Abstract
Context:
Biomarkers can be used for screening lung cancer and the clinician can decide for further invasive workup for diagnosis.
Aims:
To know the diagnostic sensitivity and specificity of Carcinoembryonic antigen (CEA) in Broncho Alveolar Lavage Fluid (BALF) and serum of bronchogenic carcinoma.
Settings and Design:
Case-Control study was conducted in the Medical College Hospital during a period of 2 years.
Methods and Material:
We randomly selected 50 cases and 50 controls subjects. Cases were the patients with proven malignancy by biopsy or cytology, and controls were other non-malignant pulmonary diseases. All patients’ CEA of Broncho Alveolar Lavage Fluid and serum was done.
Statistical Analysis:
The mean and receiver operating curve were done for CEA of serum and BAL fluid, and based on the cut-off values, sensitivity and specificity were calculated.
Results:
Mean value of CEA in both BALF and serum in non-smoker patients of the malignant lesion was significantly higher than the non-malignant lesion. Mean value of CEA in both BALF and serum in smoker patients of the malignant lesion was higher than the benign lesion, but statistically not significant. The cut-off value for Serum CEA is 1μg/l, whereas for BALF CEA is 2μg/l. Sensitivity, specificity of CEA of Serum and BALF combined were 92% and 62% respectively.
Conclusions:
Determination of CEA in the BALF and serum may be helpful as a screening tool for further workup for malignancy.
Keywords: Broncho alveolar lavage fluid, lung carcinoma, tumour marker
Introduction
Lung cancer is the most common cause of cancer mortality both in males and females.[1] As per Globocan Report 2018, 1.8 million deaths were due to lung cancer, which was 18.4% of total death worldwide.[2] In India, 8.3% of death occurred due to neoplasm, out of which death rate for Lung Cancer is 5/100000 population and the proportion of deaths due to neoplasm were higher in the 40-69 years age group.[3] More than 60% of lung cancer cases presented at stage IIB or stage IV.[4] In general, the poor prognosis of lung cancer was due to the lack of effective early detection.[5]
Tissue biopsy either by bronchoscopy or by percutaneous is the gold standard for the diagnosis. Sometimes, in view of peripherally placed lung lesions, inadequate sampling by CT guidance or by bronchoscopy to establish the diagnosis is very difficult. Tumour marker is a substance present in and produced by a tumour itself or produced by the host in response to the tumour. Tumour marker can easily be determined in body fluid like blood or Broncho Alveolar Lavage Fluid (BALF). It has a different application like diagnosis, screening, monitoring disease progress, relapse and serving as a prognostic indicator.[6] Therefore, it is very important to assess the appropriate tumour markers in BALF for diagnosis or even in predicting the recurrence of lung cancer.[7] Some studies are available using BALF as tumour marker, and some with serum as tumour marker.[8,9,10,11,12] Very few studies have been done using both BALF and serum tumour marker.[13,14] So the choice of sample type still remains contentious.
There are few studies available in India about the role of tumour marker in BALF and serum in case of bronchogenic carcinoma.[15,16] But the present study is meant to evaluate the diagnostic sensitivity and specificity of CEA in BALF of bronchogenic carcinoma, to compare BALF CEA value with corresponding serum CEA as tumour marker, and to evaluate the differences among smoker and non-smoker groups.
Methods
The Case-Control study was conducted in the Medical College Hospital during the period from January 2015 to January 2017. As per the previous study, sensitivity and specificity of serum CEA was 80% and 72.2% respectively.[16] Considering power 90%, α error 2.5% with a one-sided test, minimum sample size calculated was 45 in each group (n-Master v 2.0, BRTC, Vellore). 50 cases and 50 controls were taken as study subject.
The study was approved by the institutional ethical committee (letter no-2014/P-1-RP/14M-A-PUL-056/020) and informed consent was taken from each participant. Patient's socio-economic status was classified according to modified Kuppuswami socioeconomic status scale.[17] Study groups were patients with clinical-radiological suspicion of lung malignancy and later confirmed by computed tomography guided fine needle aspiration cytology or biopsy of lung lesion or fiber-optic bronchoscopy (FOB) guided biopsy or BAL cytology or brush cytology. Control groups were individuals admitted in Pulmonary Medicine department for various other respiratory ailments like pneumonia, diffuse parenchymal lung disease, pulmonary tuberculosis (sputum smear negative) etc., in which there was an indication for diagnostic bronchoscopy. Patient associated with unstable cardiovascular status like recent myocardial infarction, unstable angina, severe hypertension, severe carotid or cerebro-vascular disease, severe hypoxia, hypoventilation, severe bronchospasm, unstable asthma were excluded from the study. Both study group and control group were subjected to bronchoscopic BALF collection for estimation of CEA level. Bronchoscopic procedure and technique for the collection of BALF was followed as per protocol.[18] The segmental site for BALF collection was chosen based on the visible bronchoscopic abnormality or CT thorax abnormality. For diffused lesion, left lingual and right middle lobe was chosen for the collection of BALF. 20 ml of sterile normal saline per aliquot was injected through the working channel of the bronchoscope. Around 5 aliquots of normal saline were injected. Around 50 ml of BALF was collected by applying gentle suction which was filtered for removal of mucus followed by centrifugation at the rate of 2000 rpm for 10 min. Supernatant sent to the laboratory for CEA as per another published study.[19] On the same day of bronchoscopy, each patient's 10 ml blood collected for estimation of serum CEA value.
The serum prepared from collected blood and BALF from all the subjects were analysed for estimation of levels of CEA using third generation enzyme-linked immunosorbent assay (ELISA) kits (Can Ag, Canada).
Statistics
The statistical analysis was performed with the help of Dxt software (BRTC, Bagayam, CMC Vellore). Normally distributed continuous variables are presented as mean (±SD). Categorical variables are expressed as percentage. Comparison of the different mean was done with Student's t-test and comparison of different proportion was done with the Chi-square test. The receiver operating curve (ROC) was done for serum and BALF, based on the ROC, cut-off values for BALF and serum has been set for CEA. Sensitivity, specificity, positive likelihood ratio, negative likelihood ratio, positive predictive value and negative predictive value of CEA of serum and BALF were calculated. P value of 0.05 or less was considered statistically significant.
Results
The present study was carried out in 100 subjects and the following observations were noted.
Age, gender, socio-economic and smoking status were matched in both groups as evidence by P > 0.05 [Table 1]. In this study, predominantly male population (60%) was in the study group whereas predominantly female population (52%) was in the control group. Majority of study subjects were in the lower socio-economical group in both study as well as the control group. All female populations were non-smoker, both in study and control group.
Table 1.
Baseline Characteristics and Demographic profile of both case and control
| Variable | Study Group | Control Group | P |
|---|---|---|---|
| Age (Mean±SD) | 51.2±1.187 | 50.92±14.28 | 0.890 |
| Sex | |||
| Male | 30 | 24 | 0.23 |
| Female | 20 | 26 | 0.23 |
| Socio-economic Status | |||
| Upper | 1 | 4 | 0.17 |
| Upper Middle | 6 | 2 | 0.14 |
| Lower Middle | 8 | 12 | 0.32 |
| Upper Lower | 11 | 8 | 0.44 |
| Lower | 24 | 24 | 1.00 |
| Smoking Status | 14 | 10 | 0.351 |
BALF and serum CEA result of both study group and control group are presented in Table 2. The study demonstrated that the level of CEA was higher in the study group compared to the control group irrespective of smoking status. Among non-smoker population, both BALF and serum CEA statistically significant in study subjects compared to controls [Table 2].
Table 2.
Comparison of CEA profile in Serum and BALF
| BALF CEA in µg/L | Serum CEA in µg/L | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Type of Patient | Mean (SD) | t-test | P | Type of Patient | Mean (SD) | t-test | P | ||
| Smoker (n=24) | Control (n=10) | 49.33 (13.70) | 0.55 | 0.59 | Smoker (n=24) | Control (10) | 7.78 (2.09) | 1.73 | 0.097 |
| Study Group (n=14) | 59.76 (12.82) | Study Group (14) | 21.48 (6.48) | ||||||
| Non-Smoker (n=76) | Control (n=40) | 22.16 (5.97) | 4.15 | 0.000 | Non-Smoker (n=76) | Control (40) | 3.58 (0.98) | 2.38 | 0.02 |
| Study Group (n=36) | 81.63 (13.56) | Study Group (n=36) | 10.44 (2.84) | ||||||
Mean value of CEA of BALF was higher than CEA of serum among both smoker and non-smoking individuals (P < 0.0001) in malignancy group.
The performance of CEA both serum and BALF at various levels of specificity was analysed by comparing the area under ROC curves. The AUC of BALF CEA (0.77) appeared to be larger than the AUC of Serum CEA (0.72) [Figure 1]. In sub-group analysis, Non-smoker population has larger AUC. We found the best cut-off of CEA for serum was 1 μg/l and for BALF was 2 μg/l irrespective of smoking status for differentiating benign and malignant lesion. ROC could not be done in smoker population due to small sample size. The diagnostic value of CEA both serum and BALF was analysed in term of sensitivity, specificity, predicted value and like-hood ratio [Table 3].
Figure 1.
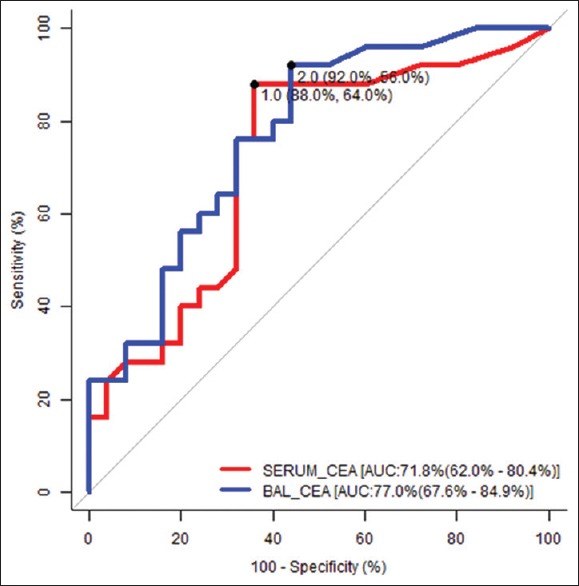
ROC of Serum and BALF CEA
Table 3.
Diagnostic accuracy of CEA
| Sensitivity (%) with 95% CI* | Specificity (%) with 95%CI* | LR +ve† with 95% CI* | LR-ve‡ with 95% CI* | PPV§ (%) with 95% CI* | NPV? (%) with 95% CI* | |
|---|---|---|---|---|---|---|
| Serum CEA Positive | 86 (73-94) | 64 (49-77) | 2.39 (1.62-3.51) | 0.22 (0.11-0.45) | 70 (57-81) | 82 (66-92) |
| BALF CEA Positive | 92 (80-98) | 56 (41-70) | 2.09 (1.51-2.89) | 0.14 (0.05-0.38) | 68 (55-78) | 87 (71-96) |
| Both Serum & BALF Positive | 92 (80-98) | 62 (46-75) | 2.39 (1.65-3.47) | 0.13 (0.05-0.35) | 71 (58-82) | 88 (72-97) |
*CI=Confidence Interval, †LR+ve=Likelihood ratio positive, ‡LR-ve=Likelihood ratio negative, §PPV=Positive predictive value, ?NPV=Negative predictive value.
Discussion
In our study, we have compared the CEA level of BALF and serum among bronchogenic carcinoma and other benign conditions. There is a definite increase level of CEA among bronchogenic carcinoma group than the control group in both serum as well as BALF. Mean value of CEA in BALF significantly higher than the serum CEA. A similar observation like the increased level of CEA among bronchogenic carcinoma as well as increased level BALF CEA higher as compared to serum was seen by other studies.[13,20] We have compared the CEA level among smoker and non-smoking individuals. The non-smoking individuals CEA level of both BALF and serum significantly increased in bronchogenic carcinoma as compared to benign lung diseases. In smoker individuals, the CEA level of both BALF and serum increased in lung malignancy than the benign lung diseases but statistically not significant. The mean CEA level of both BALF and serum was more in smoking individuals than the non-smokers, which was more marked in benign lung diseases. There was evidence of increased levels of CEA in smoking individuals than the non-smoking individual in an otherwise healthy population in another study also.[21] Probably, smoking altered the functional status of bronchial epithelium which increases the CEA level. Smoking could be a confounding factor for an increased level of CEA. So, in non-smoking individuals increased level of CEA in BALF could be an ideal tumour marker for bronchogenic carcinoma. According to de Diego A et al., there was no significant difference in the level of CEA in both serum and BALF between smokers and non-smokers.[20]
We have used ROC analysis as a tool to assess the validity of the test. AUC of both serum and BALF was higher than 0.70. Comparison between serum and BALF, it has been seen that AUC of BALF higher than serum. In the present study the cut off value of maximum sensitivity and lowest false positivity for serum CEA was 1 μg/l and for BALF CEA was 2 μg/l. That means cut-off value of BALF CEA was higher than serum CEA. In another study cut off value of BALF and serum CEA was 8 μg/l and 4.5 μg/l, respectively, where BALF cut off value was also higher than serum.[15]
In this study, based on serum CEA 1 μg/l as cut off value, sensitivity and specificity of serum CEA are 86% and 64% respectively. But as per previous studies, the sensitivity of serum CEA for the diagnosis ranges from 40.9% to 80%, whereas specificity ranges from 68% to 99.2%.[8,9,16,20,22] In this study, based on BALF CEA value 2 μg/l as cut off value, the sensitivity and specificity of BALF CEA is 92% and 56% respectively. Whereas previous studies showed, BALF CEA for diagnosis of lung cancer, sensitivity ranges from 55% to 100% and specificity ranges from 59% to 94%.[10,11,20,23,24] Combination of serum and BALF CEA levels in lung cancer, the sensitivity and specificity were 92% and 62% in our study. Previous studies showed sensitivity and specificity of 88 percent.[12] In our study BALF CEA is more sensitive and less specific to serum CEA. Previous studies showed BALF CEA had higher sensitivity than to serum CEA.[13,25] positive predictive value (PPV) is the proportion of patients with increased CEA who actually have the malignancy, and negative predictive value (NPV) is the proportion patients with normal CEA who are free of malignancy. Predictive value depends on the prevalence of disease in the community and its importance is less in rare disease. Lung malignancy is not an uncommon disease in the community, so predictive value has great importance. In our study, serum CEA had PPV of 70% and NPV of 82%, whereas BALF CEA had PPV of 68% and NPV of 87%. Studies by de Diego A et al., positive and negative predictive values were 85% and 87% in BALF and 83% and 84% in serum respectively.[20] If we considered, both serum and BALF CEA combined, the PPV was 71% and NPV was 88%. Whereas in the previous study, the author showed PPV was 66% and NPV 96% by considering both serum and BALF CEA.[12] In our study, BALF CEA predictive value did not show higher than serum CEA in contrast to another study.[15] On the basis of these high negative predictive values after considering both Serum and BALF CEA, the physician may defer further work-up like CT guided biopsy in view of complications related to the invasive procedure. The likelihood ratio (LR) refers to how much more likely someone had an increased CEA for malignancy, compared with to benign lesion which does not depend on the prevalence of diseases. In our study, serum CEA, LR+ of 2.39 and LR- of 0.22 and BALF CEA, LR+ of 2.09 and LR- of 0.14. After considering both Serum and BAL CEA combined LR+ of 2.39 and LR- of 0.13. As LR+ in both serum and BALF >2, so patient with lung cancer have approximately 2 fold higher chance of having raised CEA compared to a patient without lung cancer. On the other hand, LR- in both serum and BALF is <0.22, so patient with low CEA value the probability of lung cancer around 20% which is low enough to rule out lung cancer.
Based on our result, BALF CEA assay has almost similar yield as compared to serum CEA so far diagnostic utility is has concerned. Similar concluding remark was given by another study.[20] The study by charalabopoulos et al., pointed out that CEA of BALF alone has little value in the diagnosis of malignancy.[13] Though tissue diagnosis is the gold standard for the identification of malignancy, at times it is very difficult to get the proper sample. BALF and serum CEA can be adopted as a diagnostic tool to exclude bronchogenic carcinoma to some extent.
How the study results help family physicians in routine practice?
Estimation of serum CEA which is a relatively simple blood test could refine current lung cancer screening eligibility criteria and help identify higher-risk individuals for low-dose CT
BAL and serum CEA can become a reliable complement to imaging tests in the diagnosis of lung cancer, particularly in the doubtful case when differentiated with benign lung diseases
BAL and serum CEA may reduce the number of invasive procedures for patients without malignancy without significant delay in diagnosis of bronchogenic carcinoma.
Study limitation
The number of patients in the study group was not large. Thus, care must be taken in extrapolating the present findings to other population. We could not correlate BALF or serum CEA with stages of lung cancer. We could not analyse CEA with different histological subtype. The number of smoker population is less in comparison to the non-smoker population, both in the benign and malignant group which could be a statistical bias.
Despite these limitations, we believe that our study will be helpful in the assay of the BALF and serum CEA as tumour marker which is simple and complement to other tests in the diagnosis of lung cancer.
Conclusion
In cases of suspicious lung malignancy by clinical judgment showing negative cytology by less invasive procedure, particularly in the peripherally placed tumour and/or unsuitability for invasive procedures, the determination of tumour markers in the BALF and serum may be used as screening tool for further workup due to high sensitivity. However, more studies are required to justify it.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.International Agency for Research on Cancer [Internet] Geneva, Switzerland: World Health Organization; [Last accessed on 2018 Dec 20]. 12 September. Available from: https://www.iarc.fr/en/media-centre/2018/pdf . [Google Scholar]
- 3.Indian Council of Medical Research, Public Health Foundation of India, and Institute for Health Metrics and Evaluation. India, New Delhi, India: Health of the Nation's States-The India State-Level Disease Burden Initiative, ICMR, PHFI, and IHME; 2017. [Google Scholar]
- 4.Mäkitaro R, Pääkko P, Huhti E, Bloigu R, Kinnula VL. Prospective population-based study on the survival of patients with lung cancer. Eur Respir J. 2002;19:1087–92. doi: 10.1183/09031936.02.00048302. [DOI] [PubMed] [Google Scholar]
- 5.Sawabata N. Prognosis of lung cancer patients in Japan according to data from the Japanese Joint Committee of Lung Cancer Registry. Respir Investig. 2014;52:317–21. doi: 10.1016/j.resinv.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Strauss GM, Skarin AT. Use of tumor markers in lung cancer. Hematol Oncol Clin North Am. 1994;8:507–32. [PubMed] [Google Scholar]
- 7.Crohns M, Saarelainen S, Laine S, Poussa T, Alho H, Kellokumpu-Lehtinen P. Cytokines in bronchoalveolar lavage fluid and serum of lung cancer patients during radiotherapy-Association of interleukin 8 and VEGF with survival. Cytokine. 2010;50:30–6. doi: 10.1016/j.cyto.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 8.Okamura K, Takayama K, Izumi M, Harada T, Furuyama K, Nakanishi Y. Diagnostic value of CEA and CYFRA 21-1 tumor markers in primary lung cancer. Lung Cancer. 2013;80:45–9. doi: 10.1016/j.lungcan.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Wang B, He YJ, Tian YX, Yang RN, Zhu YR, Qiu H. Clinical utility of haptoglobin in combination with CEA, NSE and CYFRA21-1 for diagnosis of lung cancer. Asian Pac J Cancer Prev. 2014;15:9611–4. doi: 10.7314/apjcp.2014.15.22.9611. [DOI] [PubMed] [Google Scholar]
- 10.Sanguinetti CM, Riccioni G, Marchesani F, Pela R, Cecarini L. Bronchoalveolar lavage fluid level of carcinoembryonic antigen in the diagnosis of peripheral lung cancer. Monaldi Arch Chest Dis. 1995;50:177–82. [PubMed] [Google Scholar]
- 11.Li J, Chen P, Mao CM, Tang XP, Zhu LR. Evaluation of diagnostic value of four tumor markers in bronchoalveolar lavage fluid of peripheral lung cancer. Asia Pac J Clin Onco. 2014;10:141–8. doi: 10.1111/ajco.12066. [DOI] [PubMed] [Google Scholar]
- 12.de Diego A, Compte L, Sanchis J, Enguidanos MJ, Marco V. Usefulness of Carcinoembryonic antigen determination in bronchoalveolar Lavage fluid. A comparative study among patients with peripheral lung cancer, pneumonia, and healthy individuals. Chest. 1991;100:1060–3. doi: 10.1378/chest.100.4.1060. [DOI] [PubMed] [Google Scholar]
- 13.Charalabopoulos K, Karakosta A, Bablekos G, Golias C, Charalabopoulos A, Tsanou E, et al. CEA levels in serum and BAL in patients suffering from lung cancer: Correlation with individuals presenting benign lung lesions and healthy volunteers. Med Oncol. 2007;24:219–25. doi: 10.1007/BF02698043. [DOI] [PubMed] [Google Scholar]
- 14.Wang H, Zhang X, Liu X, Liu K, Li Y, Xu H. Diagnostic value of bronchoalveolar lavage fluid and serum tumor markers for lung cancer. J Can Res Ther. 2016;12:355–8. doi: 10.4103/0973-1482.162111. [DOI] [PubMed] [Google Scholar]
- 15.Ghosh I, Bhattacharjee D, Das AK, Chakrabarti G, Dasgupta A, Dey SK. Diagnostic role of tumor marker CEA, CA 15-3, CA19-9 and CA 125 in lung cancer. Ind J Clin Biochen. 2013;28:24–9. doi: 10.1007/s12291-012-0257-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu L, Lina W, Xuejun Y. The diagnostic value of serum CEA, NSE and MMP-9 for on-small cell lung cancer. Open Medicine (Wars) 2016;11:59–62. doi: 10.1515/med-2016-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mishra D, Singh HP. Kuppuswamy's socioeconomic status scale- A revision. Indian J Pediatr. 2003;70:273–4. doi: 10.1007/BF02725598. [DOI] [PubMed] [Google Scholar]
- 18.King TE., Jr . Handling and analysis of bronchoalveolar lavage specimens. In: Baughman RP, editor. Bronchoalveolar Lavage. Philadelphia: Year Book Medical Publishers; 1991. p. 3. [Google Scholar]
- 19.Yin X, Xie L, Chai Y, Fan H, Han X, Feng Z. Surfactant protein b expression in bronchoalveolar lavage fluid of full-term neonates with respiratory distress syndrome. Acta Clin Croat. 2014;53:161–5. [PubMed] [Google Scholar]
- 20.de Diego A, Compte L, Sanchis J, Enguidanos MJ, Marco V. Diagnostic value of carcinoembryonlc antigen in bronchoalveolar lavage fluid of peripheral lung cancer. Chest. 1990;97:767–8. doi: 10.1378/chest.97.3.767. [DOI] [PubMed] [Google Scholar]
- 21.Sajid KM, Parveen R, Sabih De, Chaouachi K, Naeem A, Mahmood R, et al. Carcinoembryonic antigen (CEA) levels in hookah smokers, cigarette smokers and non-smokers. J Pak Med Assoc. 2007;57:595–9. [PubMed] [Google Scholar]
- 22.Dong Y, Zheng X, Yang Z, Sun M, Zhang G, An X, et al. Serum carcinoembryonic antigen, neuron-specific enolase as biomarkers for diagnosis of non-small cell lung cancer. J Can Res Ther. 2016;12:34–6. doi: 10.4103/0973-1482.191626. [DOI] [PubMed] [Google Scholar]
- 23.Dabrowska M, Grubek-Jaworska H, Domagała-Kulawik J, Bartoszewicz Z, Kondracka A, Krenke R, et al. Diagnostic usefulness of selected tumor markers (CA125, CEA, CYFRA 21-1) in bronchoalveolar lavage fluid in patients with non-small cell lung cancer. Pol Arch Med Wewn. 2004;111:659–65. [PubMed] [Google Scholar]
- 24.Pina TC, Zapata IT, Hernandez FC, Lopez JB, Paricio PP, Hernandez PM. Tumour markers in serum, bronchoalveolar lavage and biopsy cytosol in lung carcinoma: What environment lends the optimum diagnostic yield? Clin Chim Acta. 2001;305:27–34. doi: 10.1016/s0009-8981(00)00410-1. [DOI] [PubMed] [Google Scholar]
- 25.Grunnet M, Sorensen JB. Carcinoembryonic antigen (CEA) as tumor marker in lung cancer. Lung Cancer. 2012;76:138–43. doi: 10.1016/j.lungcan.2011.11.012. [DOI] [PubMed] [Google Scholar]


