Abstract
Objectives:
Resin infiltration is a minimally invasive technique for treating noncavitated proximal caries. It slows/stops the carious lesion progression rate by creating a diffusion barrier inside the porous enamel lesion body. The aim was to evaluate the efficacy of resin infiltration on noncavitated proximal carious lesions in primary and permanent teeth.
Materials and Methods:
The records were obtained using electronic and other sources. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines were followed to ensure transparent reporting. Eligible studies were randomized controlled trials evaluating the efficacy of resin infiltration for noncavitated proximal carious lesions by comparing it with control/placebo. Each included study was assessed concerning the “risk of bias” using the Cochrane Collaboration's “risk-of-bias” assessment tool. High risk-of-bias studies were excluded from the meta-analyses due to selective reporting matters. The statistics were performed by RevMan software (The Cochrane Collaboration, The Nordic Cochrane Centre, Copenhagen, Denmark) utilizing the random effect model. The GRADE approach was implemented for assessing the quality of evidence.
Results:
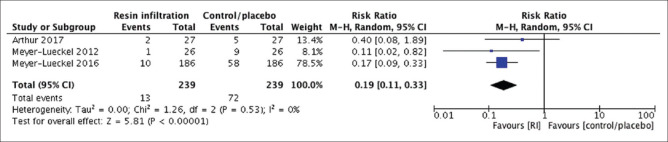
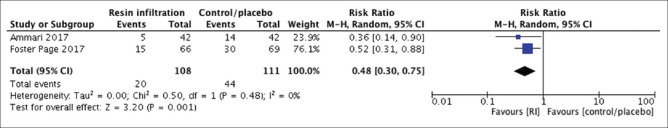
From 106 studies identified, 17 were assessed for eligibility. After “risk-of-bias” assessment, two meta-analyses were conducted to eliminate the limitation of the significant heterogeneity between trials inspecting primary teeth (n = 2) and permanent teeth (n = 3). I2 = 0% indicates the absence of statistical heterogeneity. The risk of carious lesions’ progression with resin infiltration was significantly lower in primary (risk ratio [RR]; 95% confidence interval [CI]: 0.48; 0.30–0.75, P = 0.001) and in permanent teeth (RR; 95% CI: 0.19; 0.11–0.33, P < 0.00001) compared to that of control/placebo. The GRADE approach revealed high quality of evidence.
Conclusion:
The available evidence conveys high confidence that proximal resin infiltration has superior efficacy in slowing/arresting the carious lesions’ progression rate in comparison to conventional management modalities.
KEYWORDS: Efficacy, permanent teeth, primary teeth, proximal carious lesions, resin infiltration
INTRODUCTION
Proximal dental caries is a significant health problem mainly affecting the young age group.[1,2,3] It is usually related to the difficulty of cleaning proximal surfaces relative to other smooth surfaces, and the high patient commitment required for adherence to proximal hygienic measures[2] which might be sparse in such age category. Aggravating the problem, using rotary burs, and cutting instruments in the conventional invasive treatment of proximal lesions sacrifice substantial amounts of sound tooth structure. This violates the concept of minimally invasive dentistry (MID)[4,5] which involves excavating the least possible amount of dental tissues.[6] Consequently, resin infiltration was invented.[7] This method is considered a micro-invasive treatment, a subcategory of MID.
Micro-invasive treatment entails conditioning noncavitated proximal lesions (NCPLs) by organic acids that result in the loss of few micrometers of the tooth substance.[3] Resin infiltration, in particular, implies etching NCPL/s that has/have a radiographic depth of E2 (caries reaching the inner half of enamel) or D1 (caries ending in the outer third of dentin) by 15% hydrochloric acid gel for 120 s and soaking the lesion with a low-viscosity resin.[8] This technique slows/stops the NCPLs’ progression rate by creating a diffusion barrier inside the porous enamel lesion body rather than on top of it like other sealants, i.e., bonding adhesives.[3]
Furthermore, esthetics can be enhanced by this technique as it is used for masking white-spot lesions (WSLs). The pores of WSLs are usually filled with saliva that has a refractive index (RI) of 1.33. On the other hand, the RI of enamel is 1.62. This difference in light scattering between WSLs and the surrounding enamel is masked when those pores are infiltrated with resin (RI = 1.46), which has similar optical properties to enamel.[9,10]
In spite of the growing clinical use of resin infiltrates, its efficacy on noncavitated proximal caries in primary and permanent teeth is not fully reported. The present review was undertaken to answer the question – Is resin infiltration of NCPLs an efficacious method for carious lesions’ control in primary and permanent teeth? We assumed that proximal resin infiltration might yield positive outcomes. Hence, the aim of this study was to evaluate the efficacy of resin infiltration on NCPL/s in primary and permanent teeth.
MATERIALS AND METHODS
The review proposal was submitted to the research center of Riyadh Elm University, and the Institutional Review Board approval was obtained (RC/IRB/2018/896).
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed to ensure transparent reporting.[11] Accordingly, the records were collected electronically and manually. Seven electronic sources were searched by the Medical Subject Headings terms and the most reported keywords. The search was conducted following the search syntax of every electronic source. The search strategy is presented in Table 1. No filters were applied during the search except for language; studies were limited to English only. Afterward, the manual search was conducted by examining the reference lists of related studies in an attempt to find relevant data that were not identified by the electronic search. The PRISMA flow diagram enhanced the records handling process. Duplicated records were automatically excluded using EndNote X8 software (Clarivate Analytics, Philadelphia, Pennsylvania, USA).[12] We modified the Population, Intervention, Comparison, Outcome, and Study Design (PICOS) criteria reported by Ammari and others[13] and referred to them in the eligibility assessment of the full-text records.
Table 1.
Literature search strategies
| Electronic source | Search strategy |
|---|---|
| EBSCOhost | Most reported terms: |
| PubMed | a. Resin infiltration OR caries infiltration AND proximal lesions OR proximal caries |
| Wiley Library | |
| Cochrane Library | b. Sealing AND resin infiltration OR caries infiltration AND proximal lesions OR proximal caries |
| Google Scholar | |
| OpenGrey | |
| OpenThesis | |
| PubMed | MeSH terms: ((((“Cariostatic agents/therapeutic use” [MAJR]) OR “Dental caries/prevention and control” [MAJR]) AND “Disease Progression” [MeSH terms]) AND “Humans” [MeSH terms]) AND “Resins, synthetic/therapeutic use” [MAJR] |
MeSH=Medical Subject Headings
The PICOS inclusion criteria were as follows:
Population (P): Humans with NCPL/s
Intervention (I): Resin infiltration of proximal carious lesions
Comparison (C): Resin infiltration compared to placebo or different material/technique (control groups)
Outcome (O): NCPLs’ progression rate assessed by bitewing radiographs
Study design (S): Randomized controlled trials (RCTs) with a minimum of a 12-month follow-up.
If one or more of these criteria was missing, the study was excluded.
The risk of bias of the included studies was assessed using the Cochrane Collaboration “risk-of-bias” assessment tool.[14] This tool has seven domains, which are the random sequence generation, where each included study was assessed by evaluating the method of randomization that allocates the groups of intervention and control. The allocation concealment was inspected to assure the random allocation. After that, blinding of operators, participants, and outcome examiner/s to the allocation was assessed. We graded studies as having a low risk in the “incomplete outcome data” domain when they had ≤25% dropout rate, and the reasons for participant dropout were clarified. To evaluate “reporting bias,” the selective reporting domain was examined; in which failure to mention, the prespecified outcomes was considered a high-bias risk. Moreover, each trial was evaluated as a whole to ensure the absence of other sources of bias. Any study with a high risk of bias in one or more domains was excluded from the meta-analysis.
Heterogeneity assessment was performed by analyzing “clinical,” “statistical,” and “methodological” heterogeneity. For clinical heterogeneity, studies were assessed in terms of diversity between them by identifying the following in each study: participants’ age, setting (primary care or secondary care), teeth types (primary or permanent teeth), moisture control (rubber dam isolation or cotton roll isolation), the operative procedure, and the variation of the comparator between studies (fluoridated toothpaste, dental floss, fluoride varnish, and no treatment). Furthermore, I2 and Chi-square tests were used to measure the statistical heterogeneity. Methodological heterogeneity was examined by assessing the difference in bias risk between the included studies in each meta-analysis.
The statistics were performed by RevMan software (Review Manager, version 5.3; the Cochrane Collaboration, Copenhagen, Denmark).[15] Subsequently, the attained evidence was appraised by implementing the GRADE approach.[16]
RESULTS
SEARCH RESULTS
The flow diagram of the study selection is shown in Figure 1. At first, 106 records were identified. After removing duplicates automatically by Endnote X8 software (Clarivate Analytics, Philadelphia, Pennsylvania, USA),[12] forty-six records remained for screening. This phase comprised inspecting the titles and abstracts for relevance to our PICOS inclusion criteria. Accordingly, 29 records were excluded; 28 of them were not RCTs, and one record was excluded because it was not written in English. Afterward, the full texts of the 17 remaining records were also assessed for eligibility in reference to our PICOS criteria. Seven trials met the criteria and were included in the qualitative synthesis (systematic review). The characteristics of the included studies are present in Table 2, and the reasons for exclusion of the other ten papers are justified in Figure 1. Aside from the PICOS criteria, two studies were excluded at this step since they were published as abstracts only; the missing data hindered further appraisal.
Figure 1.
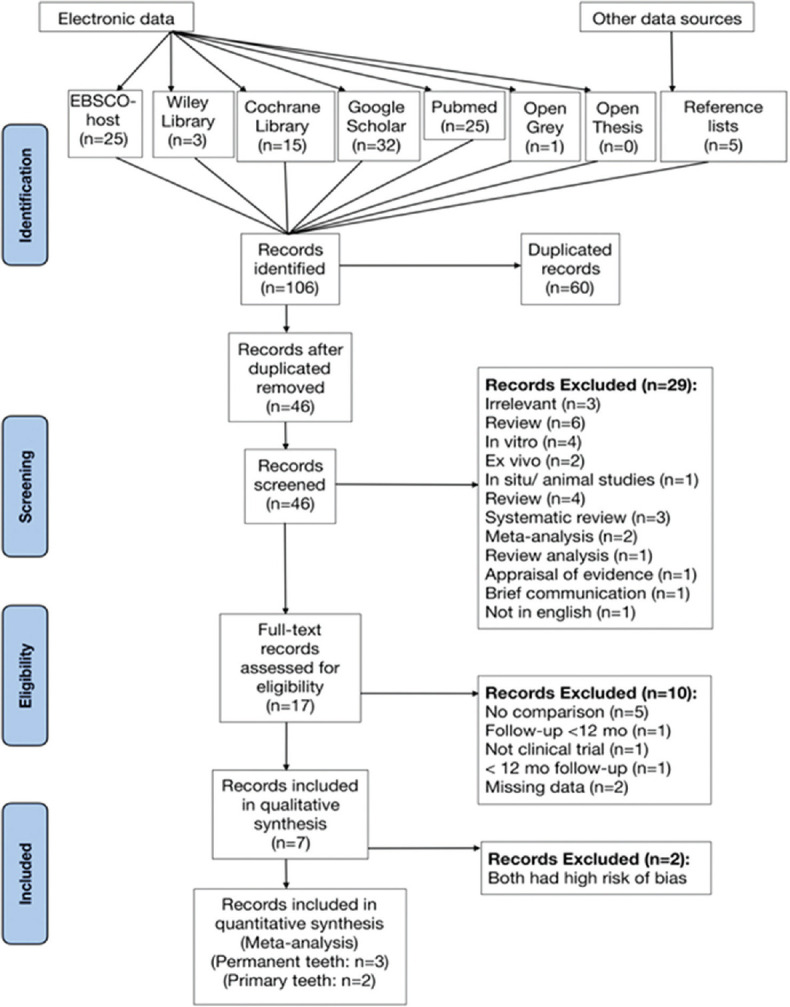
Flow diagram showing study selection
Table 2.
Characteristics of studies included in the qualitative synthesis (systematic review)
| Authors and year of publication | Study design | Population (followed up participants) | Follow-up period | Intervention | Comparison | Outcome assessment method |
|---|---|---|---|---|---|---|
| Ammari et al., 2017 | Split-mouth controlled randomized clinical trial | 42 children (42 lesion pairs) Age range of children at baseline: 5-8 years old | 6 and 12 months | Resin infiltration of NCPLs in primary molars Infiltrant: Icon®, (DMG, Hamburg, Germany) | Test group (fluoridated toothpaste + flossing + infiltration) Control group (fluoridated toothpaste + flossing) | Pairwise radiographic comparison of baseline and follow-up bitewing radiographs |
| Arthur et al., 2017 | Double-blind split-mouth placebo-controlled randomized clinical trial | 17 participants (27 lesion pairs) Age range at baseline: 16-41 years old | 36 months | Resin infiltration of NCPLs Infiltrant: Icon® (DMG, Hamburg, Germany) | Placebo treatment | Pairwise radiographic comparison of baseline and follow-up bitewing radiographs |
| Ekstrand, Bakhshandeh and Martignon 2010 | Split-mouth controlled randomized clinical trial | 39 children | 6 months and 12 months | Resin infiltration of NCPLs Infiltrant resin: Triethylene glycol dimethacrylate- based resin, camphorquinone, additives; DMG, Hamburg, Germany | Test lesion: resin infiltration followed by FV Control lesion: Only FV | Scoring caries progression in bitewing radiographs |
| Foster Page et al., 2017 | Split-mouth placebo-controlled randomized controlled trial | 69 children Age range at baseline: 7-9 years old | 6, 12, and 24 months | Resin infiltration of NCPLs Infiltrant: DMG Icon preproduct | Test group (infiltration: DMG Icon preproduct and FV) Control group (FV) | Pairwise radiographic comparison of baseline and follow-up bitewing radiographs |
| Martignon et al., 2012 | Split-mouth controlled randomized clinical trial | 37 participants (each had at least 3 NCPLs) Age range at baseline: 16-35 years old | 36 months (annual follow-up) | Resin infiltration of NCPLs Infiltrant: ICON® preproduct (DMG, Hamburg, Germany) | Test- A (Infiltration: ICON-preproduct; DMG) Test-B (Sealing: Prime-Bond NT; Dentsply) Control-C (Placebo) | Pairwise radiographic comparison of baseline and follow-up bitewing radiographs |
| Meyer-Lueckel, Bitter and Paris 2012 | Split-mouth placebo-controlled randomized clinical trial | 19 young adults (25 lesion pairs) | 18 and 36 months | Resin infiltration of NCPLs Infiltrant: Icon, preproduct; DMG, Hamburg | Placebo treatment | Pairwise radiographic comparison and DSR of baseline and follow-up bitewing radiographs |
| Meyer-Lueckel et al., 2016 | Split-mouth placebo-controlled randomized clinical trial | 79 participants (218 lesion pairs) Age range at baseline: 17-29 years old | 10 and 18 months | Resin infiltration of NCPLs Infiltrant: Icon; DMG, Hamburg, Germany | Placebo treatment | Pairwise radiographic comparison of baseline and follow-up bitewing radiographs |
NCPLs=Noncavitated proximal lesion/s, FV=Fluoride varnish, DSR=Digital subtraction radiography, DMG=Dental milestones guaranteed company
RISK-OF-BIAS ASSESSMENT
Following the criteria listed in the Cochrane Collaboration's risk-of-bias assessment tool,[14] each of the seven included trials was examined for “risk of bias” concerning seven domains [Figure 2].
Figure 2.
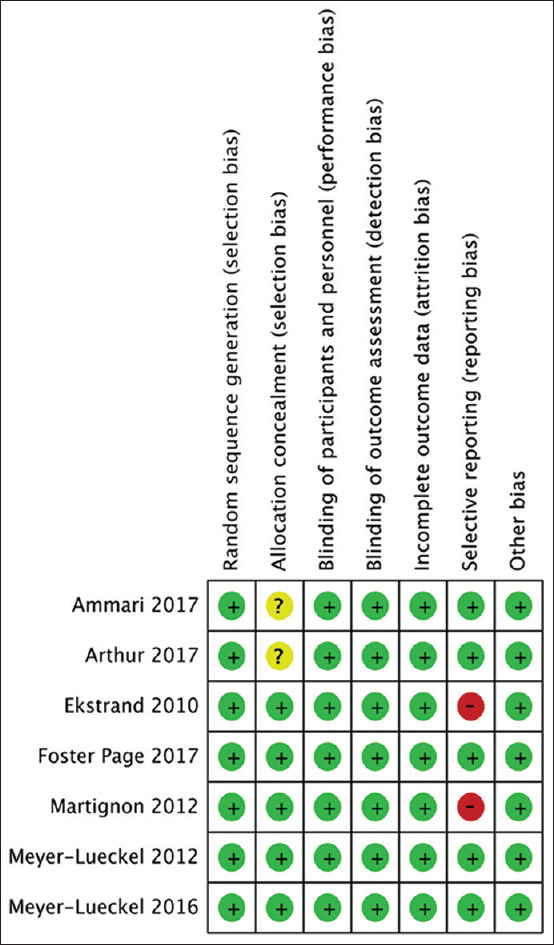
Risk-of-bias assessment summary
Random sequence generation
All the trials had a split-mouth design. Consequently, every participant in each study received both intervention (resin infiltration) and control/placebo. The distribution method of intervention and its comparator on NCPLs within each participant ensured randomization. Therefore, we judged all the trials to be at low risk of bias for this domain.
Allocation concealment
Four out of the seven papers were at low risk of bias. They reported adequate concealment measures for random allocation of intervention and control/placebo to NCPLs. Such measures were placing the random sequence inside sealed envelopes[17,18,19] and letter coding of each NCPL management strategy,[20] whereas the remaining three studies did not report any means of allocation concealment and therefore were judged to have an unclear risk of bias. However, they were not excluded because this was not likely to affect the outcomes.
Blinding of participants and personnel
Considering the significant difference between the procedures of resin infiltration and control/placebo, blinding of operators was not applicable.[3] Moreover, unblinding of participants was not likely to affect the outcome since we depended only on bitewing radiographs for outcome assessment and not on the participants’ subjective statements. As a consequence, and following the Cochrane Collaboration's criteria, all the included RCTs were appraised as having a low risk of bias.
Blinding of outcome assessment
We judged all the seven trials as having a low risk of bias concerning this domain, as reasonable blinding of outcome investigator/s to intervention and comparator allocation was described. This was implemented either by directly blinding outcome investigator/s to the allocation[17,18,21,22,23] or by involving an outer radiography specialist to assess the pairwise radiographs. As reported, this specialist was blinded to the study design.[20]
Incomplete outcome data
The dropout rate of participants in the seven studies was ≤25%. The reasons for dropout were sufficiently clarified in all the trials. Besides, the split-mouth design of the seven RCTs reduced the chance of missing a comparator to the intervention or vice versa. As a result, this domain was judged to be at low risk of bias in all the seven studies.
Selective reporting
Five studies reported all the prespecified outcomes and therefore were judged to have a low risk of bias.[17,18,19,22,23] In contrast, two trials were at high risk of bias since they failed to mention all their prespecified outcomes. Ekstrand et al.[20] did not report the results of their 6-month clinical assessment, and the radiographic evaluation was incomplete. Meanwhile, Martignon et al.[21] aimed at assessing the NCPLs’ progression rate annually for 3 years. However, they only reported the results of the 1st-year follow-up. Consequently, those two were excluded from the meta-analysis.
Other bias
There was no indication for other sources of bias in all the included studies, so they were judged to be at low risk for this domain.
HETEROGENEITY ASSESSMENT
Two of the remaining five studies had an unclear risk of bias when the allocation concealment domain was assessed. Therefore, “methodological heterogeneity” with respect to the risk of bias existed between the five studies. In addition, the diversity of teeth types reflected “clinical heterogeneity;” two trials assessed the efficacy of resin infiltration on NCPLs in primary teeth, and the other three evaluated that in permanent teeth. As a matter of fact, primary teeth have lower mineral content[24] relative to permanent teeth.[25] Accordingly, two meta-analyses were conducted separating the included RCTs based on teeth types in order to preserve the coherence of evidence. Another demonstration of clinical heterogeneity is the dissimilarity of comparators among studies. The studies included in the meta-analyses compared the resin infiltration to each of the following:
Fluoridated toothpaste and dental floss (n = 1)
Fluoride varnish (n = 1)
No treatment (n = 3).
STATISTICAL ANALYSIS
We conducted both meta-analyses using the random effect model due to the presence of “methodological heterogeneity” in terms of bias risk. Each meta-analysis had one study with an unclear risk of bias concerning allocation concealment. Meanwhile, the remainder of the included trials had a low-bias risk. In the meta-analysis of permanent teeth [Figure 3], the follow-up of studies ranged from 18 to 36 months. Similarly, the primary tooth meta-analysis [Figure 4] had a follow-up range of 12–24 months. In both meta-analyses, the included trials measured the carious lesions’ progression by radiographic pairwise comparison. Moreover, one RCT utilized two outcome assessment methods: radiographic pairwise comparison and digital subtraction radiography (DSR).[17] Despite the higher accuracy of DSR over the pairwise comparison, the outcome obtained by the pairwise comparison was the one included in our meta-analysis, to maintain homogeneity between the included trials. Technically, in both forest plots, the effect measure was the risk ratio (RR), and the effect estimate of each NCPL management modality was assessed by inspecting the caries progression rate of each included trial. The weight of each study in each meta-analysis was gauged by the Mantel-Haenszel method because it has better statistical features when the events are few.[14] As a result, the risk of NCPLs’ progression with resin infiltration was significantly lower in primary (RR; 95% confidence interval [CI]: 0.48; 0.30–0.75, P = 0.001) and in permanent teeth (RR; 95% CI: 0.19; 0.11–0.33, P < 0.00001) compared to that of control/placebo. The I2 = 0% in both forest plots indicates the absence of statistical heterogeneity.
Figure 3.
Forest plot of comparison: Proximal resin infiltration versus control/placebo, outcome: Caries progression rate in permanent teeth (pairwise, 18–36 months’ follow-up)
Figure 4.
Forest plot of comparison: Proximal resin infiltration versus control/placebo, outcome: Caries progression rate in primary teeth (pairwise, 12–24 months’ follow-up)
QUALITY OF EVIDENCE
The GRADE approach[16] revealed high quality of evidence [Table 3].[26]
Table 3.
Quality of evidence by the GRADE approach
| Summary of findings: | ||||||
| Resin infiltration compared to Control/placebo for slowing/arresting caries progression rate | ||||||
| Patient or population: slowing/arresting caries progression rate Setting: Secondary care setting Intervention: Resin infiltration Comparison: Control/placebo | ||||||
| Outcomes | Anticipated absolute effects*(95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with Control/placebo | Risk with Resin infiltration | |||||
| Caries progression rate in permanent teeth assessed with: Pairwise comparison follow up: range 18 months to 36 months | 301 per 1,000 | 57 per 1,000 (33 to 99) |
RR 0.19 (0.11 to 0.33) |
478 (3 RCTs) | ⊕⊕⊕⊕HIGH | |
| Caries progression rate in primary teeth assessed with: Secondary care setting follow up: range 12 months to 24 months |
436 per 1,000 |
209 per 1,000 (131 to 327) |
RR 0.48 (0.30 to 0.75) |
209 (2 RCTs) | ⊕⊕⊕⊕HIGH | |
*The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. GRADE Working Group grades of evidence. High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect
DISCUSSION
This evidence confirms the results of former in vitro[27] and in situ studies[28] which found that resin infiltration is efficacious in arresting/slowing the NCPLs’ progression rate. More importantly, our meta-analyses revealed that proximal resin infiltration had superior efficacy over conventional management methods in preventing further NCPLs’ progression. Such methods were fluoridated toothpaste and dental floss, fluoride varnish, and no treatment. This could be attributed to the fact that such methods demand a high level of patient compliance. On the contrary, proximal resin infiltration does not require any effort from the patient. Furthermore, the resin infiltrant will continuously act as a barrier inside the NCPL. Meanwhile, conventional management modalities are intermittent in nature, which might allow more demineralization/remineralization cycles even in highly compliant patients. Based on that, the concept of hygienic measures as an adjunctive treatment for NCPLs seems more rational than the notion behind utilizing them as a particular management strategy. Furthermore, one of the excluded trials[20] found that resin infiltration, in conjunction with fluoride varnish, was efficacious in controlling the proximal caries progression in primary molars. The other excluded study[21] showed that infiltration and sealing were superior over placebo in limiting NCPLs’ progression for permanent teeth. However, there was no significant difference between infiltration and sealing.
Risk-of-bias assessment is crucial in evaluating the quality of evidence. In general, high risk-of-bias studies lower the quality of meta-analyses;[14] thus, they were excluded to protect our quality of evidence. Accordingly, selective reporting of the outcomes increased the bias risk in two of the trials. As a consequence, they were excluded. Furthermore, unclear bias risk regarding allocation concealment was identified in another two RCTs. However, this was not likely to affect the outcomes of both trials; therefore, they were not excluded.
In adherence to the GRADE approach[16] and for complete and consistent evaluation of the quality of evidence, in each study, additional aspects were appraised besides the risk of bias. Those aspects were as follows: (1) study design, (2) consistency, (3) directness, and (4) precision. The quality of evidence was ranked as high in both meta-analyses, where all the aspects evaluated displayed high quality, since (1) the study design of all the trials included in both meta-analyses was a randomized controlled design. (2) Consistency is defined as the similarity of effect estimates between studies; both meta-analyses were consistent. Technically, (3) the outcome measures, interventions, and participants were similar between studies in each meta-analysis, and by definition, this indicated directness. In addition, precision domain lowers the quality if the number of events was few. Interestingly, (4) despite the few number of studies included in each meta-analysis, the quality of evidence was not affected when the number of events was appraised using the GRADE computer program.[26]
CONCLUSION
The available evidence conveys high confidence that proximal resin infiltration has superior efficacy in slowing/arresting the NCPLs’ progression rate in comparison to conventional management modalities. Further high-quality RCTs with long-term follow-up are recommended to increase this evidence and to allow the estimation of proximal resin infiltration longevity.
FINANCIAL SUPPORT AND SPONSORSHIP
Nil.
CONFLICTS OF INTEREST
There are no conflicts of interest.
REFERENCES
- 1.Mejàre I, Stenlund H, Zelezny-Holmlund C. Caries incidence and lesion progression from adolescence to young adulthood: A prospective 15-year cohort study in Sweden. Caries Res. 2004;38:130–41. doi: 10.1159/000075937. [DOI] [PubMed] [Google Scholar]
- 2.Altarabulsi MB, Alkilzy M, Splieth CH. Clinical applicability of resin infiltration for proximal caries. Quintessence Int. 2013;44:97–104. doi: 10.3290/j.qi.a28934. [DOI] [PubMed] [Google Scholar]
- 3.Dorri M, Dunne SM, Walsh T, Schwendicke F. Micro-invasive interventions for managing proximal dental decay in primary and permanent teeth. Cochrane Database Syst Rev. 2015;11:CD010431. doi: 10.1002/14651858.CD010431.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rayapudi J, Usha C. Knowledge, attitude and skills of dental practitioners of Puducherry on minimally invasive dentistry concepts: A questionnaire survey. J Conserv Dent. 2018;21:257–62. doi: 10.4103/JCD.JCD_309_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laske M, Opdam NJ, Bronkhorst EM, Braspenning JC, van der Sanden WJ, Huysmans MC, et al. Minimally invasive intervention for primary caries lesions: Are dentists implementing this concept? Caries Res. 2018;53:204–16. doi: 10.1159/000490626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mirsiaghi F, Leung A, Fine P, Blizard R, Louca C. An investigation of general dental practitioners’ understanding and perceptions of minimally invasive dentistry. Br Dent J. 2018;225:420–4. doi: 10.1038/sj.bdj.2018.744. [DOI] [PubMed] [Google Scholar]
- 7.Meyer-Lueckel H, Paris S. Progression of artificial enamel caries lesions after infiltration with experimental light curing resins. Caries Res. 2008;42:117–24. doi: 10.1159/000118631. [DOI] [PubMed] [Google Scholar]
- 8.Peters MC, Hopkins AR, Jr, Yu Q. Resin infiltration: An effective adjunct strategy for managing high caries risk-a within-person randomized controlled clinical trial. J Dent. 2018;79:24–30. doi: 10.1016/j.jdent.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 9.Mahran A, Fahim F, Hafez H. Treatment of post-orthodontic white-spot lesions by resin infiltration. IOSR J Dent Med Sci. 2017;16:80–4. [Google Scholar]
- 10.Paris S, Schwendicke F, Keltsch J, Dörfer C, Meyer-Lueckel H. Masking of white spot lesions by resin infiltration in vitro . J Dent. 2013;41(Suppl 5):e28–34. doi: 10.1016/j.jdent.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Onex, Baring. Endnote X8. Philadelphia: Clarivate Analytics; 2017. [Google Scholar]
- 13.Ammari MM, Soviero VM, da Silva Fidalgo TK, Lenzi M, Ferreira DM, Mattos CT, et al. Is non-cavitated proximal lesion sealing an effective method for caries control in primary and permanent teeth? A systematic review and meta-analysis. J Dent. 2014;42:1217–27. doi: 10.1016/j.jdent.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 14.Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. The Cochrane Collaboration. 2011. [Last accessed on 2018 Jan 30]. Available from: http://www.handbook.cochrane.org .
- 15.Review Manager [Computer Program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration; 2014. [Google Scholar]
- 16.Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328:1490. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meyer-Lueckel H, Bitter K, Paris S. Randomized controlled clinical trial on proximal caries infiltration: Three-year follow-up. Caries Res. 2012;46:544–8. doi: 10.1159/000341807. [DOI] [PubMed] [Google Scholar]
- 18.Meyer-Lueckel H, Balbach A, Schikowsky C, Bitter K, Paris S. Pragmatic RCT on the efficacy of proximal caries infiltration. J Dent Res. 2016;95:531–6. doi: 10.1177/0022034516629116. [DOI] [PubMed] [Google Scholar]
- 19.Foster-Page LA, Beckett D, Ahmadi R, Schwass D, Leon de La Barra S, Moffat S. Resin Infiltration of caries in primary molars in a community setting: 24-Month randomized controlled trial findings. JDR Clin Transl Res. 2017;2:287–94. doi: 10.1177/2380084417699400. [DOI] [PubMed] [Google Scholar]
- 20.Ekstrand KR, Bakhshandeh A, Martignon S. Treatment of proximal superficial caries lesions on primary molar teeth with resin infiltration and fluoride varnish versus fluoride varnish only: Efficacy after 1 year. Caries Res. 2010;44:41–6. doi: 10.1159/000275573. [DOI] [PubMed] [Google Scholar]
- 21.Martignon S, Ekstrand KR, Gomez J, Lara JS, Cortes A. Infiltrating/sealing proximal caries lesions: A 3-year randomized clinical trial. J Dent Res. 2012;91:288–92. doi: 10.1177/0022034511435328. [DOI] [PubMed] [Google Scholar]
- 22.Ammari MM, Jorge RC, Souza IPR, Soviero VM. Efficacy of resin infiltration of proximal caries in primary molars: 1-year follow-up of a split-mouth randomized controlled clinical trial. Clin Oral Investig. 2018;22:1355–62. doi: 10.1007/s00784-017-2227-7. [DOI] [PubMed] [Google Scholar]
- 23.Arthur RA, Zenkner JE, d’Ornellas Pereira Júnior JC, Correia RT, Alves LS, Maltz M, et al. Proximal carious lesions infiltration-a 3-year follow-up study of a randomized controlled clinical trial. Clin Oral Investig. 2018;22:469–74. doi: 10.1007/s00784-017-2135-x. [DOI] [PubMed] [Google Scholar]
- 24.De Menezes Oliveira MA, Torres CP, Gomes-Silva JM, Chinelatti MA, De Menezes FC, Palma-Dibb RG, et al. Microstructure and mineral composition of dental enamel of permanent and deciduous teeth. Microsc Res Tech. 2010;73:572–7. doi: 10.1002/jemt.20796. [DOI] [PubMed] [Google Scholar]
- 25.Klimuszko E, Orywal K, Sierpinska T, Sidun J, Golebiewska M. Evaluation of calcium and magnesium contents in tooth enamel without any pathological changes:In vitro preliminary study. Odontology. 2018;106:369–76. doi: 10.1007/s10266-018-0353-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.GRADEpro GDT: GRADEpro Guideline Development Tool. McMaster University: Developed by Evidence Prime, Inc; 2019. [Last accessed on 2018 Apr 10]. Available from: http://www.gradepro.org . [Google Scholar]
- 27.Mueller J, Meyer-Lueckel H, Paris S, Hopfenmuller W, Kielbassa AM. Inhibition of lesion progression by the penetration of resins in vitro: Influence of the application procedure. Oper Dent. 2006;31:338–45. doi: 10.2341/05-39. [DOI] [PubMed] [Google Scholar]
- 28.Paris S, Meyer-Lueckel H. Inhibition of caries progression by resin infiltration in situ. Caries Res. 2010;44:47–54. doi: 10.1159/000275917. [DOI] [PubMed] [Google Scholar]