Abstract
Background
Dysregulation of the splicing activator, RNA-binding motif 4 (RBM4), has recently been reported to be involved in the progression of several cancers. However, the mechanisms that underpin the activity of RBM4 in gastric cancer (GC) remain unknown. The purpose of our study was to explore how RBM4 affects the biological behavior of GC through in vivo and in vitro experiments.
Material/Methods
Western blot and flow cytometry analyses were used to investigate the RBM4 protein levels in normal gastric epithelial cells and 5 types of GC cells. Cell Counting Kit-8 assay, flow cytometry analysis, wound-healing, and migration and invasion assays were evaluated in vitro in BGC823 and MGC803 GC cells. A xenograft tumor model was used to assess whether RBM4 inhibits GC growth in vivo. Mitogen-activated protein kinase (MAPK) protein levels were determined using western blot analyses.
Results
Our study revealed that RBM4 protein was downregulated in GC cells. Re-expression of RBM4 inhibited the proliferation, migration, and invasion of GC cells, while promoting apoptosis. Thus, the overexpression of RBM4 can inhibit tumor growth in GC mouse models. We also report that RBM4 was involved in the activation of MAPK-dependent signaling pathways in human GC.
Conclusions
It is hoped that these findings will improve our understanding of GC pathogenesis while also helping us to explore the feasibility of RBM4-targeted therapy for GC treatment.
MeSH Keywords: Genes, Tumor Suppressor; MAP Kinase Signaling System; Stomach Neoplasms
Background
Gastric cancer (GC) is the most common digestive system neoplasm in humans and the second leading cause of cancer-related deaths in China [1–3]. The therapeutic value of surgical resection in the treatment of GC is limited due to high rates of recurrence and metastasis even after positive surgical procedures. In many countries, the 5-year overall survival rate for GC remains below 30% despite significant improvements in diagnostic and surgical techniques over the past few decades [4,5]. Therefore, it is imperative that we identify new molecular markers that will allow us to more precisely predict survival and the risk of recurrence. It is also hoped that the elucidation of appropriate markers will permit us to provide more targeted treatment strategies for GC patients while also facilitating the development of more effective preventive measures.
RNA-binding motif 4 (RBM4) is a splicing factor mainly involved in the regulation of selective splicing (AS) and mRNA translation [6,7]. In addition, RBM4 can promote the differentiation of muscle, pancreas, and neuronal cells [8–10]. RBM4 controls the AS of human genes by binding to a set of intronic splicing regulatory elements [11]. Imbalanced AS is synonymous with some of the major hallmarks of cancer including tumor apoptosis [12], invasion, and metastasis [13] as well as epithelial-mesenchymal transition [14]. Therefore, changes in the role of the RBM4 protein are believed to be important in the dysregulation of splicing in cancer [15,16]. In 2014, Wang et al. were the first to report that the upregulation of RBM4 activity could suppress the progression of various malignancies, including cancers of the lung, breast, ovary, liver, and prostate [17]. Subsequent studies demonstrated that RBM4 was downregulated in colorectal cancer and this downregulation correlated with a poor prognosis [18]. Studies have also indicated that RBM4 influences tumor progression by controlling biological functions of cancer cells such as proliferation, apoptosis, migration, and invasion. However, scientists have yet to elucidate how RBM4 is controlled during GC tumorigenesis.
We have previously utilized immunohistochemistry (IHC) and quantitative real-time polymerase chain reaction (qRT-PCR) techniques to reveal that the expression of RBM4 is reduced in GC tissues compared with adjacent normal tissue [19]. These results indicate that downregulation of RBM4 activity might play an important role in the development of GC. In this study, we first investigated the role of RBM4 expression in both cultured cells and in a tumor xenograft model. We also studied the effect of RBM4 on the protein expression of the mitogen activated protein kinase (MAPK) pathway in human GC cell lines by western blot analyses.
Material and Methods
Cell culture and transfections
A normal gastric cell line (GES1) and 5 human gastric carcinoma cell lines (MKN28, HGC27, BGC823, MKN45, and MGC803) were purchased from the Cell Bank of ZISHI Biomart and the Chinese Academy of Sciences (Shanghai, China). Cell culture was performed using RPMI-1640 (Invitrogen, Carlsbad, CA, USA) plus 10% fetal bovine serum (Invitrogen, Shanghai, China). We purchased pEZ-Lv201-RBM4 (lentiviral vector targeting RBM4 overexpression), pEZ-Lv201-Control (negative control lentiviral vector), psi-U6-shRBM4 (shRNA vector targeting RBM4), and psi-U6-NC (negative control shRNA vector) from Gene Pharma (Shanghai, China). Transfection into GC cells was performed using Lipofectamine 2000 transfection reagent (Invitrogen, Shanghai, China) according to the operating steps in the manual.
Reverse transcriptase PCR analysis
Total RNA was isolated from approximately 1×106 cells (collected from 60-mm culture dishes) using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the operating steps in the manual. Prime Script RT Reagent Kit (Takara, China) was used for reverse transcription. The cDNA was amplified with the following primers: 5′-CGCGGATCCCGATGGTGAAGCTGTTCATC-3′ (forward) and 5′-CCGCTCGAGAAAGGCTGAGTACCGCGCCC-3′ (reverse) for RBM4; 5′-GCCGGTGCTGAGTATGTC-3′ (forward) and 5′-CTTCTGGGTGGCAGTGAT-3′ (reverse) for glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Products of the polymerase chain reaction (PCR) were visualized on a 1% agarose gel with a final concentration of 0.5 μg/mL of ethidium bromide solution. The gels were photographed and quantitated using GADPH as an internal comparison. DNA sequence was verified by cloning the PCR products into the pcDNA3.1 vector.
CCK-8 assay cell proliferation viability assay
Cells were inoculated at 5×103 cells per well in a 96-well plate, followed by 24 hours, 48 hours, and 72 hours of culture. Cell proliferation viability tests were performed using the Cell Counting Kit-8 (CCK-8, Beyotime, Shanghai, China) according to the operating steps in the manual. Subsequently, the absorbance was measured at 450 nm using Elx800 Reader (Bio-Tek, Winooski, VT, USA).
Flow cytometric analysis
GES1, MKN28, HGC27, BGC823, MKN45, and MGC803 cells (1×106 cells each) were harvested and blocked using 1% BSA; cells were then incubated with anti-human RBM4 polyclonal rabbit IgG (Proteintech, Chicago, IL, USA, dilution 1: 100) and stained with anti-rabbit IgG (Sigma, St. Louis, MO, USA) labeled with diluted fluorescence isothiocyanate (FITC). The cells were then fixed with 1% paraformaldehyde. Finally, the cells were washed twice and suspended again with phosphate-buffered saline (PBS). Fluorescence intensity was determined by flow cytometry (FACScan, BD Biosciences, USA). As a comparison, secondary antibodies were incubated with the cells at room temperature. AnnexinV-FITC/propidium iodide (PI) apoptosis detection kits (BD Biosciences) was used for Annexin V and PI staining of apoptotic cells in accordance with the manufacturer’s protocol.
Wound-healing assay
Cells were inoculated on 6-well plates in culture media at a density of 5×105 cells per well. The cells were subsequently removed to serum-free media 12 hours prior to assay initiation. The confluent monolayer was then disrupted using a cell scraper and an artificial scratch wound was generated. Serum-free media was added prior to incubation for a further 48 hours at 37°C with 5% CO2 in a humidified chamber. The wound-healing percentage was calculated as the ratio of healing width at 48 hours to the wound width at 0 hours. Experiments were carried out in triplicate.
Migration and invasion assays
We performed cell migration and invasion assays using a Transwell chamber. For migration assay, the medium containing 600 mL of 10% fetal bovine serum was generally plated to the lower chamber (i.e., the bottom of the 24-well plate), and the suspensions containing 5×104 BGC823 cells or MGC803 cells with no serum medium were added to the upper chamber with an uncoated membrane (8.0-μm pore; 24-well; BD Biosciences, San Jose, CA, USA). For the invasion assay, Matrigel membrane (1 mg/mL, BD Biosciences) was polymerized in Transwell inserts at 37°C for 2 hours. For the migration assay, cells were cultured in a humidified chamber (37°C; 5% CO2) for 12 hours, and the invasion assay for 24 hours. Cells were wiped from the membrane surface at the bottom of the upper chamber using a wet cotton swab. Then the cells on the submembrane surface were fixed with methanol at room temperature for 30 minutes. Finally, the cells were stained with crystal violet, washed with water, and counted.
Western blot analysis
Western blots were performed in accordance with the steps described previously [20]. We used the following antibodies: rabbit anti-human RBM4 (polyclonal, Proteintech, Chicago, IL, USA, dilution 1: 1000), mouse anti-β-actin and mouse anti-ERK1/2, JNK, p38, p-ERK1/2, p-JNK, p-p38 (Santa Cruz Biotech, Santa Cruz, CA, USA). ECL system (Amersham Pharmacia, Piscataway, NJ, USA) was used for signal detection. Each blot was repeated 3 times.
Immunohistochemical staining
Proteins expression were examined by streptavidin-peroxidase (SP) staining technique after antigen retrieval using microwave technology. Endogenous peroxidase activity was prevented through incubating with 3% H2O2 for 15 minutes, the cells were then washed with PBS and incubated overnight with RBM4 (Proteintech, Chicago, IL, USA) at 4°C. The samples were washed with PBS the next day and secondary antibodies were added for 30 minutes at room temperature. Finally, the samples were incubated with SP for 30 minutes. After washing with PBS for 3 times, the cells were stained with diaminobenzidine (DAB) solution, and hematoxylin was used as the control staining. For the negative control, the primary antibody was substituted of PBS. Slides stained with antibody to RBM4 were first scanned under low power (100×) to identify 5 areas with the highest RBM4 density (hot spots). These 5 areas were photographed at a magnification of 200× and 5 digital images were generated. The staining intensity was divided into 4 grades: 0, 1, 2, and 3, which respectively represent no staining, light staining, moderate staining, and deep staining. The percentage of positive cells (0–100%) was multiplied by the staining intensity score to obtain the total score. The results were determined manually by 2 experienced pathologists using a double-blind design.
Animal experiments
This study was carried out with the permission of the committee of animal research institutions. ORT-RBM4-infected BGC823 cells and lentiviral vector control cells (1×106/100 μL) were injected subcutaneously into the flank of each 4-week-old immunodeficient nude mouse (left, control; right, RBM4). The growth of tumors was evaluated weekly for 5 weeks. The nude mice were subsequently sacrificed, and xenograft tumors were measured. Tumor volume was calculated using the formula: V=πAB2/6, where the maximum diameter is A and the perpendicular diameter represents B. Finally, hematoxylin and eosin (H&E) staining and immunohistochemical analysis of RBM4 protein expression in these tumor tissues was performed.
Statistical analysis
SPSS 20.0 statistical software was used for all statistical analyses. The significance of the difference between groups was analyzed using the unpaired Student’s t-test. All of the tests that were performed were 2-sided and a P-value of <0.05 was considered statistically significant.
Results
Expression of RBM4 is reduced in GC cell lines
Western blot and flow cytometry analyses were used to investigate the RBM4 protein levels in normal gastric epithelial cells and various types of GC cells. As shown in Figure 1A and 1B, in the normal gastric epithelial cell line GES1, the RBM4 protein expression ratio reached 52.9%, Conversely, in the MKN28, HGC27, BGC823, MKN45, and MGC803 GC cell lines, the RBM4 protein expression ratio was only 43.5%, 25.4%, 17.3%, 20.4%, and 46.0%, respectively. Upon comparison with the normal gastric epithelial cell line GES1, RBM4 expression was significantly lower in the GC cell lines, especially in BGC823 cell line. The expression of RBM4 was relatively higher in the MGC803 GC cell line compared with the other GC cell lines (Figure 1C). Therefore, the BGC823 cell line and MGC803 GC cell line were chosen as the focus of our subsequent studies.
Figure 1.
Protein and mRNA expression of RBM4 in various GC cell lines. (A) Expression levels of RBM4 protein were analyzed in various GC cell lines by western blotting; β-actin was utilized as an internal control. (B, C) Expression levels of RBM4 protein were analyzed in various GC cell lines by flow cytometry. (D) The expression of RBM4 mRNA was examined in ORT-RBM4 (RBM4), and control lentiviral vector (Control)-transfected BGC823 cells, and shRBM4, and a negative control shRNA plasmid (NC)-transfected MGC803 cells by RT-PCR. GAPDH was used as an internal control.
To investigate the biological function of RBM4, we designed a control lentiviral vector and a lentiviral vector that permitted RBM4 overexpression (ORT-RBM4), We also designed a negative control shRNA plasmid and a shRNA plasmid vector targeting RBM4 (shRBM4). ORT-RBM4 and the control lentiviral vector were independently transfected into an RBM4-deficient cell line, BGC823. RT-PCR results revealed that RBM4 mRNA expression recovered in BGC823 cell lines after transfection with ORT-RBM4. Conversely, RBM4 mRNA expression was significantly lower in MGC803 cells following stable transfection with shRBM4 (Figure 1D).
RBM4 controls proliferation, apoptosis, migration and invasion of GC cells in vitro
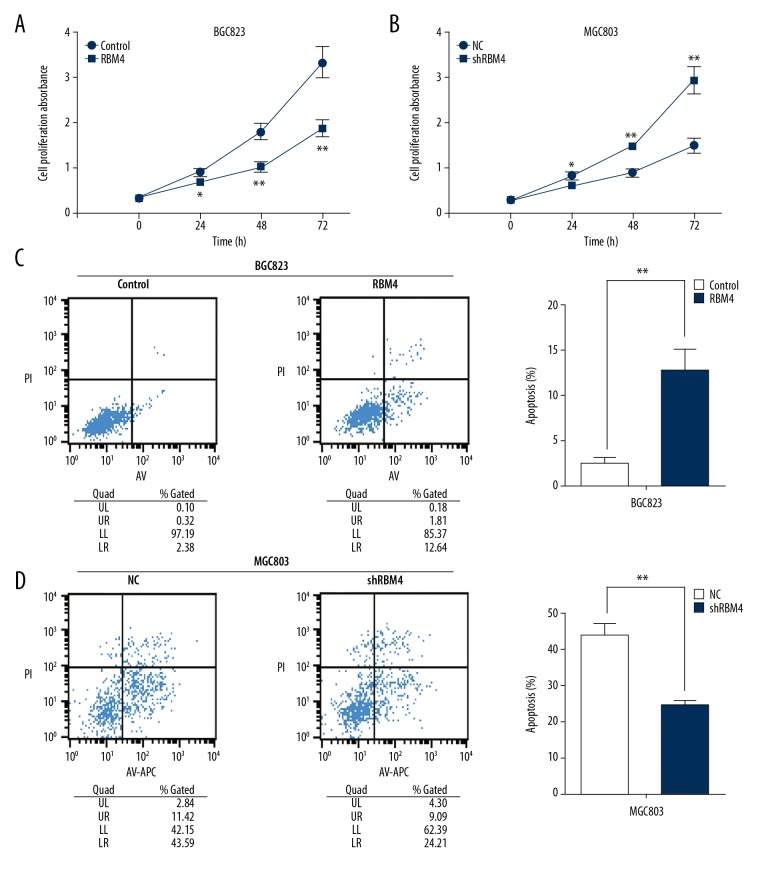
We performed a CCK-8 assay to monitor GC cell proliferation at 24 hours, 48 hours, and 72 hours after lentiviral vector and shRNA plasmid transfection. Our data reveal that ORT-RBM4 significantly inhibited cell proliferation in BGC823 cells (Figure 2A). Conversely, shRNA-mediated silencing of RBM4 significantly promoted cell proliferation in MGC803 cells (Figure 2B).
Figure 2.
RBM4 suppresses cell proliferation and promotes cell apoptosis in GC cells. (A) The viability of cell growth was assayed in Control- and RBM4-transfected BGC823 cells by CCK-8 at 0, 24 hours, 48 hours, and 72 hours. (B) The viability of cell growth was assayed in NC- and shRBM4-transfected MGC803 cells by CCK-8 at 0 hours, 24 hours, 48 hours, and 72 hours. (C) The rate of cell apoptosis was assayed in Control- and RBM4-transfected BGC823 cells by flow cytometry. (D) The rate of cell apoptosis was assayed in NC- and shRBM4-transfected MGC803 cells by flow cytometry. All experiments were carried out in triplicate. Data are shown as mean ± standard error. * P<0.05; ** P<0.01.
We subsequently performed a flow cytometry analysis to further analyze whether RBM4 inhibits proliferation of GC cells by altering the progression of apoptosis. The results revealed that ORT-RBM4 promoted cellular apoptosis in BGC823 cells (Figure 2C). Conversely, shRBM4 treatment blocked apoptosis in the MGC803 cells (Figure 2D).
Next, we investigated the role of RBM4 in the migration and invasion processes of GC cells via wound-healing, migration, and invasion assays. We observed delayed wound closure after RBM4 overexpression compared with the control transfection group (Figure 3A). Conversely, wound closure occurred significantly earlier after transfection in the shRBM4-treated group (Figure 3B). Compared with the control lentiviral vector-transfected BGC823 cells, staining images from a Transwell assay showed the migratory and invasive abilities of RBM4 overexpressed BGC823 cells were reduced (Figure 3C). However, silencing of RBM4 improved the migratory and invasive abilities of MGC803 cells compared with negative control-transfected cells (Figure 3D). These data suggest that RBM4 is involved in the metastasis of gastric cancer as a tumor suppressor gene.
Figure 3.
RBM4 inhibits GC cell motility. (A) A wound-healing assay was performed after RBM4 restoration in BGC823 cells. There was a significant delay in wound closure after RBM4 overexpression compared with the Control-transfected group. (B) A wound-healing assay was performed after RBM4 knockdown in MGC803 cells. Wound closure occurred significantly earlier after RBM4 downregulation compared with the NC-transfected group. (C) A cell migration and invasion assay was performed after RBM4 re-expression in BGC823 cells. The restoration of RBM4 resulted in a reduction in both the migration and invasive abilities of cells compared with the Control-transfected group. (D) A cell migration and invasion assay was performed after RBM4 downregulation in MGC803 cells. RBM4 knockdown promoted cell migration and invasion compared with the NC-transfected group. All experiments were carried out in triplicate. Data are shown as mean ± standard error. ** P<0.01.
Overexpression of RBM4 inhibits tumor growth in vivo
To assess whether RBM4 suppresses GC cell proliferation in vivo, we established a xenograft tumor model in nude mice using ORT-RBM4 transfected BGC823 cells or the control lentiviral vector. The transfected cells were subcutaneously injected into the flanks of nude mice. The average tumor volume associated with tumors that developed in the ORT-RBM4 group was smaller than that in the control lentiviral vector group (Figure 4A, 4B). In addition, tumors from the ORT-RBM4-transfected BGC823 cells grew more slowly than those from the control-transfected mice (Figure 4C). Following IHC staining of the xenograft tumors, we observed that the RBM4 staining signal was stronger in the ORT-RBM4 group compared with the control lentiviral vector group (Figure 4D). These data indicated that RBM4 may inhibit tumor growth in vivo.
Figure 4.
RBM4 overexpression inhibits tumor growth in vivo. (A) Photographs of representative tumor formations in nude mice. (B) Tumor xenografts 5 weeks after inoculation. (C) Tumor growth curves after injection of BGC823 cells stably transfected with Control or ORT-RBM4. The tumor volume was calculated every 7 days for 5 weeks after injection. (D) Representative immunohistochemical photographs of RBM4 protein expression in tissues of resected tumors formed from Control- or ORT-RBM4-transfected BGC823 cells. Upper: hematoxylin and eosin staining. Lower: immunostaining (400×). Scale bar, 40 um. Data are shown as mean ± standard error. * P<0.05, ** P<0.01.
RBM4 inhibits the expression of MAPK pathway protein in GC cell lines
To further investigate the potential mechanism underpinning the malignant progression associated with RBM4 activity in GC, we examined the protein expression levels of MAPK family members in BGC823 cells and MGC803 cells. This study also involved analysis of several MAPK subfamilies including ERK1/2, JNKs, and p38 [21,22]. Western blot analysis revealed that the protein expression levels of p-ERK1/2, p-JNK, and p-p38 in the ORT-RBM4 treated BGC823 cells were significantly lower than those in the control group. However, expression levels of these proteins were markedly elevated in the shRBM4-treated MGC803 cells. However, the protein expression levels of ERK1/2, JNK, and p38 were not notably different in either the ORT-RBM4-transfected BGC823 cells or the shRBM4-transfected MGC803 cells compared with the control cells (Figure 5A, 5B). Therefore, our data indicated that RBM4 might inhibit the progression of GC partly through the MAPK-dependent signaling pathway.
Figure 5.
RBM4 suppresses GC progression through MAPK-dependent signaling pathways. Representative western blotting results for ERK1/2, p-ERK1/2, JNK, p-JNK, p38, and p-p38 protein expression from Control- or ORT-RBM4-treated BGC823 cells (A) and NC- or shRBM4-treated MGC803 cells (B). All experiments were carried out in triplicate. Data are shown as mean ± standard error. * P<0.05, ** P<0.01.
Discussion
The abnormal distribution or unbalanced expression of splicing factors is regarded as a common contributing factor in carcinogenesis and hereditary diseases [23,24]. RBM4, a splicing factor that is normally expressed throughout development and has a relatively high abundance in the brain, heart, and skeletal muscle, has lately attracted increasing attention. Recent studies suggest that RBM4 is a tumor suppressor with potential involvement in malignancy progression in lung, colorectal, breast, ovarian, and liver cancers, among others [23,24]. However, the role of RBM4 in GC has not yet been closely studied. We have previously demonstrated that RBM4 is downregulated in GC tissues and patients exhibiting reduced RBM4 protein expression exhibit poor overall survival [19]. In the present study, we sought to confirm that RBM4 does indeed act as a tumor suppressor in GC.
We initially utilized western blot and flow cytometry analyses to demonstrate that RBM4 protein levels were decreased in various strains of GC cells compared with the normal gastric epithelial cell line GES1. This result was consistent with our previous findings regarding the expression of RBM4 in GC tissues [19]. However, in 2014 Lin et al. reported that the expression of RBM4 was upregulated in tumors [25]. The latter study showed that increases in SRPK1 protein levels in breast cancer cells resulted in the accumulation of RBM4 in the cytoplasm [25]. In 2016, Markus et al. [26] found that RBM4 mRNA was overexpressed in 18 different types of malignancies, including cancer of the cervix, breast, lung, colon, ovary, and rectum. Since only 10 cases of each type of tumor were studied, further investigations into the expression of RBM4 in tumors will require an expanded sample size and a more in-depth study design.
In this study, we also demonstrated that RBM4 suppressed many of the biological functions of GC in vitro – including proliferation, migration, and invasion – while also promoting the apoptosis of GC cells. These results were in line with those of Wang et al. [17] and Liang et al. [18]. However, Lin et al. [25] reported that RBM4 indirectly reduced the apoptosis of breast cancer cells by regulating a series of splicing events. The possible reasons are as follows: 1) different apoptosis detection methods were used: indirect immunofluorescence (Lin study) versus flow cytometry (our study); 2) different types of tumors were studied: breast cancer (Lin study) versus gastric cancer (our study); 3) different apoptosis molecules were detected: IR-B and MCL-1 (Lin study) versus p53 and Bcl-2 (our study). Finally, apoptosis might be affected by more signaling pathways and multiple mechanisms. Our results further implied that the loss of RBM4 might be a contributing factor in the development of GC. In addition, overexpression of RBM4 in BGC823 cells prevented tumor growth in vivo. This result supported the role of RBM4 as a tumor suppressor.
Previous studies have suggested that RBM4 antagonizes oncogenic SRSF1 to inhibit the proliferation of various cells by mTOR activation and regulates AS of Bcl-xL and Bcl-xS to induce apoptosis. [17]. Liang et al. [18] reported that an RBM4-regulated splicing cascade restrains the migration and invasion of colorectal cancer cells by modulating the activity of AKT/ERK signaling. Following previous reports, we used western blotting to further monitor the expression status of MAPK family members in ORT-RBM4-treated BGC823 cells and shRBM4-treated MGC803 cells. It is well known that the MAPK family of proteins can regulate numerous cellular responses, such as proliferation, apoptosis, migration, and invasion. Our data indicated that RBM4 mediates a reduction in protein expression of p-ERK1/2, p-JNK, and p-p38; however, protein expression of ERK1/2, JNK, and p38 were not affected. Therefore, our results suggested that RBM4 acts as a potent inhibitor, partly through the MAPK signaling pathway. Further studies are required to confirm this finding.
Conclusions
In summary, overexpression of RBM4 can lead to various changes in GC cells, including a reduction in proliferation, migration, and invasion and an increase in apoptotic capacity. In addition, overexpression of RBM4 can repress tumor growth in GC mouse models. Although RBM4 has been reported to act as a tumor suppressor, the mechanism underlying RBM4-mediated tumorigenic processes remains poorly understood. We observed for the first time that RBM4 is involved in MAPK-dependent signaling pathways in human GC. This finding further implies that the reactivation of RBM4 could be a potentially important strategy in future therapeutic approaches to treat GC.
Footnotes
Source of support: This project was supported by the National Natural Science Foundation of China (Grant number 81201596) and the Youth Medical Talent Program of Jiangsu Province (QNRC2016425)
Conflict of interest
None.
References
- 1.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. C J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 3.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 4.De Angelis R, Sant M, Coleman MP, et al. Cancer survival in Europe 1999–2007 by country and age: Results of EUROCARE-5 – a population-based study. Lancet Oncol. 2014;15:23–34. doi: 10.1016/S1470-2045(13)70546-1. [DOI] [PubMed] [Google Scholar]
- 5.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 6.Lin J, Tarn W. RBM4 down-regulates PTB and antagonizes its activity in muscle cell – specific alternative splicing. J Cell Biol. 2011;193:509–20. doi: 10.1083/jcb.201007131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin J, Tarn W, Hsieh W. Emerging role for RNA binding motif protein 4 in the development of brown adipocytes. Biochim Biophys Acta. 2014;1843:769–79. doi: 10.1016/j.bbamcr.2013.12.018. [DOI] [PubMed] [Google Scholar]
- 8.Lin JC, Tarn WY. Multiple roles of RBM4 in muscle cell differentiation. Front Biosci (Schol Ed) 2012;4:181–89. doi: 10.2741/260. [DOI] [PubMed] [Google Scholar]
- 9.Lin JC, Yan YT, Hsieh WK, et al. RBM4 promotes pancreas cell differentiation and insulin expression. Mol Cell Biol. 2012;33:319–27. doi: 10.1128/MCB.01266-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Su CH, Hung KY, Hung SC, Tarn WY. RBM4 regulates neuronal differentiation of mesenchymal stem cells by modulating alternative splicing of pyruvate kinase M. Mol Cell Biol. 2017;37 doi: 10.1128/MCB.00466-16. pii: e00466–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y, Ma M, Xiao X, Wang Z. Intronic splicing enhancers, cognate splicing factors and context-dependent regulation rules. Nat Struct Mol Biol. 2012;19:1044–52. doi: 10.1038/nsmb.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwerk C, Schulze-Osthoff K. Regulation of apoptosis by alternative pre-mRNA splicing. Mol Cell. 2005;19:1–13. doi: 10.1016/j.molcel.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 13.Ghigna C, Valacca C, Biamonti G. Alternative splicing and tumor progression. Curr Genomics. 2008;9:556–70. doi: 10.2174/138920208786847971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Warzecha CC, Jiang P, Amirikian K, et al. An ESRP-regulated splicing programme is abrogated during the epithelial-mesenchymal transition. EMBO J. 2010;29:3286–300. doi: 10.1038/emboj.2010.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bechara EG, Sebestyen E, Bernardis I, et al. RBM5, 6, and 10 differentially regulate NUMB alternative splicing to control cancer cell proliferation. Mol Cell. 2013;52:720–33. doi: 10.1016/j.molcel.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 16.Shkreta L, Bell B, Revil T, et al. Cancer-associated perturbations in alternative pre-messenger RNA splicing. Cancer Treat Res. 2013;158:41–94. doi: 10.1007/978-3-642-31659-3_3. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Chen D, Qian H, et al. The splicing factor RBM4 controls apoptosis, proliferation, and migration to suppress tumor progression. Cancer Cell. 2014;26:374–89. doi: 10.1016/j.ccr.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liang YC, Lin WC, Lin YJ, Lin JC. The impact of RNA binding motif protein 4-regulated splicing cascade on the progression and metabolism of colorectal cancer cells. Oncotarget. 2015;6:38046–60. doi: 10.18632/oncotarget.5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yong H, Zhu H, Zhang S, et al. Prognostic value of decreased expression of RBM4 in human gastric cancer. Sci Rep. 2016;6:28222. doi: 10.1038/srep28222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bai J, Yong HM, Chen FF, et al. Cullin1 is a novel marker of poor prognosis and a potential therapeutic target in human breast cancer. Ann Oncol. 2013;24:2016–22. doi: 10.1093/annonc/mdt147. [DOI] [PubMed] [Google Scholar]
- 21.Ahmad I, Iwata T, Leung HY. Mechanisms of FGFR-mediated carcinogenesis. Biochim Biophys Acta. 2012;1823:850–60. doi: 10.1016/j.bbamcr.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 22.Dienstmann R, Rodon J, Prat A, et al. Genomic aberrations in the FGFR pathway: Opportunities for targeted therapies in solid tumors. Ann Oncol. 2014;25:552–63. doi: 10.1093/annonc/mdt419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamdollah Zadeh MA, Amin EM, Hoareau-Aveilla C, et al. Alternative splicing of TIA-1 in human colon cancer regulates VEGF isoform expression, angiogenesis, tumour growth and bevacizumab resistance. Mol Oncol. 2015;9:167–78. doi: 10.1016/j.molonc.2014.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mavrou A, Brakspear K, Hamdollah-Zadeh M, et al. Serine-arginine protein kinase 1 (SRPK1) inhibition as a potential novel targeted therapeutic strategy in prostate cancer. Oncogene. 2015;34:4311–19. doi: 10.1038/onc.2014.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin JC, Lin CY, Tarn WY, Li FY. Elevated SRPK1 lessens apoptosis in breast cancer cells through RBM4-regulated splicing events. RNA. 2014;20:1621–31. doi: 10.1261/rna.045583.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Markus MA, Yang YHJ, Morris BJ. Transcriptome-wide targets of alternative splicing by RBM4 and possible role in cancer. Genomics. 2016;107:138–44. doi: 10.1016/j.ygeno.2016.02.003. [DOI] [PubMed] [Google Scholar]