Abstract
Background
We synthetized a 3D printed poly-ɛ-caprolactone (PCL) scaffold with polydopamine (PDA) coating and lithium chloride (LiCl) deposition for cartilage tissue engineering and analyzed its effect on promoting rabbit bone marrow mesenchymal stem cells (rBMSC) chondrogenesis in vitro.
Material/Methods
PCL scaffolds were prepared by 3D printing with a well-designed CAD digital model, then modified by PDA coating to produce PCL-PDA scaffolds. Finally, LiCl was deposited on the PDA coating to produce PCL-PDA-Li scaffolds. The physicochemical properties, bioactivity, and biocompatibility of PCL-PDA-Li scaffolds were accessed by comparing them with PCL scaffolds and PCL-PDA scaffolds.
Results
3D PCL scaffolds exhibited excellent mechanical integrity as designed. PDA coating and LiCl deposition improved surface hydrophilicity without sacrificing mechanical strength. Li+ release was durable and ion concentration did not reach the cytotoxicity level. This in vitro study showed that, compared to PCL scaffolds, PCL-PDA and PCL-PDA-Li scaffolds significantly increased glycosaminoglycan (GAG) formation and chondrogenic marker gene expression, while PCL-PDA-Li scaffolds showed far higher rBMSC viability and chondrogenesis.
Conclusions
3D printed PCL-PDA-Li scaffolds promoted chondrogenesis in vitro and may provide a good method for lithium administration and be a potential candidate for cartilage tissue engineering.
MeSH Keywords: Cartilage, Lithium Chloride, Printing, Tissue Scaffolds
Background
Due to avascularity and low cell density, adult articular cartilage has difficulty performing self-repair when injured. Cartilage tissue engineering offers the ability to repair cartilage by combining cells, scaffolds, and signals [1]. Scaffolds fully replicating the cartilage microenvironment are not yet available.
Three-dimensional printing (3D printing) includes rapid-prototyping and additive manufacturing. It uses a computer-assisted design (CAD) digital model to create physical samples, thus providing scaffolds with precise size, shape, and interconnectivity, as well as accurate materials compositional variations. Various studies have successfully applied 3D printed scaffolds in promoting chondrogenesis of bone mesenchymal stem cells (BMSCs) [2,3]. Further studies improved the precise and accurate 3D structures for cartilage tissue engineering [4].
Superior mechanics, bioactivity, and material processability make poly-ɛ-caprolactone (PCL) a promising material for cartilage tissue engineering [5]. In addition, PCL degrades considerably more slowly than do other aliphatic polyesters in vivo, maintaining initial mechanical support to give enough time for adequate tissue ingrowth [6]. PCL has many successful applications in cartilage regeneration. Besides pure PCL scaffolds, Fu et al. fabricated PCL-PEG-PCL scaffold [7] to improve hydrophilicity. Chen et al. developed positively charged PCL-NH2 [8] that promotes chondrogenesis. However, these modifications may change the mechanical properties and structure of scaffolds.
Surface modification helps improve cellular performance of scaffolds, such as cell adhesion, proliferation, and chondrogenetic differentiation, while maintaining or even improving the physical properties (e.g., mechanical properties) and structure. Unlike traditional surface modification, which usually requires complex preparation steps and rigorous reaction conditions, polydopamine (PDA) coating is widely used and easily prepared. Inspired by adhesive proteins found in mussels, Messersmith [9] first used the innate self-polymerization of dopamine to form PDA film. PDA possesses covalent and non-covalent bonding capabilities for a broad range of organic and inorganic substrates, making it a promising focus of tissue engineering. Subsequently, many studies applied PDA coating on scaffolds, hydrogels, and nanoparticles. Our previous studies [10] have used PDA to bind Vancomycin-loaded polylactic acid-glycolic acid (PLGA) Microspheres applied on PCL scaffolds have produced sustained antibacterial effects in vitro. We [11] also used PDA to introduce a self-assembling peptide hydrogel to the PCL scaffolds, producing superior performance for cartilage and subchondral bone repair.
Lithium chloride (LiCl) has been used to treat bipolar disorder through its regulation of inositol and Wnt signaling pathways [12]. Lithium inhibits glycogen synthase kinase 3 (GSK-3) to downregulate Wnt signaling. Wnt signaling plays an important role in chondrocyte proliferation, differentiation, and phenotype maintenance. Therefore, et al. [13] assessed the function of lithium in cartilage protection, and suggested lithium administration may be a potential therapy for arthritis. Minashima [14] and Thompson [15] also found lithium protects against cartilage degradation in osteoarthritis. Baghaban [16] used lithium as a supplement in human marrow MSC chondrogenic culture, and produced high-GAG-content cartilage mass. As the function of lithium in cartilage became increasingly clear, researchers started to use lithium to modify scaffolds in cartilage tissue engineering. Our previous study [17] introduced lithium into bioceramic by a sol-gel method, and further prepared L2C4S4 scaffolds by 3D printing. This lithium-containing bioscaffolds promoted cartilage maturation in vitro and remarkably accelerated cartilage regeneration in vivo. However, bioceramic has the disadvantage of low fracture resistance, making it less useful in cartilage tissue engineering. Better scaffolds with safe lithium administration are needed.
Given this background, we sought to develop a 3D-printed scaffold with biomimetic surfaces for cartilage regeneration. First, we printed PCL scaffolds by 3D printing with a well-designed CAD digital model, then we modified the scaffold surface by PDA coating to produce a PCL-PDA scaffold, and we deposited LiCl on the PDA coating to produce a PCL-PDA-Li scaffold. Here, we assessed the physicochemical properties, bioactivity, and biocompatibility of PCL-PDA-Li scaffold and compared these with PCL scaffold and PCL-PDA scaffold.
Material and Methods
Preparation of scaffold
3D printing
The 3D properties of PCL (molecular weight 65 000, Sigma, USA) scaffolds were designed using computer-aided design (CAD) software and then printed using a 3D layer-by-layer fused deposition modeling (FDM) 3000 system (Stratasys, Inc., USA), according to our previous studies [11]. Briefly, PCL particles were melted at 120°C in a printing chamber and were printed through a printing head (inner diameter 0.22 mm) with a lay-down pattern of 0°/60°/120° along the z-axis. The moving speed of the printing head was 6 mm/s. Finally, a cylindrical PCL scaffold with a diameter of 4 mm and height of 2 mm was fabricated. The PCL scaffold had a fiber diameter of 220 μm and pore size of 200~300 μm.
PDA coating
We dissolved 60 mg dopamine (sigma, USA) in 10 ml Tris-HCl to make a DA solution (6 mg/ml, pH 8.5). PCL scaffolds were immersed in the DA solution with vigorous stirring (300 rpm) at 37°C for 24 h, protected from light. Then, the scaffolds were washed 3 times with deionized water and then dried in a vacuum oven. These PCL scaffolds coated with PDA are referred to as PCL-PDA scaffolds.
LiCl modification
PCL-PDA scaffolds were modified by LiCl using a one-step process in an aqueous solution via a reaction with the PDA-coated surface. We dissolved 8.2 g LiCl in 10 ml deionized water to make a LiCl solution (19.3 mmol/ml). PCL-PDA scaffolds were then placed into the LiCl solution and kept at 37°C for 5 days. Then, the scaffolds were washed 3 times with deionized water and then in a vacuum oven. These scaffolds are referred to as PCL-PDA-Li scaffolds.
Physicochemical characterization studies
Morphology observation
The gross morphologies of the scaffolds were accessed by shape and color. The microstructures were observed using scanning electron microscopy (SEM, HitachiS-4800, CanScan, Hitachi, Tokyo, Japan).
Surface Hydrophobicity
Static water-contacting angles (WCA) were used to evaluate the hydrophobicity of the scaffolds. Briefly, the same amount of water was dropped onto the surface of each scaffold at room temperature, and the image was captured using a contact angle analyzer (DSA 100 Mk 2, Kruss GmbH, Hamburg, Germany). The WCA was measured as the angle formed between the liquid–solid interface and the liquid–vapor interface.
Mechanical tests
A mechanical testing machine (INSTRON 5566) with movement speed of 1.0 mm/min was used to perform compression testing.
Degradation test and lithium-releasing test
The scaffolds were weighed and immersed in Tris-HCl solution (pH 7.4) in a 37°C shaking water bath at a ratio of 200 ml/g (solution volume/scaffold weight). The scaffolds were taken out after 1, 7, 14, 21, 28, and 35 days, washed twice with deionized water, dried in a vacuum oven, and weighed again. The weight loss of scaffolds was calculated as the percentage of weight of each time point on the initial weight. The Tris-HCl solution was replaced at each time point. For PCL-PDA-Li scaffolds, the Tris-HCl solution was also collected at each time point and the average ionic concentrations of Li+ ions in the Tris-HCl solution were measured by inductively-coupled plasma atomic emission spectroscopy (ICPAES; Varian, USA).
Cell culture studies
Isolation, culturing and incubation of rabbit bone marrow mesenchymal stem cells (rBMSCs)
The use of laboratory animals was approved by the Ethics Commission of Nanjing Medical University. rBMSCs were isolated according to our previous publication [17,18]. rBMSCs were cultured with Dulbecco’s modified Eagle’s medium (DMEM; Gibco), which contains 10% fetal bovine serum (FBS, Gibco), 100 U/mL penicillin, and 100 mg/mL streptomycin (Gibco) in a 37°C, 5% CO2, 95% humidity incubator. Scaffolds were sterilized using 75% ethanol for 3 h and washed with phosphate-buffered saline (PBS) 3 times before seeding. rBMSCs were lysed and resuspended at density of 2×105/ml in a chondrogenetic medium consisting of low-glucose DMEM supplemented with 10 ng/mL recombinant human transforming growth factor-b3 (Sigma-Aldrich), 100 nM dexamethasone (Sigma-Aldrich), 50 μg/mL ascorbic acid 2-phosphate (Sigma-Aldrich), 1 mM sodium pyruvate (Sigma-Aldrich), and ITS Culture Supplement Premix (Life). We added 1 ml of cell suspension to each scaffold. The medium was changed every 2 days.
Scanning electron microscopy (SEM)
The rBMSCs attachment and morphology were assessed 7 days after seeding on the scaffolds, using SEM. rBMSCs/scaffolds composites were fixed in 4% paraformaldehyde, dehydrated in an ethanol series (70%, 80%, 90%, and 100%), and then air dried. After sputter-coating with gold-palladium, samples were scanned and micrographs were obtained by SEM (HitachiS-4800, CanScan, Hitachi, Tokyo, Japan).
Cell viability assessment
The ability of the different scaffolds to support cellular viability was analyzed using a Live/Dead kit (Life Technologies, USA) 7 days after seeding. The scaffolds were washed with phosphate-buffered saline for 5 min to remove extra media. Then, a staining solution of 4IM calcein AM and 2IM ethidium homodimer in PBS was added to the scaffolds and incubated for 30 min at 37°C in the dark. After incubation, the scaffolds were further washed in PBS and were imaged using confocal microscopy (Olympus IX 81, Japan).
Biocompatibility analysis
The biocompatibility of the scaffolds is mainly reflected by cellular attachment and cellular filtration in the structure of the scaffold. The Vybrant® MTT Cell Proliferation Assay Kit (Thermo Fisher Scientific, USA) was used to assess cell proliferation 1, 3, 5, and 7 days after seeding. We added 0.25% trypsin to BMSC/scaffolds composites to get the cells from scaffolds. Then, the solutions were centrifuged at 1200 rpm for 5 min. The supernatant was then removed and 100 μl phenol-free DMEM and 10 μl of MTT reagent were added to the cell pellets. Cell pellets were incubated at 37°C and 5% CO2 for 4 h. Then, 25 μl of dimethyl sulfoxide (DMSO) was added, and cell pellets were incubated for another 20 min. The solution was transferred to 96-well discs. Absorbance readings at 570 nm were recorded using a Benchmark Plus microplate spectrophotometer (Bio-Rad, Tokyo, Japan).
GAG assay
A dimethyl methylene blue (DMMB) GAG assay (Sigma-Aldrich, USA) was used to measure the GAG content of each rBMSCs/scaffolds composites at 7 and 14 days after seeding, as in our previous study [18]. The GAG content was calculated according to a standard curve of sulfate chondroitin from shark cartilage (Sigma-Aldrich, USA) at 530 nm on a Benchmark Plus microplate spectrophotometer (Bio-Rad, Tokyo, Japan) and subsequently divided by its corresponding DNA content to calculate GAG/DNA (mg/ng).
Gene expression evaluation
The chondrogenic gene expression was evaluated 28 days after seeding. RNA was extracted from rBMSCs/scaffolds composites by adding TRIzol Reagent (Life Technologies, USA). The cDNA was reverse-transcribed using SuperScript™ III (Thermo Fisher Scientific, USA). QRT-PCR was performed using a StepOne Real-Time PCR System (Applied Biosystems, USA) with SYBR green reaction mix (Applied Biosystems, USA). Primer sequences are listed in Table 1. GAPDH was used as an internal control for the study. The relative gene expression of each sample was normalized to the corresponding GAPDH and analyzed using the 2−ΔΔCT approach.
Table 1.
Primer sequence for chondrogenic and Wnt signaling markers.
| Species | Gene | Primer sequence | |
|---|---|---|---|
| Rabbit | GAPDH | Forward | 5′-TCACCATCTTCCAGGAGCGA-3′ |
| Reverse | 5′-CACAATGCCGAAGTGGTCGT-3′ | ||
| Rabbit | Col2 | Forward | 5′-AACACTGCCAACGTCCGAT-3′ |
| Reverse | 5′-CTGCAGCACGGTATAGGGA-3′ | ||
| Rabbit | SOX9 | Forward | 5′-GGTGCTCAAGGGCTACGACT-3′ |
| Reverse | 5′-GGGTGGTCTTTCTTGTGCTG-3′ | ||
| Rabbit | Aggrecan | Forward | 5′-AGGTCGTGGTGAAAGGTGTTG-3′ |
| Reverse | 5′-GTAGGTTCTCACGCCAGGGA-3′ | ||
| Rabbit | N-cadh | Forward | 5′-TCATCTTCGTTTCCATTGGA-3′ |
| Reverse | 5′-TAAGAACTCTGTAAGTTTTGGCAGC-3′ | ||
| Rabbit | b-catenin | Forward | 5′-GAAAATCCAGCGTGGACAATGGCTACTC-3′ |
| Reverse | 5′-ACCATAACTGCAGCCTTATTAACC-3′ |
Statistical analysis
All experiments were repeated at least 3 times and data are expressed as the mean ± standard deviation. Multiple sets of data were analyzed by one-way analysis of variance (ANOVA) using SPSS22 software. Differences between 2 groups were analyzed by t test. p<0.05 was considered statistically significant.
Results
Physicochemical properties of scaffolds
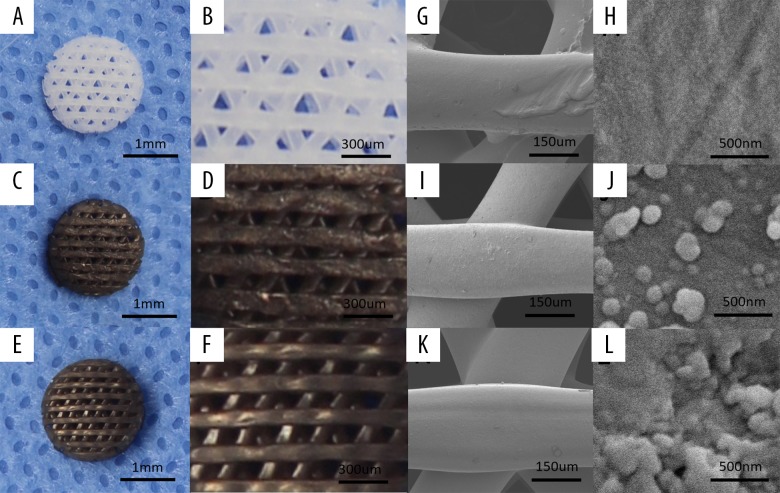
Scaffolds with the designed size and shape were successfully prepared by 3D printing. Pure PCL scaffolds appeared white (Figure 1A, 1B), which is the original color of PCL. After PDA coating, PCL-PDA and PCL-PDA-Li scaffolds appeared brown dark, which is the color of PDA (Figure 1C, 1D). Li deposition did not change the color of the scaffolds (Figure 1E, 1F). SEM clearly showed the structure of the scaffold surface. The differences between the 3 kinds of scaffolds were clearly visible. PCL scaffolds showed a smooth surface (Figure 1G, 1H), while PCL-PDA scaffolds showed small, evenly distributed granules (Figure 1I, 1J). PCL-PDA-Li scaffolds had LiCl crystals attached to the small granules (Figure 1K,1L).
Figure 1.
The morphology and surface microstructure of 3D-printed scaffolds. The lower and higher magnification of digital photographs of PCL (A, B), PCL-PDA (C, D), and PCL-PDA-Li scaffolds (E, F). The corresponding SEM images of PCL (G, H), PCL-PDA (I, J), and PCL-PDA-Li (K, L) scaffolds.
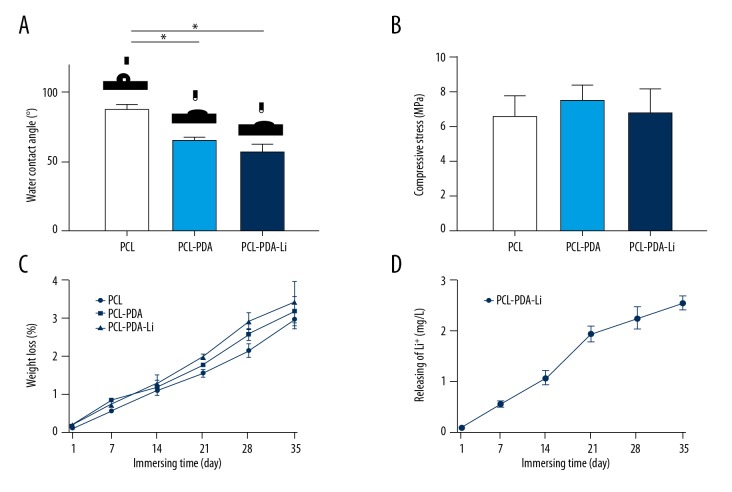
The surface hydrophilicity of scaffolds was determined by water-contact angle. The average WCAs of PCL scaffolds (88.4±3.4°) was still within the range of optimal contact angle for cell adhesion [19]. Lower WCAs of PCL-PDA (66.5±2.2°) and PCL-PDA-Li (57.5±5.1°) scaffolds showed better hydrophilicity (Figure 2A). rBMSCs were cultured in an aqueous environment. Hydrophilic scaffolds like PCL-PDA and PCL-PDA-Li scaffolds can provide better environments for cell adherence, differentiation, and proliferation.
Figure 2.
Characterizations of scaffolds. (A) Static water-contacting angles. (B) Compressive strength. (C) The degradation behavior of PCL, PCL-PDA, and PCL-PDA-Li scaffolds. (D) The average rates of Li+ release at each time point from the PCL-PDA-Li scaffolds (* p<0.05).
The results of mechanical property analysis did not show obvious differences in compressive strength between scaffolds. The compressive strength of scaffolds was 6.60, 7.57, and 6.84 MPa, respectively (Figure 2B). PDA coating and LiCl deposition did not change scaffold mechanical strength.
The degradation profiles showed that the weight loss of pure PCL, PCL-PDA, and PCL-PDA-Li scaffolds immersed in Tris-HCl solution for 5 weeks was 2.8, 3.1, and 3.5%, respectively (Figure 2C). PCL-PDA-Li scaffolds maintained a durable release of Li+ ions (Figure 2D). At day 35, the concentration of Li+ ions was 2.54±0.22 mg/L, which was under the cytotoxicity level.
Cell adherence
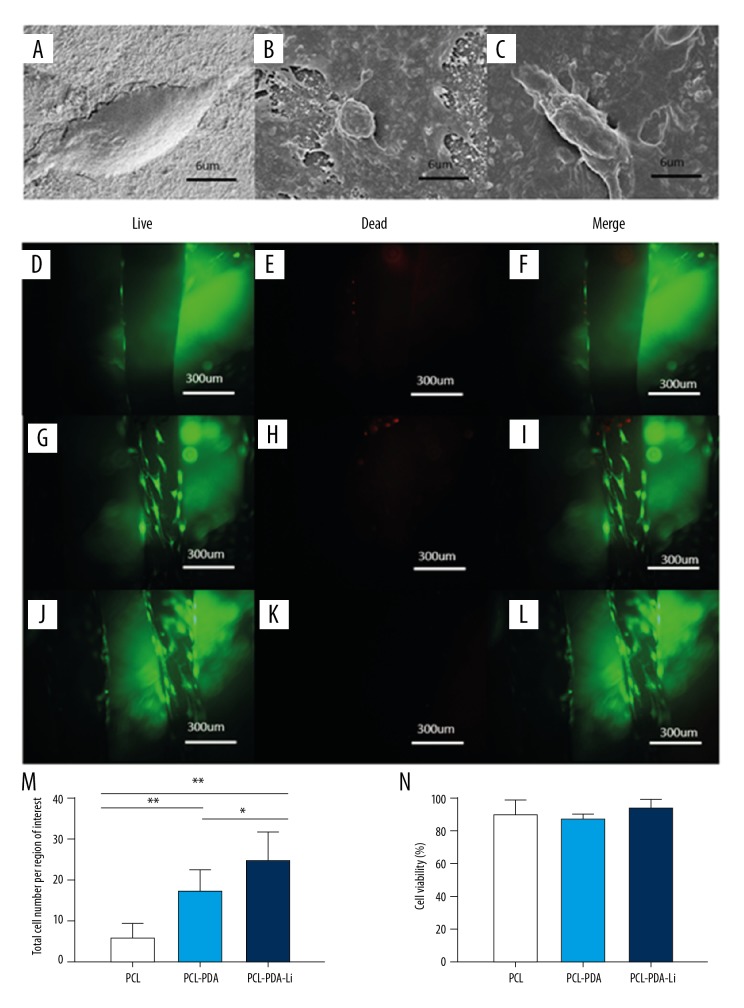
rBMSCs were seeded in different scaffolds. Cell morphology and adhesion in scaffolds were observed by SEM at 7 days after seeding (Figure 3A–3C). Cells attached in PCL scaffolds were flat, while cells in PCL-PDA and PCL-PDA-Li scaffolds had more filopodia.
Figure 3.
SEM micrograph of rBMSCs cultured on the PCL (A), PCL-PDA (B), and PCL-PDA-Li (C) scaffolds for 7 days. Live and dead staining of rBMSCs cultured on the PCL (D–F), PCL-PDA (G–I), and PCL-PDA-Li (J–L) scaffolds for 14 days. (M) Total cell number attached on the scaffolds. (N) Quantification of live and dead cells on the scaffolds. (* p<0.05, ** p<0.01)
Cell viability assessment
Cell viability was assessed by live/dead assay at 14 days after seeding rBMSCs on scaffolds. Live cells were found on all scaffolds. PCL-PDA (Figure 3G–3I) and PCL-PDA-Li scaffolds (Figure 3J–3L) had obviously more cells attached than did PCL scaffolds (Figure 3D–3F). Although there were more live cells attached on PCL-PDA-Li scaffolds (Figure 3M), there were no differences in the ratio of live cells (cell viability) among the 3 groups (Figure 3N).
Biocompatibility analysis
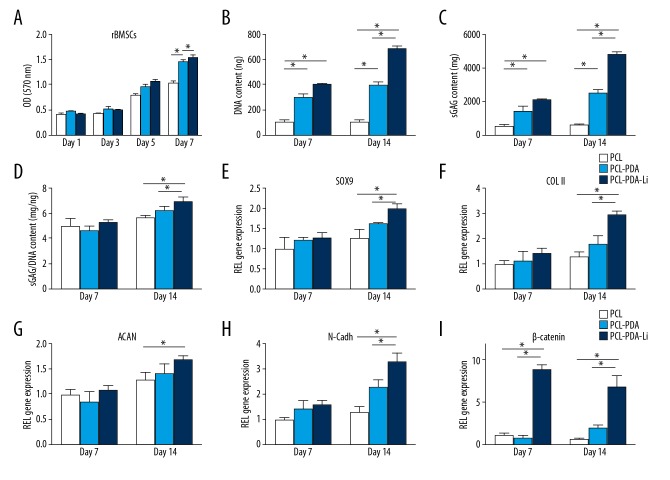
The proliferation of rBMSCs on scaffolds was assessed by MTT assay at 1, 3, 5, and 7 days after seeding (Figure 4A). As all scaffolds were prepared by 3D printing, the designed pore size and structure was suitable for cell attachment and nutrient transmission. All scaffolds showed a time-dependent increase in cell numbers. Our results showed higher cell proliferation in PCL-PDA-Li scaffolds at 5 and 7 days and higher cell proliferation in PCL-PDA scaffolds at 7 days, compared to PCL scaffolds.
Figure 4.
(A) Biocompatibility analysis of the scaffolds by MTT. DNA (B) and GAG (C) production and GAG/DNA (D) by rBMSCs cultured on scaffolds (* p<0.05). (E–I) The gene expression of rBMSCs cultured on the PCL, PCL-PDA, and PCL-PDA-Li scaffolds at 7 and 14 days. All data were normalized to the corresponding GAPDH value (* p<0.05).
GAG assay
The DNA contents and GAG production were quantified at 7 and 14 days after seeding rBMSCs on scaffolds. The DNA content and GAG production increased with time in all scaffolds and was higher in PCL-PDA and PCL-PDA-Li scaffolds than in PCL scaffolds (Figure 4B, 4C). After normalizing GAG content by the corresponding DNA, the sGAG/DNA content in the PCL-PDA-Li scaffolds was higher than that in the PCL and PCL-PDA scaffolds at 14 days (Figure 4D).
Gene expression evaluation
To investigate the chondrogenic effect of scaffolds on rBMSC, typical chondrogenic differentiation markers (SOX9, collagen II, aggrecan, and aggreca) and β-catenin were assessed at 7 and 14 days after seeding and culturing in chondrogenic medium. All chondrogenic gene expression markers (SOX9, collagen II, aggrecan, and aggreca) showed increasing trends in all scaffolds (Figure 4E–4H). SOX9, collagen II, and N-cadh gene expression levels were higher in PCL-PDA-Li scaffolds than in PCL and PCL-PDA scaffolds at 14 days. Aggrecan gene expression was higher in PCL-PDA-Li scaffolds than in PCL scaffolds at 14 days. β-catenin gene expression levels (Figure 4I) were higher in PCL-PDA-Li scaffolds than in PCL and PCL-PDA scaffolds at 7 and 14 days, although it decreased at 14 days.
Discussion
Although lithium chloride has been shown to have cartilage protection and chondrogenesis effects, a safe and effective method of lithium administration is still lacking. This study mainly aimed to develop a better lithium-releasing scaffold for cartilage tissue engineering.
The 3 critical elements for tissue regeneration are scaffolds, cells, and growth factors. Scaffolds are especially important as they provide a suitable environment for cell seeding cells and tissue regeneration. Various in vitro and in vivo studies have focused on scaffolds for cartilage regeneration and have had some success [20], but long-term results are still controversial, as chondrocyte phenotype alteration and fibrocartilage formation occur [21]. Improvement of scaffolds is needed to provide a better environment for chondrogenesis. Due to the poor expansion capacity, chondrocytes are not an ideal choice for cartilage tissue engineering. BMSCs have an extraordinary potential for proliferation and multipotential differentiation, including chondrogenesis [22], can be easily harvested by bone marrow aspirates, and can avoid immunoreaction due to the autologous source. Furthermore, the use of BMSCs better replicates the clinical scenario of microfracture, where bone marrow is induced to the defect. BMSCs are the main functional cells in bone marrow. Therefore, we chose to use rBMSCs, rather than chondrocytes, for the present study.
3D-printed PCL scaffolds with good biofunction and cell-scaffold interaction have been widely used in cartilage engineering [23,24]. Based on this, we successfully prepared a PCL-PDA-Li scaffold by a 2-step immersion method after 3D printing. Scaffolds created by CAD 3D printing have the specific parameters suitable for cartilage regeneration. With a designed pore size of about 200~300 μm, it is suitable for cell immigration, adhesion, and the exchange of nutrients. With a compressive strength around 6.84 MPa, it is suitable to withstand the forces on the joint. PDA coating improved the low surface hydrophilicity of PCL, while retaining the biodegradation ability of PCL. Lithium ion was durably released and the accumulated concentration ([Li+]=3.4 μg/mL for 28 days) was below the cytotoxicity level [19]. We also performed MTT Live/Dead analysis to further reveal that the released Li+ had no cytotoxicity.
The first foundational step for chondrogenesis of BMSCs is cell condensation. Scaffolds with PDA coating had better cell adhesion, while PCL-PDA-Li scaffolds showed the best cell adhesion, indicated by SEM scan and highest expression of cell adhesion molecules (N-cadherin). This is consistent with the bioadhesive capacity of PDA [9]. Li can further promote cell adhesion by modulating primary cilia [25].
Inducing molecules is another important factor in chondrogenesis. Lithium chloride has been shown to prevent cartilage degradation [26] and improve cartilage differentiation [27]. Many studies have tried to find safer and more effective methods of lithium administration. Our team previously used lithium in 3D-printed bioceramic scaffolds and found both chondrogenesis and osteogenesis bioactivity in vitro and a dual osteochondral regeneration effect in vivo [17]. Siwei Li et al. [28] developed a Sol-gel-derived lithium-releasing glass and induced chondrogenic differentiation in vivo. Juliana et al. [29] reported that lithia-silica glass-ceramics enhanced osteogenic effects in vitro. However, bioceramics and bioglasses have the disadvantages of high fragility and low biodegradation, and release of multiple ions makes it hard to fully analyze the synergy effect and avoid adverse effects. In contrast, PCL has better compressive strength and biodegradation, which makes it a better material for tissue engineering scaffolds. With PDA coating, lithium chloride is successfully added to the scaffold. Although scaffolds provide better compressive strength, it is important to perfectly induce new matrix deposition, of which the main part is GAG. In our study, PCL-PDA-Li scaffold had highest GAG production, which is consistent with previous studies [30,31]. Q-PCR revealed that the PCL-PDA-Li scaffold had highest expression of type II collagen, aggrecan, and SOX9. SOX9 can upregulate the expression of type II collagen and aggrecan. The enhanced expression of type II collagen and aggrecan provides better matrix synthesis for cartilage repair. Of the many signaling pathways lithium can affect [32,33], Wnt signaling is the most important in cartilage metabolism. Previous studies revealed lithium can accumulate activated β-catenin by inhibition of GSKβ [34], and higher β-catenin expression level was also observed in the PCL-PDA-Li scaffold in our study.
Conclusions
We successfully synthesized PCL-PDA-Li scaffolds by 3D printing following a simple 2-step method. PDA coating and LiCl deposition improves surface hydrophilicity without sacrificing mechanical strength. In vitro experiments showed that PCL-PDA-Li scaffolds promote better chondrocyte adhesion and cartilage matrix deposition. Our results demonstrate a better method for lithium administration and present a promising scaffold for cartilage tissue engineering.
Footnotes
Source of support: This study was supported by the National Natural Science Foundation of China (81601612, 81771985), the Key Research Program of Science & Technology Support Program of Jiangsu Province (BE 2015613, BE 2016763), and the Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYLX16_1103)
References
- 1.Armiento AR, Stoddart MJ, Alin M, et al. Biomaterials for articular cartilage tissue engineering: Learning from biology. Acta Biomater. 2018;65:1–20. doi: 10.1016/j.actbio.2017.11.021. [DOI] [PubMed] [Google Scholar]
- 2.Guo T, Noshin M, Baker HB, et al. 3D printed biofunctionalized scaffolds for microfracture repair of cartilage defects. Biomaterials. 2018;185:219–31. doi: 10.1016/j.biomaterials.2018.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wei B, Yao Q, Guo Y, et al. Three-dimensional polycaprolactone-hydroxyapatite scaffolds combined with bone marrow cells for cartilage tissue engineering. J Biomater Appl. 2015;30:160–70. doi: 10.1177/0885328215575762. [DOI] [PubMed] [Google Scholar]
- 4.Noreikait A, Antanaviciute I, Mikalayeva V, et al. Scaffold design for artificial tissue with bone marrow stem cells. Medicina (Kaunas) 2017;53:203–10. doi: 10.1016/j.medici.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Makris EA, Hadidi P, Athanasiou KA, et al. The knee meniscus: Structure-function, pathophysiology, current repair techniques, and prospects for regeneration. Biomaterials. 2011;32:7411–31. doi: 10.1016/j.biomaterials.2011.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kweon H, Yoo MK, Park IK, et al. A novel degradable polycaprolactone networks for tissue engineering. Biomaterials. 2003;24:801–8. doi: 10.1016/s0142-9612(02)00370-8. [DOI] [PubMed] [Google Scholar]
- 7.Fu N, Liao J, Lin S, et al. PCL-PEG-PCL film promotes cartilage regeneration in vivo. Cell Prolif. 2016;49:729–39. doi: 10.1111/cpr.12295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen M, Xu L, Zhou Y, et al. Poly(caprolactone)-based substrates bearing pendant small chemical groups as a platform for systemic investigation of chondrogenesis. Cell Prolif. 2016;49:512–22. doi: 10.1111/cpr.12272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee H, Dellatore SM, Miller WM, et al. Mussel-Inspired surface chemistry for multifunctional coatings. Science. 2007;318:426–30. doi: 10.1126/science.1147241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou Z, Yao Q, Li J, et al. Antimicrobial activity of 3D-printed poly(ɛ-caprolactone) (PCL) composite scaffolds presenting vancomycin-loaded polylactic acid-glycolic acid (PLGA) microspheres. Med Sci Monit. 2018;30:6934–45. doi: 10.12659/MSM.911770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li L, Li J, Guo J, et al. 3D molecularly functionalized cell-free biomimetic scaffolds for osteochondral regeneration. Adv Funct Mater. 2019;29:1807356. [Google Scholar]
- 12.Williams R, Ryves WJ, Dalton EC, et al. A molecular cell biology of lithium. Biochem Soc Tran. 2004;32:799–802. doi: 10.1042/BST0320799. [DOI] [PubMed] [Google Scholar]
- 13.Hui W, Litherland GJ, Jefferson M, et al. Lithium protects cartilage from cytokine-mediated degradation by reducing collagen-degrading MP production via inhibition of the P38 mitogen-activated protein kinase pathway. Rheumatology (Oxford) 2010;49:2043–53. doi: 10.1093/rheumatology/keq217. [DOI] [PubMed] [Google Scholar]
- 14.Minashima T, Zhang Y, Lee Y, et al. Lithium protects against cartilage degradation in osteoarthritis. Arthritis Rheum. 2014;66:1228–36. doi: 10.1002/art.38373. [DOI] [PubMed] [Google Scholar]
- 15.Thompson CL, Yasmin H, Varone A, et al. Lithium chloride prevents interleukin-1 induced cartilage degradation and loss of mechanical properties. J Orthop Res. 2015;33:1552–59. doi: 10.1002/jor.22913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baghaban M, Karimi N, Shahhoseini M, et al. Enhancement of glycosaminoglycan-rich matrix production in human marrow-derived mesenchymal stem cell chondrogenic culture by lithium chloride and SB216763 treatment. Cell J. 2011;13:117–26. [PMC free article] [PubMed] [Google Scholar]
- 17.Xu Y, Xu G, Tang C, et al. Preparation and characterization of bone marrow mesenchymal stem cell-derived extracellular matrix scaffolds. J Biomed Mater Res Part B. 2015;103:670–78. doi: 10.1002/jbm.b.33231. [DOI] [PubMed] [Google Scholar]
- 18.Tang C, Jin C, Xu Y, et al. Chondrogenic differentiation could be induced by autologous bone marrow mesenchymal stem cell-derived extracellular matrix scaffolds without exogenous growth factor. Tissue Eng Part A. 2016;22:222–32. doi: 10.1089/ten.TEA.2014.0491. [DOI] [PubMed] [Google Scholar]
- 19.Tamada Y, Ikada Y. Cell adhesion to plasma-treated polymer surfaces. Polymer. 1993;34:2208–12. [Google Scholar]
- 20.Fisher MB, Henning EA, Soegaard NB, et al. Maximizing cartilage formation and integration via a trajectory-based tissue engineering approach. Biomaterials. 2014;35:2140–48. doi: 10.1016/j.biomaterials.2013.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ng JJ, Wei Y, Zhou B, et al. Recapitulation of physiological spatiotemporal signals promotes in vitro formation of phenotypically stable human articular cartilage. Proc Natl Acad Sci. 2017;114:25–61. doi: 10.1073/pnas.1611771114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tian S, Yan Y, Qi X, et al. Treatment of type II collagen-induced rat rheumatoid arthritis model by interleukin 10 (IL10)-mesenchymal stem cells (BMSCs) Med Sci Monit. 2019;25:2923–34. doi: 10.12659/MSM.911184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dao T, Vu N, Pham L, et al. In vitro production of cartilage tissue from rabbit bone marrow-derived mesenchymal stem cells and polycaprolactone scaffold. Adv Exp Med Biol. 2018 doi: 10.1007/5584_2017_133. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 24.Zhang Z, Wang S, Zhang J, et al. 3D-printed poly(ɛ-caprolactone) scaffold augmented with mesenchymal stem cells for total meniscal substitution: A 12- and 24-week animal study in a rabbit model. 2017;45:1497–511. doi: 10.1177/0363546517691513. [DOI] [PubMed] [Google Scholar]
- 25.Thompson C, Wiles A, Poole C, et al. Lithium chloride modulates chondrocyte primary cilia and inhibits Hedgehog signaling. FASEB J. 2016;2:716–26. doi: 10.1096/fj.15-274944. [DOI] [PubMed] [Google Scholar]
- 26.Zhang WV, Jullig M, Connolly AR, et al. Early gene response in lithium chloride induced apoptosis. Apoptosis. 2005;10:75–90. doi: 10.1007/s10495-005-6063-x. [DOI] [PubMed] [Google Scholar]
- 27.Yano F, Kugimiya F, Ohba S, et al. The canonical Wnt signaling pathway promotes chondrocyte differentiation in a Sox9-dependent manner. Biochem Biophys Res Commun. 2005;333:1300–8. doi: 10.1016/j.bbrc.2005.06.041. [DOI] [PubMed] [Google Scholar]
- 28.Li S, Maçon A, Jacquemin M, et al. Sol-gel derived lithium-releasing glass for cartilage regeneration. J Biomater Appl. 2017;32:104–13. doi: 10.1177/0885328217706640. [DOI] [PubMed] [Google Scholar]
- 29.Daguano J, Milesi M, Rodas A, et al. In vitro biocompatibility of new bioactive lithia-silica glass-ceramics. Mater Sci Eng C Mater Biol Appl. 2019;94:117–25. doi: 10.1016/j.msec.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 30.Eslaminejad MB, Karimi N, Shahhoseini M, et al. Chondrogenic differentiation of human bone marrow-derived mesenchymal stem cells treated by GSK-3 inhibitors. Histochem Cell Biol. 2013;140:623–33. doi: 10.1007/s00418-013-1121-x. [DOI] [PubMed] [Google Scholar]
- 31.Tanthaisong P, Imsoonthornruksa S, Ngernsoungnern A, et al. Enhanced chondrogenic differentiation of human umbilical cord Wharton’s jelly derived mesenchymal stem cells by GSK-3 inhibitors. PLoS One. 2017;12:0168059. doi: 10.1371/journal.pone.0168059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peng Z, Ji Z, Mei F, et al. Lithium inhibits tumorigenic potential of PDA cells through targeting hedgehog-GLI signaling pathway. PLoS One. 2013;8:61457. doi: 10.1371/journal.pone.0061457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stambolic V, Ruel L, Woodgett JR, et al. Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Curr Biol. 1996;6:1664–68. doi: 10.1016/s0960-9822(02)70790-2. [DOI] [PubMed] [Google Scholar]
- 34.Nemoto T, Kanai T, Yanagita T, et al. Regulation of Akt mRNA and protein levels by glycogen synthase kinase-3beta in adrenal chromaffin cells: Effects of LiCl and SB216763. Eur J Pharmacol. 2008;586:82–89. doi: 10.1016/j.ejphar.2008.02.075. [DOI] [PubMed] [Google Scholar]