Abstract
Background
Alzheimer’s disease (AD) is an age-associated neurodegenerative disorder. This study aimed to investigate effects of acupuncture administration on cognitive function and associated mechanisms.
Material/Methods
Senescence-accelerated prone 8 (SAM-P8) mice were randomly divided into 3 groups: the SAM-P8 group (P8-CN), the SAM-P8 administrating with acupuncture (P8-Acup) group, and the SAM-P8 administrating without acupuncture (P8-Sham) group. Morris water maze test was conducted to evaluate cognitive functions (memory and learning ability). PDK1, nPKC, and Rac1 inhibitors were used to treat SAM-P8 mice. Transmission electron microscope analysis was used to examine nuclear damage hippocampal tissues. Hematoxylin and eosin (H&E) staining was employed to evaluate inflammation. Western blot was used to detect PI3K, PDK1, nPKC, and Rac 1 expression in hippocampal tissues.
Results
Acupuncture administration significantly reduced PI3K, PDK1, nPKC, and Rac 1 levels compared to P8-CN group (P<0.05). Both acupuncture and enzyme inhibitors (NSC23766, Rottlerin, OSU03012) significantly improved cognitive functions, reduced inflammation, and alleviated nuclear damages of SAM-P8 mice compared to P8-CN group (P<0.05). Acupuncture significantly enhanced effects of inhibitors on inflammation and nuclear damages compared to inhibitor treatment single (P<0.05). Acupuncture significantly enhanced down-regulative effects of OSU03012 on PI3K and PDK1 levels, increased down-regulative effects of Rottlerin on nPKC and Rac 1 levels and enhanced effects of Rottlerin on Rac 1 compared to P8-CN group (P<0.05).
Conclusions
Acupuncture administration improved cognitive functions and alleviated inflammatory response and nuclear damage of SAM-P8 mice, by downregulating PI3K/PDK1/nPKC/Rac 1 signaling pathway. This study could provide potential insight for treating cognitive dysfunction and aging of AD patients.
MeSH Keywords: Acupuncture, Aging, Alzheimer Disease, Cognition
Background
Alzheimer’s disease (AD) is a clinically common and age-associated neurodegenerative disorder that is characterized by deposition of neurofibrillary tangles/senile plaques and pathological characteristics of neuron loss, which finally cause the progressive decline of cognitive function [1,2]. AD is the leading reason for the dementia-related mortality and morbidity of elderly population and affects appropriate 25 million people globally [3,4]. In clinical settings, the etiology of AD is also elusive and AD-modifying strategies remain beyond reach until now [2]. There are 2 main approved strategies for AD, including N-methyl-D-aspartate receptor antagonist memantine and the acetylcholinesterase inhibitors, however, both of which cannot affect the potential pathological processes and only target symptoms [5]. These 2 strategies also mainly target the tangles or senile plaques in pathological processes of AD, and have been proven to be unsuccessful across clinical trials at the late stage of the disease [6]. Therefore, the discovering an approach for clearing the tangles or senile plaques holds great promising for the AD treatment.
Due to the theory of traditional Chinese medicine, acupuncture (Acup) could refurbish the flow of Qi (vital force) by stimulating acupuncture points with fine needles along the energy channels in the organs of human body [7]. Acupuncture has been applied for treating many diseases of internal organs in China for thousands of years, and also has been approved by the World Health Organization (WHO) and US National Institutes of Health (NIH) as a kind of therapeutic strategy for some disorders [8,9]. A previous study [10] reported that acupuncture stimulation mainly targets the autonomic nervous system because acupuncture triggers interconnection between the external somatosensory inputs and the internal organ response. Actually, acupuncture has been reported to be a safe, well tolerated, and effective approach for improving cognitive functions in the AD patients. However, the specific mechanism has not been fully clarified [11–13]. Therefore, in this study, we hypothesized that acupuncture might improve cognitive functions and explored the protective mechanism that might be triggered by acupuncture in SAM-P8 mice.
A previous study [14] described that phosphatidylinositol 3 kinase (PI3K)/phosphoinositol-dependent kinase 1 (PDK1)/novel protein kinase C (nPKC)/Rac 1 was involved in the pathological processes and neuro-toxicity of neurofibrillary tangles/senile plaques in AD mice. Therefore, in the present study, we employed the senescence-accelerated prone 8 (SAM-P8) mouse model [2,15,16], acting as a model of AD-like dementia and accelerated aging, to investigate the effects of the acupuncture strategy on the cognitive function and associated mechanisms.
Material and Methods
Animals and trial grouping
The SAM-P8 mice (male, weighting from 25 to 20 g) and the senescence accelerated mouse resistant 1 (SAM-R1) mice (male, weighting from 25 to 20 g) were provided by Professor Hui Xie at The First People’s Hospital of Chenzhou, Hunan, China. All of the mice were housed with the conditions of 55±5% humidity, 22±1°C, 12-hour light/12-hour dark cycle and free access to the food and water. This study was approved by the Ethics Committee of The First People’s Hospital of Chenzhou, Hunan, China. All of the procedures were performed according to the Guidelines for National Institute of the Health Animal Care and Use Committee.
The SAM-P8 mice were randomly divided into 3 groups, including SAM-P8 control group (P8-CN, n=3), SAM-P8 administrating with acupuncture group (P8-Acup, n=3), and SAM-P8 administrating without acupuncture group (P8-Sham, n=3). Meanwhile, SAM-R1 mice were employed as the control group (R1-CN, n=3).
Acupuncture administration
Before acupuncture administration, the mice were anaesthetized by intraperitoneally injecting with 3.5% chloral hydrate (Shanghai Aladdin Bio-Chem. Tech. Co. Ltd., Shanghai, China) at final concentration of 5 mL/kg body weight. Then, the needles (Mode: 0.2×7 mm, Taixing Tianhe Med. Ins. Co. Ltd., Taixing, China) were inserted into Tanzhong point, Zhongwan point, Guanyuan point, Xuehai point, and Zusanli point (bilateral), at a depth of 2 to 3 mm (Figure 1). Acupuncture needles were slowly rotated for 30 seconds every 5 minutes and lasted for 15 minutes. For the sham mice, the sites under the seasonal ribs were selected as the non-acupuncture points. The aforementioned administration of acupuncture was conducted once a day, for continuous 15 days and with an interval at day 8. In this study, the acupuncture administration was invented and performed by a Traditional Chinese medical doctor, Prof. Jingxian Han, who had many years of experience.
Figure 1.
Detailed procedures for administrating acupuncture and hippocampal tissues isolating.
Morris water maze test
In this study, the Morris water maze test was performed following the method reported by de Senna et al. [17]. Briefly, the locomotor activity and spatial memory were tested with a water maze circular pool (with diameter of 150 cm, height of 50 cm, filling with water to 30 to 40 cm depth). Meanwhile, a circular platform with diameter of 8 cm was also hided at 1 cm under the water surface. The water maze circular pool was divided into 4 equally-sized quadrants, and the platform could be placed in each quadrant. The water temperature was designed from 23°C to 25°C and the water injection and drainage equipment worked better. Finally, the number of line crossing, total distance travelled and number of entries to IL zone were evaluated and analyzed. All of the data were captured with a camera placed over the water maze circular pool and traveling track of mice were collected by using video tracking software (Mode: EthoVision XT, Noldus Information Tech., Wageningen, Netherlands).
PDK1/nPKC/Rac1 inhibitor treatment and trial grouping
In this study, the specific inhibitors for the PDK1 (OSU03012) [18], nPKC (Rottlerin) [19], and Rac 1 (NSC23766) [20] were used to treat the SMA-R1 and SMA-P8 mice. The total of the other 45 SAM-P8 mice were divided into 15 groups, including SMA-P8 control group (SMA-CN), SMA-P8 mice treated with OSU03012 (P8+OSU03012, Selleck Bio, Houston, TX, USA), SMA-P8 mice treated with Rottlerin (P8+Rottlerin, Sigma-Aldrich, St. Louis, MO, USA), SMA-P8 mice treated with NSC23766 (P8+USC23766, Selleck Bio, Houston, TX, USA), SMA-P8 mice treating with saline (P8+Saline, Hebei Tiancheng Pharm Co., Ltd., Shijiazhuang, China), SMA-P8 administrating with acupuncture (P8+Acup), P8+OSU03012 mice administrating with acupuncture (P8+OSU03012+Acup), P8+Rottlerin mice administrating with acupuncture (P8+Rottlerin+Acup), P8+USC23766 mice administrating with acupuncture (P8+USC23766+Acup), P8+Saline mice administrating with acupuncture (P8+Saline+Acup), SMA-P8 OSU03012 stimulating non-acupuncture points (P8+Sham), P8+OSU03012 mice stimulating non-acupuncture points (P8+OSU03012+Sham), P8+Rottlerin mice stimulating non-acupuncture points (P8+Rottlerin+Sham), P8+USC23766 mice stimulating non-acupuncture points (P8+Sham+Acup), P8+Saline mice stimulating non-acupuncture points (P8+Saline+Sham) group. Meanwhile, the SAM-R1 mice were employed as the control group (R1-CN, n=3).
Brain stereotaxic localization and drug administration
All of the mice were placed in a stereotaxic head holder (Mode: JK023, JYKCSolar, Beijing, China) and anesthetized by using 3.5% chloral hydrate (Shanghai Aladdin Bio-Chem. Tech. Co. Ltd., Shanghai, China) at final concentration of 5 mL/kg body weight. For the cerebral drug administration, the skull was exposed and the high-speed cranial drill (Mode: ZH-GSZ, Anhui Zhenghua Bio. Ins. Co. Ltd., Hefei, China) was used to direct toward the nucleus ventralis posterolateralis and penetrate the skull (lateral to midline 1.5 mm, anterior-posterior to bregma −0.6 mm, ventral to skull surface −1.7 mm). The drugs (including OSU03012, Rottlerin, USC23766 and saline) were microinjected into the ventriculus dexter cerebri at speed of 0.5 μL/minute and dosage of 3 μL per mouse. Post injecting the syringe stayed for 2 minutes to avoid overflow of drugs. Finally, the syringe was removed, the bone wax was smeared, and the outer skin was sutured.
Transmission electron microscope analysis
The hippocampal tissues were fixed with 4% glutaraldehyde (Beyotime Biotech. Shanghai, China) for 2 hours and with 1% osmium tetroxide (OsO4) for 25 to 30 minutes, and washed with phosphate-buffered solution (PBS, ZSGB Bio. Tech., Beijing, China) for 2 times, 10 minutes per time. Then, the hippocampal tissues were dehydrated in graded ethanol (Sigma-Aldrich, St. Louis, MO, USA), including 50% and 70% ethanol for 1 time, 90% ethanol for 2 times and 100% ethanol for 3 time, for 12 minutes per time. The hippocampal tissues were continuously embedded in acetone and embedding regent (Cat. No. EPON812, Sigma-Aldrich, St. Louis, MO, USA). The hippocampal tissues were sliced into sections with ultramicrotome (Mode: OMU3, Leica Reichert, Germany) and stained with uranyl acetate (Beyotime Biotech. Shanghai, China) and lead citrated (ZSGB Bio. Tech., Beijing, China). Eventually, the tissue sections were observed with Philips TECNAI-10 transmission electron microscope (Mode: TECNAI-10, Philips, Holland, Switzerland) as the previous report described [21].
Hematoxylin and eosin (H&E) staining
The hippocampal tissues were isolated and treated by using 4% formaldehyde (Sangon Biotech. Co. Ltd., Shanghai, China) in PBS (ZSGB Bio. Tech., Beijing, China). The histological characteristics of hippocampal tissues were evaluated by using hematoxylin regent (Nanjing Jiancheng Bio. Tech. Co. Ltd., Shanghai, China) and eosin (Beyotime Biotech. Shanghai, China), according to the previously published H&E staining method [22]. Finally, H&E stained hippocampal tissues were observed with an inverted microscope (Mode: IX71, Olympus, Japan). Magnification of H&E staining images, 40×.
Western blot assay
The hippocampal tissues were digested and lysed with radioimmunoprecipitation assay solution (RIPA, Beyotime Biotech., Shanghai, China) and centrifuged at 12 000 r/min for 10 minutes. The obtained products were separated by using 15% SDS-PAGE (Sangon Biotech. Co. Ltd., Shanghai, China) and electro-transferred onto the PVDF membranes (Bio-Rad Laboratories (Hercules, CA, USA) by using a Trans-Blot Electrophoretic Transfer (Mode: 170–3940, Bio-Rad Laboratories). The rabbit anti-mouse monoclonal antibody (1: 3000; Cat. No. ab183957), rabbit anti-mouse PDK1 monoclonal antibody (1: 3000, Cat. No. ab52893), rabbit anti-mouse nPKC monoclonal antibody (1: 3000, Cat. No. ab179523), rabbit anti-mouse Rac 1 polyclonal antibody (1: 2000, Cat. No. ab97732) and rabbit anti-mouse β-actin polyclonal antibody (1: 2000; Cat. No. ab5694) were used to incubate PVDF membranes at 4°C overnight. All of the first antibodies were purchased from Abcam Biotech. (Cambridge, MA, USA). PVDF membranes were incubated with horse radish peroxidase (HRP)-labeled goat anti-rabbit IgG (1: 1000, Cat. No. AQ132P, Sigma-Aldrich, St. Louis, MO, USA) for 2 hours at 37°. Then, the PVDF membranes were treated using the ECL enhanced chemiluminescence detection kit (Cat. No. 32019, Thermo Scientific Pierce, Rockford, IL, USA) at room temperature for 2 minutes in the dark. Finally, western blotting bands were captured and analyzed by using Labworks™ Analysis Software 4.0 (Labworks, Upland, CA, USA).
Statistical analysis
All of the data were represented as the mean ± standard deviation (SD) and analyzed with SPSS software 19.0 (SPSS Inc., Chicago, IL, USA). The Student’s t test was employed to analyze the differences between 2 groups and the one-way ANOVA was used to analyze the differences among the multiple groups. All of the data were obtained from at least 3 independent tests or experiments. A P<0.05 was defined as statistical significance.
Results
Acupuncture inhibited enzymes levels in SAM-P8 mice
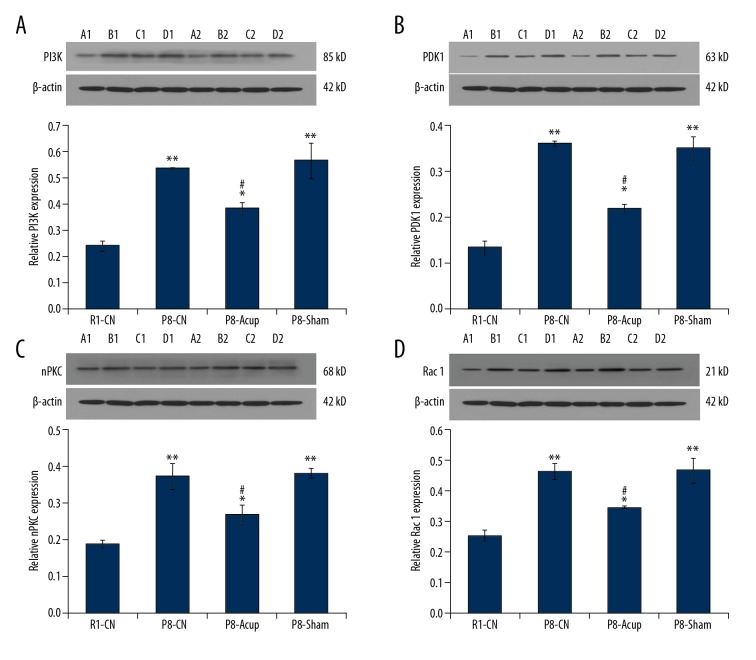
Due to the changes of enzyme activities, such as PI3K, PDK1, nPKC, and Rac 1, that are associated with pathological processes of AD, these enzymes were evaluated in the SAM-P8 mice. The results indicated that the PI3K levels (Figure 2A), PDK1 levels (Figure 2B), nPKC levels (Figure 2C), and Rac 1 levels (Figure 2D) in the P8-CN group were significantly higher compared to the R1-CN group (all P<0.05). Meanwhile, the acupuncture administration significantly reduced the PI3K levels (Figure 2A), PDK1 levels (Figure 2B), nPKC levels (Figure 2C), and Rac 1 levels (Figure 2D) compared to the P8-CN group (all P<0.05), but was also higher compared to the R1-CN group (all P<0.05). Moreover, there were no effects of treatment of P8-Sham group on the enzyme levels compared to the P8-CN group (Figure 2, P>0.05).
Figure 2.
Evaluation for the effects of acupuncture on the PI3K, PDK1, nPKC, and Rac 1 expression by using western blot assay. (A) Western blot and statistical analysis for PI3K expression. (B) Western blot and statistical analysis for PDK1 expression. (C) Western blot and statistical analysis for nPKC expression. (D) Western blot and statistical analysis for PI3K expression. * P<0.05, ** P<0.01 versus R1-CN group. # P<0.05 versus P8-CN group.
Acupuncture improved the cognitive functions of SAM-P8 mice
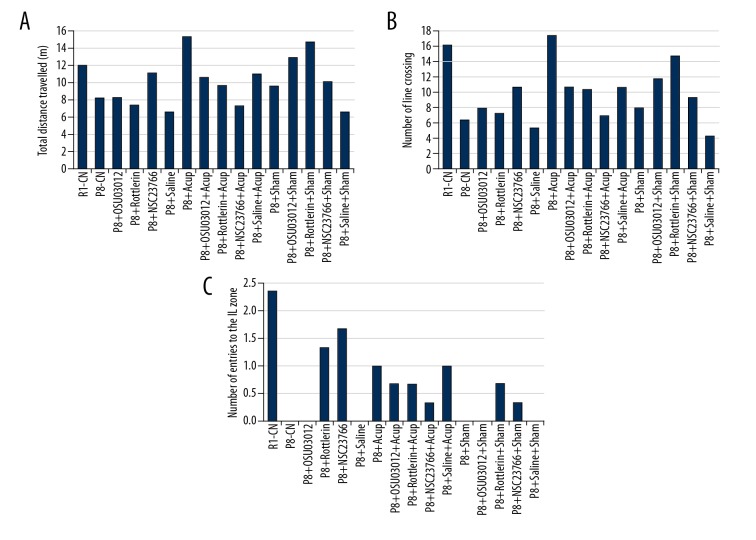
The analysis of the Maze software showed that the acupuncture administrated mice (P8+Acup) presented the enhanced locomotor activity compared with the P8 mice without acupuncture administration (P8-CN) as shown by the increased distance travelled (Figure 3A, P<0.05) and higher number of the line crossing mice (Figure 3B, P<0.05). Meanwhile, acupuncture administration significantly enhanced the number of entries to IL zone compared to that of P8-CN group (Figure 3C, P<0.05).
Figure 3.
Evaluation for the locomotor activity and spatial memory by using a mirror water maze test. (A) Total distance travelled (m). (B) Number of line crossing. (C) Number of entries to the IL zone.
Enzyme inhibitors improved cognitive functions of SAM-P8 mice
According to the aforementioned results that PI3K, PDK1, nPKC, and Rac 1 were involved in the pathogenesis of AD, the inhibitors of these enzymes were administrated to the SAM-P8 mice. The results showed that Rac 1 inhibitor (NSC23766) significantly increased distance travelled (Figure 3A), nPKC inhibitor (Rottlerin) and PDK1 inhibitor (OSU03012) significantly enhanced (Figure 3C) the number of line crossing mice compared with that of the P8-CN group. However, all of 3 inhibitors demonstrated no effect on the number of line crossings in SAM-P8 mice (Figure 3B).
Acupuncture and enzyme inhibitors administration reduced inflammation of hippocampal tissues in SAM-P8 mice
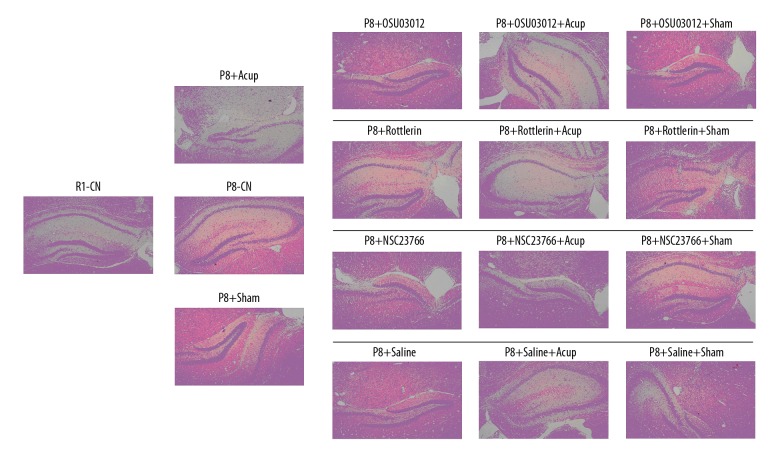
The H&E findings showed that inflammation was obviously decreased in the P8+Acup group compared to the P8-CN group and the P8-Sham group (Figure 4). The single treatment of OSU03012, Rottlerin, and NSC23766 only exhibited slight effects on the inflammation compared to the P8-CN group (Figure 4). Moreover, compared with the P8+CN group, the acupuncture administration demonstrated a slight additional effects on inflammation of OSU03012, Rottlerin, and NSC23766 in SAM-P8 mice (Figure 4).
Figure 4.
Hematoxylin and eosin staining determination for the inflammation of the hippocampal tissues in SAM-P8 mice.
Acupuncture and enzyme inhibitors improved nuclear ultrastructure of neuron in SAM-P8 mice
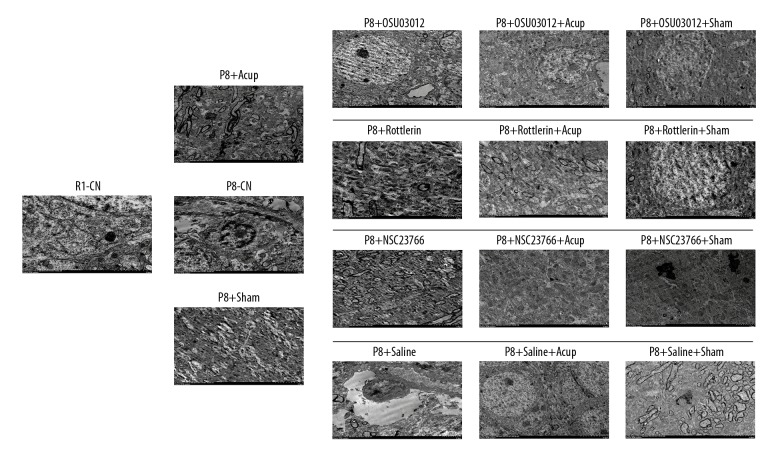
According to the nuclear ultrastructure in Figure 5, the neuron unclear in R1-CN group illustrated complete and regular nucleus, enriched mitochondria, endoplasmic reticulum, Golgi body, and ribosome. However, the neuron unclear in P8-CN group exhibited unregular nucleus, increased heterochromatin condensation in the nucleus, and decreased amounts and damaged structure of organelle. However, acupuncture administration obviously repaired the damages and increased the amounts of organelle, and improved the morphology of the nucleus. Meanwhile, the enzyme inhibitors also remarkably improved the status of the nucleus compared to the P8-CN group. Interestingly, the combining of acupuncture and enzyme inhibitor treatments demonstrated better improvement for nucleus status compared to that in both the single acupuncture and the enzyme inhibitor groups.
Figure 5.
Evaluation for the nuclear ultrastructure of hippocampal tissues in SAM-P8 mice by using the transmission electron microscope analysis.
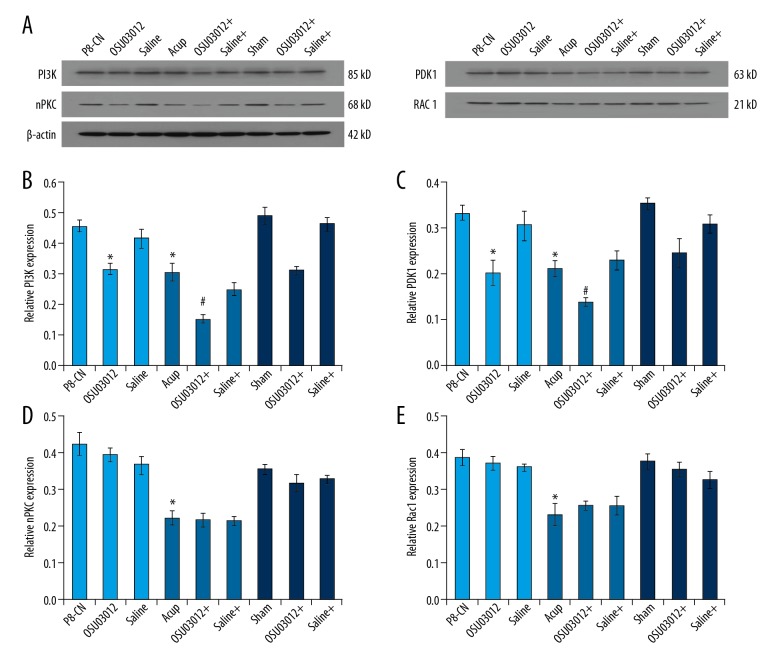
Acupuncture enhanced the down-regulative effects of OSU03012 on PI3K and PDK1 levels
In order to evaluate the assistive effects of acupuncture, the enzyme levels in OSU03012 treated mice were examined by using western blot assay (Figure 6A). The results indicated that both OSU03012 and acupuncture treatment significantly decreased PI3K levels (Figure 6B), PDK1 levels (Figure 6C), nPKC levels (Figure 6D), and Rac 1 levels (Figure 6E) compared to that of the P8-CN group (P<0.05). Meanwhile, acupuncture administration significantly enhanced the down-regulative effects of OSU03012 on PI3K levels (Figure 6B) and PDK1 levels (Figure 6C) compared to that of the single OSU03012 treatment group (P<0.05). However, there were no additional effects of acupuncture on nPKC levels (Figure 6D) and Rac 1 levels (Figure 6E) compared to the OSU03012 treatment group (P>0.05).
Figure 6.
(A) Determination of the PI3K/PDK1/nPKC/Rac 1 signaling pathway molecules in hippocampal by using western blot assay. (B) Western blot assay and statistical analysis for the PI3K expression in hippocampal. (C) Western blot assay and statistical analysis for the PDK1 expression in hippocampal. (D) Western blot assay and statistical analysis for the nPKC expression in hippocampal. (E) Western blot assay and statistical analysis for the Rac 1 expression in hippocampal. * P<0.05 versus R1-CN group. # P<0.05 versus P8-Acup group.
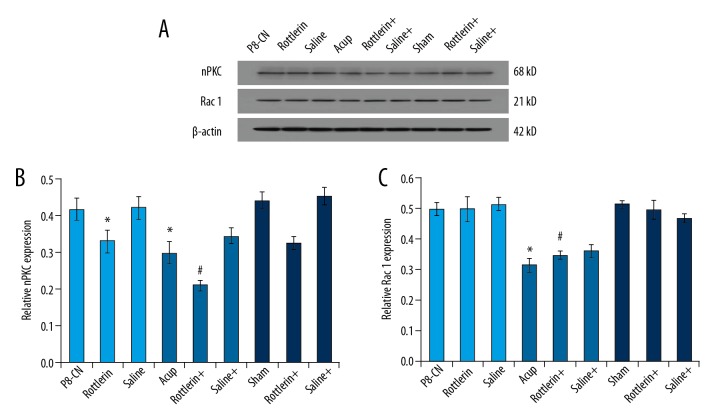
Acupuncture increased the downregulatory effects of Rottlerin on nPKC and Rac 1 levels
The western blot assay (Figure 7A) also illustrated that acupuncture and Rottlerin significantly decreased the nPKC levels (Figure 7B) and Rac 1 levels (Figure 7C) compared to that of the P8-CN group (P<0.05). Furthermore, the acupuncture administration significantly increased the downregulatory effects of Rottlerin on nPKC levels (Figure 7B) and Rac 1 levels (Figure 7C) compared to that of the single Rottlerin treatment group (P<0.05).
Figure 7.
(A) Examination of the nPKC and Rac 1 molecules in hippocampal by using western blot assay. (B) Western blot assay and statistical analysis for the nPKC expression in hippocampal. (C) Western blot assay and statistical analysis for the Rac 1 expression in hippocampal. * P<0.05 versus R1-CN group. # P<0.05 versus P8-Acup group.
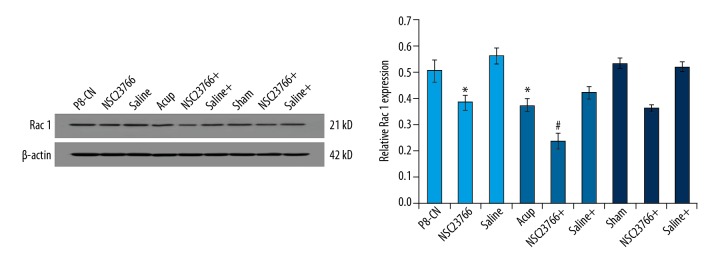
Acupuncture combining Rottlerin suppressed Rac 1 levels
The results indicated that the Rac 1 levels in both acupuncture and Rottlerin treatment group were significantly decreased compared to that of P8-CN group (Figure 8, p<0.05). Meanwhile, the acupuncture administrated significantly enhanced the down-regulative effects of Rottlerin on Rac 1 levels, compared to that of the single Rottlerin treatment group (Figure 8, P<0.05).
Figure 8.
Evaluation for the Rac 1 molecules in hippocampal by using western blot assay. * P<0.05 versus R1-CN group. # P<0.05 versus P8-Acup group.
Discussion
Acupuncture is a critical therapeutic strategy in Traditional Chinese Medicine and has been extensively applied for treating many diseases [23–25]. The previous study [26] reported that acupuncture is a promising approach to relieve cognitive impairment post stroke. However, the neuro-protective effects of acupuncture on cognitive functions and the associated mechanisms have not been fully clarified. In this study, we found that acupuncture administration reduced PI3K, PDK1, nPKC, and Rac 1 levels. Acupuncture enhanced effects of inhibitors on inflammation and nuclear damages and significantly enhanced the down-regulative effects of OSU03012 on PI3K and PDK1 levels, increased down-regulative effects of Rottlerin on nPKC and Rac 1 levels and enhanced effects of Rottlerin on Rac 1 levels.
Aging is the most important risk factor for the pathogenesis of AD [27]. Recent studies [28,29] have shown that abnormalities of the cerebral metabolism in the AD patients were closely associated with the aging process. The hippocampus is the most critical cerebral region associated with the memory and learning in the AD pathological processes [30]. The senescence-accelerated mouse 8 (SAM-8) is a commonly used animal model for aging, which is characterized with a significantly shortened life span compared to that of SAM-R1 mouse [31]. The SAM-P8 mouse exhibits a variety of hippocampus-associated memory and learning deficits during the aging process, according to cognitive technique evaluations [32]. Therefore, in this study, we employed the SAM-P8 mice to investigate the neuro-protective effects of acupuncture on the aging processes.
In the nervous system, the GTPases, belonging to the Rho family, participate in the modulation or control of cytoskeleton dynamics and synaptic plasticity in the neurons [33]. However, the partners of GTPases in the aging process have not been discovered in aging process of AD. In the present study, the results showed that acupuncture administration induced a remarkable Rac 1 down-regulation in SAM-P8 mice, triggering a series of signaling cascades. This study indicated that the PI3K, PDK1, and nPKC levels were also down-regulated in the acupuncture administrated SAM-P8 mice. What was most important, this study identified the PI3K/PDK1/nPKC signaling pathway as a specific upstream regulatory signaling pathway for the Rac 1 in acupuncture administrated SAM-P8 mice. Actually, the PI3K/PDK1/nPKC/Rac 1 signaling pathway has been proven to be the control point for preventing the Aβ1–42 induced toxicity in neurons [14]. To our knowledge, the best-characterized proliferation signaling pathway of neurons is the PI3K/Akt cascade in the neuro-degenerative disorders, such as AD [34]. This study proved that acupuncture activated the PI3K/Akt cascade in the aging process of SAM-P8 mice. PDK1 can also activate and phosphorylate other intracellular signaling pathway associated molecules, such as novel PKC (nPKC) and PKA [35]. Our study showed that nPKC also participated in the neuro-protective effects of acupuncture in the SAM-P8 mice. Therefore, we believed that the PI3K, PDK1, nPKC, and Rac 1 molecules were involved in the neuro-degenerative and aging processes in SAM-P8 mice.
In order to confirm the effects of acupuncture on the PDK1/nPKC/Rac 1 signaling pathway in SAM-P8 mice, inhibitors including OSU03012, Rottlerin, and NSC23766 [14] were administrated to the SAM-P8 mice in this study. The Morris water maze test was performed to evaluate the effects of acupuncture on the memory and learning of SAM-P8 mice undergoing the aforementioned inhibitors treatment. The results showed that acupuncture improved the cognitive functions of SAM-P8 mice by increasing distance travelled and increasing the number of line crossing mice and mice entries to the IL zone. Meanwhile, the enzyme inhibitors also improved cognitive functions of SAM-P8 mice by increasing distance travelled through regulating PDK1, and by enhancing the number of line crossing mice via modulating nPKC and Rac 1. Moreover, the H&E staining results indicated that acupuncture and enzyme inhibitors administration reduced the inflammation of hippocampal tissues in SAM-P8 mice. Compared with the P8+CN group, the acupuncture administration demonstrated a slight additional effects on inflammation of OSU03012, Rottlerin, and NSC23766 in SAM-P8 mice. The transmission electron microscope analysis also illustrated that acupuncture and enzyme inhibitors improved nuclear ultrastructure of neurons in SAM-P8 mice and the combination of acupuncture and enzyme inhibitor treatment demonstrated better improvement for nucleus status. Therefore, the H&E staining and transmission electron microscope results suggest that acupuncture protects the cognitive impairment and alleviates the inflammation and nuclear damage of SAM-P8 mice, all of which have not been discussed in previous studies [36,37].
Furthermore, our study also proved that acupuncture enhanced the down-regulative effects of OSU03012 on PI3K and PDK1 levels, increased down-regulative effects of Rottlerin on nPKC and Rac 1 levels and increased the down-regulative effects of Rottlerin on Rac 1 levels. These results suggest that acupuncture administration improves cognitive functions and alleviates inflammation and nuclear damages by reducing the molecule levels in PI3K/PDK1/nPKC/Rac 1 signaling pathway.
Actually, the non-pharmacological therapeutic approaches have been applied for regulating the hypothalamic-pituitary-adrenal axis. Lanza et al. [38] reported that Shiatsu might improve depression in AD patients, thus enhancing the positive effect of physical activity on depressed mood. However, we have not investigated the effects of acupuncture administration on cognitive dysfunction via the hypothalamic-pituitary-adrenal axis pathway, which was a limitation of this study. Therefore, in future studies, we would also explore the improvement of cognitive functions in AD patients by using other approaches. Meanwhile, this study also had the other limitations. First, abnormalities of cerebral metabolism have been proven to be involved in AD pathogenesis and aging processes, and are risk factors for development and progression of neuro-cognitive disorders [39–42]. Also, acupuncture is known to effect blood pressure levels and contributes to blood pressure regulation [43]. However, the effects of acupuncture on pressure associated cognitive dysfunction have not been explored. Second, inflammatory mechanisms play a critical role in mediating damage of the central nervous system [44]. This study proved the anti-inflammatory effects of acupuncture, however, the peripheral biomarkers of inflammation [44–46], such as neutrophil-to-lymphocyte ratio, ESR, IL-6, were not examined. In future studies, we would verify these aforementioned biomarkers to confirm the effects of acupuncture.
Conclusions
Acupuncture administration significantly improved cognitive functions and alleviated the inflammatory response and nuclear damage in SAM-P8 mice by down-regulating molecules in the PI3K/PDK1/nPKC/Rac 1 signaling pathway. In clinical settings, this study could provide potential insight for treating cognitive dysfunction and aging of AD patients. Further studies investigating the effects of acupuncture on cognitive impairment are required to verify our conclusions.
Footnotes
Source of support: This study was granted by the National Natural Science Foundation Youth Project (Grant No.81403471) and the National Natural Science Foundation General Program (Grant No. 81674070 and 81473766)
Conflict of interest
None.
References
- 1.Kitzlerova E, Fisar Z, Lelkova P, et al. Interactions among polymorphisms of susceptibility loci for Alzheimer’s disease or depressive disorder. Med Sci Monit. 2018;24:2599–619. doi: 10.12659/MSM.907202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang J, Cheng X, Zeng J, et al. LW-AFC effects on N-glycan profile in senescence-accelerated mouse prone 8 strain, a mouse model of Alzheimer’s disease. Aging Dis. 2017;8:101–14. doi: 10.14336/AD.2016.0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qian W, Li H, Pan N, et al. Curcumin treatment is associated with increased expression of the N-methyl-D-aspartate receptor (NMDAR) subunit, NR2A, in a Rat PC12 cell line model of Alzheimer’s disease treated with the acetyl amyloid-beta peptide (25–35) Med Sci Monit. 2018;24:2693–99. doi: 10.12659/MSM.906933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hansen HH, Fabricius K, Barkholt P, et al. The GLP-1 receptor agonist liraglutide improves memory function and increases hippocampal CA1 neuronal numbers in a senescence-accelerated mouse model of Alzheimer’s disease. J Alzheimers Dis. 2015;46:877–88. doi: 10.3233/JAD-143090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gauthier S, Feldman HH, Schneider LS, et al. Efficacy and safety of tau-aggregation inhibitor therapy in patients with mild or moderate Alzheimer’s disease: A randomised, controlled, double-blind, parallel-arm, phase 3 trial. Lancet. 2016;388:2873–84. doi: 10.1016/S0140-6736(16)31275-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mullane K, Williams M. Alzheimer’s therapeutics: Continued clinical failures question the validity of the amyloid hypothesis-but what lies beyond? Biochem Pharmacol. 2013;85:289–305. doi: 10.1016/j.bcp.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 7.Tian T, Sun Y, Wu H, et al. Acupuncture promotes mTOR-independent autophagic clearance of aggregation prone proteins in mouse brain. Sic Rep. 2016;6:19714. doi: 10.1038/srep19714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belivani M, Dimitroula C, Katsiki N, et al. Acupuncture in the treatment of obesity: A narrative review of the literature. Acupunct Med. 2013;31:88–97. doi: 10.1136/acupmed-2012-010247. [DOI] [PubMed] [Google Scholar]
- 9.Torres-Rosas R, Yehia G, Pena G, et al. Dopamine mediates vagal modulation of the immune system by electroacupuncture. Nat Med. 2014;20:291–95. doi: 10.1038/nm.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kavoussi B, Ross BE. The meuroimmune basis of anti-inflammatory acupuncture. Integr Cancer Ther. 2007;6:251–57. doi: 10.1177/1534735407305892. [DOI] [PubMed] [Google Scholar]
- 11.Jia Y, Zhang X, Yu J, et al. Acupuncture for patients with mild to moderate Alzheimer’s disease: A randomized controlled trial. BMC Complement Altern Med. 2017;17:556. doi: 10.1186/s12906-017-2064-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou J, Peng W, Xu M, et al. The effectiveness and safety of acupuncture for patients with Alzheimer disease: A systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore) 2015;94:e933. doi: 10.1097/MD.0000000000000933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cao Y, Zhang LW, Wang J, et al. Mechanisms of acupuncture effect in Alzheimer’s disease in animal, based researches. Curr Top Med Chem. 2016;16:574–78. doi: 10.2174/1568026615666150813144942. [DOI] [PubMed] [Google Scholar]
- 14.Manterola L, Hernando-Rodriguez M, Ruiz A, et al. 1–42-beta amyloid peptide requires PDK1/nPKC/Rac 1 pathway to induce neuronal death. Transl Psychiatry. 2013;3:e219. doi: 10.1038/tp.2012.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morley JE, Farr SA, Kumar VB, et al. The SAMP8 mouse: A model to develop therapeutic interventions for Alzheimer’s disease. Curr Pharm Des. 2012;18:1123–30. doi: 10.2174/138161212799315795. [DOI] [PubMed] [Google Scholar]
- 16.Ren HL, Lv CN, Xing Y, et al. Downregulated nuclear factor E2-relatee factor 2 (Nrf2) aggravates cognitive impairments via neuroinflammation and synaptic plasticity in the senescence-accelerated mouse prone 8 (SAMP8) mouse: A model of accelerated senescence. Med Sci Monit. 2018;24:1132–44. doi: 10.12659/MSM.908954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Senna PN, Ilha J, Baptista PP, et al. Effects of physical exercise on spatial memory and astroglial alterations in the hippocampus of diabetic rats. Metab Brain Dis. 2011;26:269–79. doi: 10.1007/s11011-011-9262-x. [DOI] [PubMed] [Google Scholar]
- 18.Alcantara-Hernandez R, Hernandez-Mendez A, Garcia-Sainz JA. The phosphoinositide-dependent protein kinase 1 inhibitor, UCN-01, induces fragmentation: Possible role of metalloproteinases. Eur J Pharmacol. 2014;740:88–96. doi: 10.1016/j.ejphar.2014.06.057. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi K, Murata T, Hori M, et al. Prostaglandin E2 prostanoid EP3 signal induces vascular contraction via nPKC and ROCK activation in rat mesenteric artery. Eur J Pharmacol. 2011;660:375–80. doi: 10.1016/j.ejphar.2011.03.032. [DOI] [PubMed] [Google Scholar]
- 20.Carrizzo A, Forte M, Lembo M, et al. Rac-1 as new therapeutic target in cerebro- and cardio-vascular diseases. Curr Drug Targets. 2014;15:1231–36. doi: 10.2174/1389450115666141027110156. [DOI] [PubMed] [Google Scholar]
- 21.Cai DX, Quan Y, He PJ, et al. Dynamic perfusion culture of human outgrowth endothelial progenitor cells on demineralized bone matrix in vitro. Med Sci Monit. 2016;22:4037–45. doi: 10.12659/MSM.897884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qiu Z, Yang J, Deng G, et al. Angiopoietin-like 4 attenuates brain edema and neurological deficits in a mouse model of experimental intracerebral hemorrhage. Med Sci Monit. 2018;24:880–90. doi: 10.12659/MSM.907939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fu SP, Hong H, Lu SF, et al. Genome-wide regulation of electro-acupuncture on the neural Stat5-loss-induced obese mice. PLoS One. 2017;12:e0181948. doi: 10.1371/journal.pone.0181948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Becel S, Sezgin Y, Akcay F. Evaluation of the effectiveness of acupuncture therapy by verbal pain scale in patients with abdominal pain of familial mediterranean fever. J Acupunct Meridian Stud. 2016;9:264–66. doi: 10.1016/j.jams.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 25.Wang B, Hu J, Zhang N, et al. [Clinical study of the acupuncture with centro-square needles for knee osteoarthritis]. Zhongguo Zhen Jiu. 2017;37:463–66. doi: 10.13703/j.0255-2930.2017.05.003. [in Chinese] [DOI] [PubMed] [Google Scholar]
- 26.Suh SW, Shin BS, Ma H, et al. Glucose and NADPH oxidase drive neuronal superoxide for formation in stroke. Ann Neurol. 2008;64:654–63. doi: 10.1002/ana.21511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang Y, Xu B, Chen J, et al. Micro-RNA-137 inhibits tau hyperphosphorylation in Alzheimer’s disease and targets the CACNA1C gene in transgenic mice and human neuroblastoma SH-SY5Y cells. Med Sci Monit. 2018;24:5635–44. doi: 10.12659/MSM.908765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takano Y, Kunitoki K, Tatewaki Y, et al. Posterior associative and cingulate Cortex involvement of brain single-photon emission computed tomography (SPECT) imaging in semantic dementia with probable Alzheimer disease pathology: A case report. Am J Case Rep. 2018;19:153–57. doi: 10.12659/AJCR.907799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naulik M, Westaway D, Jhamandas JH, et al. Role of cholesterol in APP metabolism and its significance in Alzheimer’s disease pathogenesis. Mol Neurobiol. 2013;47:37–63. doi: 10.1007/s12035-012-8337-y. [DOI] [PubMed] [Google Scholar]
- 30.Wang H, Lian K, Han B, et al. Age-related alterations in the metabolic profile in the hippocampus of senescence-accelerated mouse prone 8: A spontaneous Alzheimer’s disease mouse model. J Alzheimers Dis. 2014;39:841–48. doi: 10.3233/JAD-131463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takahashi R. [Anti-aging studies on the senescence accelerated mouse (SAM) strains]. Yakugaku Zasshi. 2010;130:11–18. doi: 10.1248/yakushi.130.11. [in Japanese] [DOI] [PubMed] [Google Scholar]
- 32.Ma Q, Qiang J, Gu P, et al. Age-related autophagy alterations in the brain of senescence accelerated mouse prone 8 (SAMP8) mice. Exp Gerontol. 2011;46:533–41. doi: 10.1016/j.exger.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 33.Ramakers GJ, Wolfer D, Rosenberger G, et al. Dysregulation of Rho GTPase in the pix/Arhgef6 mouse model of X-linked intellectual disability is paralled by impaired structural and synaptic plasticity and cognitive deficits. Hum Mol Genet. 2012;21:268–86. doi: 10.1093/hmg/ddr457. [DOI] [PubMed] [Google Scholar]
- 34.Brunet A, Datta SR, Greenberg ME. Transcription-dependent and -independent control of neuronal survival by the PI3K-Akt signaling pathway. Curr Opin Neurobiol. 2011;11:297–305. doi: 10.1016/s0959-4388(00)00211-7. [DOI] [PubMed] [Google Scholar]
- 35.Gao T, Toker A, Newton AC. The carboxyl terminus of protein C provides a switch to regulate its interaction with the phosphoinositide-dependent kinase PDK1. J Biol Chem. 2001;276:19588–96. doi: 10.1074/jbc.M101357200. [DOI] [PubMed] [Google Scholar]
- 36.Du SQ, Wang XR, Zhu W, et al. Acupuncture inhibits TXNIP-associated oxidative stress and inflammation to attenuate cognitive impairment in vasvcular dementia rats. CNS Neurosci Ther. 2018;24:39–46. doi: 10.1111/cns.12773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang J, You X, Liu W, et al. Electroacupuncture ameliorating post-stroke cognitive impairments via inhibition of peri-infarct astroglial and microglial/macrophage P2 purinoceptors-mediated neuroinflammation and hyperplasia. BMC Complement Altern Med. 2017;17:480. doi: 10.1186/s12906-017-1974-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lanza G, Centonze SS, Destro G, et al. Shiatsu as adjuvant therapy for depression in patients with Alzheimer’s disease: A pilot study. Complement Ther Med. 2018;38:74–78. doi: 10.1016/j.ctim.2018.04.013. [DOI] [PubMed] [Google Scholar]
- 39.Lattanzi S, Carbonari L, Pagliariccio G, et al. Neurocognitive functioning and cerebrovascular reactivity after carotid endarterectomy. Neurology. 2018;90:e307–15. doi: 10.1212/WNL.0000000000004862. [DOI] [PubMed] [Google Scholar]
- 40.Lattanzi S, Brigo F, Vernieri F, et al. Visit-to-visit variability in blood pressure and Alzheimer’s disease. J Clin Hypertens (Greenwich) 2018;20:918–24. doi: 10.1111/jch.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lattanzi S, Vernieri F, Silvestrini M. Blood pressure variability and neurocognitive functioning. J Clin Hypertens (Greenwich) 2018;20:645–47. doi: 10.1111/jch.13232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lattanzi S, Luzzi S, Provinciali L, et al. Blood pressure variability in Alzheimer’s disease and frontotemporal dementia: The effect on the rate of cognitive decline. J Alzheimers Dis. 2015;45:387–94. doi: 10.3233/JAD-142532. [DOI] [PubMed] [Google Scholar]
- 43.Li DZ, Zhou Y, Yang YN, et al. Acupuncture for essential hypertension: A meta-analysis of randomized sham-controlled clinical trials. Evid Based Complement Alternat Med. 2014;2014 doi: 10.1155/2014/279478. 279478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lattanzi S, Cagnetti C, Rinaldi C, et al. Neutrophil-to-lymphocyte ratio improves outcome prediction of acute intracerebral hemorrhage. J Neuro Sci. 2018;387:98–102. doi: 10.1016/j.jns.2018.01.038. [DOI] [PubMed] [Google Scholar]
- 45.Lattanzi S, Cagnetti C, Provinciali L, et al. Neutrophil-to-lymphocyte ratio and neurological deterioration following acute cerebral hemorrhage. Oncotarget. 2017;8:57489–94. doi: 10.18632/oncotarget.15423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rubio-Perez JM, Morillas-Ruiz JM. A review: Inflammatory process in Alzheimer’s disease, role of cytokines. ScientificWorldJournal. 2012;2012 doi: 10.1100/2012/756357. 756357. [DOI] [PMC free article] [PubMed] [Google Scholar]