status patients were significantly worse than for the “good” collateral status patients. “Poor” collateral status patients persistently performed worse in cognitive function test of VFT (P=0.001), SDMT (P=0.002), and PASAT (P=0.001) than “moderate” collateral status patients (Table 2). Cognitive changes in 3 leptomeningeal collateral status stratified by the side of MCAS are presented in Table 3. There was no significant difference in cognitive function between the MCAS subgroups of patients.
Table 2.
Cognitive test between patients with MCAS and controls.
| Cognitive Test and Domain | Control group (N=42) | Leptomeningeal collateral status of MCAS | F | P | ||
|---|---|---|---|---|---|---|
| Good (N=48) | Moderate (N=66) | Poor (N=59) | ||||
| Executive function VFT | 21.68±1.87 | 20.63±2.28 | 18.85±1.91ab | 15.95±1.36abc | 58.441 | <0.001 |
| Stroop interference task | 46.34±4.81 | 44.04±5.19 | 39.47±3.67ab | 37.59±3.25ab | 27.428 | <0.001 |
| Information processing speed SDMT | 45.76±1.85 | 43.82±2.07 | 36.50±1.55ab | 33.84±2.26abc | 247.595 | <0.001 |
| Stroop task | 94.91±6.97 | 90.71±7.21 | 82.89±6.29ab | 79.91±5.01ab | 34.997 | <0.001 |
| Episodic memory | ||||||
| AVLT immediate recall | 9.41±1.50 | 9.27±1.04 | 9.02±1.45 | 8.52±0.99 | 2.648 | 0.052 |
| AVLT delayed free recall | 8.59±1.40 | 8.35±1.07 | 8.08±1.47 | 7.83±1.05 | 2.113 | 0.102 |
| Attention PASAT | 37.73±3.19 | 36.50±2.58 | 31.48±1.53ab | 29.84±1.54abc | 92.214 | <0.001 |
VFT – verbal fluency task; SDMT – symbol-digit modalities test; AVLT – auditory verbal learning test; PASAT – paced auditory serial addition test.
Significantly different from control group, P<0.05;
Significantly different from good leptomeningeal collateral, P<0.05;
Significantly different from moderate leptomeningeal collateral, P<0.05.
Table 3.
Cognitive changes in the three leptomeningeal collateral status between MCAS subgroups.
| Good collateral status | Moderate collateral status | Poor collateral status | |||||||
|---|---|---|---|---|---|---|---|---|---|
| L-MCAS (N=21) | R-MCAS (N=27) | P | L-MCAS (N=31) | R-MCAS (N=35) | P | L-MCAS (N=32) | R-MCAS (N=27) | P | |
| PASAT | 36.55±2.97 | 36.46±2.33 | 0.931 | 31.86±1.54 | 31.01±1.38 | 0.102 | 30.01±1.58 | 29.60±1.54 | 0.418 |
| SDMT | 43.36±2.03 | 44.21±2.10 | 0.331 | 36.61±1.75 | 36.27±1.32 | 0.536 | 33.85±2.16 | 33.79±2.36 | 0.927 |
| VFT | 20.27±2.15 | 20.92±2.43 | 0.499 | 19.22±1.99 | 18.25±1.81 | 0.147 | 15.79±1.23 | 16.10±1.45 | 0.478 |
| AVLT immediate recall | 9.04±1.07 | 9.46±1.02 | 0.331 | 9.31±1.46 | 8.56±1.43 | 0.142 | 8.36±1.00 | 8.77±1.03 | 0.211 |
| AVLT delayed free recall | 8.15±1.07 | 8.53±1.08 | 0.412 | 8.42±1.47 | 7.56±1.44 | 0.098 | 7.74±1.45 | 7.90±1.01 | 0.616 |
| Stroop task | 88.73±7.11 | 92.38±7.14 | 0.223 | 84.14±5.91 | 80.81±6.80 | 0.137 | 80.05±5.47 | 79.86±4.56 | 0.903 |
| Stroop interference task | 42.77±4.92 | 45.12±5.35 | 0.280 | 40.08±3.47 | 38.41±3.97 | 0.198 | 37.37±3.60 | 37.88±2.92 | 0.622 |
L – left; R – right. MCAS – middle cerebral artery stenosis. VFT – verbal fluency task; SDMT – symbol-digit modalities test; AVLT – auditory verbal learning test; PASAT – paced auditory serial addition test.
For “good” collateral status patients, there was no significant difference in cognitive test during the 2-year follow-up period. A significant reduction in VFT (P=0.012), Stroop task (P=0.049), SDMT (P=0.009), and PASAT (P=0.002) was observed in the patients with “moderate” collateral status between baseline and 2-year cognitive evaluation (Table 4). VFT (P<0.001), Stroop task (P=0.005), Stroop interference task (P=0.001), SDMT (P<0.001), and PASAT (P<0.001) were also significantly worse in “poor” collateral status patients after 2 years (Table 4).
Table 4.
Cognitive changes after 2-year in the three leptomeningeal collateral status MCAS groups.
| Good collateral status group | 2-year follow-up | Moderate collateral status group | 2-year follow-up | Poor collateral status droup | 2-year follow-up | |
|---|---|---|---|---|---|---|
| PASAT | 36.50±2.58 | 35.88±3.10 | 31.48±1.53 | 29.63±1.31a | 29.84±1.54 | 25.42±1.88b |
| SDMT | 43.82±2.07 | 42.71±2.17 | 36.50±1.55 | 34.11±1.75a | 33.84±2.26 | 29.17±1.35b |
| VFT | 20.63±2.28 | 20.08±2.48 | 18.85±1.91 | 17.18±1.99a | 15.95±1.36 | 12.36±1.42b |
| AVLT immediate recall | 9.27±1.04 | 8.79±0.85 | 9.02±1.45 | 8.40±1.48 | 8.52±0.99 | 8.08±1.01 |
| AVLT delayed free recall | 8.35±1.07 | 8.01±1.06 | 8.08±1.47 | 7.74±1.45 | 7.83±1.05 | 7.32±1.21 |
| Stroop task | 90.71±7.21 | 89.54±6.28 | 82.89±6.29 | 80.33±5.61 | 79.91±5.01 | 72.64±4.48b |
| Stroop interference task | 44.04±5.19 | 42.58±5.19 | 39.47±3.67 | 37.62±3.86a | 37.59±3.25 | 31.56±3.02b |
VFT – verbal fluency task; SDMT – symbol-digit modalities test; AVLT – auditory verbal learning test; PASAT – paced auditory serial addition test.
Moderate collateral circulation baseline vs. 2-year follow-up, P<0.05;
Poor collateral circulation baseline vs. 2-year follow-up, P<0.05.
Multiple regression analysis in MCAS patients
The results of the multiple regression models were adjusted for influencing factor from the multivariable-adjusted models, for cognitive domains, with different grades of collateral circulation as categorical variables. There was a significant association of “moderate” collateral circulation with VFT (OR=0.56, 95% CI: 0.41–0.77), Stroop task (OR=0.77, 95% CI: 0.66–0.87), and Stroop interference task (OR=0.77, 95% CI: 0.66–0.90). There was a significant association of “poor” collateral circulation with VFT (OR=0.20, 95% CI: 0.16–0.23), Stroop task (OR=0.57, 95% CI: 0.47–0.69), Stroop interference task (OR=0.46, 95% CI: 0.34–0.61), PASAT (OR=0.02, 95% CI: 0.002–0.20), and SDMT (OR=0.30, 95% CI: 0.002–0.46) (Table 5).
Table 5.
Multiple regression analysis in MCAS patients.
| Cognitive test | Collateral circulation, OR (95%CI) | |||
|---|---|---|---|---|
| Moderate (n=66) | P value* | Poor (n=59) | P value* | |
| PASAT | 0.45 (0.30–0.67) | 0.053 | 0.04 (0.002–0.18) | 0.005 |
| SDMT | 0.69 (0.52–0.94) | 0.109 | 0.30 (0.002–0.46) | 0.002 |
| VFT | 0.56 (0.41–0.77) | 0.000 | 0.20 (0.16–0.23) | 0.000 |
| AVLT immediate recall | 0.82 (0.54–1.23) | 0.335 | 0.54 (0.35–0.84) | 0.065 |
| AVLT delayed free recall | 0.72 (0.46–1.13) | 0.155 | 0.63 (0.40–0.97) | 0.085 |
| Stroop task | 0.76 (0.66–0.87) | 0.000 | 0.57 (0.47–0.69) | 0.000 |
| Stroop interference task | 0.77 (0.66–0.90) | 0.000 | 0.46 (0.34–0.61) | 0.000 |
For difference in cognitive function at baseline using analysis of covariance, adjusted for age, silent brain infarct and leukoaraiosis.
Discussion
In this prospective study, patients who had severe MCAS with “moderate” and “poor” collateral circulation status in the previous 2 years had function impairment in at least 1 cognitive domain. The most affected cognitive domains were attention, executive function, and information-processing speed, unrelated to the presence and severity of WMH and silent brain infarct. Episodic memory remained relatively intact. However, the cognitive function of severe MCAS patients with “good” collateral circulation status was not significantly affected. These findings are consistent with previous reports [30,31] suggesting that intracranial atherosclerotic stenosis is an important contributor to cognitive decline. However, the mechanisms by which large-vessel disease causes cognitive impairment, or whether this is only an association, are unclear.
Current theories on pathogenesis of cognitive decline suggest that there is a continuum between cerebral large-vascular disease and cognitive impairment [32]. There is increasing recognition that atherosclerosis in large vessels is driven by the mechanisms of microcirculatory disturbance. Moreover, the probable pathophysiology of cognitive decline is chronic hypoperfusion in the brain parenchyma [9]. Intracranial atherosclerotic stenosis exacerbates cerebral hypoperfusion, which may subsequently cause brain atrophy, cognitive decline, and dementia [33]. Our results indicate that severe MCAS is associated with cognitive impairment.
Leptomeningeal collaterals are a subsidiary vascular network of small blood vessels connecting distal regions of the intracerebral arterial system [34–36]. Collaterals formation is dependent on natural, genetically determined differences in the affected tissue and the vigor of the molecular mechanisms [37,38]. The preservation of flow through leptomeningeal collaterals is known to protect brain tissue against ischemia damage after proximal arterial stenosis or occlusion, such as MCA. The deficiency in leptomeningeal collaterals circulation is related to cognitive decline. One possible explanation is that severe MCAS with “good” collateral status ensures sufficient blood supply to the MCA-region, making “good” collateral status a better indicator of cognitive performance. Previous data on cognitive function in asymptomatic severe MCAS are scarce and heterogeneous. Furthermore, cognitive assessment, such as the Montreal Cognitive Assessment and Mini-Mental State Examination, are primary screening tools, which have low sensitivity to mild cognitive decline and cannot evaluate changes in specific cognitive domains [39]. However, herein, a sensitive neuropsychological test, assessing the major cognitive domains defined by international standards [40], was performed. Therefore, conclusions on domain-specific cognition were drawn. The cognitive profile in severe MCAS with poor collaterals was obviously impaired in information-processing speed, executive function, and attention, but not episodic memory. The impairment of non-amnestic cognition was similar to that of vascular cognition. The profile was possibly caused by disruption of subcortical-frontal connections involved in cognitive processing. The cognitive profile of asymptomatic severe MCAS patients not been studied, except for one study that reported a patient with severe MCAS, in which Mini-Mental State Examination instead of comprehensive neuropsychological evaluation was performed [41].
Although the present study has some interesting results, it has some limitations. First, the time course between cognitive function and severe MCAS could not be established due to the cross-sectional design. Second, 2-year follow-up is not long enough to monitor cognitive changes. Severe MCAS patients do not report subjective cognitive complaints more frequently than healthy individuals. Finally, the subjects of our study may not be representative of the whole severe MCAS population due to our limited sample size. Future studies with larger and demographically varied samples are warranted.
Conclusions
In conclusion, MCAS patients with moderate and poor collateral circulation have impairment of ≥1 cognitive domain over time. Cognitive function decline in MCAS patients is a dynamic process in which (especially) attention, executive function, and information-processing speed are insufficient after collateral compensation. The affected domains are consistent with the profile of vascular cognitive impairment. Good collateral circulation plays a vital role in sustaining blood flow to the ischemic areas after MCA stenosis and seems to be associated with less risk for vascular cognitive impairment.
Footnotes
Source of support: Departmental sources
Conflict of interest
None.
References
- 1.Libby P. Vascular biology of atherosclerosis: Overview and state of the art. Am J Cardiol. 2003;91:3A–6A. doi: 10.1016/s0002-9149(02)03143-0. [DOI] [PubMed] [Google Scholar]
- 2.Barquera S, Pedroza-Tobias A, Medina C, et al. Global overview of the epidemiology of atherosclerotic cardiovascular disease. Arch Med Res. 2015;46:328–38. doi: 10.1016/j.arcmed.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 3.Gorelick PB, Wong KS, Bae HJ, Pandey DK. Large artery intracranial occlusive disease: A large worldwide burden but a relatively neglected frontier. Stroke. 2008;39:2396–99. doi: 10.1161/STROKEAHA.107.505776. [DOI] [PubMed] [Google Scholar]
- 4.Wong KS, Huang YN, Gao S, et al. Intracranial stenosis in Chinese patients with acute stroke. Neurology. 1998;50:812–13. doi: 10.1212/wnl.50.3.812. [DOI] [PubMed] [Google Scholar]
- 5.Zaidat OO, Klucznik R, Alexander MJ, et al. The NIH registry on use of the Wingspan stent for symptomatic 70–99% intracranial arterial stenosis. Neurology. 2008;70:1518–24. doi: 10.1212/01.wnl.0000306308.08229.a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miao Z, Jiang L, Wu H, et al. Randomized controlled trial of symptomatic middle cerebral artery stenosis: Endovascular versus medical therapy in a Chinese population. Stroke. 2012;43:3284–90. doi: 10.1161/STROKEAHA.112.662270. [DOI] [PubMed] [Google Scholar]
- 7.Sztriha LK, Nemeth D, Sefcsik T, Vecsei L. Carotid stenosis and the cognitive function. J Neurol Sci. 2009;283:36–40. doi: 10.1016/j.jns.2009.02.307. [DOI] [PubMed] [Google Scholar]
- 8.Tian L, Yue X, Xi G, et al. Multiple intracranial arterial stenosis influences the long-term prognosis of symptomatic middle cerebral artery occlusion. BMC Neurol. 2015;15:68. doi: 10.1186/s12883-015-0326-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kimura S, Ogata T, Watanabe J, et al. Does cerebral large-artery disease contribute to cognitive impairment? eNeurologicalSci. 2017;8:5–8. doi: 10.1016/j.ensci.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liebeskind DS. Collateral circulation. Stroke. 2003;34:2279–84. doi: 10.1161/01.STR.0000086465.41263.06. [DOI] [PubMed] [Google Scholar]
- 11.van Raamt AF, Mali WP, van Laar PJ, van der Graaf Y. The fetal variant of the circle of Willis and its influence on the cerebral collateral circulation. Cerebrovasc Dis. 2006;22:217–24. doi: 10.1159/000094007. [DOI] [PubMed] [Google Scholar]
- 12.Liebeskind DS, Cotsonis GA, Saver JL, et al. Collateral circulation in symptomatic intracranial atherosclerosis. J Cereb Blood Flow Metab. 2011;31:1293–301. doi: 10.1038/jcbfm.2010.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Seeters T, Biessels GJ, Kappelle LJ, et al. Determinants of leptomeningeal collateral flow in stroke patients with a middle cerebral artery occlusion. Neuroradiology. 2016;58:969–77. doi: 10.1007/s00234-016-1727-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brunner F, Tomandl B, Hanken K, et al. Impact of collateral circulation on early outcome and risk of hemorrhagic complications after systemic thrombolysis. Int J Stroke. 2014;9:992–98. doi: 10.1111/j.1747-4949.2012.00922.x. [DOI] [PubMed] [Google Scholar]
- 15.Menon BK, Smith EE, Modi J, et al. Regional leptomeningeal score on CT angiography predicts clinical and imaging outcomes in patients with acute anterior circulation occlusions. Am J Neuroradiol. 2011;32:1640–45. doi: 10.3174/ajnr.A2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lau AY, Wong EH, Wong A, et al. Significance of good collateral compensation in symptomatic intracranial atherosclerosis. Cerebrovasc Dis. 2012;33:517–24. doi: 10.1159/000337332. [DOI] [PubMed] [Google Scholar]
- 17.Seet RC, Wijdicks EF, Rabinstein AA. Stroke from acute cervical internal carotid artery occlusion: Treatment results and predictors of outcome. Arch Neurol. 2012;69:1615–20. doi: 10.1001/archneurol.2012.2569. [DOI] [PubMed] [Google Scholar]
- 18.van Seeters T, Biessels GJ, Kappelle LJ, et al. CT angiography and CT perfusion improve prediction of infarct volume in patients with anterior circulation stroke. Neuroradiology. 2016;58:327–37. doi: 10.1007/s00234-015-1636-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hansen CK, Christensen A, Ovesen C, et al. Stroke severity and incidence of acute large vessel occlusions in patients with hyper-acute cerebral ischemia: Results from a prospective cohort study based on CT-angiography (CTA) Int J Stroke. 2015;10:336–42. doi: 10.1111/ijs.12383. [DOI] [PubMed] [Google Scholar]
- 20.Samuels OB, Joseph GJ, Lynn MJ, et al. A standardized method for measuring intracranial arterial stenosis. Am J Neuroradiol. 2000;21:643–46. [PMC free article] [PubMed] [Google Scholar]
- 21.Miteff F, Levi CR, Bateman GA, et al. The independent predictive utility of computed tomography angiographic collateral status in acute ischaemic stroke. Brain. 2009;132:2231–38. doi: 10.1093/brain/awp155. [DOI] [PubMed] [Google Scholar]
- 22.Dodrill CB. A neuropsychological battery for epilepsy. Epilepsia. 1978;19:611–23. doi: 10.1111/j.1528-1157.1978.tb05041.x. [DOI] [PubMed] [Google Scholar]
- 23.Houx PJ, Jolles J, Vreeling FW. Stroop interference: Aging effects assessed with the Stroop Color-Word Test. Exp Aging Res. 1993;19:209–24. doi: 10.1080/03610739308253934. [DOI] [PubMed] [Google Scholar]
- 24.Parmenter BA, Weinstock-Guttman B, Garg N, et al. Screening for cognitive impairment in multiple sclerosis using the Symbol digit Modalities Test. Mult Scler. 2007;13:52–57. doi: 10.1177/1352458506070750. [DOI] [PubMed] [Google Scholar]
- 25.Maj M, D’Elia L, Satz P, et al. Evaluation of two new neuropsychological tests designed to minimize cultural bias in the assessment of HIV-1 seropositive persons: Aa WHO study. Arch Clin Neuropsychol. 1993;8:123–35. [PubMed] [Google Scholar]
- 26.Gronwall DM, Sampson H. The psychological effects of concussion. Neuroscience. 1974;1:231. [Google Scholar]
- 27.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002;52:69–77. doi: 10.1016/s0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 28.Jeong SH, Ahn SS, Baik M, et al. Impact of white matter hyperintensities on the prognosis of cryptogenic stroke patients. PLoS One. 2018;13:e196014. doi: 10.1371/journal.pone.0196014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Helenius J, Mayasi Y, Henninger N. White matter hyperintensity lesion burden is associated with the infarct volume and 90-day outcome in small subcortical infarcts. Acta Neurol Scand. 2017;135:585–92. doi: 10.1111/ane.12670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia: A statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42:2672–713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jellinger KA, Attems J. Neuropathological evaluation of mixed dementia. J Neurol Sci. 2007;257:80–87. doi: 10.1016/j.jns.2007.01.045. [DOI] [PubMed] [Google Scholar]
- 32.Dearborn JL, Zhang Y, Qiao Y, et al. Intracranial atherosclerosis and dementia: The Atherosclerosis Risk in Communities (ARIC) Study. Neurology. 2017;88:1556–63. doi: 10.1212/WNL.0000000000003837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hilal S, Xu X, Ikram MK, et al. Intracranial stenosis in cognitive impairment and dementia. J Cereb Blood Flow Metab. 2017;37:2262–69. doi: 10.1177/0271678X16663752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marshall NS, Wong KK, Cullen SR, et al. Sleep apnea and 20-year follow-up for all-cause mortality, stroke, and cancer incidence and mortality in the Busselton Health Study cohort. J Clin Sleep Med. 2014;10:355–62. doi: 10.5664/jcsm.3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yildirim T, Alp R. The role of oxidative stress in the relation between fibromyalgia and obstructive sleep apnea syndrome. Eur Rev Med Pharmacol Sci. 2017;21:20–29. [PubMed] [Google Scholar]
- 36.Faber JE, Chilian WM, Deindl E, et al. A brief etymology of the collateral circulation. Arterioscler Thromb Vasc Biol. 2014;34:1854–59. doi: 10.1161/ATVBAHA.114.303929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chalothorn D, Faber JE. Formation and maturation of the native cerebral collateral circulation. J Mol Cell Cardiol. 2010;49:251–59. doi: 10.1016/j.yjmcc.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sealock R, Zhang H, Lucitti JL, et al. Congenic fine-mapping identifies a major causal locus for variation in the native collateral circulation and ischemic injury in brain and lower extremity. Circ Res. 2014;114:660–71. doi: 10.1161/CIRCRESAHA.114.302931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suministrado M, Shuang E, Xu J, et al. Poststroke cognitive decline is independent of longitudinal changes in cerebral hemodynamics parameters. J Neuroimaging. 2017;27:326–32. doi: 10.1111/jon.12395. [DOI] [PubMed] [Google Scholar]
- 40.Hachinski V, Iadecola C, Petersen RC, et al. National Institute of Neurological Disorders and Stroke-Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke. 2006;37:2220–41. doi: 10.1161/01.STR.0000237236.88823.47. [DOI] [PubMed] [Google Scholar]
- 41.Rosario M, Tartar S, Spiegel G, McCullough L. Clinical reasoning: A case of progressive cognitive decline reversed by middle cerebral artery stent placement. Neurology. 2011;76:e52–56. doi: 10.1212/WNL.0b013e318211c1b1. [DOI] [PMC free article] [PubMed] [Google Scholar]