Abstract
Wakefulness and sleep arise from global changes in brain physiology that may also govern the flow of neural activity between cortical regions responsible for perceptual processing versus planning and action. To test whether and how the sleep/wake cycle affects the overall propagation of neural activity in large-scale brain networks, we applied single-pulse electrical stimulation (SPES) in patients implanted with intracranial EEG electrodes for epilepsy surgery. SPES elicited cortico-cortical spectral responses at high-gamma frequencies (CCSRHG, 80–150 Hz), which indexes changes in neuronal population firing rates. Using event-related causality (ERC) analysis, we found that the overall patterns of neural propagation among sites with CCSRHG were different during wakefulness and different sleep stages. For example, stimulation of frontal lobe elicited greater propagation toward parietal lobe during slow-wave sleep than during wakefulness. During REM sleep, we observed a decrease in propagation within frontal lobe, and an increase in propagation within parietal lobe, elicited by frontal and parietal stimulation, respectively. These biases in the directionality of large-scale cortical network dynamics during REM sleep could potentially account for some of the unique experiential aspects of this sleep stage. Together these findings suggest that the regulation of conscious awareness and sleep is associated with differences in the balance of neural propagation across large-scale frontal-parietal networks.
Keywords: brain waves, human electrocorticography, high-gamma activity, effective connectivity, causal interactions
Statement of Significance.
While awake, different areas of the brain are involved in perception of the environment versus planning and organizing behavior, but they are in constant communication. While asleep, the brain processes information gathered during wakefulness, but how different brain areas interact during sleep, is poorly understood. To elucidate these interactions, we activated the brain with weak electrical pulses during wakefulness and sleep, and we analyzed how this activation spread through the brain. We observed greater propagation of activity from frontal to parietal lobe during slow-wave sleep, and decreased propagation within frontal lobe, but increased propagation within parietal lobe, during REM sleep. These suggest that wakefulness and sleep are associated with different patterns of propagation of neural activity across brain networks.
Introduction
The profound neurophysiological changes that occur during the sleep-wake cycle may modify the overall direction by which neural activity is propagated among various areas. For example, previous studies have shown that low-frequency oscillations characteristic of slow wave, or non-REM, sleep tend to travel in an anteroposterior direction [1–3]. In contrast, this flow may reverse during wakefulness [1]. Reminiscent of the tides of the ocean, circadian rhythms in the brain may be accompanied by cyclical variations in the kind and strength of neural interactions across large-scale brain networks [4–6]. However, little is known whether these changes in neural interactions can only be observed at low frequencies, and how they are linked to brain function. Modulation of causal relationships between neural networks may be one of the mechanisms that the human brain employs, not only for efficient processing of information [7, 8], but also for regulation of sleep and wakefulness. Furthermore, if significant differences exist in the predominant direction of flows in non-REM sleep, in which we are usually unconscious, versus wakefulness, these differences may reflect mechanisms regulating conscious awareness.
To address these questions, we retrospectively analyzed the data obtained in our previous study [9]. We applied single-pulse electrical stimulation (SPES) through subdural electrodes that were implanted for presurgical evaluation in patients with intractable partial epilepsy, and we recorded electrophysiological responses to SPES in the rest of the implanted electrodes. SPES of the human cortex has been shown to evoke electrophysiological responses at distant sites. These responses may include cortico-cortical evoked potentials (CCEP) [10], which consist of phase-locked responses revealed by averaging signals in the time domain, and cortico-cortical spectral responses (CCSRs) [11], which are obtained by averaging in the frequency domain and are usually dominated, particularly at high frequencies, by non-phase-locked responses. Nowadays, many researchers are using SPES to expand our understanding about human cortical networks and their input/output characteristics, often using CCEPs to estimate effective connectivity between stimulus sites and recording sites [12–15]. CCSRs, a term firstly introduced by Gkogkidis et al. [11], occur at a variety of frequencies, including high-gamma (HG) bands. For example, Maliia et al. recently investigated HG responses to SPES in human physiologic and pathological networks [16]. HG activity is also widely used as an index of changes in neuronal population firing rates [17–19]. Thus, a CCSRHG induced by SPES reflects changes in neuronal firing rates elicited by simulated external input, via direct or indirect anatomical connections with the stimulation site [9, 20–26]. CCSRHG generator mechanisms are still debated [27], but the changes in neural behavior reported in stimulation studies with single unit recordings or dye imaging are similar to those of CCSRHG [28–30]. Because SPES is task-free and does not require patient cooperation, CCEP and CCSR recorded by SPES can be used to study how sleep alters the dynamic characteristics of human brain networks.
We used event-related causality (ERC) analysis of HG activity induced by SPES [31–35] to estimate the magnitude and directionality of neural propagation within and between different brain lobes, and we compared the overall patterns of these induced neural propagations during wakefulness and different sleep stages. ERC analysis, a multichannel extension of the Granger causality concept [36], has previously been used to study the dynamics of neural propagation during word production tasks [8, 37–39], and to study both ictal and interictal activity in epileptogenic networks [40]. Here, we applied it to HG activity induced by SPES. To the best of our knowledge, this is not only the first application of ERC analysis to stimulus-induced HG activity, but as such, it is the first study to measure the predominant directionality of internally generated neural propagation in human cortical networks during wakefulness and sleep.
Methods
This study is based largely on the dataset that were used in our previous studies [9, 26]. The method of recording SPES data across wakefulness and sleep, sleep staging, and the anatomic localization of electrodes, are described in detail in our previous publications [9, 31, 41]. The outline of this study is depicted in Figure 1. We have determined sleep stages according to the standard Rechtchaffen & Kales (R&K) sleep staging criteria [42]. Data analysis of high-gamma activity and ERC analysis were performed using a custom data analysis pipeline (using Fortran77, C++, and R) developed at Johns Hopkins. All other analyses were performed using in-house scripts in Matlab software (version 2018a, MathWorks, Natick, MA).
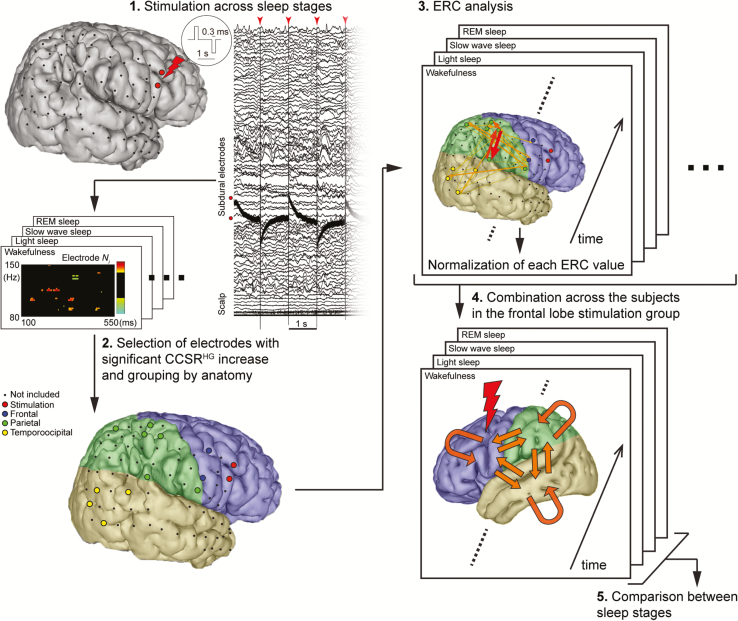
Figure 1.
The schema of analysis. (1) Single-pulse electrical stimulation (SPES) at a fixed frequency of 1 Hz with alternating polarity was applied to frontal or parietal cortex (this representative figure shows one of the frontal stimulation group) via two neighboring subdural electrodes across sleep stages (wakefulness, light sleep, slow-wave sleep, and REM sleep.). (2) High-gamma cortico-cortical spectral responses (CCSRHG) were defined as statistically significant increases in high-gamma (80–150 Hz) power within 100–550 ms of stimulation, relative to baseline (−300 to −100 ms before stimulation). Any ECoG site with a CCSRHG during wakefulness or any sleep stage was considered to be part of network dynamics elicited by SPES. These ECoG sites were grouped by anatomy into frontal, parietal, and temporo-occipital lobe for further analysis. (3) Patterns of high-gamma propagation within the network were analyzed by the event-related causality (ERC) method in each sleep stage. ERC values were normalized for group analysis. (4) The data were pooled across subjects in each stimulation group (frontal or parietal) in each sleep stage. (5) We investigated whether ERC values between sleep stages change in each propagation pattern.
Patients
Thirteen patients who underwent invasive presurgical evaluation for intractable partial epilepsy since February 2010 through October 2013 in Kyoto University Hospital provided written informed consent to the study. The protocol conformed to the Declaration of Helsinki and was approved by the Ethics Committee of the Institute (IRB#443). Among them, eight patients were eligible for this study (see Table 1 for the patients’ profiles) according to the criteria mentioned below in detail. The locations of the electrodes were confirmed by an MRI image after implantation, and they were linearly co-registered into a pre-implantation image. For the purpose of 3D display in figures in each patient, the gray matter segmentation was done for pre-implantation image using Freesurfer software (http://surfer.nmr.mgh.harvard.edu/) and presented in FSL view (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FslView).
Table 1.
Patient profile
| Patient No, age / gender, handedness | Epilepsy classification / etiology | Drug | Electrode-implanted side / No. of recording electrodes | Stimulus intensity (mA) | Midpoint of Stimulus | No. of electrodes that showed significant CCSRHG increase and were used for ERC analysis | ||
|---|---|---|---|---|---|---|---|---|
| Frontal | Parietal | Temporo-Occipital | ||||||
| Frontal stimulation group | ||||||||
| 1. 22F, R | R. FLE / FCD | CBZ, LTG | R, L / 50 | 12 | L MFG | 5 | 5 | |
| 2. 22M, R | L FLE, TLE / FCD | CBZ, VPA | L / 60 | 10 | L FP | 3 | 1 | 4 |
| 3-1. 34M, R | R PLE, TLE / Traumatic injury, HS | CBZ, LEV, TPM, ZNS | R / 73 | 4 | R preCG | 2 | 4 | 6 |
| 3-2. | R / 73 | 10 | R PMv | 2 | 6 | 4 | ||
| 4-1. 27F, R | R PLE / tumor | CBZ, CZP, LEV | R / 54 | 10 | R MFG | 10 | 1 | |
| 5. 27F, L | R PLE / FCD | CBZ, LEV, fosPHT, PHT | R / 51 | 8 | R SMA | 2 | 1 | 5 |
| 6. 45M, R | L FLE / FCD | PHT, PB, CLB, LEV | L / 52 | 10 | L PM | 3 | 2 | |
| Parietal stimulation group | ||||||||
| 7-1. 44M, R | R FLE / tumor | CBZ, LEV, TPM, VPA | R / 44 | 10 | R AG | 6 | 2 | |
| 7-2. | R / 44 | 10 | R precuneus | 6 | 3 | 1 | ||
| 8-1. 29M, L | L mesial TLE, TLE / HS | CLB, PHT, ZNS | L / 98 | 10 | L SMG | 4 | 2 | 7 |
| 8-2. | L / 98 | 10 | L AG | 5 | 1 | 7 | ||
| 4-2. | See 4-1 | R / 54 | 10 | R postCG | 4 | 4 | ||
Epilepsy classification / etiology: frontal/parietal/temporal lobe epilepsy; FCD, focal cortical dysplasia; HS, hippocampal sclerosis. Drugs: CBZ, carbamazepine; LTG, lamotrigine; VPA, valproic acid; LEV, levetiracetam; TPM, topiramate; ZNS, zonisamide; CZP, clonazepam; CLB, clobazam; PHT, phenytoin; PB, phenobarbital. Stimulus site: MFG, middle frontal gyrus; FP, frontal pole; preCG, precentral gyrus; PMv, ventral part of premotor area; SMA, supplementary motor area; AG, angular gyrus; SMG, supramarginal gyrus; postCG, postcentral gyrus.
Recording signals induced by SPES during sleep
During the daytime, direct electrical stimulation was applied in a bipolar manner to a pair of adjacently placed subdural platinum electrodes (2.3 mm in diameter, 1 cm in interelectrode distance, AD-TECH, Wisconsin), using a constant-current stimulator (MS-120B/MEE-1232, Nihon Kohden, Tokyo, Japan). The single-pulse electrical stimuli (square wave pulse: 0.3-ms duration) were delivered in alternating polarity at a fixed frequency of 1 Hz. CCEPs obtained by stimulating the majority of the implanted electrodes during daytime were used for identifying the electrodes to be stimulated during sleep. One or two stimulation sites were selected for the night-time CCEP recordings in each patient according to the following criteria: (1) sites away from the epileptic focus and (2) sites that produced discrete CCEP responses in the adjacent (local CCEP field) and/or distant (remote CCEP fields) regions. Thus, SPES was delivered to the cortical surface during wakefulness, light sleep, slow-wave sleep, and REM sleep. In each recorded electrode of each stimulus sites, we calculated CCSRHGs. We used the highest intensity (maximum: 12 mA) at which (1) patients did not notice the stimulation and no symptoms were evoked, (2) the adjacent electrodes did not show excessive artifacts over a dynamic range that would prohibit recording, and (3) no afterdischarges were induced. Intensity was adjusted in increments of 1 or 2 mA. One stimulation set included 50–100 pulses. In general, 200–400 electrical pulses were delivered during each sleep stage that is judged on-line according to aforementioned sleep staging criteria. Electrocorticograms (ECoGs) were referenced to a scalp electrode placed on the skin over the mastoid process contralateral to the implantation site and were sampled at 1000 Hz (in Subject 4 and 8) or 2000 Hz (in the other subjects) with a bandpass filter of 0.08–300 Hz or 0.08–600 Hz, respectively. ECoG signals were aligned to SPES (analysis window: −300 to +700 ms) offline. Examples of CCSRHGs at two recording sites with corresponding CCEPs during different sleep stages are shown in Supplementary Figure S1.
Selection of the SPES-affected sites for ERC analysis
The following criteria were used for selecting eligible patients and sites (channels) to be analyzed with multivariate autoregressive modeling (MVAR). Finally, the patients in whom SPES was delivered to the cortical surface in frontal (seven sites in six patients in total) or parietal lobe (five sites in three patients in total) across sleep stages were eligible for this study (Table 1). In one patient, SPES was delivered independently to frontal and parietal lobes.
First, for ERC analysis at least 50 trials were required, and the number of trials had to be the same across sleep stages in one patient. It reassured us that the analyses were not biased by different or insufficient numbers of trials. Trials were separated according to the stimulation polarity. Therefore, for each sleep stage at least 100 trials were used (50 of each polarity), and the first N trials (N = the least number of eligible trials across sleep stages) within each sleep stage were used for analysis, after removing trials with artifacts.
Second, we chose patients with midpoints of stimulation sites in frontal or parietal lobe to further investigate anterior–posterior interactions in sleeping brain. Patients in whom the pair of electrodes used for stimulation traversed central sulcus were excluded, because more than one lobe could be stimulated at the same time, which would lead to ambiguous results.
Third, to diminish the potential influence of pathological activities, we did not include recordings from sites that were identified by certified clinicians as seizure onset zone, or containing frequent spikes (>1/120 seconds), or that showed baseline shift, or artifacts.
Data sampled at 2000 Hz were down-sampled to 1000 Hz. Then, recorded signals were band-passed filtered at 70–170 Hz with a Butterworth filter of order 6. Next, the time-frequency energy distribution of SPES-induced activity, that is, CCSR, at time intervals between +100 ms and +550 ms after stimulation were computed using matching pursuit (MP) algorithm [43]. Baseline was set at −300 to −100 ms before stimulation. Data between −100 ms and +100 ms were excluded from analyses to avoid artifacts introduced by stimulation pulse. The sites that showed significant CCSRHG increase (between 80 and 150 Hz) (p < .05 by MP) in any sleep stage and in either stimulation polarity were included in ERC analyses.
We previously showed that SPES elicits CCSRHG decrease right after stimulation at recording sites that had strong connectivity to the stimulation site (measured by CCEPs) and that these HG decreases are potentiated during sleep [9, 20]. We excluded sites showing significantly decreased CCSRHG immediately after stimulation. This reduced the risk of including sites with prolonged post-stimulus inhibition (HG decrease), which would have affected the baseline.
ERC analysis
ERC method is a multichannel extension of Granger causality concept, which states that an observed time series x(t) causes another series y(t), if knowledge of x(t)’s past significantly improves prediction of y(t). ERC gives an estimate of changes in the intensity and direction of neural activity propagation between recording sites as a function of frequency, in comparison to baseline activity. A greater ERC value X->Y infers that HG activity from site X causally influences site Y by Granger criteria [8, 37]. To detect propagation between two sites, the ERC method analyzes the similarity of signals recorded at both sites and the time delays between them (for simplicity, one can think about delayed, or lagged, correlations). If HG power is suppressed in one of the sites after SPES, it can reflect a decrease of neural input to the site, or inhibition of local activity caused by an increase of neural input to the site. As it operates on the activity of an entire neuronal population, the ERC method cannot distinguish between these two situations. We think that it is unlikely that SPES-induced HG power suppression would be propagated to other sites, and that if this were to happen at all, it would occur early in our analysis window. For these reasons, we excluded sites in which SPES induced HG power suppression.
For each dataset, MVAR model order was determined using Akaike Information Criterion (AIC), and the coefficients were computed for 20 ms shifting window, overlapped by 5 ms over 650 ms trial (including baseline −300 to −100 ms and response period +100 to +550 ms). For each set of trials with the same stimulation polarity in each site, ERC values of post-stimulus period were compared to baseline epoch [8].
Statistical group analysis
In each patient, all ERC values were normalized to the highest ERC value, resulting in the highest value of ERC being 1, which allowed averaging over patients. Normalized ERC values reaching significance (p < .05, Bonferroni corrected) for flows within and between three regions of interest (network nodes); frontal, parietal, and temporal-occipital, were pooled across patients, in frontal or parietal stimulation group separately, and used as an index of directed interaction between the nodes (Supplementary Figure S1 shows the examples of ERC values calculated between two electrodes). Our previous study did not show the difference of propensity of CCEP/CCSRs between different stimulation intensity [9]. Therefore ERC results obtained for different stimulation intensities (4–12 mA) were pooled together in this study (Table 1).
Our null hypothesis is that no difference in HG propagation patterns is found between sleep stages. We used nonparametric permutation test for statistical analysis [44]. First, we randomly shuffled ERC values between four sleep stages within each directed connection. Second, paired t test between any two sleep stages was applied across time points (100–500 ms) and selected all the samples where the t value satisfied p < .01 lasting for 20 ms or more. Third, we calculated the sum of the t values in each cluster and made cluster-level statistics. Lastly, only the highest statistic from all the comparisons between any two sleep stages was taken. This procedure was repeated 10 000 times and the null distribution of the maximum cluster-level statistics was compared with the original cluster-level statistics in each propagation, respectively. A p-value threshold was defined < .05. See Supplementary Figure S2 for an illustrative pipeline of the analysis.
Results
The ERC results of eight patients were combined after normalization of data (see Table 1 for the patients’ profile and Figure 2 for electrodes that were included for analysis) and statistically analyzed. Significant SPES-induced changes in the propagation of HG activity within and between regions of interest, were compared between sleep stages (Figures 3 and 4).
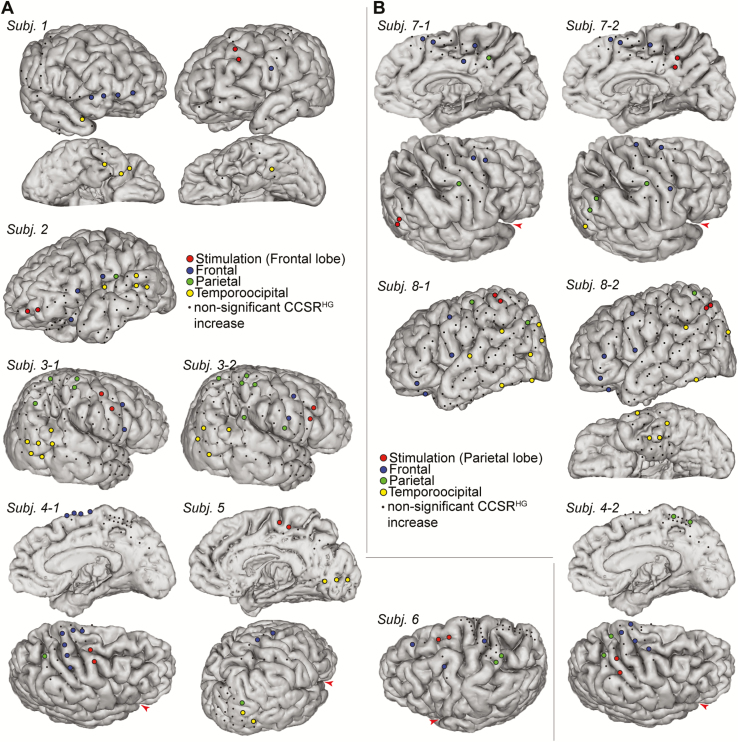
Figure 2.
The electrodes in which CCSRHG increase was found from +100 to +550 ms after stimulation, (A) in the frontal stimulation group, (B) in the parietal stimulation group. The subdural electrodes were all linearly co-registered onto MRI taken before implantation in each subject. Each angle of the brain view was arranged so that all analyzed electrodes could be seen. The Sylvian fissure is indicated by a red arrowhead in some subjects for better understanding of the orientation.
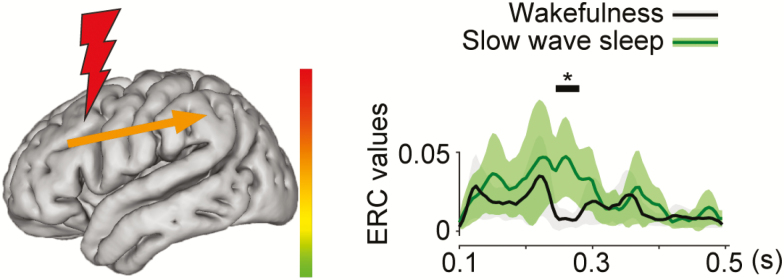
Figure 3.
Schematic illustrates a category of neural propagation, induced by SPES (frontal or parietal stimulation), that was significantly different between sleep stages. Stimulation sites are shown by a red lightning bolt. Right graphs depict the time course in unit of seconds after stimulation (x-axis) of ERC values (y-axis) of each specific propagation within or between regions. Each waveform is shown by mean ± 1.96*S.E.M. Black bar with an asterisk above the waveforms shows the period that showed significant difference by permutation test using cluster-level statistics. The average magnitude of significant differences (min = −0.246, max = 0.085), during the time interval marked with black line and asterisk, is shown by the color and thickness (same thickness for the positive and negative changes) of the arrow in the left figure. Note that herein we found that sleep stage changed ERC values in frontal to parietal propagation after frontal stimulation (N = 82) and that it significantly occurred between wakefulness and slow-wave sleep.
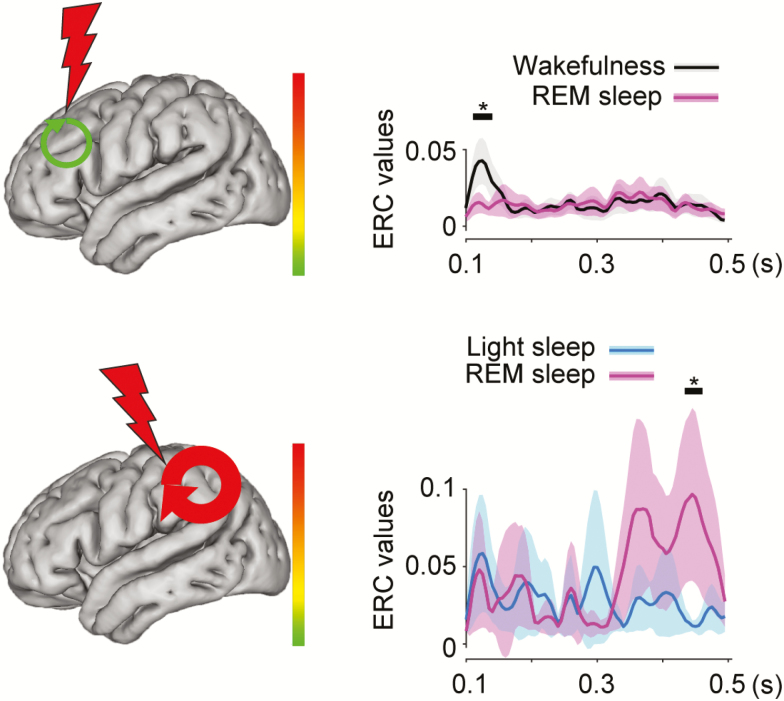
Figure 4.
Schematic illustrates categories of neural propagation, induced by SPES, that were significantly different between sleep stages (N = 256 for propagation within the frontal lobe after frontal stimulation; N = 118 for propagation within the parietal lobe after parietal stimulation). The other conventions are the same as in Figure 3.
Changes in the pattern of induced neural propagation during slow-wave sleep
Stimulation of frontal lobe during slow-wave sleep, induced greater propagation from frontal to parietal lobe (F→P, 245–280 ms, p < .05 by permutation test), as compared to the stimulation during wakefulness (Figure 3). Examples in which we did not observe any significant ERC values between wakefulness and other sleep stages in F→P are shown in Supplementary Figure S3. No other significant differences in the patterns of neural propagation were found between slow-wave sleep and wakefulness.
The results of stimulating parietal lobe during slow-wave sleep were not significantly different from those during wakefulness.
Changes in the pattern of evoked neural interactions during REM sleep
Compared to wakefulness, frontal stimulation during REM sleep induced less HG propagation within frontal lobe (at 110–140 ms, Figure 4, top). During wakefulness SPES induced immediate propagation within frontal network, while the frontal network did not exhibit a significant increase in propagation during REM. No other significant changes in neural propagation were found between REM and wakefulness.
Compared to light sleep, parietal stimulation during REM sleep resulted in greater propagation within parietal lobe (at 435–460 ms, Figure 4, bottom). Interestingly, no significant difference in propagation was found when compared to wakefulness. Overall, these findings suggest that the frontal network is “mute” during REM, while the parietal network is active.
Discussion
We observed changes in the overall pattern of neural propagation across large-scale human cortical networks when SPES was applied to frontal or parietal lobe during wakefulness and sleep stages. We found that different sleep stages affected the overall pattern of neural propagation among brain network. For example, stimulation of frontal lobe elicited larger early propagation from frontal to parietal lobe (F→P) during slow-wave sleep as compared to wakefulness (Figure 3). This result is in line with previous studies of passive scalp EEG showing propagation of low-frequency activity (<30 Hz) from anterior to posterior regions during slow-wave sleep [1–3], and extends those findings to HG propagation. It is important to note that HG activity has been shown to reflect the overall firing rates in neural populations [19]. Thus, our findings suggest a natural brain tide, reflecting a bias in propagation between frontal and parietal neuronal populations, changing according to sleep stage. During slow-wave sleep, slow-wave activities originate in the frontal lobe and tend to travel posteriorly [1–3, 45] although they are partly produced and regulated locally [6, 46–48]. Source modeling of slow-wave activity using a high-density EEG showed that this flow is mediated by a cingulate highway [3]. It is difficult to directly relate our results to slow-wave activity itself. However, our results support the notion that in slow-wave sleep, the readout from processing in the frontal lobe is conveyed in a top–down direction to the parietal lobe. This may facilitate the modification of synaptic strengths to consolidate processed information [49, 50]. In frontal lobe epilepsy, seizures occur predominantly during slow-wave sleep [51, 52]. Propagation of neuronal activity from frontal lobe to parietal lobe during slow-wave sleep, as observed here, may also facilitate seizure propagation in this sleep stage in frontal lobe epilepsy.
On the other hand, when we are awake, information from the periphery is largely processed in a bottom-up manner, and is encoded in widespread cortical regions [6]. REM sleep is similar to wakefulness in terms of its cortico-cortical connectivity, and cortical excitability [9, 53]. Indeed, humans may be partially conscious during the dreams of REM sleep [54]. However, there are clear experiential differences between wakefulness and REM sleep, and previous reports have attempted to account for these differences. For example, a positron emission tomography study showed decreased hypometabolism in frontal association areas including lateral orbital and dorsolateral prefrontal cortices during REM sleep [55]. In the present study, we observed a reduction in early SPES-induced neural propagations within the frontal lobe during REM sleep (Figure 4). Consistent with the PET study, this suggests that few active processes are running in frontal cortex. Moreover, we observed enhanced network interactions within parietal lobe during REM sleep. Together, these findings may explain the complex and realistic egocentric experiences that characterize dreaming [56] during REM sleep, as well as some of their bizarre characteristics, perhaps owing to the lack of top–down executive control on these experiences.
In previous studies, we and other researchers have observed inhibition of HG activity immediately after stimulation, and decreased phase-locked activity between low-gamma and stimulation-induced slow waves during slow-wave sleep [9, 23], suggesting that loss of information integration leads to unconsciousness during slow-wave sleep, based on information integration theory [9, 23, 57]. However, our present results suggest that causal interactions could occur across “frontal-parietal” networks [58] during sleep. Siclari et al. recently found evidence for “hot zones” in the posterior human brain that when activated, elicit dream-like experiences, whether during non-REM or REM sleep [54]. They did not analyze how neuronal activity propagated from the hot zones, though. Humans are unconsciousness and/or amnestic during slow-wave sleep. Based on our findings in the present study, we speculate that the propagation of neural processing from frontal to parietal lobes, observed to be greater during slow-wave sleep than during wakefulness, reflects processes that are either incompatible with conscious awareness or simply do not support it. However, the mechanisms responsible for this difference in the overall tides of neural propagation are unknown.
It would be noteworthy to compare the SPES-induced HG propagation to our CCEP results [9]. However, the two techniques are more complementary than comparable. The first sharp negative deflection of CCEP, called the N1, at latencies of 10–50 ms, is believed to reflect direct cortico-cortical connection [59]. It was impossible to analyze SPES-induced HG propagation earlier than 100 ms after stimulation in this study, because the MVAR model used by ERC method requires stationarity of the modeled process, while SPES introduces a strong perturbation. With this in mind, our analysis focused on connectivity among cortical networks involved in SPES-induced activities although we often observe CCSRHG decrease early after stimulation. A more sophisticated method is required in the future to look at induced activities earlier after stimulation.
A few more concerns need to be addressed in this study. First, all our participants were patients with intractable partial epilepsy. Furthermore, they were all treated with different regimens of anti-seizure drugs, and it has recently been reported that some of these drugs can influence TMS-evoked EEG responses in healthy subjects [60]. Both confounds could have influenced our results independently. Second, we did not define sleep stages throughout all sleep, but only during SPES sessions. Comparisons between ERC with and without SPES during particular sleep stages may be considered in future analyses but are beyond the scope of this report. Lastly, to reduce the risk of our baseline being affected by preceding SPESs at 1-second intervals, we excluded sites within the seizure onset zone, in which stronger and more prolonged inhibition of HG can sometimes be elicited by SPES [16]. We also excluded sites with frequent epileptiform discharges. Nevertheless, previous studies have failed to show significant differences in CCEPs and CCSRs for SPES at 1 Hz versus 0.5 Hz [12, 14, 23]. The duration of interstimulus intervals for SPES is constrained by the amount of time available for testing during sleep. Establishing an efficient and patient-friendly SPES protocol during sleep is an important goal for future studies.
Herein we studied, for the first time, the strength and directionality of neural propagation, estimated with ERC analyses, elicited among different brain regions in response to external input delivered artificially during wakefulness and various sleep stages. We observed differences between interlobar neural propagations elicited during wakefulness and different sleep stages. Although slow-wave sleep is believed to be accompanied by a breakdown of connectivity across cortex, we observed greater propagation from frontal lobe to parietal lobe during this sleep stage than during wakefulness, suggesting that this pattern of network interaction is either counterproductive to conscious awareness or reflects the absence of bottom-up input to executive areas in frontal lobe from perceptual and integrative areas in parietal lobe. Moreover, during REM sleep, we observed a decrease in propagation within frontal lobe, and an increase in propagation within parietal lobe, relative to wakefulness and light sleep, respectively. These differences could potentially account for some of the unique experiential aspects of this sleep stage. Together our findings suggest that the regulation of conscious awareness and sleep is associated with differences in the balance of neural propagation across large-scale frontal-parietal networks. Our results may help to understand the human physiology of sleep in terms of directed neural propagation among large-scale cortical networks, and could help recast the pathophysiological mechanisms governing sleep-related behavioral disorders and the effects of sleep on epilepsy in these terms. Further investigations of causal interaction across large-scale cortical networks during wakefulness and different sleep stages are needed, including analyses of the effects of lesions, neurobehavioral disorders, and sleep disorders on these interactions.
Funding
This research was partly funded by National Institute of Neurological Disorders and Stroke (R01NS091139), Kyoto University Foundation, the Japan Epilepsy Research Foundation, and MEXT KAKENHI 15H05874, 16K19510, 17H05907, JSPS KAKENHI 17K16120, 18H06087.
Conflict of interest statement. None declared.
Supplementary Material
Acknowledgments
I acknowledge Dr. Piotr J. Franaszczuk (Johns Hopkins University School of Medicine) for insightful mathematical advice, and Dr. Jumpei Togawa, Dr. Hirofumi Takeyama, and Dr. Takuro Nakae (Kyoto University) for data collection. The Department of Epilepsy, Movement Disorders and Physiology is an Industry-Academia Collaboration Course supported by a grant from Eisai Company, NIHON KOHDEN CORPORATION, Otsuka Pharmaceutical Co., and UCB Japan Co., Ltd.
References
- 1. Kamiński M, et al. . Topographic analysis of coherence and propagation of EEG activity during sleep and wakefulness. Electroencephalogr Clin Neurophysiol. 1997;102(3):216–227. [DOI] [PubMed] [Google Scholar]
- 2. Massimini M, et al. . The sleep slow oscillation as a traveling wave. J Neurosci. 2004;24(31):6862–6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Murphy M, et al. . Source modeling sleep slow waves. Proc Natl Acad Sci U S A. 2009;106(5):1608–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Diekelmann S, et al. . The memory function of sleep. Nat Rev Neurosci. 2010;11(2):114–126. [DOI] [PubMed] [Google Scholar]
- 5. Maquet P. The role of sleep in learning and memory. Science. 2001;294(5544):1048–1052. [DOI] [PubMed] [Google Scholar]
- 6. Tononi G, et al. . Sleep and the price of plasticity: from synaptic and cellular homeostasis to memory consolidation and integration. Neuron. 2014;81(1):12–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bassett DS, et al. . Network neuroscience. Nat Neurosci. 2017;20(3):353–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Korzeniewska A, et al. . Dynamics of event-related causality in brain electrical activity. Hum Brain Mapp. 2008;29(10):1170–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Usami K, et al. . Sleep modulates cortical connectivity and excitability in humans: direct evidence from neural activity induced by single-pulse electrical stimulation. Hum Brain Mapp. 2015;36(11):4714–4729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Matsumoto R, et al. . Single pulse electrical stimulation to probe functional and pathological connectivity in epilepsy. Seizure. 2017;44:27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gkogkidis CA, et al. . Closed-loop interaction with the cerebral cortex using a novel micro-ECoG-based implant: the impact of beta vs. gamma stimulation frequencies on cortico-cortical spectral responses. Brain-Computer Interfaces. 2017;4(4):214–224. [Google Scholar]
- 12. Entz L, et al. . Evoked effective connectivity of the human neocortex. Hum Brain Mapp. 2014;35(12):5736–5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Keller CJ, et al. . Intrinsic functional architecture predicts electrically evoked responses in the human brain. Proc Natl Acad Sci U S A. 2011;108(25):10308–10313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Keller CJ, et al. . Corticocortical evoked potentials reveal projectors and integrators in human brain networks. J Neurosci. 2014;34(27):9152–9163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Keller CJ, et al. . Mapping human brain networks with cortico-cortical evoked potentials. Philos Trans R Soc Lond B Biol Sci. 2014;369(1653). doi:10.1098/rstb.2013.0528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mălîia MD, et al. . High frequency spectral changes induced by single-pulse electric stimulation: comparison between physiologic and pathologic networks. Clin Neurophysiol. 2017;128(6):1053–1060. [DOI] [PubMed] [Google Scholar]
- 17. Buzsáki G, et al. . Mechanisms of gamma oscillations. Annu Rev Neurosci. 2012;35:203–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Crone NE, et al. . Cortical γ responses: searching high and low. Int J Psychophysiol. 2011;79(1):9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ray S, et al. . Neural correlates of high-gamma oscillations (60-200 Hz) in macaque local field potentials and their potential implications in electrocorticography. J Neurosci. 2008;28(45):11526–11536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kobayashi K, et al. . Different mode of afferents determines the frequency range of high frequency activities in the human brain: direct electrocorticographic comparison between peripheral nerve and direct cortical stimulation. PLoS One. 2015;10(6):e0130461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kobayashi K, et al. . High frequency activity overriding cortico-cortical evoked potentials reflects altered excitability in the human epileptic focus. Clin Neurophysiol. 2017;128(9):1673–1681. [DOI] [PubMed] [Google Scholar]
- 22. Matsuzaki N, et al. . Cortico-cortical evoked potentials and stimulation-elicited gamma activity preferentially propagate from lower- to higher-order visual areas. Clin Neurophysiol. 2013;124(7):1290–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pigorini A, et al. . Bistability breaks-off deterministic responses to intracortical stimulation during non-REM sleep. Neuroimage. 2015;112:105–113. [DOI] [PubMed] [Google Scholar]
- 24. van ‘t Klooster MA, et al. . Evoked versus spontaneous high frequency oscillations in the chronic electrocorticogram in focal epilepsy. Clin Neurophysiol. 2017;128(5):858–866. [DOI] [PubMed] [Google Scholar]
- 25. Usami K, et al. . Cortical responses to input from distant areas are modulated by local spontaneous alpha/beta oscillations. Cereb Cortex. 2019;29(2):777–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Usami K, et al. . Phasic REM transiently approaches wakefulness in the human cortex - a single-pulse electrical stimulation study. Sleep. 2017;40(8). doi:10.1093/sleep/zsx077. [DOI] [PubMed] [Google Scholar]
- 27. Valentín A, et al. . Single pulse electrical stimulation and high-frequency oscillations, a complicated marriage. Clin Neurophysiol. 2017;128(6):1026–1027. [DOI] [PubMed] [Google Scholar]
- 28. Alarcón G, et al. . In vivo neuronal firing patterns during human epileptiform discharges replicated by electrical stimulation. Clin Neurophysiol. 2012;123(9):1736–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kozyrev V, et al. . Voltage-sensitive dye imaging of transcranial magnetic stimulation-induced intracortical dynamics. Proc Natl Acad Sci U S A. 2014;111(37):13553–13558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. LI CL, et al. . Cortical intracellular synaptic potentials and direct cortical stimulation. J Cell Comp Physiol. 1962;60:1–16. [DOI] [PubMed] [Google Scholar]
- 31. Matsumoto R, et al. . Functional connectivity in the human language system: a cortico-cortical evoked potential study. Brain. 2004;127(Pt 10):2316–2330. [DOI] [PubMed] [Google Scholar]
- 32. Greenlee JD, et al. . A functional connection between inferior frontal gyrus and orofacial motor cortex in human. J Neurophysiol. 2004;92(2):1153–1164. [DOI] [PubMed] [Google Scholar]
- 33. Matsumoto R, et al. . Functional connectivity in human cortical motor system: a cortico-cortical evoked potential study. Brain. 2007;130(Pt 1):181–197. [DOI] [PubMed] [Google Scholar]
- 34. Lacruz ME, et al. . Frontal and temporal functional connections of the living human brain. Eur J Neurosci. 2007;26(5):1357–1370. [DOI] [PubMed] [Google Scholar]
- 35. Jiménez-Jiménez D, et al. . Incidence of functional bi-temporal connections in the human brain in vivo and their relevance to epilepsy surgery. Cortex. 2015;65:208–218. [DOI] [PubMed] [Google Scholar]
- 36. Granger CWJ. Investigating causal relations by econometric models and cross-spectral methods. Econometrica. 1969;37(3):424–438. [Google Scholar]
- 37. Flinker A, et al. . Redefining the role of Broca’s area in speech. Proc Natl Acad Sci U S A. 2015;112(9):2871–2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Korzeniewska A, et al. . Dynamics of large-scale cortical interactions at high gamma frequencies during word production: event related causality (ERC) analysis of human electrocorticography (ECoG). Neuroimage. 2011;56(4):2218–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nishida M, et al. . Brain network dynamics in the human articulatory loop. Clin Neurophysiol. 2017;128(8):1473–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Korzeniewska A, et al. . Ictal propagation of high frequency activity is recapitulated in interictal recordings: effective connectivity of epileptogenic networks recorded with intracranial EEG. Neuroimage. 2014;101:96–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Matsumoto R, et al. . Left anterior temporal cortex actively engages in speech perception: a direct cortical stimulation study. Neuropsychologia. 2011;49(5):1350–1354. [DOI] [PubMed] [Google Scholar]
- 42. Rechtschaffen A, et al. . A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. Los Angeles, CA: UCLA Brain Information Service/Brain Research Institute; 1968. [Google Scholar]
- 43. Mallat SG, et al. . Matching pursuits with time-frequency dictionaries. Ieee T Signal Proces. 1993;41(12):3397–3415. [Google Scholar]
- 44. Maris E, et al. . Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods. 2007;164(1):177–190. [DOI] [PubMed] [Google Scholar]
- 45. Steriade M, et al. . A novel slow (< 1 Hz) oscillation of neocortical neurons in vivo: depolarizing and hyperpolarizing components. J Neurosci. 1993;13(8):3252–3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Huber R, et al. . Arm immobilization causes cortical plastic changes and locally decreases sleep slow wave activity. Nat Neurosci. 2006;9(9):1169–1176. [DOI] [PubMed] [Google Scholar]
- 47. Landsness EC, et al. . Sleep-dependent improvement in visuomotor learning: a causal role for slow waves. Sleep. 2009;32(10):1273–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nir Y, et al. . Regional slow waves and spindles in human sleep. Neuron. 2011;70(1):153–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Steriade M, et al. . Neuronal plasticity in thalamocortical networks during sleep and waking oscillations. Neuron. 2003;37(4):563–576. [DOI] [PubMed] [Google Scholar]
- 50. Esser SK, et al. . Sleep homeostasis and cortical synchronization: I. Modeling the effects of synaptic strength on sleep slow waves. Sleep. 2007;30(12):1617–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Herman ST, et al. . Distribution of partial seizures during the sleep–wake cycle: differences by seizure onset site. Neurology. 2001;56(11):1453–1459. [DOI] [PubMed] [Google Scholar]
- 52. Kaleyias J, et al. . Sleep-wake patterns of seizures in children with lesional epilepsy. Pediatr Neurol. 2011;45(2):109–113. [DOI] [PubMed] [Google Scholar]
- 53. Massimini M, et al. . Cortical reactivity and effective connectivity during REM sleep in humans. Cogn Neurosci. 2010;1(3):176–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Siclari F, et al. . The neural correlates of dreaming. Nat Neurosci. 2017;20(6):872–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Braun AR, et al. . Dissociated pattern of activity in visual cortices and their projections during human rapid eye movement sleep. Science. 1998;279(5347):91–95. [DOI] [PubMed] [Google Scholar]
- 56. Hobson JA, et al. . Dreaming and the brain: toward a cognitive neuroscience of conscious states. Behav Brain Sci. 2000;23(6):793–842; discussion 904. [DOI] [PubMed] [Google Scholar]
- 57. Tononi G. Consciousness as integrated information: a provisional manifesto. Biol Bull. 2008;215(3):216–242. [DOI] [PubMed] [Google Scholar]
- 58. Bor D, et al. . Consciousness and the prefrontal parietal network: insights from attention, working memory, and chunking. Front Psychol. 2012;3:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yamao Y, et al. . Intraoperative dorsal language network mapping by using single-pulse electrical stimulation. Hum Brain Mapp. 2014;35(9):4345–4361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Premoli I, et al. . Lamotrigine and levetiracetam exert a similar modulation of TMS-evoked EEG potentials. Epilepsia. 2017;58(1):42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.