Abstract
The bladder urothelium plays an important role of barrier function to prevent influx of urinary toxic substance and bacteria. When there is insult to the urinary bladder, the urothelium will start to regenerate on injury. However, several factors might affect the regenerative function of bladder urothelium, including aging, chronic inflammation, and system diseases such as diabetes and chronic kidney diseases (CKDs). Impairment of bladder mucosal regenerative function might result in defective urothelial cell differentiation as well as barrier function, which might be the underlying pathophysiology of interstitial cystitis/bladder pain syndrome (IC/BPS) and recurrent bacterial cystitis. Our previous immunohistochemistry (IHC) study and electron microscopic study revealed that the loss of normal umbrella cells and defective junction proteins in IC/BPS and recurrent cystitis. Platelet-rich plasma (PRP) has been previously used in many medical aspects as regenerative medicine therapy. PRP is rich in many growth factors and cytokines which modulate the process of inflammation and regeneration in the wound healing process. Recent pilot studies have shown that intravesical PRP injections improve IC symptoms and yield a success rate of 70% at 3 months after treatment. The results highly suggest that PRP injection could improve urothelial regenerative function and reduce chronic inflammation in IC patients. This article reviews recently published researches on the urothelial dysfunction biomarkers, urothelial cell differentiation, and urinary regenerative and inflammatory proteins in patients with IC/BPS or recurrent bacterial cystitis. The pathophysiology of the insufficient urothelial regeneration and differentiation; and chronic inflammation may induce urothelial dysfunction and further affect the regenerative ability of the diseased bladder urothelium in IC/BPS and recurrent bacterial cystitis are discussed.
KEYWORDS: Bladder urothelium, Interstitial cystitis, Platelet-rich plasma
PHYSIOLOGY AND PATHOPHYSIOLOGY OF BLADDER UROTHELIUM
The urinary bladder is an organ of storage of urine and periodic emptying with time. As the bladder is not a vital organ, the bladder urothelium is essentially quiescent. It is estimated that the turnover rate of the human bladder urothelium is as low as 0.12% [1]. When there is insult to the urinary bladder such as toxin, chemical, surgical trauma, or bacterial infection, the urothelium will regenerate readily on injury [2,3,4]. The processes of the bladder urothelial regeneration are initiated from the proliferation of progenitor cells in the basal or intermediate layers, and differentiate to the mature apical umbrella cells. The hexagonal umbrella cells connect each other through tight junction proteins, including claudin and zonula occludens-1 (ZO-1) [5]. The apical urothelial cells secret glycoproteins such as uroplakin (Upk) proteins that restrict permeability of water, solutes, and toxins into the urothelium [6]. The superficial umbrella cells also contribute to the widely variable distension of the bladder mucosal surface through the endocytosis and exocytosis of fusiform vesicles underneath the cell membrane [7]. In normal condition, the bladder urothelium will regenerate within 24 h on insult and complete the urothelial repair within 72 h [5]. The rapid regenerative process enables the injured urothelium to prevent the influx of urinary solutes such as potassium and hydrogen ion into the suburothelium and evoke neurogenic inflammation and pain.
In human bladder urothelium, the apical umbrella cells, intermediate cells, and basal cells can be distinguished by molecular markers. The mature apical cells express low-molecular-weight cytokeratin 20 (Krt20) and several Upk but not the high-molecular-weight cytokeratin Krt5, transcription factor p63, and signaling molecular Sonic Hedgehog (Shh). Intermediate cells express p63, Shh, and Upk, but no Krt5, Krt 14, or Krt20. Basal cells express Krt5, Shh, and p63, but not Upk or Krt20 [4,8]. Heterogeneity of urothelial cells was also noted that around 14% Krt5-positive basal cells also express Krt14, which are considered as important progenitor cells in urothelial regeneration. These combinations of urothelial specific markers can be used to differentiate the maturation of urothelial regeneration in different bladder disorders [9].
PREVIOUS RESEARCHES ON CHRONIC INTERSTITIAL CYSTITIS – DEFECTIVE UROTHELIAL PROLIFERATION DUE TO INFLAMMATION AND ANTIPROLIFERATIVE FACTOR
The pathophysiology of interstitial cystitis/bladder pain syndrome (IC/BPS) has not been well elucidated; denudation of the bladder urothelium has been widely accepted as the common histopathological change of IC/BPS bladders [10]. Increased number of urothelial cell apoptosis and decrease of cell proliferation have been found, which suggest altered homeostasis of IC/BPS bladder urothelium [11,12]. Abnormally, low expression of the tight junction and barrier proteins, such as ZO-1, Upk, and chondroitin, has also been found in IC/BPS bladders [13,14,15]. Increased antiproliferative factor (APF) and lower expression of interleukin-8 have been found in IC/BPS bladders, which might contribute to the pathophysiology of IC/BPS [16,17,18,19]. Altered regeneration and differentiation of urothelium might result in the increase of permeability and decrease of the barrier function of IC/BPS bladder [20]. The increase of urothelial permeability could result in a leaky urothelium and cause bladder irritative symptoms in patients with IC/BPS and other bladder dysfunction [21].
It has been proposed that chronic suburothelial inflammation might be the main cause of urothelial dysfunction in patients with IC/BPS. The inflammation involves the infiltration of several inflammatory cells such as macrophages, eosinophils, and mast cells in IC/BPS urothelium. The eosinophils involved in IC/BPS pathogenesis is also supported by the increase of urinary eosinophil cationic protein in the urine cytology [22,23]. There is also substantive evidence that mitochondria, which are the major cellular organelle responsible for energy provision to all cells, can increase production of reactive oxygen species and are actively involved in the inflammatory signaling and cell apoptosis. In the IC/BPS patients with ESSIC type 3C clinical characteristics, an increase of expression of B-cell and T-cell biomarkers, decrease of expression of urothelial cell markers, focal lymphoid aggregation in the suburothelium, and higher immunoglobulin concentration in urine have been found [24]. Chronic inflammation of suburothelium might alter normal urothelial basal cell proliferation and affect barrier function of the apical urothelium. The apoptotic signaling molecules, including Bax, cleaved caspase-3, and Bad have been found to increase in IC/BPS bladders [25]. Abnormal cell differentiation in the IC/BPS bladder urothelium had also been noted with impaired E-cadherin expression and altered cellular differentiation markers [26]. Previous studies also showed decrease of E-cadherin expression is associated with recurrent bacterial cystitis and IC/BPS in women [27,28,29]. Put together, the pathophysiology of IC/BPS urothelium is likely to initiate with chronic inflammation, followed by an aberrant differentiation program that results in the deficient synthesis of proteoglycans, tight junction proteins, cell adhesive proteins, and bacterial defense molecules such as GP51 [30,31,32].
IMPAIRMENT OF REGENERATIVE ABILITY MIGHT UNDERLIE THE PATHOPHYSIOLOGY OF PATIENTS WITH BLADDER DYSFUNCTION
The role of bladder urothelium is a barrier to prevent injurious stimuli, toxins, or microorganism from invasion into the stroma and upper urinary tract [33]. Histopathological evidence has shown that bladder urothelium was absent or defective in patients with IC/BPS and recurrent urinary tract infection (UTI) [34]. In these diseased bladders, the mature apical umbrella cells were largely replaced by the immature intermediate cells which lack tight cell junction and adhesive proteins. Investigations have demonstrated that altered proliferative ability due to a slower rate of proliferation of the cultured urothelial cells from IC/BPS bladder mucosa [35,36]. Abnormal increase of the APF production was believed to inhibit human urothelial cell proliferation in these diseased bladders [35].
The epithelium integrity in the urinary bladder is regulated by the proliferation and differentiation of the stem cells and progenitor cells. Although the urothelial cells in the bladder mucosa undergo little or no cell division, injury with chemicals, toxins, or bacterial infection induces rapid proliferation [4]. The basal cells of the urothelium include stem cells which can be marked by expression of the secreted protein Sonic Hedgehog (Shh) are considered as the progenitor cells for this urothelial repair on injury [4]. The Shh expression in the basal cells increases on injury to the urothelium, then the stromal expression of Wnt protein signals increase and stimulate urothelial and stromal cell proliferation. After infection by pathogenic Escherichia coli, the proliferative marker Ki67 increased within 24 h and basal urothelial cells proliferation increase to increase the urothelial cell layers, increased exfoliation of the infected apical urothelial cells, in which the intracellular bacterial communities (IBC) are present.
Histopathology of IC/BPS includes mast cell infiltration, suggesting that IC/BPS is mediated by abnormal immune system and result in chronic inflammation [10,37]. There is a consensus that the defects in the urothelial integrity play the most important role in IC/BPS [38]. Our previous study has also revealed that the chronic suburothelial inflammation might contribute to increase of urothelial cell apoptosis [11]. The severity of mast cell infiltration also significantly correlated with urothelial cell apoptosis in the IC/BPS bladders [29]. Moreover, further research had shown that the urothelial cell apoptosis could involve with up-regulation of the pro-inflammatory signals, including p38 mitogen-activated protein kinase and tumor necrosis factor-α [25]. The findings from the study demonstrate that apoptosis is present in the urothelium of patients with recurrent bacterial cystitis and is possibly mediated by the inflammatory pathway. Recently, we also found that Hunner ulcer type IC/BPS bladder urothelium had a higher grade of moderate or severe eosinophil infiltration and urothelium denudation than those in the nonulcer IC/BPS bladders [39]. Compared with nonulcer IC/BPS, the E-cadherin expression in the urothelium was significantly lower, and endothelial nitric oxide synthase expression was significantly higher in Hunner IC/BPS bladder.
Chronic inflammation in the IC/BPS bladder might affect the proliferation ability of these basal cells function, altered proliferation rate and result in defective urothelial apical cells and barrier function [36]. Similarly, in the bladders of recurrent bacterial cystitis, the basal cell proliferative function might also be affected. Decrease proliferation of basal cells result in the continuing presence of IBC, and recurrent cystitis may ensue after cessation of antibiotics treatment [40]. Improvement of basal cell proliferation in diseased bladders such as IC/BPS and recurrent bacterial cystitis is crucial to restore normal bladder mucosal barrier.
UROTHELIAL DYSFUNCTION AND CHRONIC INFLAMMATION ARE HIGHLY PREVALENT IN PATIENTS WITH INCREASED BLADDER SENSATION DUE TO DIFFERENT DISEASES
In addition to the urothelial dysfunction in IC/BPS, our previous studies also revealed that urothelial dysfunction is frequently encountered in patients with sensory bladder dysfunction due to bladder outlet obstruction (BOO), detrusor underactivity (DU), diabetes-associated overactive bladder, recurrent UTI, chronic renal insufficiency, and chronic spinal cord injury (SCI). Increase of urothelial inflammation and increased urothelial cell apoptosis seem to have common pathophysiology of various lower urinary tract dysfunctions (LUTDs) that cause similar bladder symptoms such as frequency urgency and bladder discomfort [41].
In one study of urothelial dysfunction between patients with IC/BPS and overactive bladder (OAB), we have found chronic inflammation of urothelium is involved in both patients with IC/PBS and OAB. Tight junction protein ZO-1 and adhesive protein E-cadherin are down-regulated in IC/PBS, but not in OAB, compared with the controls, suggesting that deficiency of bladder barrier is involved in the pathophysiology of IC/BPS but not OAB. The different urothelial dysfunction also accounts for the difference of clinical characteristics between patients with IC/PBS and OAB [29]. In patients with DU, the urothelial dysfunction increased suburothelial inflammation and altered sensory protein expressions in the bladder mucosa were also prominent. Impaired urothelial signaling and sensory transduction pathways are likely to contribute to the pathophysiology of DU [42]. In patients with BOO, urothelial dysfunction, increase of inflammation and urothelial cell apoptosis, and alterations of sensory proteins are also remarkable. Impaired urothelial signaling and sensory transduction pathway might also be involved in bladder dysfunction and DU in BOO patients [43].
In patients with diabetes associated OAB, the E-cadherin expression, mast cells count, apoptotic cell level, and ZO-1 expression are comparable between patients with and without DM. In addition, M3 muscarinic receptors in the OAB bladders with and without DM were significantly higher than that in the controls [44]. We also found that these urothelial dysfunction and impaired barrier function could contribute to the pathophysiology of recurrent bacterial cystitis in women. It is possible that chronic inflammation might reside in the bladder mucosa after antibiotic treatment, which might contribute to the continuing urothelial dysfunction and defective barrier function. Since the IBC remains and the urothelial exfoliation ability is impaired, bacterial cystitis might recur after cease of antibiotics in these patients [28]. Bladder urothelial dysfunction and chronic inflammation are also noted in patients with end-stage renal disease (ESRD) or CKD. Increase of chronic inflammation markers and defective barrier function are prominent in ESRD/CKD bladders with bladder oversensitivity [45]. In patients with chronic SCI, decrease of urothelial adhesive proteins and junction proteins, and increase of urothelial inflammation and cellular apoptosis have been noted in chronic SCI patients with different injured level [46]. Such mechanisms might contribute to the impairment of urothelial proliferation and regeneration on urothelial insult such as surgical injury or toxin, and cause SCI patients to have recurrent bacterial infections [47].
Chronic stress can exacerbate symptoms of most pain disorders including IC/BPS. In this regard, evidence has shown that chronic stress (animal model) can result in significant changes in urothelial “mitochondrial” functions. This can impact the barrier function and also signaling which may contribute to bladder dysfunction and pain. Shown is example of a cultured urothelial cell and a dye that is associated with the mitochondrial membrane potential (an indicator of mitochondrial and cellular health). In the chronic stress urothelial cell, this is diminished indicative of alteration in urothelial health and function.
Put these findings together, the urothelial dysfunction and underlying chronic inflammation might be the down-stream pathogenesis of increased bladder sensation, bladder pain, and recurrent UTI in the LUTDs due to the bladder, bladder outlet, or systemic diseases. Elimination of chronic inflammation might improve urothelial regeneration and differentiation and rebuilt the defense mechanism of the diseased bladder.
INADEQUATE UROTHELIAL REGENERATION MIGHT BE THE CAUSE OF RECURRENT BACTERIAL CYSTITIS
Recurrent bacterial cystitis is one of the most common diseases which annoyed women and is difficult to treat [48]. According to the the International Urogynecological association/International Continence Society joint report on the terminology for female pelvic floor dysfunction, women with recurrent bacterial cystitis should have at least three symptomatic and clinical diagnosed bacterial cystitis in the past 12 months. Our previous studies have shown that patients with recurrent bacterial cystitis had increased nerve growth factor in urine, suggesting that chronic inflammation is present in the bladder after adequate treatment of cystitis [49]. Therefore, we suggested that the chronic inflammation raised by bacterial cystitis might still reside in the bladder mucosa or submucosa, which lead to urothelial dysfunction and subsequent defective barrier function. Bacterial cystitis might be easier to recur in these patients with persistent and unresolved chronic bladder inflammation [28]. Immunofluorescence staining also showed significantly lower E-cadherin and higher mast cell expression in the bladder tissue of recurrent bacterial cystitis in comparison with the controls. Urothelial cell apoptosis by TUNEL staining also revealed a significantly higher numbers of apoptotic cells in the bladder tissue of recurrent bacterial cystitis compared with the control bladders. The expressions of tryptase and Bax by the western blots also increased in recurrent UTI specimens. These findings all suggest that chronic inflammation, urothelial cell apoptosis, and impairment of barrier function of urothelial cells might contribute to recurrent bacterial cystitis in women.
In bacterial cystitis, the pathogenic E. coli will form IBC in the urothelial cells after antibiotic treatment. In normal condition, bacterial infection will stimulate rapid urothelial regeneration and differentiation, which increases the exfoliation of the urotheial cells and expels the intracellular bacteria cells [40]. In the women who have impaired urothelial regeneration under bacterial infection, this defense mechanism might not be adequate, and the bacteria in IBC might invade into unshed cells and seed a new acute bacterial infection [9]. To eliminate the bacteria hidden in the dysfunctional urothelial cells, improve urothelial regeneration, and differentiation is essential by all means.
ELECTRON MICROSCOPY (EM) STUDY AND IMMUNOHISTOCHEMISTRY RESEARCHES ON UROTHELIAL DYSFUNCTION IN INTERSTITIAL CYSTITIS/BLADDER PAIN SYNDROME AND RECURRENT BACTERIAL CYSTITIS
The urothelial dysfunction has been widely accepted as one of the pathogenesis of IC/BPS and become the therapeutic target [50]. Researches have shown the deficiency of mucosal glycosaminoglycan resulting in increase of urothelial permeability are the main causes for painful bladder symptoms in IC/BPS patients [20,51]. IHC investigation also revealed the cellular tight junction ZO-1 and adhesive protein E-cadherin were down-regaulated in the IC/BPS bladders [29]. In addition, up-regulation of purinergic receptor P2 × 3 and increase of adenosine triphosphate released from urothelium have been found in IC/BPS bladders [52,53]. Currently, the dysfunction of the urothelium has been considered one of the key pathogeneses of IC/BPS.
Electron microscopy (EM) provides ultrastructural investigation of the bladder urothelial cells in patients with IC/BPS and other bladder disorders for >40 years [54]. Deficiency of the cellular tight junction, epithelial cell pleomorphism, microvilli of the urothelial cell membrane, and mast cells in the IC/BPS bladders have been reported in several previous studies [55,56]. In our recent EM study, we also found a significant decrease of the cell layers and decrease of apical umbrella cells in IC/BPS urothelium [34]. Compared with the controls, IC/BPS urothelium had significantly more severely deficient urothelial cell layers and loss of the integrity of apical umbrella cells in transmission EM (TEM). In scanning EM (SEM), increase of umbrella cell pleomorphism and decrease of microplicae of the apical cell membrane are noted in IC/BPS bladders, and both EM defective features are significantly greater than controls. Patients with urothelial deficiency and impaired umbrella cell integrity also had higher degree of bladder pain and smaller maximal bladder capacity (MBC) during cystoscopic hydrodistention. Patients with moderate-to-severe deficiency in microplicae of cell membrane also had significantly lower MBC and cystometric bladder capacity.
These EM findings suggest the urothelial dysfunction might result from defective umbrella cells coverage and cause more severe bladder pain scores in IC/BPS patients. Loss of apical umbrella cells of urothelium results in barrier function deficit, leading to influx of the urinary solutes or ionic substances across the defective urothelium to the suburothelial tissue, and directly elicit bladder pain in IC/BPS patients [14]. The microplicae or ridges in the umbrella cell membrane usually become flatten during bladder distension and may play an important role in the normal bladder physiology [57]. Decrease of microplicae of the apical cell membrane is considered to associate with the immature apical cells (actually, the intermediate cells but not umbrella cells) and may restrict the extent of bladder distention, causing small functional bladder capacity (FBC) and frequency symptom. In addition, the apical cell pleomorphism is also noted on SEM, suggesting the partial loss of umbrella cells and were replaced by the immature intermediate cells. The findings of the EM study suggest the urothelium deficiency, especially in the loss of mature umbrella cells, may play an important role in the pathogenesis of IC/BPS [Figures 1–4].
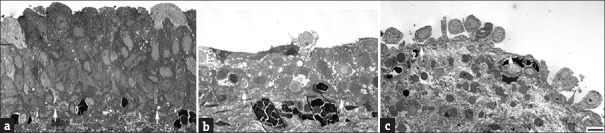
Figure 1.
(a) Normal urothelial cell composition in the control bladder, and (b) defective urothelium and (c) loss of normal cell integrity in the interstitial cystitis/bladder pain syndrome bladder
Figure 4.
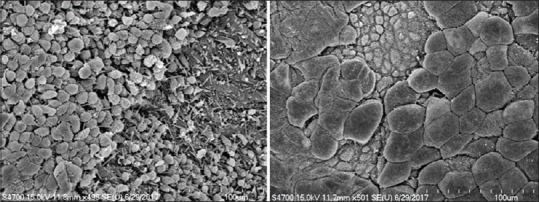
The defective umbrella cell coverage in interstitial cystitis/bladder pain syndrome bladder urothelium
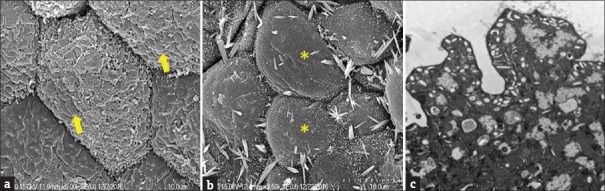
Figure 2.
The normal (a, arrows) and defective (b: *) structure of uroplakins on the umbrella cell surface, and normal fusiform vesicles in control bladder urothelium (c)
Figure 3.
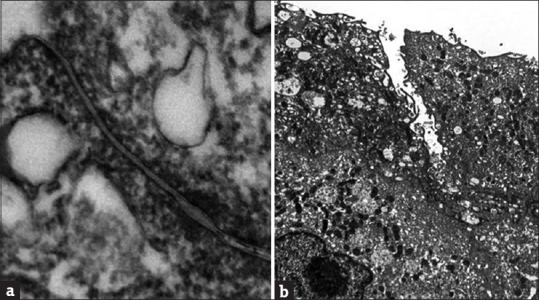
The normal tight junction (a) and defective tight junction in interstitial cystitis/bladder pain syndrome bladder urothelium (b)
THERAPEUTIC EFFICACY OF PLATELET-RICH PLASMA ON SOFT TISSUE REGENERATION AND CHRONIC INFLAMMATION
Urothelial dysfunction resulting in barrier defects, chronic inflammation, increase of urothelial cell apoptosis, nociceptive receptor up-regulation, mast cell activation, and somatic functional syndrome constitute the pathophysiology of IC/BPS [25,37]. In the ultrastructural investigation, loss of mature apical umbrella cells and defects of the cellular junction proteins are the fundamental urothelial dysfunction of IC/BPS [34]. With persistent suburothelial inflammation, the increased urothelial cell apoptosis and decreased cell proliferation result in impaired mucosal integrity and increased urothelial permeability, and causing bladder irritative symptoms [11]. These pathophysiologies of IC/BPS might result from impaired regenerative ability of the urothelial cells. If the progenitor cell regeneration can be improved, the bladder urothelial barrier might be rebuilt and IC/BPS symptoms might also be eliminated.
Platelet-rich plasma (PRP) has been previously used in many medical aspects as a regenerative medicine therapy [58]. PRP is rich in several kinds of growth factors, including platelet-derived growth factor, epidermal growth factor (EGF), and transforming growth factor, the injured tissue cells will increase the rate of proliferation and differentiation with these growth factors and repair the tissue defects, leading to early wound healing [59]. PRP also secrets several kinds of cytokines that can initiate a new inflammatory process and facilitate the resolution of the unsolved inflammation, resulting in eliminating the neurogenic pain caused by the previous old inflammation [60].
In addition to hemostasis and thrombosis, platelets might also modulate tissue inflammation, cell regeneration, and promote wound healing [61]. PRP had been used in the treatment of osteoarthritis through modulation of inflammation by releasing platelet-related growth factors [62]. In addition, PRP has also been demonstrated to promote cell proliferation and skeletal muscle cells migration in vitro [63]. In SCI rats, PRP had also been used to promote tissue angiogenesis and neuron regeneration [64]. In drug-resistant bacterial infected wound, PRP had been shown to improve the healing process [65]. Through the increase of vascular endothelial growth factor (VEGF), PRP has been found to effectively treat avascular necrosis of the femoral head in rabbits [66].
Biologically active molecules can be released from activated platelets during the wound healing process. The rationale for using PRP in treating IC/BPS is the ease of obtaining material, the concentration of PRP, and delivering of bioactive molecules into the bladder wall [59]. Recent research has demonstrated that PRP can act as an immunomodulator of the inflammatory response. Platelet releasing growth factors can promote tissue angiogenesis, increase blood flow, and improve oxygenation in the wound [67]. PRP also contains small amount of stem that might also contribute to the wound healing process [68]. The macrophages and neutrophils recruited by the platelets may also play important roles in modulating the inflammation as well as infection [68]. Platelet-recruited macrophages and neutrophils play important roles in inducing the neural hypersensitivity of the wound, then switching to an anti-inflammatory phenotype and release anti-inflammatory factors [69]. In cyclophosphamide-induced hemorrhage rat model, intravesical instillation of PRP has been shown to increase the mitotic index and decreased bladder bleeding, suggesting that PRP can improve cell proliferation and urothelial deficiency due to chemical cystitis [70]. However, the histological study did not show significant histological changes in single PRP treated rat bladders [71]. The histological changes in effectively treated cystitis model might still occur after repeated PRP treatments.
In addition, PRP has been shown to eliminate the refractory neuropathic pain through the platelet releasing factors which initiate the wound healing process [60]. The effect of PRP on neuropathic pain might start from inducing a new inflammation in the wound healing process, followed by tissue remodeling, axon regeneration, and finally result in the elimination of neuropathic pain and facilitating wound healing [60]. IC/BPS is a condition of inflammation and urothelial defects. We might consider the bladder dysfunction in IC/BPS is an unresolved wound healing process and the defective urothelium as a wound to induce bladder pain, PRP administration into the bladder urothelium could initiate and complete the wound healing process, produce a new inflammation, lead to the relief of neurogenic pain. Put together, the activated PRP might induce a locally new inflammation which may override the unsolved inflammatory, promote wound healing process, and increase tissue regeneration.
The EGF can be produced by the injured urothelial cells to promote cell proliferation and tissue regeneration in the urothelium [72]. Sustained release of fibroblast growth factor 2 from a bladder acellular matrix can promote VEGF production and improve tissue angiogenesis in the graft [73]. Autologous PRP has been widely applied to treat soft tissue injury and promote wound healing [59]. Platelet-released factors can promote angiogenesis and increase blood flow and oxygenation in the wound [67]. PRP also contains small amount of mesenchymal stem cells that may have contribution to wound healing process [68]. It is possible that adding PRP into the suburothelium and release of growth factors might promote urothelial regeneration and repair of the defective urothelium, therefore, the urothelial dysfunction in IC/BPS and recurrent bacterial cystitis could be gradually improved.
POTENTIAL THERAPEUTIC EFFICACY OF PLATELET-RICH PLASMA ON TREATMENT OF RECURRENT BACTERIAL CYSTITIS
The urothelium provides a barrier between urine and the underlying bladder submucosa so that the urinary solutes and toxins cannot penetrate into the circulation [74]. To prevent urine leak in, the bladder urothelium secrets mucus containing highly anionic polysaccharide components including glycosamnoglycans, which are extremely hydrophilic and form a barrier at the interface between urine and the bladder [13]. The highly impermeable urothelium serves as the key barrier for the bladder interstitium. Under several conditions such as trauma, infection or irradiation, the urothelial barrier might be disrupted and result in a cascade of events in the bladder and lead to bladder irritative symptoms. Intravesical instillation of solution containing potassium may depolarize suburothelial nerves and muscles and provoke bladder inflammation and tissue injury [75]. Previous studies have shown that IC/BPS, irradiation cystitis, and bacterial cystitis have a positive potassium chloride sensitivity test and cause symptoms of bladder pain [74]. Conditions that cause mucus deficiency and urothelial dysfunction are likely to be the pathogenesis for the painful bladder syndrome [76].
Recurrent bacterial cystitis is commonly occurred in women. Deficiency of the mucosal barrier and specific factors of uropathogenic bacteria must be present before the bacteria adherence to the urothelium and causing an infection. Uropathogenic E. coli (UPEC) adherence to the bladder urothelium can induce a rapid apoptosis and exfoliation of the terminally differentiated urothelial apical cells. Expression of Upk III is essential in the UPEC-induced urothelial cell death and enhanced urothelial cell differentiation [77]. Currently, there is no definite prophylactic method to prevent recurrent bacterial cystitis in women except continuous antibiotics treatment. Recurrent bacterial cystitis is also commonly seen in patients with IC/PBS and chemical cystitis such as by ketamine or chemotherapeutic agents. Recent studies found the most common finding in the bladder of IC/PBS and ketamine cystitis is denudation of the bladder urothelium, suggesting altered regulation of the urothelial homeostasis might contribute to the bladder diseases [10,78,79]. It is possible that the denuded urothelium provides a chance for UPEC to adhere on the urothelium, and there induce recurrent bacterial cystitis in IC/PBS or ketamine cystitis bladder.
The binding of UPEC to the urothelial mucosal surface is an important initial event for bacterial cystitis because the bacteria can adhere on the urothelial surface without being removed by the micturition. The Upk Ia serves as the receptor on the urothelium for the adherence of Type 1-fimbriated E. coli. The binding of UPEC to Upk Ia may play the key role in mediating the urothelial responses to bacterial adherence and invasion [80]. The UPEC in IBC within the bladder urotheial cells might be persistent as a quiescent or semi-quiescent state regardless of multiple antibiotic treatments [81]. To eradicate these IBC, increase urothelial proliferation, differentiation and maturation to facilitate exfoliation of the most superficial urothelial cells that contain IBC is essential. Treatment based on multiple antibiotics might not be adequate in expelling these UPEC in IBC. In this consideration, intravesical PRP injections might play an important role, especially in the women who are old, frail, immune compromised and having systemic or bladder diseases that interfere normal urothelial regeneration under previous bacterial invasion.
POTENTIAL THERAPEUTIC EFFICACY OF PLATELET-RICH PLASMA ON TREATMENT OF INTERSTITIAL CYSTITIS/BLADDER PAIN SYNDROME
Urothelial dysfunction resulting in barrier deficiency, increased of urothelial cell apoptosis, nociceptive receptors up-regulation, and neurogenic inflammation [25]. Under EM study, loss of apical umbrella cells and defective tight junction are considered the main urothelial dysfunction in IC/BPS [34]. These histopathological changes of the urothelium result in impaired integrity of the urolthelial cell lining and increase bladder wall permeability [11]. Treatment to improve the urothelial progenitor cells regeneration may improve the urothelial barrier function and improve bladder symptoms in IC/BPS.
The animal study revealed intravesical instillation of PRP in rabbit bladders treated with hydrochloride could protect the bladder wall from hemorrhagic cystitis [70]. The average mitotic and proliferative indices were observed to increase in PRP treated bladders. The mitotic index is a sign of tissue regeneration. Direct contact of PRP with the damaged urothelium, initiate cell proliferations above basement membrane and protect the urothelium. Recently, a pilot study was performed to test whether PRP can effectively treat IC/BPS patients and improve bladder pain symptoms. Four monthly PRP injections were given to 20 IC/BPS patients and we found the bladder pain VAS score decreased in about 70% of patients [82]. All patients completed the trial and no adverse event was reported. This successful pilot study encourages us to go further. It is likely that this novel treatment provide therapeutic benefit to IC/BPS patients who are refractory to conventional therapies.
In a recently published clinical trial using 4 monthly PRP intravesical injections to treat refractory IC/BPS patients, we also demonstrated a high success rate. Forty patients completed the four PRP injections and the post-treatment visits. GRA improved was reported after the 1st PRP treatment and the satisfaction to treatment persists up to 3 months after the 4th PRP treatment [83]. The success rate was 45% at 1st, 52% at 2nd, 70% at 3rd, and 70% at 4th PRP injection, and 67.5% at 3 months after the 4th PRP injection. The IC symptom score, including O’Leary Sant symptom score and visual analog pain score also significantly improved after PRP treatment. The postvoid residual did not increase after PRP treatment, the FBC increased, and the daily frequency and nocturia episodes recorded in 3-day voiding diary all decreased after PRP treatments. During the treatment and follow-up periods, no patient reported difficulty in urination and all were free of UTI. This study has demonstrated that PRP injections could provide an alternative therapy for IC/BPS patients who have failed conventional therapies. The treatment is effective and also safe. The disadvantage is the need for anesthesia during PRP injection.
OTHER THERAPEUTIC POTENTIAL OF PLATELET-RICH PLASMA ON TREATMENT OF INFLAMMATORY BLADDER DISORDERS
In addition to recurrent bacterial cystitis and IC/BPS, there are several bladder inflammatory disorders that the bladder urothelium might also have deficient regenerative ability. Patients with radiation cystitis, ketamine cystitis, CKD and end-stage renal disease, and chronic cystitis due to systemic autoimmune diseases such as systemic lupus erythematosus, Sjögren's syndrome, and chemical cystitis after systemic chemotherapy might also have LUTS refractory to OAB medication or nonsteroid anti-inflammatory drugs. The urothelium of bladder disorders also showed increase of chronic inflammation and deficient junction and barrier protein expressions [41,45]. If chronic inflammation can result in defective bladder urothelial regeneration, PRP injections to the urothelium of these bladder disorders might provide beneficial therapeutic effects.
However, although PRP has been widely applied in treatment of different local inflammatory diseases, there has been no standard preparation for a good quality of PRP solution for different aspects of regenerative therapy [84]. The most popular and standard procedure for PRP preparation is an initial soft spin to obtain the platelet-containing plasma, followed by a hard spin to obtain the platelet pellets. However, several conditions during PRP preparation such as temperature, leukocyte, and plasma volume which might influence therapeutic results have not been well determined. One recent investigation showed that adding saline instead of plasma (which might contain anti-platelet factors) to the platelet pellets improved the therapeutic efficacy on the wound healing and angiogenesis in an animal model [58]. In future investigation of the therapeutic potentials of PRP on lower urinary tract disorders, it is mandatory to search for the best concentration for the optimal therapeutic results, especially in patients with IC/BPS and recurrent bacterial cystitis.
CONCLUSIONS
Evidence reveals that the urothelial regeneration and differentiation are insufficient in IC/BPS and recurrent bacterial cystitis. Chronic inflammation may induce urothelial dysfunction and further affect the regenerative ability of the diseased bladder urothelium. Intravesical injection of PRP is safe and effective to decrease bladder inflammation, reverse the urothelial dysfunction to a normal condition. Clinical evidences have shown that PRP injections can improve symptoms and decrease of urinary inflammatory proteins in IC/BPS. In future perspectives, PRP might also have therapeutic potentials on several bladder disorders that are caused by defective urothelial regenerative function and urothelial cell differentiation, such as recurrent bacterial cystitis, radiation cystitis, chemical cystitis as well as bladder disorders due to systemic diseases.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Hainau B, Dombernowsky P. Histology and cell proliferation in human bladder tumors. An autoradiographic study. Cancer. 1974;33:115–26. doi: 10.1002/1097-0142(197401)33:1<115::aid-cncr2820330118>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 2.Gandhi D, Molotkov A, Batourina E, Schneider K, Dan H, Reiley M, et al. Retinoid signaling in progenitors controls specification and regeneration of the urothelium. Dev Cell. 2013;26:469–82. doi: 10.1016/j.devcel.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mysorekar IU, Isaacson-Schmid M, Walker JN, Mills JC, Hultgren SJ. Bone morphogenetic protein 4 signaling regulates epithelial renewal in the urinary tract in response to uropathogenic infection. Cell Host Microbe. 2009;5:463–75. doi: 10.1016/j.chom.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shin K, Lee J, Guo N, Kim J, Lim A, Qu L, et al. Hedgehog/Wnt feedback supports regenerative proliferation of epithelial stem cells in bladder. Nature. 2011;472:110–4. doi: 10.1038/nature09851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kreft ME, Sterle M, Veranic P, Jezernik K. Urothelial injuries and the early wound healing response: Tight junctions and urothelial cytodifferentiation. Histochem Cell Biol. 2005;123:529–39. doi: 10.1007/s00418-005-0770-9. [DOI] [PubMed] [Google Scholar]
- 6.Wu XR, Medina JJ, Sun TT. Selective interactions of UPIa and UPIb, two members of the transmembrane 4 superfamily, with distinct single transmembrane-domained proteins in differentiated urothelial cells. J Biol Chem. 1995;270:29752–9. doi: 10.1074/jbc.270.50.29752. [DOI] [PubMed] [Google Scholar]
- 7.Yu W, Khandelwal P, Apodaca G. Distinct apical and basolateral membrane requirements for stretch-induced membrane traffic at the apical surface of bladder umbrella cells. Mol Biol Cell. 2009;20:282–95. doi: 10.1091/mbc.E08-04-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pignon JC, Grisanzio C, Geng Y, Song J, Shivdasani RA, Signoretti S, et al. P63-expressing cells are the stem cells of developing prostate, bladder, and colorectal epithelia. Proc Natl Acad Sci U S A. 2013;110:8105–10. doi: 10.1073/pnas.1221216110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balsara ZR, Li X. Sleeping beauty: Awakening urothelium from its slumber. Am J Physiol Renal Physiol. 2017;312:F732–43. doi: 10.1152/ajprenal.00337.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sant GR, Kempuraj D, Marchand JE, Theoharides TC. The mast cell in interstitial cystitis: Role in pathophysiology and pathogenesis. Urology. 2007;69:34–40. doi: 10.1016/j.urology.2006.08.1109. [DOI] [PubMed] [Google Scholar]
- 11.Shie JH, Kuo HC. Higher levels of cell apoptosis and abnormal E-cadherin expression in the urothelium are associated with inflammation in patients with interstitial cystitis/painful bladder syndrome. BJU Int. 2011;108:E136–41. doi: 10.1111/j.1464-410X.2010.09911.x. [DOI] [PubMed] [Google Scholar]
- 12.Lee JD, Lee MH. Activation of extrinsic apoptotic pathway from bladder biopsy in patients with interstitial cystitis/painful bladder syndrome. Urology. 2013;82:1451.e7–11. doi: 10.1016/j.urology.2013.08.042. [DOI] [PubMed] [Google Scholar]
- 13.Southgate J, Varley CL, Garthwaite MA, Hinley J, Marsh F, Stahlschmidt J, et al. Differentiation potential of urothelium from patients with benign bladder dysfunction. BJU Int. 2007;99:1506–16. doi: 10.1111/j.1464-410X.2007.06795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeng Y, Wu XX, Homma Y, Yoshimura N, Iwaki H, Kageyama S, et al. Uroplakin III-delta4 messenger RNA as a promising marker to identify nonulcerative interstitial cystitis. J Urol. 2007;178:1322–7. doi: 10.1016/j.juro.2007.05.125. [DOI] [PubMed] [Google Scholar]
- 15.Hauser PJ, Dozmorov MG, Bane BL, Slobodov G, Culkin DJ, Hurst RE, et al. Abnormal expression of differentiation related proteins and proteoglycan core proteins in the urothelium of patients with interstitial cystitis. J Urol. 2008;179:764–9. doi: 10.1016/j.juro.2007.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim J, Keay SK, Dimitrakov JD, Freeman MR. P53 mediates interstitial cystitis antiproliferative factor (APF)-induced growth inhibition of human urothelial cells. FEBS Lett. 2007;581:3795–9. doi: 10.1016/j.febslet.2007.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang CO, Li ZL, Kong CZ. APF, HB-EGF, and EGF biomarkers in patients with ulcerative vs.non-ulcerative interstitial cystitis. BMC Urol. 2005;5:7. doi: 10.1186/1471-2490-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keay S, Kaczmarek P, Zhang CO, Koch K, Szekely Z, Barchi JJ, Jr, et al. Normalization of proliferation and tight junction formation in bladder epithelial cells from patients with interstitial cystitis/painful bladder syndrome by d-proline and d-pipecolic acid derivatives of antiproliferative factor. Chem Biol Drug Des. 2011;77:421–30. doi: 10.1111/j.1747-0285.2011.01108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tseng-Rogenski S, Liebert M. Interleukin-8 is essential for normal urothelial cell survival. Am J Physiol Renal Physiol. 2009;297:F816–21. doi: 10.1152/ajprenal.90733.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Graham E, Chai TC. Dysfunction of bladder urothelium and bladder urothelial cells in interstitial cystitis. Curr Urol Rep. 2006;7:440–6. doi: 10.1007/s11934-006-0051-8. [DOI] [PubMed] [Google Scholar]
- 21.Parsons CL. The role of a leaky epithelium and potassium in the generation of bladder symptoms in interstitial cystitis/overactive bladder, urethral syndrome, prostatitis and gynaecological chronic pelvic pain. BJU Int. 2011;107:370–5. doi: 10.1111/j.1464-410X.2010.09843.x. [DOI] [PubMed] [Google Scholar]
- 22.Dodd LG, Tello J. Cytologic examination of urine from patients with interstitial cystitis. Acta Cytol. 1998;42:923–7. doi: 10.1159/000331969. [DOI] [PubMed] [Google Scholar]
- 23.Bouchelouche K, Kristensen B, Nordling J, Horn T, Bouchelouche P. Increased urinary leukotriene E4 and eosinophil protein X excretion in patients with interstitial cystitis. J Urol. 2001;166:2121–5. [PubMed] [Google Scholar]
- 24.Gamper M, Viereck V, Eberhard J, Binder J, Moll C, Welter J, et al. Local immune response in bladder pain syndrome/interstitial cystitis ESSIC type 3C. Int Urogynecol J. 2013;24:2049–57. doi: 10.1007/s00192-013-2112-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shie JH, Liu HT, Kuo HC. Increased cell apoptosis of urothelium mediated by inflammation in interstitial cystitis/painful bladder syndrome. Urology. 2012;79:484.e7–13. doi: 10.1016/j.urology.2011.09.049. [DOI] [PubMed] [Google Scholar]
- 26.Lee CL, Jiang YH, Kuo HC. Increased apoptosis and suburothelial inflammation in patients with ketamine-related cystitis: A comparison with non-ulcerative interstitial cystitis and controls. BJU Int. 2013;112:1156–62. doi: 10.1111/bju.12256. [DOI] [PubMed] [Google Scholar]
- 27.van Roy F, Berx G. The cell-cell adhesion molecule E-cadherin. Cell Mol Life Sci. 2008;65:3756–88. doi: 10.1007/s00018-008-8281-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chuang FC, Kuo HC. Increased urothelial cell apoptosis and chronic inflammation are associated with recurrent urinary tract infection in women. PLoS One. 2013;8:e63760. doi: 10.1371/journal.pone.0063760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu HT, Shie JH, Chen SH, Wang YS, Kuo HC. Differences in mast cell infiltration, E-cadherin, and zonula occludens-1 expression between patients with overactive bladder and interstitial cystitis/bladder pain syndrome. Urology. 2012;80:225.e13–8. doi: 10.1016/j.urology.2012.01.047. [DOI] [PubMed] [Google Scholar]
- 30.Slobodov G, Feloney M, Gran C, Kyker KD, Hurst RE, Culkin DJ. Abnormal expression of molecular markers for bladder impermeability and differentiation in the urothelium of patients with interstitial cystitis. J Urol. 2004;171:1554–8. doi: 10.1097/01.ju.0000118938.09119.a5. [DOI] [PubMed] [Google Scholar]
- 31.Min G, Zhou G, Schapira M, Sun TT, Kong XP. Structural basis of urothelial permeability barrier function as revealed by cryo-EM studies of the 16 nm uroplakin particle. J Cell Sci. 2003;116:4087–94. doi: 10.1242/jcs.00811. [DOI] [PubMed] [Google Scholar]
- 32.Shupp Byrne DE, Sedor JF, Soroush M, McCue PA, Mulholland SG. Interaction of bladder glycoprotein GP51 with uropathogenic bacteria. J Urol. 2001;165:1342–6. doi: 10.1097/00005392-200104000-00081. [DOI] [PubMed] [Google Scholar]
- 33.Hicks RM. The mammalian urinary bladder: An accommodating organ. Biol Rev Camb Philos Soc. 1975;50:215–46. doi: 10.1111/j.1469-185x.1975.tb01057.x. [DOI] [PubMed] [Google Scholar]
- 34.Jhang JF, Ho HC, Jiang YH, Lee CL, Hsu YH, Kuo HC. Electron microscopic characteristics of interstitial cystitis/bladder pain syndrome and their association with clinical condition. PLoS One. 2018;13:e0198816. doi: 10.1371/journal.pone.0198816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keay S, Zhang CO, Kagen DI, Hise MK, Jacobs SC, Hebel JR, et al. Concentrations of specific epithelial growth factors in the urine of interstitial cystitis patients and controls. J Urol. 1997;158:1983–8. doi: 10.1016/s0022-5347(01)64198-3. [DOI] [PubMed] [Google Scholar]
- 36.Tomaszewski JE, Landis JR, Russack V, Williams TM, Wang LP, Hardy C, et al. Biopsy features are associated with primary symptoms in interstitial cystitis: Results from the interstitial cystitis database study. Urology. 2001;57:67–81. doi: 10.1016/s0090-4295(01)01166-9. [DOI] [PubMed] [Google Scholar]
- 37.Keay S. Cell signaling in interstitial cystitis/painful bladder syndrome. Cell Signal. 2008;20:2174–9. doi: 10.1016/j.cellsig.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 38.Homma Y, Ueda T, Tomoe H, Lin AT, Kuo HC, Lee MH, et al. Clinical guidelines for interstitial cystitis and hypersensitive bladder updated in 2015. Int J Urol. 2016;23:542–9. doi: 10.1111/iju.13118. [DOI] [PubMed] [Google Scholar]
- 39.Jhang JF, Hsu YH, Jiang YH, Lee CL, Kuo HC. Histopathological characteristics of ketamine-associated uropathy and their clinical association. Neurourol Urodyn. 2018;37:1764–72. doi: 10.1002/nau.23514. [DOI] [PubMed] [Google Scholar]
- 40.Mysorekar IU, Hultgren SJ. Mechanisms of uropathogenic Escherichia coli persistence and eradication from the urinary tract. Proc Natl Acad Sci U S A. 2006;103:14170–5. doi: 10.1073/pnas.0602136103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu HT, Jiang YH, Kuo HC. Alteration of urothelial inflammation, apoptosis, and junction protein in patients with various bladder conditions and storage bladder symptoms suggest common pathway involved in underlying pathophysiology. Low Urin Tract Symptoms. 2015;7:102–7. doi: 10.1111/luts.12062. [DOI] [PubMed] [Google Scholar]
- 42.Jiang YH, Kuo HC. Urothelial barrier deficits, suburothelial inflammation and altered sensory protein expression in detrusor underactivity. J Urol. 2017;197:197–203. doi: 10.1016/j.juro.2016.07.071. [DOI] [PubMed] [Google Scholar]
- 43.Jiang YH, Lee CL, Kuo HC. Urothelial dysfunction, suburothelial inflammation and altered sensory protein expression in men with bladder outlet obstruction and various bladder dysfunctions: Correlation with urodynamics. J Urol. 2016;196:831–7. doi: 10.1016/j.juro.2016.02.2958. [DOI] [PubMed] [Google Scholar]
- 44.Wang CC, Kuo HC. Urothelial dysfunction and chronic inflammation in diabetic patients with overactive bladder. Low Urin Tract Symptoms. 2017;9:151–6. doi: 10.1111/luts.12126. [DOI] [PubMed] [Google Scholar]
- 45.Cheng SF, Jiang YH, Kuo HC. Urothelial dysfunction and chronic inflammation are associated with increased bladder sensation in patients with chronic renal insufficiency. Int Neurourol J. 2018;22:S46–54. doi: 10.5213/inj.1832814.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jiang YH, Liu HT, Kuo HC. Urothelial dysfunction and chronic inflammation in patients with spinal cord injuries at different levels and correlation with urodynamic findings. Neurourol Urodyn. 2015;34:757–62. doi: 10.1002/nau.22650. [DOI] [PubMed] [Google Scholar]
- 47.Kullmann FA, Clayton DR, Ruiz WG, Wolf-Johnston A, Gauthier C, Kanai A, et al. Urothelial proliferation and regeneration after spinal cord injury. Am J Physiol Renal Physiol. 2017;313:F85–102. doi: 10.1152/ajprenal.00592.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haylen BT, de Ridder D, Freeman RM, Swift SE, Berghmans B, Lee J, et al. An international urogynecological association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn. 2010;29:4–20. doi: 10.1002/nau.20798. [DOI] [PubMed] [Google Scholar]
- 49.Liu HT, Tyagi P, Chancellor MB, Kuo HC. Urinary nerve growth factor but not prostaglandin E2 increases in patients with interstitial cystitis/bladder pain syndrome and detrusor overactivity. BJU Int. 2010;106:1681–5. doi: 10.1111/j.1464-410X.2009.08851.x. [DOI] [PubMed] [Google Scholar]
- 50.Jhang JF, Kuo HC. Pathomechanism of interstitial cystitis/bladder pain syndrome and mapping the heterogeneity of disease. Int Neurourol J. 2016;20:S95–104. doi: 10.5213/inj.1632712.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cervigni M. Interstitial cystitis/bladder pain syndrome and glycosaminoglycans replacement therapy. Transl Androl Urol. 2015;4:638–42. doi: 10.3978/j.issn.2223-4683.2015.11.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sun Y, Chai TC. Up-regulation of P2X3 receptor during stretch of bladder urothelial cells from patients with interstitial cystitis. J Urol. 2004;171:448–52. doi: 10.1097/01.ju.0000099660.46774.3c. [DOI] [PubMed] [Google Scholar]
- 53.Sun Y, Keay S, Lehrfeld TJ, Chai TC. Changes in adenosine triphosphate-stimulated ATP release suggest association between cytokine and purinergic signaling in bladder urothelial cells. Urology. 2009;74:1163–8. doi: 10.1016/j.urology.2009.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Collan Y, Alfthan O, Kivilaakso E, Oravisto KJ. Electron microscopic and histological findings on urinary bladder epithelium in interstitial cystitis. Eur Urol. 1976;2:242–7. doi: 10.1159/000472019. [DOI] [PubMed] [Google Scholar]
- 55.Anderström CR, Fall M, Johansson SL. Scanning electron microscopic findings in interstitial cystitis. Br J Urol. 1989;63:270–5. doi: 10.1111/j.1464-410x.1989.tb05188.x. [DOI] [PubMed] [Google Scholar]
- 56.Theoharides TC, Sant GR, el-Mansoury M, Letourneau R, Ucci AA, Jr, Meares EM., Jr Activation of bladder mast cells in interstitial cystitis: A light and electron microscopic study. J Urol. 1995;153:629–36. doi: 10.1097/00005392-199503000-00021. [DOI] [PubMed] [Google Scholar]
- 57.Wong YC, Martin BF. A study by scanning electron microscopy of the bladder epithelium of the guinea pig. Am J Anat. 1977;150:237–45. doi: 10.1002/aja.1001500203. [DOI] [PubMed] [Google Scholar]
- 58.Etulain J, Mena HA, Meiss RP, Frechtel G, Gutt S, Negrotto S, et al. An optimised protocol for platelet-rich plasma preparation to improve its angiogenic and regenerative properties. Sci Rep. 2018;8:1513. doi: 10.1038/s41598-018-19419-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mussano F, Genova T, Munaron L, Petrillo S, Erovigni F, Carossa S. Cytokine, chemokine, and growth factor profile of platelet-rich plasma. Platelets. 2016;27:467–71. doi: 10.3109/09537104.2016.1143922. [DOI] [PubMed] [Google Scholar]
- 60.Kuffler DP. Platelet-rich plasma and the elimination of neuropathic pain. Mol Neurobiol. 2013;48:315–32. doi: 10.1007/s12035-013-8494-7. [DOI] [PubMed] [Google Scholar]
- 61.Etulain J. Platelets in wound healing and regenerative medicine. Platelets. 2018;29:556–68. doi: 10.1080/09537104.2018.1430357. [DOI] [PubMed] [Google Scholar]
- 62.Louis ML, Magalon J, Jouve E, Bornet CE, Mattei JC, Chagnaud C, et al. Growth factors levels determine efficacy of platelets rich plasma injection in knee osteoarthritis: A randomized double blind noninferiority trial compared with viscosupplementation. Arthroscopy. 2018;34:1530–40.e2. doi: 10.1016/j.arthro.2017.11.035. [DOI] [PubMed] [Google Scholar]
- 63.Propert KJ, Mayer RD, Wang Y, Sant GR, Hanno PM, Peters KM, et al. Responsiveness of symptom scales for interstitial cystitis. Urology. 2006;67:55–9. doi: 10.1016/j.urology.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 64.Tsai WC, Yu TY, Chang GJ, Lin LP, Lin MS, Pang JS. Platelet-rich plasma releasate promotes regeneration and decreases inflammation and apoptosis of injured skeletal muscle. Am J Sports Med. 2018;46:1980–6. doi: 10.1177/0363546518771076. [DOI] [PubMed] [Google Scholar]
- 65.Chen NF, Sung CS, Wen ZH, Chen CH, Feng CW, Hung HC, et al. Therapeutic effect of platelet-rich plasma in rat spinal cord injuries. Front Neurosci. 2018;12:252. doi: 10.3389/fnins.2018.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Çetinkaya RA, Yenilmez E, Petrone P, Yılmaz S, Bektöre B, şimsek B, et al. Platelet-rich plasma as an additional therapeutic option for infected wounds with multi-drug resistant bacteria: In vitro antibacterial activity study? Eur J Trauma Emerg Surg. 2018 doi: 10.1007/s00068-018-0957-0. doi:10.1007/S00068-018-0957-0. [Epub of ahead of print] [DOI] [PubMed] [Google Scholar]
- 67.Roy S, Driggs J, Elgharably H, Biswas S, Findley M, Khanna S, et al. Platelet-rich fibrin matrix improves wound angiogenesis via inducing endothelial cell proliferation. Wound Repair Regen. 2011;19:753–66. doi: 10.1111/j.1524-475X.2011.00740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu Y, Wang J, Scott PG, Tredget EE. Bone marrow-derived stem cells in wound healing: A review. Wound Repair Regen. 2007;15(Suppl 1):S18–26. doi: 10.1111/j.1524-475X.2007.00221.x. [DOI] [PubMed] [Google Scholar]
- 69.Scull CM, Hays WD, Fischer TH. Macrophage pro-inflammatory cytokine secretion is enhanced following interaction with autologous platelets. J Inflamm (Lond) 2010;7:53. doi: 10.1186/1476-9255-7-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dönmez Mİ, İnci K, Zeybek ND, Doğan HS, Ergen A. The early histological effects of intravesical instillation of platelet-rich plasma in cystitis models. Int Neurourol J. 2016;20:188–96. doi: 10.5213/inj.1632548.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ozyuvali E, Yildirim ME, Yaman T, Kosem B, Atli O, Cimentepe E, et al. Protective effect of intravesical platelet-rich plasma on cyclophosphamide-induced hemorrhagic cystitis. Clin Invest Med. 2016;39:27514. [PubMed] [Google Scholar]
- 72.Varley C, Hill G, Pellegrin S, Shaw NJ, Selby PJ, Trejdosiewicz LK, et al. Autocrine regulation of human urothelial cell proliferation and migration during regenerative responses in vitro. Exp Cell Res. 2005;306:216–29. doi: 10.1016/j.yexcr.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 73.Kanematsu A, Yamamoto S, Noguchi T, Ozeki M, Tabata Y, Ogawa O. Bladder regeneration by bladder acellular matrix combined with sustained release of exogenous growth factor. J Urol. 2003;170:1633–8. doi: 10.1097/01.ju.0000084021.51099.8a. [DOI] [PubMed] [Google Scholar]
- 74.Parsons CL. The role of the urinary epithelium in the pathogenesis of interstitial cystitis/prostatitis/urethritis. Urology. 2007;69:9–16. doi: 10.1016/j.urology.2006.03.084. [DOI] [PubMed] [Google Scholar]
- 75.Soler R, Bruschini H, Freire MP, Alves MT, Srougi M, Ortiz V. Urine is necessary to provoke bladder inflammation in protamine sulfate induced urothelial injury. J Urol. 2008;180:1527–31. doi: 10.1016/j.juro.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 76.Teichman JM, Moldwin R. The role of the bladder surface in interstitial cystitis/painful bladder syndrome. Can J Urol. 2007;14:3599–607. [PubMed] [Google Scholar]
- 77.Thumbikat P, Berry RE, Schaeffer AJ, Klumpp DJ. Differentiation-induced uroplakin III expression promotes urothelial cell death in response to uropathogenic E.coli. Microbes Infect. 2009;11:57–65. doi: 10.1016/j.micinf.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Van De Merwe JP, Arendsen HJ. Interstitial cystitis: A review of immunological aspects of the aetiology and pathogenesis, with a hypothesis. BJU Int. 2000;85:995–9. doi: 10.1046/j.1464-410x.2000.00646.x. [DOI] [PubMed] [Google Scholar]
- 79.Saban R, Saban MR, Maier J, Fowler B, Tengowski M, Davis CA. Urothelial expression of neuropilins and VEGF receptors in control and interstitial cystitis patients. Am J Physiol Renal Physiol. 2008;295:F1613–23. doi: 10.1152/ajprenal.90344.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhou G, Mo WJ, Sebbel P, Min G, Neubert TA, Glockshuber R. Uroplakin ia is the urothelial receptor for uropathogenic Escherichia coli: Evidence from in vitro fimH binding. J Cell Sci. 2001;114:4095–103. doi: 10.1242/jcs.114.22.4095. [DOI] [PubMed] [Google Scholar]
- 81.Blango MG, Mulvey MA. Persistence of uropathogenic Escherichia coli in the face of multiple antibiotics. Antimicrob Agents Chemother. 2010;54:1855–63. doi: 10.1128/AAC.00014-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jhang JF, Wu SY, Lin TY, Kuo HC. Repeated intravesical injections of platelet-rich plasma are effective in the treatment of interstitial cystitis: A case control pilot study? Low Urin Tract Symptoms. 2017 doi: 10.1111/luts.12212. doi:10.1111/luts.12212. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 83.Jhang JF, Lin TY, Kuo HC. Intravesical injections of platelet-rich plasma is effective and safe in treatment of interstitial cystitis refractory to conventional treatment-A prospective clinical trial. Neurourol Urodyn. 2019;38:703–9. doi: 10.1002/nau.23898. [DOI] [PubMed] [Google Scholar]
- 84.Amable PR, Carias RB, Teixeira MV, da Cruz Pacheco I, Corrêa do Amaral RJ, Granjeiro JM. Platelet-rich plasma preparation for regenerative medicine: Optimization and quantification of cytokines and growth factors. Stem Cell Res Ther. 2013;4:67. doi: 10.1186/scrt218. [DOI] [PMC free article] [PubMed] [Google Scholar]