Abstract
Study Objectives
Intraindividual variability (IIV) in sleep may be a risk factor for disease above the influence of mean sleep. Associations between IIV in sleep and risk for a comprehensive set of common medical and mental health conditions have not been assessed in a representative sample.
Methods
This study examined mean and IIV in total sleep time (TST), sleep quality (SQ), sleep efficiency (SE), and circadian midpoint (CM) in 771 adults recruited for an epidemiological study. Participants completed 14 days of sleep diaries to assess TST, SQ, SE, and CM, after which they reported on medical conditions and mental health symptoms. Data were analyzed using logistic regression, and models controlled for gender, body mass index, age, and race.
Results
Lower mean TST, SQ, and SE were related to increased odds of having gastrointestinal problems, depression, and anxiety. IIV in TST was related to increased odds of having neurological, breathing, and gastrointestinal problems, as well as pain and depression; all results held controlling for mean sleep and adjusting for false discovery rate. IIV in SQ and SE was not associated with odds of having any medical or mental health conditions after adjusting for false discovery rate, nor was IIV in CM or mean CM.
Conclusions
Confirming previous research, mean TST, SQ, and SE are related to risk for gastrointestinal problems, depression, and anxiety. IIV in TST may be a unique facet of disturbed sleep that is associated with increased risk for a diverse cluster of medical and mental health conditions.
Keywords: intraindividual variability, epidemiology, sleep diaries, medical conditions, public health, total sleep time
Statement of Significance.
This is the first study to investigate intraindividual variability (IIV; i.e. within-person night-to-night variation) in total sleep time, sleep quality, sleep efficiency, and circadian midpoint and comorbidity with common medical and mental health conditions in a racially/ethnically and age representative sample. Increased IIV in total sleep time was associated with elevated odds of having breathing, gastrointestinal, and neurological problems, as well as depression and pain, independently of mean sleep, age, body mass index, gender, and race. Future research should continue examining IIV in sleep as a distinct facet of disturbed sleep. Theoretical models and prospective, longitudinal studies are needed to understand the psychobiological pathways by which IIV in sleep may relate to medical conditions over time.
Introduction
Approximately 30% US adult population is chronically sleep deprived (i.e. sleeping < 7 hours per 24 hours) [1, 2], and approximately 30% regularly report at least one symptom of poor quality sleep (i.e. difficulty initiating or maintaining sleep, waking up too early, or nonrestorative sleep) [3]. Insufficient and poor quality sleep is robustly linked to elevated risk for disease, including coronary heart disease, depression, stroke, arthritis, diabetes, and all-cause mortality [4–9]. In the United States alone, it is estimated that insufficient and poor quality sleep cost approximately $63.2 billion per year in lost productivity and $411 billion per year in gross domestic product, underscoring the enormous impact that impaired sleep has on public health and the American economy [10, 11].
The majority of epidemiological research on sleep and disease has focused exclusively on habitual sleep (i.e. average sleep over the past weeks or months, reported retrospectively). Recent research has highlighted the important role that fluctuations in sleep from night to night also may play in disease risk (see Bei et al. [12] for a review). These fluctuations are referred to as intraindividual variability (IIV) and quantify the degree to which an individual’s nightly values are different from another across time. IIV in sleep can be measured prospectively using repeated assessments of sleep (e.g. via sleep diaries or actigraphy) and adds important information that is obscured when only the mean level across these values is examined [12]. For example, two individuals may have the same intraindividual mean (hereafter referred to as “mean” for simplicity) sleep duration across a week, but one individual may remain relatively constant in their sleep patterns from night to night, whereas another individual may fluctuate greatly, obtaining long sleep durations some nights and short sleep durations other nights. This first individual exhibits low IIV in sleep duration, whereas the second individual exhibits high IIV in sleep duration (Figure 1).
Figure 1.
Two hypothetical individuals with the same intraindividual mean (IIM) total sleep time but with different levels of IIV.
Greater IIV in sleep timing, duration, and/or quality appears to be a risk factor for disease, even after accounting for the influence of mean sleep [12]. For example, greater IIV in sleep timing (i.e. bedtime and risetime), sleep duration (both nightly sleep duration and daytime nap duration), and sleep quality (SQ) and fragmentation are associated with reports of more physical health conditions (e.g. diabetes, obesity, and heart conditions) [13–15], kidney disease [16], higher body mass index (BMI) and weight gain [14], insomnia [17, 18], asthma and sleep-disordered breathing [19, 20], and greater depressive symptoms and negative mood [15, 21, 22]. Greater IIV in sleep timing, sleep efficiency (SE), time in bed, and sleep duration also is associated with flatter cortisol diurnal slopes [23], higher levels of systemic inflammation [24], and greater allostatic load [23]. Similarly, rotating shift work, which is characterized by high IIV in sleep/wake cycles, is associated with elevated risk for cardiovascular disease [25], obesity [26], type 2 diabetes [27, 28], and depression [29], as well as higher levels of systemic inflammation [30, 31] and dysregulated cortisol [32]. Together, these studies suggest that inconsistency in sleep/wake patterns may be an important correlate of physiological dysregulation and disease risk.
Despite growing attention toward IIV in sleep and disease, research on this topic has several limitations (see Bei et al. [12] for a discussion). First, most previous studies examining associations between IIV in sleep and disease typically only measure one facet of sleep (e.g. duration) over a short period of time (i.e. ≤ 7 days), which may not reliably capture a holistic view of sleep or IIV. Second, most studies use post-hoc analyses and do not carefully control for potentially confounding variables (including mean sleep). Third, most previous work on IIV in sleep has not been conducted in large, representative samples. Finally, no studies have examined IIV in multiple dimensions of sleep (i.e. duration, quality, efficiency, and timing) as a risk factor for a wide array of common medical conditions. Systematic characterization of IIV in multiple facets of sleep and risk for medical conditions in a representative sample, therefore, is needed. Such results will provide essential epidemiological information about the potential disease risks associated with IIV in sleep and may help identify more precise targets for intervention and prevention efforts.
The Current Study
To address these gaps in the literature, we examined IIV in sleep and its associations with comorbid medical conditions in age and racially/ethnically diverse sample of adults using a rigorous methodological approach. We hypothesized that greater IIV in self-reported total sleep time (TST), SQ, SE, and circadian midpoint (CM; i.e. the midpoint between bedtime and risetime) assessed across 14 days would be associated with elevated odds of having comorbid medical conditions (i.e. heart disease, cancer, high blood pressure, neurological problems, breathing problems, urinary problems, diabetes, pain, gastrointestinal problems, depression, and anxiety), controlling for age, gender, BMI, race, and the mean values of each facet of sleep. Together, these results will provide a robust epidemiological characterization of the specific medical conditions associated with IIV in sleep, and guide future prevention and intervention efforts.
Methods
Procedure
This study is a secondary analysis of data collected as part of an epidemiological study that sought to characterize age, gender, and racial/ethnic differences in sleep norms [33]. Potential participants living in Shelby County, Tennessee were contacted using random digit dialing. To ensure adequate sampling of sex and age, a stratified random sampling method was used to obtain data from at least 50 men and 50 women in each decade from ages 20 to +80. Unrestricted random sampling was used until a cell was filled, on which it was closed for additional sampling. Given that the population of the area was divided nearly equally between African Americans (48.6%) and Caucasian Americans (47.3%) at the time of data collection (2000) [34], large numbers of participants from each of these racial/ethnic groups were obtained. Brief telephonic interviews were conducted to inquire about participation in the study. Those who agreed to participate were mailed an informed consent, a 14 day paper and pencil sleep diary, and a health survey. On receipt, participants began completing the sleep diary and, at the end of the 14 days, completed the remainder of the material in the packet.
Participants
Of the 1769 volunteers recruited, an adjusted response rate of 37.7% was obtained. Adjustment was necessary because, as the study progressed, some cells filled more quickly than others (e.g. men older than 80 years of age were the last to fill). Greater detail about adjustment methods is published elsewhere [33]. The final sample included 771 participants, which consisted of 380 men and 391 women from the ages of 20–98 years. The racial/ethnic representation included 539 Caucasians, 222 African Americans, 7 Asians, 1 Hispanic/Latino, and 2 whose racial/ethnic information was not reported (Table 1).
Table 1.
Participant characteristics
| M or n | SD or % | |
|---|---|---|
| Age (years) | 53.78 | 19.81 |
| Body mass index | 26.64 | 5.60 |
| Gender | ||
| Female | 391 | 50.71% |
| Male | 380 | 49.29% |
| Race | ||
| White | 539 | 69.91% |
| Black | 222 | 28.79% |
| Asian | 7 | 0.91% |
| Hispanic | 1 | 0.13% |
| Missing | 2 | 0.26% |
| Medical conditions | ||
| Heart disease | 98 | 12.71% |
| Cancer | 43 | 5.58% |
| High blood pressure | 190 | 24.64% |
| Neurological problems | 20 | 2.59% |
| Breathing problems | 85 | 11.02% |
| Urinary problems | 102 | 13.23% |
| Diabetes | 58 | 7.52% |
| Pain | 222 | 28.79% |
| Gastrointestinal problems | 126 | 16.34% |
| Total number of medical conditions | 1.22 | 1.38 |
| Clinically significant depression (BDI scores > 18) | 74 | 9.60% |
| Clinically significant anxiety (STAI scores > 52) | 63 | 8.17% |
| Intraindividual mean in sleep | ||
| Total sleep time (hours) | 6.99 | 1.16 |
| Sleep quality (1 = very poor to 5 = excellent) | 3.41 | 0.68 |
| Sleep efficiency (%) | 86.06 | 9.14 |
| Circadian midpoint | 3:20 am | 1.61 hours |
| Intraindividual variability in sleep | ||
| Total sleep time (hours) | 1.52 | 0.67 |
| Sleep quality (1 = very poor to 5 = excellent) | 0.69 | 0.33 |
| Sleep efficiency (%) | 7.34 | 4.97 |
| Circadian midpoint (hours) | 1.06 | 1.47 |
N = 771.
Measures
Nightly sleep
Participants completed the paper and pencil sleep diary every morning on awakening for 14 consecutive days, which was used to determine TST (time elapsed from reported bedtime to risetime minus time reported awake, in hours), SQ (Likert-type scale ranging from 1 = very poor to 5 = excellent), SE (i.e. TST divided by time in bed multiplied by 100), and CM (i.e. midpoint between bedtime and risetime; e.g. if bedtime = 11:00 pm and risetime = 6:00 am, then CM = 2:30 am; times were converted to numeric values by taking hours plus minutes as a fraction of 60, e.g. 2:30 am = 2.50). These particular measures were chosen to comprehensively assess the facets of sleep that have shown to be most relevant for health (i.e. duration, timing, quality, and efficiency) [35].
Medical conditions
These were determined by asking participants to indicate with a check mark whether they had the following health conditions: heart disease, cancer, human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS), high blood pressure, neurological disease (e.g. seizures and Parkinson’s disease), breathing problems (e.g. asthma and emphysema), urinary problems (e.g. kidney disease and prostate problems), diabetes, pain (e.g. arthritis, back pain, and migraines), and gastrointestinal problems (e.g. stomach, irritable bowel syndrome, and ulcers).
Depression and anxiety
Depression was assessed using the 21-item Beck Depression Inventory (BDI) [36]. BDI is a widely used measure of depression and has good internal consistency (α = .91) and convergent validity with other depression scales and psychiatric assessments [37]. Participants were categorized as having minimal depression (BDI < 10) or clinically significant depression (moderate to severe; BDI > 18). These cutoff scores were based on values derived by independent raters of patients with psychiatric disorder in an inpatient environment [37]. Anxiety was assessed using the 20-item State-Trait Anxiety Inventory (STAI), Form Y Trait Scale [38]. The STAI is a widely used measure of trait anxiety and has adequate reliability and validity [39]. Participants were categorized as having minimal anxiety (trait score of ≤52) or significant anxiety (trait score of >52). Scores of 52 or greater represent 2 SD above the mean for adults.
Covariates
Baseline age, gender (1 = male, 2 = female), race (0 = white and 1 = any other race, given that the majority of the sample was white), and BMI (weight in pounds divided by squared height in inches, multiplied by 703) were used as covariates in all models, as these variables have been shown to affect both sleep and risk for medical conditions [12, 33, 40]. Mean sleep (i.e. an individual’s sleep averaged across the 14 days) also was included in all analyses in alignment with recommendations from previous research on IIV in sleep [12].
Analysis plan
All statistical analyses were conducted using the open-source statistical program R (RStudio, version 1.0.153) [41]. Intraclass correlation coefficients (ICCs; i.e. the ratio of between-person variability to the sum of between-person variability plus within-person variability) first were calculated for all four sleep parameters (TST, SQ, SE, and CM) to examine the amount of within-person, IIV across time. Next, for each sleep parameter, intraindividual means were calculated by summing daily values across the 14 days and dividing by the total number of days a sleep diary was completed for each participant. Likewise, intraindividual SDs (i.e. the measure of IIV) were calculated across the 14 days using the R package varian [12, 42]. Logistic regression analyses then were performed using R base packages. Each medical condition (i.e. heart disease, cancer, high blood pressure, neurologic disease, breathing problems, urinary problems, diabetes, chronic pain, gastrointestinal problems, depression, and anxiety) was analyzed separately as the dependent variable, and the IIV of TST, SQ, SE, or CM was analyzed separately as the independent variable. All models controlled for age, gender, BMI, race, and the mean of TST, SQ, SE, or CM. Although HIV/AIDS was measured, it was not examined statistically, as only one participant reported having this condition. Odds ratios (OR) were calculated for each medical condition by exponentiating log odds. In alignment with established guidelines [43], ORs between 1.49 and 3.45 were considered small effects; ORs between 3.46 and 8.99 were considered medium effects; and ORs at least 9 were considered large effects. With the sample size of 771, power was at 88% to achieve a small effect size (i.e. OR) of 1.7 (α = .05, two-tailed, binomial distribution). Given the large number of statistical relationships assessed, in alignment with recommendations from Benjamini and Hochberg [44], false discovery rate was accounted for by ranking each of the 308 p-values obtained (i.e. 11 medical conditions × 4 sleep parameters × 7 variables in each model) from smallest to largest, dividing each ranking by 308 (i.e. the number of statistical relationships assessed), and then multiplying this value by an expected false discovery rate of 15%. p-Values that remained less than .05 after adjusting for false discovery rate were considered to be indicative of statistically robust effects.
Results
Descriptive results
Participants completed an average of 13.64 of 14 possible daily surveys, for an average compliance rate of 97%. More than 96% participants (n = 742) completed all 14 days of daily surveys, resulting in a nominal amount of missing data. Across the 14 days, participants reported mean TSTs of 6.99 ± 1.16 hours, SQ ratings of 3.41 ± 0.68, sleep efficiencies of 86.06% ± 9.14%, and CMs of 3:20 am ± 1.61 hours (Table 1). Participants reported having an average of 1.22 ± 1.38 medical conditions (excluding depression and anxiety), with 13% reporting having heart disease, 6% having cancer, 25% having high blood pressure, 3% having neurological problems, 11% having breathing problems, 13% having urinary problems, 8% having diabetes, 29% having pain, 16% having gastrointestinal problems, 10% having clinically significant depression, and 8% having clinically significant anxiety (Table 1).
Amount of IIV in sleep parameters
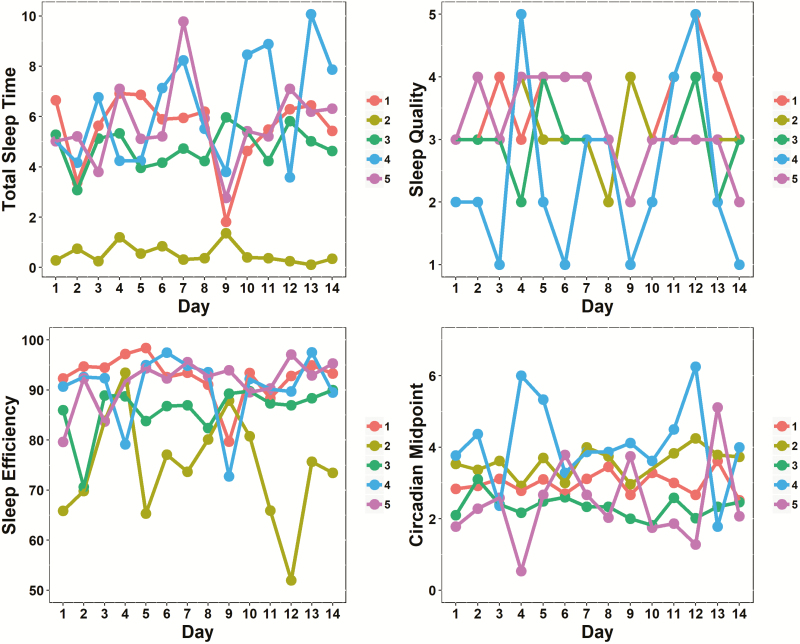
Examination of ICCs revealed that for TST, 61% variability could be attributed to within-person fluctuations, and 39% could be attributed to between-person fluctuations. For SQ, 58% variability was due to within-person fluctuations, and 42% was due to between-person fluctuations. For SE, 50% variability was due to within-person fluctuations, and 50% was due to between-person fluctuations. For CM, 49% variability was due to within-person fluctuation, and 51% was due to between-person fluctuations. These results suggest a high degree of within-person, IIV in all four sleep parameters across the 14 days. For an illustration of how the four sleep measures varied over the 14 days for five sample participants, see Figure 2.
Figure 2.
Spaghetti plots of total sleep time (in hours; top left), sleep efficiency (%; bottom left), sleep quality ratings (1 = very poor, 5 = excellent; top right), and circadian midpoint values (numeric time; bottom right) for five sample participants across 14 days. Each color represents a different participant.
Intraindividual mean and variability in TST and odds of medical and mental health conditions
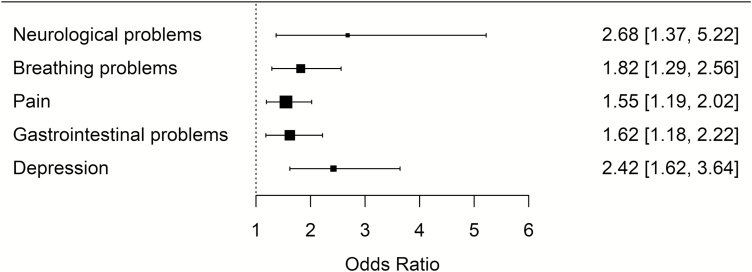
For every 1 hour increase in IIV in TST, odds of having the following medical or mental health conditions increased by the following magnitudes: neurological problems by 2.68 (95% CI = 1.37 to 5.22), breathing problems by 1.82 (95% CI = 1.29 to 2.56), pain by 1.55 (95% CI = 1.19 to 2.02), gastrointestinal problems by 1.62 (95% CI = 1.18 to 2.22), depression by 2.42 (95% CI = 1.62 to 3.64), and anxiety by 1.77 (95% CI = 1.18 to 2.65; Table 2). For every 1 hour increase in mean TST, the odds of having gastrointestinal problems decreased by 0.72 (95% CI = 0.60 to 0.86), and the odds of having anxiety decreased by 0.54 (95% CI = 0.42 to 0.70; Table 2). After adjusting for false discovery rate, the relationships between IIV in TST and odds of having neurological problems, breathing problems, pain, gastrointestinal problems, and depression (see Figure 3 for a forest plot of ORs for these significant effects), as well as the relationships between mean TST and odds of having gastrointestinal problems and anxiety remained significant at the p value of less than .05 level (Table 2).
Table 2.
Intraindividual mean and intraindividual variability in total sleep time, sleep quality, sleep efficiency, and circadian midpoint and odds [95% CI] of having medical or mental health conditions
| Total sleep time | Sleep quality | Sleep efficiency | Circadian midpoint | |||||
|---|---|---|---|---|---|---|---|---|
| IIM | IIV | IIM | IIV | IIM | IIV | IIM | IIV | |
| Odds ratio [95% CI] | Odds ratio [95% CI] | Odds ratio [95% CI] | Odds ratio [95% CI] | |||||
| Heart | 0.89 [0.73 to 1.08] | 1.41 [0.96 to 2.06] | 0.67* [0.47 to 0.95] | 0.77 [0.36 to 1.67] | 0.98 [0.95 to 1.01] | 1.02 [0.96 to 1.08] | 1.03 [0.88 to 1.18] | 0.96 [0.78 to 1.15] |
| Cancer | 0.84 [0.65 to 1.09] | 1.11 [0.65 to 1.87] | 0.75 [0.46 to 1.23] | 1.96 [0.68 to 5.78] | 0.95* [0.92 to 0.99] | 0.98 [0.90 to 1.06] | 1.00 [0.79 to 1.17] | 1.06 [0.80 to 1.31] |
| HTN | 0.95 [0.81 to 1.10] | 1.08 [0.81 to 1.45] | 0.83 [0.63 to 1.09] | 1.56 [0.86 to 2.84] | 0.98 [0.96 to 1.01] | 1.01 [0.96 to 1.07] | 0.98 [0.86 to 1.10] | 0.98 [0.85 to 1.13] |
| Neuro | 0.79 [0.54 to 1.12] | 2.68** [1.37 to 5.22] | 0.98 [0.49 to 2.00] | 1.04 [0.23 to 4.63] | 0.95 [0.90 to 1.00] | 1.06 [0.96 to 1.17] | 1.01 [0.70 to 1.25] | 0.88 [0.37 to 1.30] |
| Breathing | 0.93 [0.76 to 1.12] | 1.82** [1.29 to 2.56] | 0.62** [0.43 to 0.88] | 2.61* [1.21 to 5.69] | 0.96* [0.93 to 0.99] | 1.00 [0.94 to 1.06] | 0.98 [0.83 to 1.12] | 1.06 [0.89 to 1.25] |
| Urinary | 0.99 [0.82 to 1.20] | 1.47 [1.00 to 2.15] | 0.63** [0.44 to 0.89] | 0.84 [0.39 to 1.80] | 0.98 [0.95 to 1.01] | 0.99 [0.93 to 1.05] | 1.00 [0.86 to 1.14] | 1.18* [1.00 to 1.38] |
| Diabetes | 1.06 [0.84 to 1.34] | 1.53 [0.99 to 2.34] | 0.84 [0.55 to 1.28] | 1.83 [0.73 to 4.64] | 1.01 [0.97 to 1.05] | 1.04 [0.97 to 1.12] | 1.02 [0.83 to 1.18] | 1.02 [0.82 to 1.22] |
| Pain | 0.88 [0.76 to 1.02] | 1.55** [1.19 to 2.02] | 0.69** [0.53 to 0.89] | 1.66 0.96 to 2.87] | 0.98 [0.95 to 1.00] | 1.03 [0.99 to 1.08] | 1.02 [0.92 to 1.13] | 1.05 [0.92 to 1.19] |
| GI | 0.72** [0.60 to 0.86] | 1.62** [1.18 to 2.22] | 0.45** [0.33 to 0.62] | 2.27* [1.15 to 4.53] | 0.96* [0.93 to 0.99] | 1.03 [0.98 to 1.09] | 1.06 [0.94 to 1.19] | 0.98 [0.83 to 1.14] |
| Depression | 0.93 [0.74 to 1.16] | 2.42** [1.62 to 3.64] | 0.22** [0.14 to 0.35] | 3.08* [1.26 to 7.73] | 0.93** [0.89 to 0.96] | 1.03 [0.96 to 1.10] | 1.12 [0.98 to 1.28] | 1.06 [0.90 to 1.24] |
| Anxiety | 0.54** [0.42 to 0.70] | 1.77** [1.18 to 2.65%] | 0.12** [0.07 to 0.21] | 1.01 [0.38 to 2.69%] | 0.91** [0.87 to 0.94] | 0.99 [0.92 to 1.06] | 1.13 [0.99 to 1.23] | 1.07 [0.91 to 1.25] |
*p < .05, **p < .01. Bold values represent significant values (p < .05) after adjusting for false discovery rate using the Benjamini–Hochberg procedure. All models adjusted for gender (1 = male, 2 = female), body mass index, and race (0 = white, 1 = other race) and examined IIV and intraindividual mean (IIM) simultaneously. Total sleep time was measured in hours. Sleep quality was measured on a scale of 1 = very poor to 5 = excellent. Sleep efficiency was measured as a percentage from 0% to 100%. Circadian midpoint was calculated as the midpoint between bedtime and risetime in numeric time. Heart = heart disease, HTN = hypertension, Neuro = neurological problems, Breathing = breathing problems, Urinary = urinary problems, GI = gastrointestinal problems.
Figure 3.
Forest plot depicting significant associations between intraindividual variability in total sleep time (in hours) and odds of having medical conditions. Columns on the right indicate odds ratios and 95% confidence intervals. All associations control for age, gender, body mass index, race, and mean total sleep time, and remained significant after adjusting for false discovery rate using the Benjamini–Hochberg procedure.
Intraindividual mean and variability in SQ and odds of medical and mental health conditions
For every one-unit increase in IIV in SQ, the odds of having the following medical or mental health conditions increased by the following magnitudes: breathing problems by 2.61 (95% CI = 1.21 to 5.69), gastrointestinal problems by 2.27 (95% CI = 1.15 to 4.53), and depression by 3.08 (95% CI = 1.26 to 7.73; Table 2). However, these relationships did not hold after adjusting for false discovery rate. For every one-unit increase in mean SQ, the odds of having the following medical or mental health conditions decreased by the following magnitudes: heart disease by 0.67 (95% CI = 0.47 to 0.95), breathing problems by 0.62 (95% CI = 0.43 to 0.88), urinary problems by 0.63 (95% CI = 0.44 to 0.89), pain by 0.69 (95% CI = 0.53 to 0.89), gastrointestinal problems by 0.45 (95% CI = 0.33 to 0.62), depression by 0.22 (95% CI = 0.14 to 0.35), and anxiety by 0.12 (95% CI = 0.07 to 0.21; Table 2). After adjusting for false discovery rate, only the relationships between mean SQ and odds of having pain, gastrointestinal problems, depression, and anxiety remained significant at the p value of less than .05 level.
Intraindividual mean and variability in SE and odds of medical and mental health conditions
IIV in SE was not associated with increased odds of having any medical or mental health conditions, either before or after adjusting for false discovery rate (Table 2). Higher mean SE was associated with decreased odds of having cancer, breathing problems, gastrointestinal problems, and depression: For every 1% increase in mean SE, the odds of having each of these conditions decreased by the following magnitudes: cancer by 0.95 (95% CI = 0.92 to 0.995), breathing problems by 0.96 (95% CI = 0.93 to 0.99), gastrointestinal problems by 0.96 (95% CI = 0.93 to 0.99), depression by 0.93 (95% CI = 0.89 to 0.96), and anxiety by 0.97 (95% CI = 0.87 to 0.94; Table 2). After adjusting for false discovery rate, only the relationships between mean SE and depression and anxiety remained significant at the p value of less than .05 level.
Intraindividual mean and variability in CM and odds of medical and mental health conditions
IIV in CM only was associated with increased odds of having urinary problems, but not any other medical or mental health conditions: For every 1 hour increase in IIV in CM, the odds of having urinary problems increased by 1.18 (95% CI = 1.003 to 1.378; Table 2). However, after adjusting for false discovery rate, this relationship was no longer significant. Mean CM was not associated with elevated odds of having any medical or mental health conditions, either before or after adjusting for false discovery rate (Table 2).
Discussion
In their 2016 systematic review of 53 studies examining correlates of IIV in sleep, Bei et al. [12] report that the limitations of research on this topic included (1) unsystematic and post-hoc analyses, (2) not controlling for mean sleep, and (3) a lack of careful consideration of potentially confounding variables. In contrast to previous research, this study specified specific hypotheses a priori (reporting both significant and null findings), corrected for false discovery rate, controlled for mean sleep in all analyses, and carefully included other covariates that may have been confounding (i.e. age, gender, BMI, and race). This also was the first study to examine IIV in multiple facets of sleep (i.e. TST, SQ, SE , and CM) as predictors of multiple comorbid medical conditions in an age and racially/ethnically diverse sample. Adjusting for false discovery rate, we found that greater IIV in self-reported TST (but not IIV in SQ, SE, or CM) is a distinct risk factor for a diverse cluster of medical conditions (neurological, breathing, gastrointestinal, depression, and pain) and that these effects are independent of the influence of age, BMI, race, and mean TST.
IIV in TST as a unique correlate of depression, pain, and GI, breathing and neurological conditions
Our results support a growing body of evidence suggesting that IIV in TST may be a distinct correlate of neurological problems and depressive symptoms [14, 15, 21, 45]. We found that every hour increase in IIV in TST was associated with 2.7 times greater odds of having neurological problems. Similarly, Dautovich et al. [13] found that greater IIV in nap duration is associated with a greater number of health conditions (including neurological problems) in older adults. Greater IIV in TST also has been related to poorer white matter integrity in adolescents [46], which may reflect underlying neurological problems and poor cognitive development. We also found that every hour increase in IIV in TST was associated with 2.4 times greater odds of having depression. Supporting our findings, other studies have shown that greater IIV in TST, as well as IIV time in bed, sleep onset latency, and sleep fragmentation (all facets of SE), is associated with depressive symptoms and negative mood [20, 22, 47]. Expanding on the existing literature, our study was the first to show that IIV in TST also may be a correlate of self-reported gastrointestinal problems, breathing problems, and pain. Together, these results suggest the need for future research to examine if reducing IIV in TST sleep may lead to more favorable health outcomes.
Null results with IIV in TST and other medical conditions
In contrast to findings from other studies [13–16], in this study, IIV in TST was not associated with elevated odds of having cardiometabolic disease (hypertension, diabetes, or heart disease), cancer, urinary problems, or anxiety. This may be attributed to the self-report, epidemiological nature of this study, and therefore, the possible underdiagnosis of these medical conditions. There also may be something unique about the specific medical conditions with which IIV in TST was associated; for example, depression, pain, and gastrointestinal, breathing, and neurological problems all tend to involve elevated levels of systemic inflammation, with which IIV in TST may be uniquely associated. Finally, the fact that IIV in TST was related to these specific medical conditions and not others may also have something to do with the specific demographic characteristics of this sample (e.g. age, gender, race/ethnicity, income, and education levels); future studies should seek to replicate our results in other samples and examine demographic moderators of the associations between IIV in sleep and medical conditions.
Null results with IIV in other sleep parameters and medical conditions
Contrary to our expected hypotheses, we found that IIV in SQ, SE, and CM was not associated with elevated odds of any medical or mental health conditions. Overall, IIV in TST appeared to be the most robust and consistent sleep-related risk factor for medical and mental health conditions. Even relative to other factors such as age, which is typically considered one of the most robust risk factors for disease morbidity, IIV in TST was associated with substantially elevated odds of having medical and mental health conditions. It is unclear, at this point, why the TST IIV results did not extend to SE, CM, or SQ. It may be that mean values of SQ, SE, or CM are more important than IIV, as discussed later. It might also be that TST is more health relevant in general than SE, CM, or SQ, or that TST is more validly self-reported by participants than other sleep parameters (a finding that has been supported by previous studies) [48, 49]. Finally, the null findings with IIV in SQ, SE, and CM also may be attributed to a relative restriction of range in these values and lower within-person variability in these measures across time. To our knowledge, no other studies have examined within-person variability in CM over time; however, in a nationally representative sample, Knutson et al. [50] showed that SE is relatively stable from one year to the next (r = .90); whereas TST is more labile (r = .76). In concordance with these results, in this study, there was more within-person variation in TST (61%) than in SE (50%), CM (49%) or SQ (58%) over the 14 day study period. More studies are needed to replicate and extend these findings before strong conclusions can be made about the relative importance of IIV in TST versus IIV in other sleep parameters across time.
The relative importance of IIV in sleep compared to mean sleep
In addition to IIV in TST, lower means of some sleep parameters (TST, SQ, and SE) were related to increased odds of having gastrointestinal problems, depression, and anxiety. These results are in concordance with findings from other daily and epidemiological studies that show that shorter mean TST and lower mean SE and SQ are associated with poorer mental health outcomes and gastrointestinal problems [51, 52]. However, it is notable that the effect of IIV in TST on these medical conditions was stronger than the effect of mean TST. Although the mean and IIV in sleep parameters often are correlated and may be driven by similar physiological processes (i.e. the homeostatic sleep drive and the internal circadian clock, see further discussion later), IIV in sleep likely better captures day-to-day situational changes in mood, work schedules, stress, or illness symptomatology that are obscured when only the mean in sleep is computed across time. It is therefore possible that the mean and IIV in sleep parameters may capture related but somewhat distinct phenomena, possibly with partially divergent physiological effects.
Potential mechanisms linking IIV in sleep and health outcomes
Alterations in hypothalamic–pituitary–adrenal axis and autonomic nervous system functioning
There are a number of potential biobehavioral mechanisms to explain how IIV in sleep may be related to adverse health outcomes. First, it may be that the health effects of IIV in sleep are mediated by alterations in hypothalamic–pituitary–adrenal (HPA) axis and autonomic nervous system (ANS) activity (e.g. blunted parasympathetic and increased sympathetic nervous system [SNS] activity). The HPA axis and the ANS regulate the immune response, metabolism, and cardiometabolic function [53–55], and the byproducts of these systems (i.e. cortisol, norepinephrine, epinephrine, and inflammatory cytokines) have been associated with IIV in sleep [23, 24]. During a night of sleep loss, the SNS branch of the ANS releases norepinephrine and epinephrine, which stimulate the upregulation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and the production of pro-inflammatory biomarkers such as interleukin-6 (IL-6) and tumor necrosis factor alpha (TNF-α) [56, 57]. Together, this heightened pro-inflammatory state may make an individual more tired on awakening, causing rebound sleep the next night and increasing IIV in sleep from night to night [58, 59].
Previous studies have indeed shown that greater IIV in sleep duration and fragmentation are related to increased nocturnal norepinephrine in insomniacs or individuals under chronic stress [20, 60]. In midlife adults, greater IIV in sleep timing and TST is associated with flatter diurnal cortisol slopes, and greater IIV in sleep onset latency and wake after sleep onset (both facets of SE) is associated with higher allostatic load (i.e. multisystem physiological dysregulation), both of which suggest dysregulated HPA axis functioning [23]. Greater IIV in wake time and time in bed also has been associated with higher IL-6 among good sleepers, and greater IIV in bedtime has been associated with higher TNF-α in both good and poor sleepers [24]. Importantly, greater systemic inflammation and dysregulation of the HPA axis and ANS are prospectively implicated in the development of a number of chronic diseases, including respiratory, neurological, and gastrointestinal disease, as well as pain and depression [61–71]. Although it remains to be empirically tested, these findings provide some evidence to suggest that the relationships we observed between IIV in sleep and these diseases may be mediated by systemic inflammation and dysfunction of the HPA axis and ANS.
Alterations in circadian processes
IIV in sleep also may lead to poor health outcomes via disruption of circadian rhythms. Misalignment of biological and behavioral circadian rhythms is involved in the pathogenesis of multiple health morbidities, including depression [72], metabolic syndrome [73], and cancer [74]. The circadian clock regulates critical aspects of cell growth and survival, including the cell cycle, cellular senescence, and metabolism [75]. Evidence from experimental circadian misalignment and simulated night shift work studies suggests that greater variability in simulated sleep schedules is associated with elevated inflammation, dysregulated cortisol, and insulin resistance [32, 76, 77]. Circadian misalignment also can lead to suppression of nocturnal melatonin [78]. Melatonin is a potent antioxidant, has anti-inflammatory effects, and inhibits leukocytes from adhering to endothelial cells [79]; therefore, its suppression may be associated with elevations in chronic inflammation and tissue damage that is characteristic of many chronic diseases (e.g. depression, cardiovascular disease, neurodegenerative disease, and chronic pain).
Alterations in behavioral processes
Greater IIV in sleep may also be related to poor health outcomes via behavioral pathways. After a night of poor sleep, individuals may engage in maladaptive health behaviors during the subsequent day (e.g. taking naps, drinking caffeine or alcohol, and using sleeping medications), in attempts to compensate for lost sleep. These behaviors may impair the ability for the individual to initiate sleep the following night, unintentionally promoting a cycle of instability and high IIV in sleep, and possibly impairing other physiological processes (e.g. metabolism, cognition, and cardiovascular function). Individuals with high IIV in TST may also engage in other emotional coping behaviors (e.g. unhealthy eating, using nicotine, alcohol or illicit drugs, avoiding social or work situations, and worrying about sleep), all in attempts to cope with lost or variable sleep, but which could result in direct negative physiological consequences. More comprehensive theoretical models are needed to explain the specific behavioral and physiological pathways by which IIV in sleep may contribute to specific disease risks, or over what time frame these effects may occur. A better understanding of specific biomarkers (e.g. heart rate variability, catecholamines, cortisol, blood pressure, and immune biomarkers) and health behaviors (e.g. caffeine usage, napping, and medication use) associated with increased IIV in sleep will facilitate understanding of how IIV may unfold as a disease risk factor.
Limitations and future directions
Despite this study’s unique contribution of the literature, there are some limitations that warrant further research. First, given that this was a large, epidemiological study, all measures were assessed via self-report. Results should be replicated using more objective measures of sleep (i.e. via actigraphy or at-home polysomnography) and physician-diagnosed medical conditions to address potential self-report biases. Second, although we assessed IIV in sleep over a longer period than most previous studies, more reliable estimates of IIV are obtained with more measurement occasions. A previous study demonstrated that 1 week of sleep diaries resulted in reliable estimates of intraindividual means in TST and SE [80], but comparatively, little work has been done to estimate the optimal number of measurements to obtain reliable measures of IIV.
Finally, causality between IIV in sleep and comorbid medical conditions cannot be determined with this study design. Although evidence from experimental and prospective studies suggests that mean sleep disturbances often precede the development of medical conditions and impaired physiological functioning [30, 76, 77, 81–84], no studies have examined the prospective relationships between IIV in sleep and subsequent risk for medical conditions. It is highly likely that the relationship between IIV in sleep and risk for medical conditions is bidirectional or cyclical. Greater IIV in sleep may lead to the development of or exacerbate preexisting medical conditions. Medical conditions also may lead to the development of greater IIV in sleep as a result of increased discomfort and sleep/wake alterations associated with disease processes. It also is possible that IIV in sleep and medical conditions share a common genetic or environmental influence (e.g. stress).
Evidence from experimental circadian misalignment and simulated rotating night shift work studies suggests that greater variability in simulated sleep schedules is in turn associated with elevated inflammation, dysregulated cortisol, and insulin resistance [32, 76, 77]. However, these types of simulated laboratory studies likely result in a different type of IIV in sleep than naturally occurring night-to-night fluctuations in sleep experienced in everyday life. Prospective, longitudinal studies of naturally occurring IIV in sleep and markers of disease risk are needed to infer the directionality of these associations, over what time frame the effects may unfold, and what “dosage” of IIV in sleep may be a risk factor for disease. A better understanding of the distinct social, psychological, and biological causes of IIV in sleep (e.g. social jetlag, sleep debt, shift work or work schedule, parenting or caregiving, stress, aging, and genetics) also may help delineate when greater IIV in sleep becomes a disease risk factor. Future studies should examine if IIV in sleep is a unique risk factor for morbidity beyond these variables. Finally, although it was not a goal of this study, examination of a priori, theoretically grounded demographic moderators (e.g. age, gender, and race/ethnicity) of the relationship between IIV in sleep parameters and medical and mental health conditions also is warranted to better understand who may be most at risk. For example, previous studies (including one using the same sample as this study [15]) have shown that racial and ethnic minorities, women, and younger and older adults (compared to midlife adults) tend to have greater IIV in sleep [15, 20, 50, 85, 86].
Conclusion
In summary, results from this study suggest that greater IIV in TST may be a distinct correlate of a diverse set of medical conditions, including neurological problems, gastrointestinal problems, breathing problems, pain, and depression. As we transition to an increasingly 24 hour society, the burden of IIV in sleep on disease risk is likely to become an even greater public health concern. As such, future research should continue to examine IIV in sleep as a specific facet of disturbed sleep above the influence of mean sleep. Prospective, longitudinal, and experimental studies will help better infer the directionality of the relationship between IIV and disease and elucidate physiological mechanisms by which IIV in sleep may confer disease risk over time.
Funding
None.
Conflict of interest statement. None declared
Acknowledgements
This research supported by National Institute on Aging grants AG12136 and AG14738.
References
- 1. Krueger PM, et al. Sleep duration in the United States: a cross-sectional population-based study. Am J Epidemiol. 2009;169(9):1052–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Institute of Medicine. Sleep Disorders and Sleep Deprivation: An Unmet Public Health Problem. Washington D.C.: The National Academies Press; 2006. [PubMed] [Google Scholar]
- 3. Roth T. Insomnia: definition, prevalence, etiology, and consequences. J Clin Sleep Med. 2007;3(5 Suppl):S7–S 10. [PMC free article] [PubMed] [Google Scholar]
- 4. Dew MA, et al. Healthy older adults’ sleep predicts all-cause mortality at 4 to 19 years of follow-up. Psychosom Med. 2003;65(1):63–73. [DOI] [PubMed] [Google Scholar]
- 5. Knutson KL, et al. The metabolic consequences of sleep deprivation. Sleep Med Rev. 2007;11(3):163–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Knutson KL. Sleep duration and cardiometabolic risk: a review of the epidemiologic evidence. Best Pract Res Clin Endocrinol Metab. 2010;24(5):731–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Patel SR, et al. A prospective study of sleep duration and mortality risk in women. Sleep. 2004;27(3):440–444. [DOI] [PubMed] [Google Scholar]
- 8. Liu Y, et al. Association between perceived insufficient sleep, frequent mental distress, obesity and chronic diseases among US adults, 2009 behavioral risk factor surveillance system. BMC Public Health. 2013;13:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shankar A, et al. Insufficient rest or sleep and its relation to cardiovascular disease, diabetes and obesity in a national, multiethnic sample. PLoS One. 2010;5(11):e14189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kessler RC, et al. Insomnia and the performance of US workers: results from the America insomnia survey. Sleep. 2011;34(9):1161–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hafner M, et al. Why sleep matters-the economic costs of insufficient sleep: a cross-country comparative analysis. Rand Health Q. 2017;6(4):11. [PMC free article] [PubMed] [Google Scholar]
- 12. Bei B, et al. Beyond the mean: a systematic review on the correlates of daily intraindividual variability of sleep/wake patterns. Sleep Med Rev. 2016;28:108–124. [DOI] [PubMed] [Google Scholar]
- 13. Dautovich ND, et al. Day-to-day variability in nap duration predicts medical morbidity in older adults. Health Psychol. 2012;31(5):671–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Patel SR, et al. ; Osteoporotic Fractures in Men (MrOS); Study of Osteoporotic Fractures (SOF) Research Groups The association between sleep patterns and obesity in older adults. Int J Obes (Lond). 2014;38(9):1159–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dillon HR, et al. Variability in self-reported normal sleep across the adult age span. J Gerontol B Psychol Sci Soc Sci. 2015;70(1):46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Roumelioti ME, et al. Sleep quality, mood, alertness and their variability in CKD and ESRD. Nephron Clin Pract. 2010;114(4):c277–c287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Molzof HE, et al. Intraindividual sleep variability and its association with insomnia identity and poor sleep. Sleep Med. 2018;52:58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Buysse DJ, et al. Night-to-night sleep variability in older adults with and without chronic insomnia. Sleep Med. 2010;11(1):56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Krietsch KN, et al. Influence of asthma status on sleep variability in overweight/obese youth. J Asthma. 2017;54(4):383–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mezick EJ, et al. Intra-individual variability in sleep duration and fragmentation: associations with stress. Psychoneuroendocrinology. 2009;34(9):1346–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vanderlind WM, et al. Sleep and sadness: exploring the relation among sleep, cognitive control, and depressive symptoms in young adults. Sleep Med. 2014;15(1):144–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bei B, Manber R, Allen NB, Trinder J, Wiley JF. Too long, too short, or too variable? Sleep intraindividual variability and its associations with perceived sleep quality and mood in adolescents during naturalistically unconstrained sleep. Sleep. 2017;40(2): zsw067. [DOI] [PubMed] [Google Scholar]
- 23. Bei B, Seeman TE, Carroll JE, Wiley JF. Sleep and physiological dysregulation: a closer look at sleep intraindividual variability. Sleep. 2017;40(9):zsx109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Okun ML, et al. Sleep variability, health-related practices, and inflammatory markers in a community dwelling sample of older adults. Psychosom Med. 2011;73(2):142–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vyas MV, et al. Shift work and vascular events: systematic review and meta-analysis. BMJ. 2012;345:e4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ramin C, et al. Night shift work at specific age ranges and chronic disease risk factors. Occup Environ Med. 2015;72(2):100–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gan Y, et al. Shift work and diabetes mellitus: a meta-analysis of observational studies. Occup Environ Med. 2015;72(1):72–78. [DOI] [PubMed] [Google Scholar]
- 28. Pan A, et al. Rotating night shift work and risk of type 2 diabetes: two prospective cohort studies in women. PLoS Med. 2011;8(12):e1001141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kalmbach DA, et al. Shift work disorder, depression, and anxiety in the transition to rotating shifts: the role of sleep reactivity. Sleep Med. 2015;16(12):1532–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Morris CJ, et al. Circadian misalignment increases C-reactive protein and blood pressure in chronic shift workers. J Biol Rhythms. 2017;32(2):154–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sookoian S, et al. Effects of rotating shift work on biomarkers of metabolic syndrome and inflammation. J Intern Med. 2007;261(3):285–292. [DOI] [PubMed] [Google Scholar]
- 32. Wright KP Jr, et al. Influence of sleep deprivation and circadian misalignment on cortisol, inflammatory markers, and cytokine balance. Brain Behav Immun. 2015;47:24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lichstein KL, Durrence HH, Riedel BW, Taylor DJ, Bush AJ.. Epidemiology of Sleep: Age, Gender, and Ethnicity. New York, NY: Psychology Press; 2004. [Google Scholar]
- 34. U.S. Census Bureau. Census 2000 ;2000. [Google Scholar]
- 35. Buysse DJ. Sleep health: can we define it? Does it matter? Sleep. 2014;37(1):9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Beck AT, Steer RA, Brown GK.. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 37. Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clin Psychol Rev. 1988;8:77–100. [Google Scholar]
- 38. Spielberger CD, Gorsuch RL, Lushene R.. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1970. [Google Scholar]
- 39. Spielberger CD, Gorsuch RL, Lushene PR, Vagg PR, Jacobs GA.. Manual for the State-Trait Anxiety Inventory (Form Y). Redwood City, CA: Mind Garden; 1983. [Google Scholar]
- 40. National Center for Health Statistics. Health, United States 2015: With Special Feature on Racial and Ethnic Health Disparities. Hyattsville, MD;2016. [PubMed] [Google Scholar]
- 41. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2013. [Google Scholar]
- 42. varian: Variability Analysis in R. Version 0.2.2; 2016. [Google Scholar]
- 43. Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Lawrence Earlbaum Associates; 1988. [Google Scholar]
- 44. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57(1):289–300. [Google Scholar]
- 45. Lemola S, et al. Variability of sleep duration is related to subjective sleep quality and subjective well-being: an actigraphy study. PLoS One. 2013;8(8):e71292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Telzer EH, et al. Sleep variability in adolescence is associated with altered brain development. Dev Cogn Neurosci. 2015;14:16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Suh S, et al. Clinical significance of night-to-night sleep variability in insomnia. Sleep Med. 2012;13(5):469–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lichstein KL, et al. Actigraphy validation with insomnia. Sleep. 2006;29(2):232–239. [PubMed] [Google Scholar]
- 49. Williams JM, Taylor DJ, Slavish DC, et al. Validity of actigraphy in young adults with insomnia. Behav Sleep Med. 2018. [DOI] [PubMed] [Google Scholar]
- 50. Knutson KL, et al. Intra-individual daily and yearly variability in actigraphically recorded sleep measures: the CARDIA study. Sleep. 2007;30(6):793–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Spira AP, et al. Anxiety symptoms and objectively measured sleep quality in older women. Am J Geriatr Psychiatry. 2009;17(2):136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Fass R, et al. Sleep disturbances in clinic patients with functional bowel disorders. Am J Gastroenterol. 2000;95(5):1195–2000. [DOI] [PubMed] [Google Scholar]
- 53. Buckley TM, et al. On the interactions of the hypothalamic-pituitary-adrenal (HPA) axis and sleep: normal HPA axis activity and circadian rhythm, exemplary sleep disorders. J Clin Endocrinol Metab. 2005;90(5):3106–3114. [DOI] [PubMed] [Google Scholar]
- 54. Burgess HJ, et al. Sleep and circadian influences on cardiac autonomic nervous system activity. Am J Physiol. 1997;273(4 Pt 2):H1761–H1768. [DOI] [PubMed] [Google Scholar]
- 55. Chrousos GP, et al. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA. 1992;267(9):1244–1252. [PubMed] [Google Scholar]
- 56. Irwin MR, et al. Sleep health: reciprocal regulation of sleep and innate immunity. Neuropsychopharmacology. 2017;42(1):129–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Irwin MR. Why sleep is important for health: a psychoneuroimmunology perspective. Annu Rev Psychol. 2015;66:143–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Pejovic S, et al. Effects of recovery sleep after one work week of mild sleep restriction on interleukin-6 and cortisol secretion and daytime sleepiness and performance. Am J Physiol Endocrinol Metab. 2013;305(7):E890–E896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Faraut B, et al. Immune, inflammatory and cardiovascular consequences of sleep restriction and recovery. Sleep Med Rev. 2012;16(2):137–149. [DOI] [PubMed] [Google Scholar]
- 60. Irwin M, et al. Nocturnal catecholamines and immune function in insomniacs, depressed patients, and control subjects. Brain Behav Immun. 2003;17(5):365–372. [DOI] [PubMed] [Google Scholar]
- 61. Bose M, et al. Stress and obesity: the role of the hypothalamic-pituitary-adrenal axis in metabolic disease. Curr Opin Endocrinol Diabetes Obes. 2009;16(5):340–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Pariante CM, et al. The HPA axis in major depression: classical theories and new developments. Trends Neurosci. 2008;31(9):464–468. [DOI] [PubMed] [Google Scholar]
- 63. Chang L, et al. Dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis in irritable bowel syndrome. Neurogastroenterol Motil. 2009;21(2):149–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Blackburn-Munro G. Hypothalamo-pituitary-adrenal axis dysfunction as a contributory factor to chronic pain and depression. Curr Pain Headache Rep. 2004;8(2):116–124. [DOI] [PubMed] [Google Scholar]
- 65. Magri F, et al. Stress and dementia: the role of the hypothalamicpituitary-adrenal axis. Aging Clin Exp Res. 2006;18(2):167–170. [DOI] [PubMed] [Google Scholar]
- 66. Abelson JL, et al. HPA axis, respiration and the airways in stress–a review in search of intersections. Biol Psychol. 2010;84(1):57–65. [DOI] [PubMed] [Google Scholar]
- 67. Smith AL, et al. Basal and stress-activated hypothalamic pituitary adrenal axis function in postmenopausal women with overactive bladder. Int Urogynecol J. 2016;27(9):1383–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Miller AH, et al. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65(9):732–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Pradhan AD, et al. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286(3):327–334. [DOI] [PubMed] [Google Scholar]
- 70. Marx J. Cancer research. Inflammation and cancer: the link grows stronger. Science. 2004;306(5698):966–968. [DOI] [PubMed] [Google Scholar]
- 71. Coussens LM, et al. Inflammation and cancer. Nature. 2002;420(6917):860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Levandovski R, et al. Depression scores associate with chronotype and social jetlag in a rural population. Chronobiol Int. 2011;28(9):771–778. [DOI] [PubMed] [Google Scholar]
- 73. Scheer FAJL, et al. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci USA. 2009;106(11):4453–4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Davis S, et al. Night shift work, light at night, and risk of breast cancer. J Natl Cancer Inst. 2001;93(20):1557–1562. [DOI] [PubMed] [Google Scholar]
- 75. Sahar S, et al. Metabolism and cancer: the circadian clock connection. Nat Rev Cancer. 2009;9(12):886–896. [DOI] [PubMed] [Google Scholar]
- 76. Morris CJ, et al. Circadian misalignment increases cardiovascular disease risk factors in humans. Proc Natl Acad Sci USA. 2016;113(10):E1402–E1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Leproult R, et al. Circadian misalignment augments markers of insulin resistance and inflammation, independently of sleep loss. Diabetes. 2014;63(6):1860–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Sephton S, et al. Circadian disruption in cancer: a neuroendocrine-immune pathway from stress to disease? Brain Behav Immun. 2003;17(5):321–328. [DOI] [PubMed] [Google Scholar]
- 79. Reiter RJ, et al. Melatonin and its relation to the immune system and inflammation. Ann N Y Acad Sci. 2000;917:376–386. [DOI] [PubMed] [Google Scholar]
- 80. Wohlgemuth WK, et al. How many nights are enough? The short-term stability of sleep parameters in elderly insomniacs and normal sleepers. Psychophysiology. 1999;36(2):233–244. [PubMed] [Google Scholar]
- 81. Baglioni C, et al. Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. J Affect Disord. 2011;135(1-3):10–19. [DOI] [PubMed] [Google Scholar]
- 82. Troxel WM, et al. Sleep symptoms predict the development of the metabolic syndrome. Sleep. 2010;33(12):1633–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Gregory AM, et al. Prospective longitudinal associations between persistent sleep problems in childhood and anxiety and depression disorders in adulthood. J Abnorm Child Psychol. 2005;33(2):157–163. [DOI] [PubMed] [Google Scholar]
- 84. Finan PH, et al. The association of sleep and pain: an update and a path forward. J Pain. 2013;14(12):1539–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Shoji KD, et al. Beyond mean values: quantifying intraindividual variability in pre-sleep arousal and sleep in younger and older community-dwelling adults. Sleep Sci. 2015;8(1):24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. van Hilten JJ, et al. Ambulatory activity monitoring during sleep: an evaluation of internight and intrasubject variability in healthy persons aged 50-98 years. Sleep. 1993;16(2):146–150. [DOI] [PubMed] [Google Scholar]