Abstract
Current sleep analyses have used electroencephalography (EEG) to establish sleep intensity through linear and nonlinear measures. Slow wave activity (SWA) and entropy are the most commonly used markers of sleep depth. The purpose of this study is to evaluate changes in brain EEG connectivity during sleep in healthy subjects and compare them with SWA and entropy. Four different connectivity metrics: coherence (MSC), synchronization likelihood (SL), cross mutual information function (CMIF), and phase locking value (PLV), were computed focusing on their correlation with sleep depth. These measures provide different information and perspectives about functional connectivity. All connectivity measures revealed to have functional changes between the different sleep stages. The averaged CMIF seemed to be a more robust connectivity metric to measure sleep depth (correlations of 0.78 and 0.84 with SWA and entropy, respectively), translating greater linear and nonlinear interdependences between brain regions especially during slow wave sleep. Potential changes of brain connectivity were also assessed throughout the night. Connectivity measures indicated a reduction of functional connectivity in N2 as sleep progresses. The validation of connectivity indexes is necessary because they can reveal the interaction between different brain regions in physiological and pathological conditions and help understand the different functions of deep sleep in humans.
Keywords: electroencephalography, slow wave activity, entropy, functional connectivity
Statement of Significance.
In this study, we analyzed four of the most important connectivity measures to assess their efficacy measuring sleep depth. We compared the time-course results with SWA and entropy, two metrics that were previously used as biomarkers of sleep intensity. We concluded that these measures are effective to evaluate the changes in brain connectivity during sleep. These metrics are a potential tool to support other biomarkers of sleep depth and to evaluate the interactions between different brain regions.
Introduction
Sleep is a physiological state characterized by changes in neural oscillations. During sleep, periods of electrocortical synchronization are followed by phases of desynchronized activity. Polysomnographic (PSG) recordings allow the differentiation of at least three different states: wakefulness, non-REM (NREM), and REM sleep. NREM and REM alternate rhythmically during sleep in a cyclic fashion. REM sleep is characterized by low-amplitude mixed-frequency brain waves that are similar to the awake brain. NREM sleep is more synchronized, resulting in slow frequency waves of high voltage. NREM sleep is further divided into three stages ordered by sleep depth: N1 and N2, also known as light sleep, and N3 often referred to as deep sleep or slow wave sleep (SWS). SWS has been recognized as pivotal for the restorative effects of sleep. Several studies have shown that cognitive functions such as attention [1], and memory consolidation are associated with slow wave activity (SWA) [2], possibly due to synaptic downscaling and relevant neuronal circuitry changes [3]. Additionally, SWS has been associated with several physiological functions such as restoration of brain energy [4] and cerebral blood flow, hormonal regulation [5], immune system homeostasis [6], and parasympathetic tone [7]. Given the importance of deep sleep, a better characterization, beyond the current gold standard of electroencephalography (EEG) sleep staging, through the evaluation of connectivity patterns would broaden our understanding of its physiological functions and reveal new approaches to improve sleep quality.
Traditionally, SWA measured as the power in the delta band (0.5–4 Hz) and derived from linear EEG oscillatory activity analysis, has been considered the best indicator of sleep intensity. An additional approach to analyzing sleep depth is the study of cyclic alternating patterns (CAPs), which focus on sleep microstructure [8]. CAP technique can be seen as EEG activity that may indicate sleep instability, sleep disturbance, or both. The CAP sequence could be conceptualized as an arousal phenomenon and it can occur also in association with identifiable sleep pathophysiology (e.g. sleep-disordered breathing and periodic leg movement activity) [8]. CAP patterns are interpreted as an unstable/arousable stage and non-CAP patterns are interpreted as a deeper/lees arousable sleep [9]. However, it has long been known that brain oscillations are not a linear combination of arbitrary frequency components, a property called “nonlinearity.” Nonlinear alternatives to analyze sleep intensity based on entropy measures have been proposed and show a parallel depth-of-sleep continuum [9, 10]. Entropy is a measure of unpredictability of information content in a message, or time series data—patterns with low predictability are assigned high entropy, whereas highly ordered, regular signals (e.g. sine waves with fixed frequency) contain very little entropy [11]. Several studies have shown that the brain tends toward larger complexity and entropy in wakefulness as compared with the altered states of consciousness [11–14]. In particular, there is an inverse relationship between sleep depth and the complexity of the EEG signal (i.e. deeper NREM sleep is less complex than lighter NREM sleep) because the lack of arrival of multiple sensory inputs during unconscious states causes changes in neural firing frequencies [9, 11, 12, 14].
A novel way to measure sleep depth is through the study of brain EEG connectivity, representing the capacity of certain groups of neurons to affect the function of other groups of neurons within a system [15]. For example, coherence, one measure of connectivity, is a mathematical method used to determine if two or more brain regions share similar neuronal oscillatory activity. Broadly, coherence is high during NREM, especially in the range of sleep spindles [16], it is both decreased and increased in REM sleep, depending on the brain areas involved [17], and it peaks both at 14 Hz and at slower SWS frequencies [18]. The decline of cortical connectivity during NREM has been proposed to parallel the consciousness decay observed during some sleep stages [19]. Moreover, some studies indicate that sleep EEG synchronization is highly dependent on CAP conditions and sleep stages [20, 21]. Although EEG brain connectivity measures are able to differentiate between the different sleep states, they are generally not used as markers of sleep depth. They may however constitute important tools to better describe different patterns of sleep depth, especially given that growing evidence suggests that synchronization of neuronal groups may be in the root of both physiological and abnormal brain activity.
The purpose of this study was to analyze the changes in brain connectivity measures during sleep, focusing on the time correlation between connectivity indexes and sleep intensity, and their relationship with the currently more established markers of sleep depth. Four different connectivity mapping metrics: magnitude squared coherence (MSC), synchronization likelihood (SL), cross mutual information function (CMIF), and phase locking value (PLV), were tested to take into account different underlying mathematical assumptions of linear and nonlinear dependency. Secondary objectives were to include the analysis of the modulation of the connectivity patterns between sleep stages and throughout the night.
Methods
Database acquisition and sleep staging
Thirty healthy young volunteers (28.93 ± 4.56 years-old) underwent PSG recordings at the Drug Research Centre of the Hospital de la Santa Creu i Sant Pau in Barcelona, Spain. All subjects were screened 3 weeks before the study through a clinical interview, physical examination, and laboratory testing (including hematology, urinalysis, and serological tests). Subjects with history of medical or psychiatric disorders or any sleep disturbance (Pittsburg Sleep Quality Index > 5), were excluded [22]. In the 24 h before the experimental session, volunteers were not allowed to smoke, drink alcohol or coffee, practice strenuous physical exercise, or take naps. Subjects were asked to maintain their regular sleep-wake schedule and to avoid traveling throughout the study. The trial was approved by the Hospital’s Research Ethics Committee, and was conducted following the principles stated in the Declaration of Helsinki and subsequent revisions. All participants gave written informed consent before participating in the study.
The experimental session consisted of two sequential nights in the laboratory. The first night was used for adaptation and only data from the second night was processed. On both nights, participants were under continuous supervision while PSG was recorded, in a sound-attenuated and temperature-regulated individual room. Data were acquired by means of Compumedics E-Series System, and included 19 electroencephalographic channels following the 10–20 International System (namely Fp1, Fp2, F7, F3, Fz, F4, F8, T3, C3, Cz, C4, T4, T5, P5, Pz, P4, T6, O1, and O2, referenced to the averaged mastoids [A1 and A2]); left and right electrooculographic (EOG) channels to detect ocular movements in horizontal and vertical directions; one submental electromyographic channel (EMG) to assess muscle tone; two EMG channels to monitor limb movements; and three channels to monitor the respiratory function (chest band, nasal thermistor, and finger SaO2). Electrodes were gold-plated and calibrated before each recording, keeping their impedances below 10 kΩ. Sampling frequency was set to 256 Hz, and the amplifiers were configured with an analog bandwidth of 0.1–75 Hz and a notch filter set to 50 Hz to attenuate electrical noise.
Two independent experts visually scored the sleep recordings following the American Academy of Sleep Medicine (AASM) guidelines [23] and using the Profusion Sleep software (Compumedics PSG3 version 3.4). A third expert from the same laboratory solved discrepancies between the two scorers. Sleep cycles were determined following the criteria by Feinberg et al. [24], where a sleep cycle is considered as a NREM episode starting from stage 2 (N2), with at least 15 min of N2 or SWS (N3) and followed by a REM episode lasting at least 5 min.
Signal preprocessing
Even with careful setup of the equipment and environment used for the study, the acquired signals presented several artifacts originated by noise, movement, and external interferences. EEG signals were segmented into 5-s epochs. Artifact epochs containing saturation or muscular activity were automatically identified and eliminated by a procedure based on time and frequency domain features. Once this was completed, each artifact-free segment was zero-phase filtered between 0.5 and 35 Hz using a type II Chebyshev filter of order 8. Sample entropy and SWA as the absolute power in the delta band (0.5–3.5 Hz), were calculated for each artifact-free 5-s epoch.
Measures of cerebral connectivity
In this study, we used the following different measures to assess brain connectivity: coherence, phase-locking value, SL, and CMIF.
Although all these methods quantify the strength of connectivity between two nodes, they measure different aspects of functional connectivity. MSC is a linear mathematical method that measures the linear dependence between a pair of nodes in the frequency domain, and this method can provide a measure that is more robust against noise. PLV measures the coupling between the phases of two signals, even though their amplitudes may remain uncorrelated. The other connectivity measures used in this work are based on nonlinear techniques. CMIF is the nonlinear analogue of the cross-correlation function between two time series, but it is much more robust to noise and outliers. It quantifies the amount of information that can be obtained about a random variable by observing another as a function of the delay between them. Finally, SL can capture nonlinear interconnections by measuring the generalized synchronization, that is, to what extent one of the variables is synchronized with a general function of the other variable. SL assumes a fixed phase relationship between time series. This choice of connectivity measures allows the evaluation of functional connectivity from four different and most common approaches: classical spectral measures (MSC), phase synchronization measures (PLV), generalized synchronization measures (SL), and information theoretic measures (CMIF).
Coherence
The magnitude squared coherence (MSC), or simply called coherence, is a measure of the degree of synchronicity between neuronal systems. It quantifies the level of dependence between EEG signals from different neuronal groups, based on the correlation between their EEG frequencies [25, 26]. MSC is commonly obtained via nonparametric spectral estimators, such as the Welch’s periodogram and the minimum variance distortionless response (MVDR) spectrum based on the Capon’s approach [27]. While the former can overestimate the MSC and is dependent on the amplitude of the signals, MVDR has the advantage of providing a higher resolution and no overestimated values (providing null coherence) in the frequency ranges from 0 to 0.5 Hz and >35 Hz, as expected after zero-phase filtering. The significance level for the MSC was obtained through surrogate data, by means of 20 sets of amplitude adjusted and Fourier transformed surrogates [28]. Only significant values of MSC were considered for subsequent analysis. MSC was calculated for different frequency bands: delta (0.5–3.5 Hz), theta (3.5–7.5 Hz), alpha (7.5–13 Hz), beta1-2 (13–20 Hz), and high beta (23–35 Hz).
Cross mutual information function
The CMIF can be understood as the nonlinear counterpart of the cross correlation function, as it measures the nonlinear correlation between two EEG signals in the time domain. It essentially quantifies the amount of information transferred between two signals that is common for both of them (coupling). In this work, CMIF was obtained for a maximum delay of 52 samples (20% of the sampling frequency), as reported in previous studies [29, 30]. Finally, the CMIF was defined by the calculation of its average value [31, 32].
Synchronization likelihood
The SL is a measure of generalized synchronization, based on both linear and nonlinear dependencies between EEG signals. It was developed by Stam et al. [33] to overcome some of the limitations of other techniques used for detecting statistical dependencies between time series, that allows dealing with multivariate data and nonstationary dynamics. In short, SL measures the amount of synchronization between one channel and all others, by representing the state of a dynamic system in a given time window, and this state is usually obtained through the Takens’ time-delay embedding theorem [34].
SL is used to detect simultaneously occurring patterns and depends on several parameters that, when chosen incorrectly, can bias the results toward detecting patterns in specific frequency bands.
Phase-locking value
Phase-locking value (PLV) is a highly sensitive measure of neural synchronization in the EEG, and it can range from 0 (no synchrony) to 1 (full synchrony). PLV was introduced by Lachaux et al. [35] as a measure of the stability of phases between pairs of electrodes in the same frequency band. Since then, it has been widely used to investigate a multitude of different functional brain networks [36]. Besides the vast experience with this measure, one of the main advantages of PLV is that it does not depend on stationarity and it is sensitive to small amplitude oscillations [37].
To calculate PLV, it is necessary to constrain the frequency spectrum to a narrow bandwidth and extract the instantaneous phase of every signal [35]. Therefore, in our study, firstly EEG data were band-pass filtered in each previously defined frequency band, and secondly, the phase and amplitude of each band-pass signal were obtained via Hilbert transform.
Standardization of sleep cycles and statistical analysis
Each connectivity measure was obtained for all artifact-free segments resulting from the signal preprocessing. Segments of consecutive artifact-free 20 s were considered for robust estimation of the measures. As each volunteer presented different durations of sleep cycles, the temporal course of each connectivity measure was standardized, to average sleep time courses across subjects. Each NREM cycle was divided into nine equal segments, and each REM cycle into three.
Given the chance of significant results by the effect of multiple comparisons, statistical significance was assessed using a statistical threshold p < 0.05 corrected by the false discovery rate (FDR), which reduces the rate of type I errors [38].
Results
Three of the initial 30 participants were excluded from posterior analysis due to low sleep efficiency (47.65%, 62.60%, and 63.40%). The remaining participants showed good sleep efficiency values (higher than 80%), with an average of 7 h of total sleep, with a good sleep efficiency and no evidence of obstructive sleep apnea. Table 1 shows PSG variables related to sleep initiation and maintenance as well as sleep architecture.
Table 1.
Sleep initiation, maintenance, and sleep architecture characteristics of the study group (mean ± SD; n = 27)
| Sleep initiation and maintenance | Sleep architecture | ||
|---|---|---|---|
| Time in bed (min) | 482.63 ± 4.14 | Number of sleep cycles | 3.52 ± 1.01 |
| Total sleep time (min) | 426.24 ± 24.06 | Stage N1 (min) | 23.94 ± 15.30 |
| Sleep onset latency (min) | 23.77 ± 20.09 | Stage N1 (%) | 4.97 ± 3.19 |
| REM latency (min) | 116.43 ± 33.77 | Stage N2 (min) | 190.69 ± 49.54 |
| Sleep efficiency (%) | 88.29 ± 4.89 | Stage N2 (%) | 39.25 ± 10.25 |
| Stage N3 (min) | 134.28 ± 43.22 | ||
| Stage N3 (%) | 27.85 ± 9.07 | ||
| REM (min) | 77.13 ± 19.93 | ||
| REM (%) | 15.98 ± 4.13 |
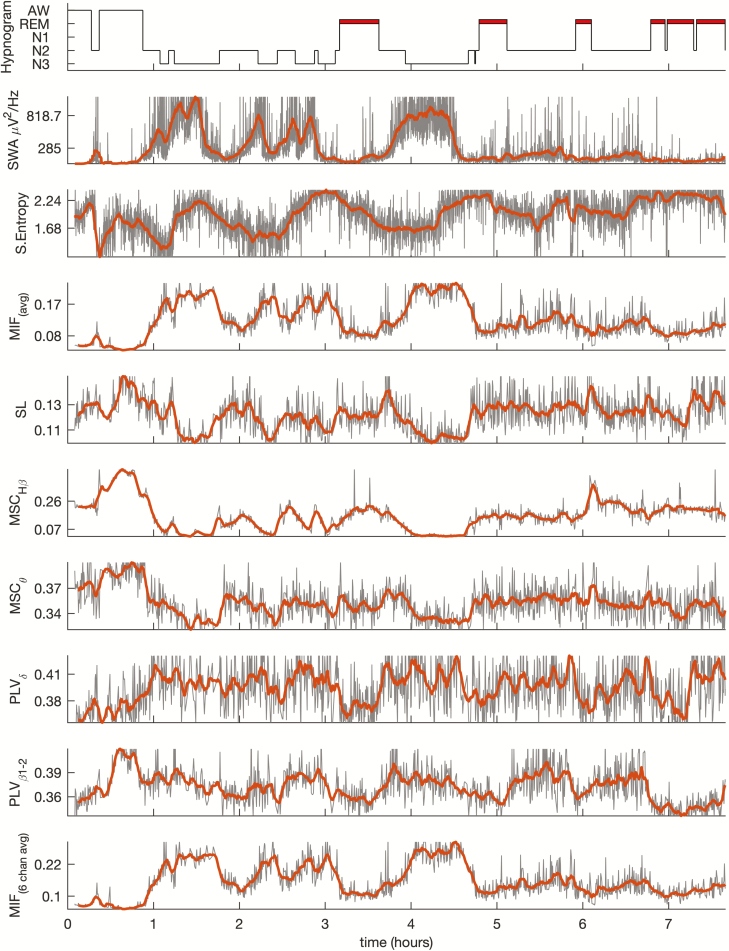
Artifact rejection procedure discarded 17.52% ± 11.00% of the 5-s epochs for posterior EEG processing. Figure 1 shows an example of the hypnogram and the time course of the EEG connectivity measures used in this study (MSC, SL, CMIF, and PLV), during the night for one volunteer. SWA, widely considered the gold-standard marker for sleep depth, and sample entropy, were also included in the figure. All connectivity indexes followed the morphology of the hypnogram, especially the average value of CMIF and the coherence (MSC), in the high beta band (23–35 Hz). Averaged CMIF and PLV in delta and beta bands increased as sleep deepened, decreasing again to lower values in REM sleep. Conversely, SL and MSC measures in theta and high beta bands presented the opposite behavior, with higher values during NREM and decreases during REM. Other frequency bands of SL and MSC showed time courses poorly correlated with the hypnogram.
Figure 1.
Time course of the hypnogram, SWA, sample entropy, and different averaged connectivity measures (MIF, SL, MSC, and PLV) obtained for a subject during the night as an example. Thick lines in the graphs were calculated by means of a moving average filter using a 5 min sliding window. All the connectivity measures represent the average value from the 19 electrodes, except for MIF, which were also calculated averaging only six electrodes (Fp1, Fp2, C3, C4, O1, and O2).
A reduced number of EEG electrodes is commonly used in sleep studies. To increase external validity, a configuration including only six EEG electrodes (Fp1, Fp2, C3, C4, O1, and O2) was considered. The averaged CMIF graphic of all possible pairs of these six EEG electrodes is represented in the same figure, showing that the behavior of time course of 6-channel CMIF was very similar to that obtained with 19 EEG electrodes.
Correlation coefficients between the time course of SWA and sample entropy and other connectivity measures are shown in Table 2. High averaged correlation values were obtained for CMIF and SL. The minimum value of correlation coefficient between SWA and CMIF (0.59 and 0.65 for SWA and sample entropy, respectively) for one subject, evidenced the robustness and consistency of CMIF to reproduce the time courses of SWA and entropy, and therefore to be an indicator of sleep intensity.
Table 2.
Maximum value of normalized cross-correlation function between established markers of sleep depth (SWA and entropy) and different connectivity measures averaged for the entire study group (n = 27)
| Connectivity measure | SWA | Sample entropy | ||
|---|---|---|---|---|
| Mean | Range | Mean | Range | |
| CMIF | 0.78 | [0.59 to 0.91] | −0.84 | [−0.65 to −0.96] |
| CMIF6 channels | 0.75 | [0.55 to 0.90] | −0.82 | [−0.60 to −0.95] |
| SL | −0.65 | [−0.46 to −0.86] | 0.66 | [0.48 to 0.80] |
| MSC high beta | −0.53 | [−0.23 to −0.86] | 0.57 | [0.31 to 0.89] |
| MSC theta | −0.50 | [−0.25 to −0.86] | 0.53 | [0.24 to 0.88] |
| PLV delta | 0.49 | [0.25 to 0.88] | −0.53 | [−0.31 to −0.94] |
| PLV beta12 | 0.46 | [0.21 to 0.72] | −0.49 | [−0.29 to −0.73] |
Range statistic showing the smallest and largest values of correlation was included.
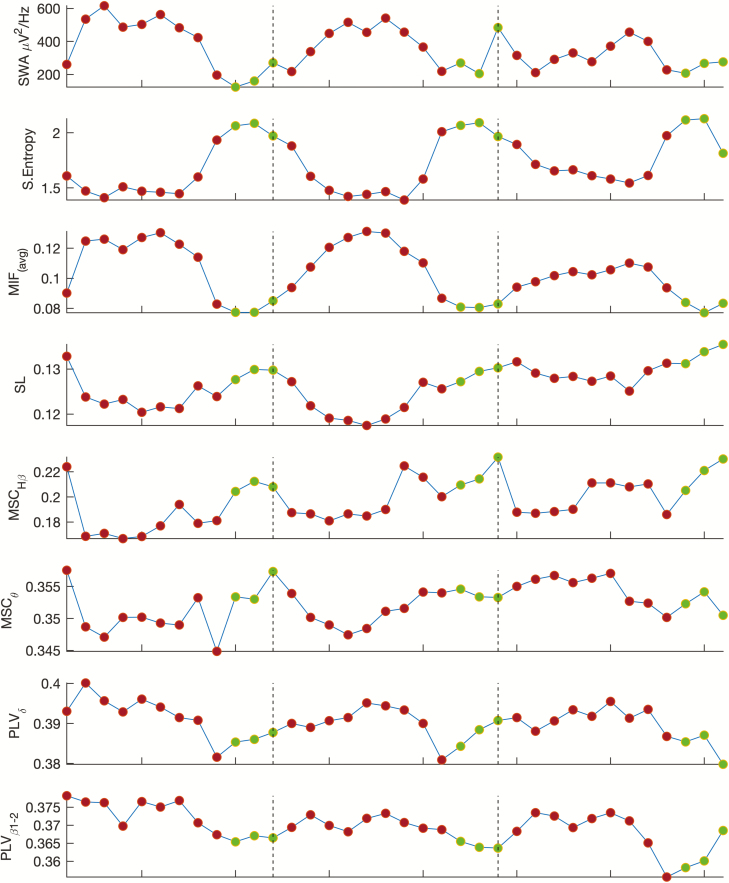
Figure 2 shows averaged SWA once the duration of sleep NREM-REM cycles was normalized. SWA presented its typical declining trend over consecutive NREM episodes. Averaged connectivity measures included in the figure showed different values between NREM and REM periods. SL and MSC measures exhibited a U-shaped pattern, with lowest values coinciding with higher SWA, and highest values toward transitions to REM episodes.
Figure 2.
Time courses of SWA (0.5–4 Hz), sample entropy and different connectivity measures obtained as the grand mean average of 19 EEG electrodes and 27 subjects. To compensate for the individual differences in occurrence and duration of the NREM-REM cycles, individual NREMs and REMs episodes were subdivided in nine and three equal time bins, respectively for each subject. Red points represent averaged NREM bins and green points REM values. Dashed vertical lines delimit NREM-REM cycles.
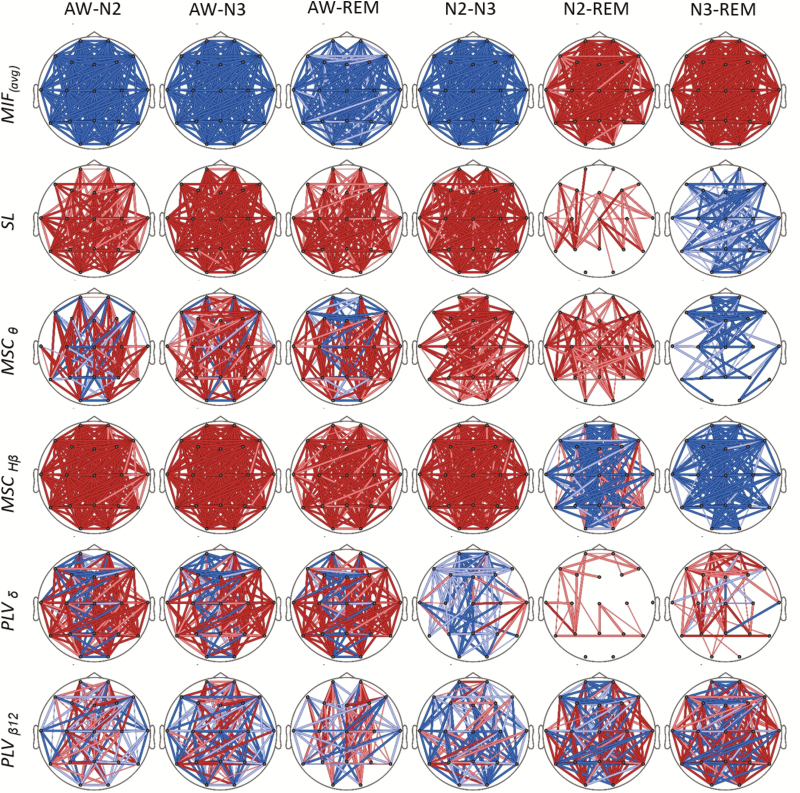
Alterations in the connectivity measures between sleep stages, focused in the first NREM-REM cycle, are represented in Figure 3. Statistical probability maps (SPM) show differences for the different connectivity measures between wakefulness, NREM and REM stages. Most significant changes were obtained for CMIF, whose maps indicate that information shared by EEG channels was increased as sleep became deeper, showing statistically significant differences in connectivity patterns between all the sleep stages. It is noteworthy to mention that CMIF connectivity patterns for REM differed from those obtained for awake and stages N2 and N3. SL connectivity maps were statistically different between all sleep stages and wakefulness. Coherence (MSC) and PLV measures revealed less clear connectivity changes, with both increases and decreases occurring in some cases.
Figure 3.
SPMs showing statistical changes on connectivity measures between sleep stages. Increases and decreases of connectivity values are indicated with hot and cold colors. Color intensity and line thickness were related to the associated probability value of significant differences (p < 0.01 dark and thick; 0.01 < p < 0.05 light and thick; 0.05 < p < 0.10 light and thin). FDR-based multiple comparison correction procedure was applied.
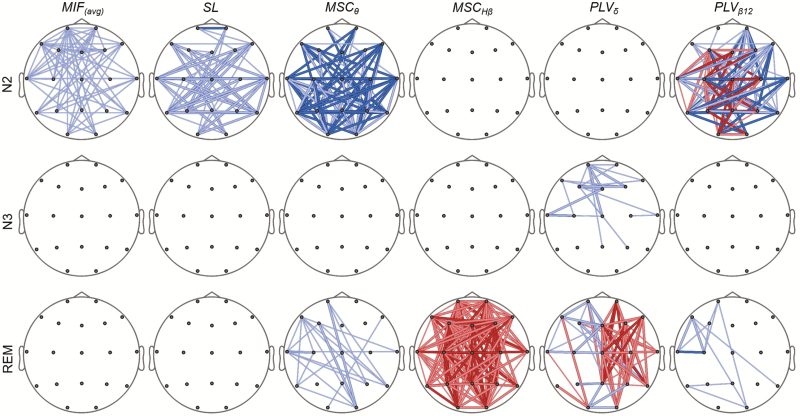
Finally, the potential changes of brain connectivity for a specific sleep stage throughout the night were analyzed. Figure 4 displays the statistical differences in the connectivity patterns of stages N2, N3, and REM between the first and the last sleep cycles. Only first and last sleep cycles located in the first and last thirds of the night were considered for the analysis. Connectivity measures indicated a reduction of functional connectivity in stage N2 as sleep progresses. There were almost no statistical differences for stage N3 after applying multiple comparison correction by FDR. Coherence in high beta band increased in REM in the last sleep cycle compared with the first one.
Figure 4.
SPM showing statistical connectivity alterations after FDR correction in sleep stages N2, N3, and REM between the first and the last NREM-REM cycles. Only first and last sleep cycles located at the first and last thirds of the night were considered (n = 27 for N2 and REM, and n = 17 for N3). For more details on line meanings, see the legend of Figure 3.
Discussion
In this study, we evaluated the behavior of four brain connectivity measures (MSC, SL, CMIF, and PLV) during different sleep stages, aiming to better characterize the relationships between brain areas during sleep and to assess how well they correlated with sleep staging, SWA, and entropy.
Functional connectivity measures the existence of linear or nonlinear covariances between two neurophysiological signals. The choice of these four connectivity measures allows the evaluation of functional connectivity using different approaches. Most commonly used connectivity methodologies in the neuroscientific literature are classical measures, such as MSC. These indexes are well-known and fast to compute but they only detect linear dependences between signals. Phase synchronization measures, such as PLV, estimate if signals are coupled even though their amplitudes remain uncorrelated [39]. PLV has shown more accurate estimation of oscillatory synchronization across a wide range of different synchronization regimes than spectral coherence, that can fail to robustly reflect changes in synchrony-mediated information [39]. The synchronization approach is only valid when the time series are approximately oscillatory. In the early 1990s, it was demonstrated that two interacting chaotic systems can display synchronization phenomena [33]. This phenomenon was called generalized synchronization and refers to a situation where the response of a system Y appears as a function of another system X. The most important feature of these measures is that time series do not need to resemble each other, neither in amplitude nor in phase. SL is arguably the most popular index to estimate generalized synchronization between two time-series [40]. Finally, the fourth popular functional connectivity approach, CMIF, is based in the information theory. These measures use the Shannon entropy to quantify the amount of information of a discrete random variable. It is noteworthy that CMIF is based on probability distributions and does not rely on any specific model of the data, allowing the detection of high-order correlations that cannot be measured with the aforementioned techniques. Each of these measures evaluate the connectivity between time-series considering different mathematical approaches. Therefore, we expected to find different functional changes between the different sleep stages and sleep intensity. All connectivity measures, especially CMIF and SL (that assume nonlinear interactions between sensors), revealed to have functional changes between the different sleep stages, raising the possibility for their development as alternative markers of sleep depth.
Sleep and wake states emerge from dynamic interactions throughout the sleep/wake circuitry pathways: bottom-up and top-down mechanisms [41]. The bottom-up pathway originates in the brainstem and relays to cortical areas by thalamo-cortical transfer. This pathway is integrated in the ascending reticular activating system (ARAS) playing a role of an on-off switch related to the two-process model of sleep regulation: ARAS is highly activated during wakefulness and decreased during NREM [42]. Reduced activation of anterior cortical areas by ARAS during NREM sleep facilitates an oscillatory interaction between cortex and thalamus, revealing the slow oscillation patterns typical of NREM sleep. Recent studies have shown that this loop would be orchestrated by cortical sleep-active neurons to thalamus to entrain a synchronized oscillatory circuit [41, 43]. Thus, slow oscillations have an important weight in EEG connectivity measures that could model this top-down pathway, indicating the dependences and couplings by cortical areas observed at the scalp. In this way, some studies have explained slow oscillations as traveling waves, originating from the frontal cortex and propagating along the anterior-posterior axis [44]. A local feedback network composed by thalamo-cortical neurons and reticular thalamic neurons serving as time arbitrators/moderators during NREM sleep can explain other oscillation transients in the cortico-thalamic-cortical loop, as sleep spindles and K-complexes [45].
Mutual information analysis allowed us to detect changes in the dependence between cortical regions associated with different sleep stages. CMIF was significantly increased in deeper sleep, translating greater linear and nonlinear interdependence between cerebral regions in SWS, showing differences scattered among the scalp. This global effect indicates a reduction of the complexity of cerebral activity and an increase of shared information in all areas occurs during SWS.
On the other hand, SL was lower in deeper sleep stages. This reduction of synchronization might seem counterintuitive, but a similar result was obtained by Ferri et al. [20]. As explained clearly by Montez et al. [46], SL is a measure of generalized synchronization that can be extremely sensitive in tracing the emergence of complex patterns of synchronized activity. Nevertheless, it is a statistical estimate of coupling only applicable under the assumption that certain patterns are detected repeatedly in different sensors, and a lack of such consistency will result in lower SL values. It is important to note that, by using the frequency priors for the calculation of SL, which avoids bias toward patterns in certain bands; only patterns of at most one cycle of the lowest frequency are sought. So, considering the whole band of EEG signals, the increase of CMIF indicated a longer dependence among signals, possibly because of autocorrelation effects (patterns varying slowly with respect to sampling frequency), but the lower SL showed a clear reduction of the recurrence of temporal signal patterns.
Conversely, when comparing light sleep with REM stages, CMIF increased, suggesting that during REM the interdependence is lower (more independent signals), but SL did not show significant differences (no temporal recurrence). During REM sleep, fast spiking neurons are even more active than during waking, with additional activation of limbic and occipital structures. Therefore, it can be assumed that during REM the brain presents more complex iterations that reduce the temporal synchronization of the electrophysiological activity [47]. This, in turn, may lead to understanding that the increase of interdependence of EEG signals during light sleep can be related to a higher temporal synchronization (more recurrence of signal patterns).
In light of the obtained results, the averaged CMIF seems to be the more robust measure to observe connectivity differences among sleep stages. SL and PLV in the beta band show a similar behavior that allows to distinguish among stages but not between light sleep and REM. The CMIF increase does not seem to be related to higher temporal recurrence, therefore reinforcing the idea that autocorrelation effects are key in the changes evidenced by CMIF, because they are not affecting SL estimation.
MSC measures showed the least significant differences among sleep stages. Although the course of average coherence somewhat followed the different sleep stages, MSC did not show a clear pattern, with mixed increases and decreases found even in the best bands (Figure 3).
Regarding the changes associated to the sleep cycles, there was an overall decrease of cortical connectivity in stage N2. During REM sleep, high beta coherence showed a greater amount of common information transmitted between channels in more advanced cycles.
Our analysis was performed using 19 EEG channels, and further analysis of average CMIF curves based on only the classic six channels showed similar results, proving that a reduced number of electrodes used in many standard PSG’s was enough to obtain a connectivity index as a reflection of sleep intensity.
Although small differences were detected in our analysis, they can be considered conclusive, as significant changes in the temporal course of the connectivity measures were clearly observed even in this small dataset.
In conclusion, all connectivity measures used in this study were effective representing the differences between sleep stages and cycles. Particularly, averaged CMIF could constitute a potential indicator of sleep depth, as it exhibited a robust and consistent behavior similar to SWA and entropy measures.
Increasing evidence is implicating deep sleep in processes of brain and systemic homeostasis. SWS is involved in cognitive processes of attention and memory consolidation. Important physiological functions such as brain energy preservation, hormonal regulation, immune system homeostasis, and cardiovascular regulation, have consistently been linked to SWS. Additionally, several studies are showing that the clearance of toxic brain metabolites produced during the day (e.g. Aβ42 involved in Alzheimer’s disease), is influenced by the amount of SWS during the night. So far, the currently prevalent EEG sleep stages analysis, has allowed the description of sleep depth based on simple linear measures of brain activity. The validation of new methods that translate patterns of connectivity between different brain areas is necessary both to support these commonly used methods as well as to expand the current knowledge about complex system interactions and their role on the different functions of SWS in humans.
Funding
This study has been partially supported by the Ministry of Economy and Competitiveness (MINECO), Spain, under contract DPI2017-83989-R and Fundación BBVA grants for researchers and cultural creators 2016. CIBER-BBN is an initiative of the Instituto de Salud Carlos III, Spain. Joan F. Alonso is a Serra Húnter Fellow. Salaries for Drs Andrade, Osorio, and Varga were supported by grants from the National Institute of Health (R21AG055002, R01AG056031, R01AG056531, R01AG056682, and R21AG059179).
Conflict of interest statement. None declared.
References
- 1. Walsh JK, et al. Tiagabine is associated with sustained attention during sleep restriction: evidence for the value of slow-wave sleep enhancement? Sleep. 2006;29(4):433–443. [PubMed] [Google Scholar]
- 2. Marshall L, et al. Boosting slow oscillations during sleep potentiates memory. Nature. 2006;444(7119):610–613. [DOI] [PubMed] [Google Scholar]
- 3. Tononi G, et al. Sleep function and synaptic homeostasis. Sleep Med Rev. 2006;10(1):49–62. [DOI] [PubMed] [Google Scholar]
- 4. Benington JH, et al. Restoration of brain energy metabolism as the function of sleep. Prog Neurobiol. 1995;45(4):347–360. [DOI] [PubMed] [Google Scholar]
- 5. Born J, et al. Hypothalamus-pituitary-adrenal activity during human sleep: a coordinating role for the limbic hippocampal system. Exp Clin Endocrinol Diabetes. 1998;106(3):153–163. [DOI] [PubMed] [Google Scholar]
- 6. Lange T, et al. Effects of sleep and circadian rhythm on the human immune system. Ann N Y Acad Sci. 2010;1193:48–59. [DOI] [PubMed] [Google Scholar]
- 7. Cabiddu R, et al. Modulation of the Sympatho-Vagal balance during sleep: frequency domain study of heart rate variability and respiration. Front Physiol. 2012;3:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Terzano MG, et al. Atlas, rules, and recording techniques for the scoring of cyclic alternating pattern (CAP) in human sleep. Sleep Med. 2001;2(6):537–553. [DOI] [PubMed] [Google Scholar]
- 9. Mariani S, et al. Analysis of the sleep EEG in the complexity domain. Conf Proc IEEE Eng Med Biol Soc. 2016;2016:6429–6432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Burioka N, et al. Approximate entropy in the electroencephalogram during wake and sleep. Clin EEG Neurosci. 2005;36(1):21–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Miskovic V, et al. Changes in EEG multiscale entropy and power-law frequency scaling during the human sleep cycle. Hum Brain Mapp. 2019;40(2):538–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mateos DM, et al. Measures of entropy and complexity in altered states of consciousness. Cogn Neurodyn. 2018;12(1):73–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bruce EN, et al. Sample entropy tracks changes in electroencephalogram power spectrum with sleep state and aging. J Clin Neurophysiol. 2009;26(4):257–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee GM, et al. Electroencephalogram approximate entropy influenced by both age and sleep. Front Neuroinform. 2013;7:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee L, et al. A report of the functional connectivity workshop, Dusseldorf 2002. NeuroImage. 2003;19(2 Pt 1):457–465. [DOI] [PubMed] [Google Scholar]
- 16. Achermann P, et al. Coherence analysis of the human sleep electroencephalogram. Neuroscience. 1998;85(4):1195–1208. [DOI] [PubMed] [Google Scholar]
- 17. Corsi-Cabrera M, et al. Rapid eye movement sleep dreaming is characterized by uncoupled EEG activity between frontal and perceptual cortical regions. Brain Cogn. 2003;51(3):337–345. [DOI] [PubMed] [Google Scholar]
- 18. Duckrow RB, et al. Coherence of the electroencephalogram during the first sleep cycle. Clin Neurophysiol. 2005;116(5):1088–1095. [DOI] [PubMed] [Google Scholar]
- 19. Massimini M, et al. Breakdown of cortical effective connectivity during sleep. Science. 2005;309(5744):2228–2232. [DOI] [PubMed] [Google Scholar]
- 20. Ferri R, et al. Dynamics of the EEG slow-wave synchronization during sleep. Clin Neurophysiol. 2005;116(12):2783–2795. [DOI] [PubMed] [Google Scholar]
- 21. Ferri R, et al. Small-world network organization of functional connectivity of EEG slow-wave activity during sleep. Clin Neurophysiol. 2007;118(2):449–456. [DOI] [PubMed] [Google Scholar]
- 22. Buysse DJ, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989. doi: 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- 23. Iber C, et al. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. In: AASM Manual for Scoring Sleep. Darien, Illinois: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 24. Feinberg I, et al. Systematic trends across the night in human sleep cycles. Psychophysiology. 1979;16(3):283–291. [DOI] [PubMed] [Google Scholar]
- 25. Alonso JF, et al. Cross-conditional entropy and coherence analysis of pharmaco-EEG changes induced by alprazolam. Psychopharmacology (Berl). 2012;221(3):397–406. [DOI] [PubMed] [Google Scholar]
- 26. Horschig JM, et al. Directed communication between nucleus accumbens and neocortex in humans is differentially supported by synchronization in the Theta and Alpha band. PLoS One. 2015;10(9):e0138685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Benesty J, et al. Estimation of the coherence function with the MVDR approach. In: 2006 IEEE International Conference on Acoustics Speed and Signal Processing Proceedings Vol. 3 Touluse, France: IEEE; 2006:III-500–III-503. [Google Scholar]
- 28. Schreiber T, et al. Surrogate time series. Phys D Nonlinear Phenom. 2000;142(3–4):346–382. doi: 10.1016/S0167-2789(00)00043-9 [DOI] [Google Scholar]
- 29. Ramanand P, et al. Mutual information analysis of EEG signals indicates age-related changes in cortical interdependence during sleep in middle-aged versus elderly women. J Clin Neurophysiol. 2010;27(4):274–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Alonso JF, et al. Stress assessment based on EEG univariate features and functional connectivity measures. Physiol Meas. 2015;36(7):1351–1365. [DOI] [PubMed] [Google Scholar]
- 31. Na SH, et al. The effects of total sleep deprivation on brain functional organization: mutual information analysis of waking human EEG. Int J Psychophysiol. 2006;62(2):238–242. [DOI] [PubMed] [Google Scholar]
- 32. Alonso JF, et al. Drug effect on EEG connectivity assessed by linear and nonlinear couplings. Hum Brain Mapp. 2010;31(3):487–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stam CJ, et al. Synchronization likelihood: an unbiased measure of generalized synchronization in multivariate data sets. Phys D Nonlinear Phenom. 2002;163(3–4):236–251. doi: 10.1016/S0167-2789(01)00386-4 [DOI] [Google Scholar]
- 34. Takens F. Detecting strange attractors in turbulence. In: Dynamical Systems and Turbulence, Lecture Notes in Mathematics. Vol. 898. Springer, Berlin: Springer-Verlag; 1981:366–381. ISBN 978-3-540-11171-9. [Google Scholar]
- 35. Lachaux JP, et al. Measuring phase synchrony in brain signals. Hum Brain Mapp. 1999;8(4):194–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Canolty RT, et al. Multivariate phase-amplitude cross-frequency coupling in neurophysiological signals. IEEE Trans Biomed Eng. 2012;59(1):8–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Spencer KM, et al. Abnormal neural synchrony in schizophrenia. J Neurosci. 2003;23(19):7407–7411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Benjamini Y, et al. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B. 1995:289–300. [Google Scholar]
- 39. Lowet E, et al. Quantifying neural oscillatory synchronization: a comparison between spectral coherence and phase-locking value approaches. PLoS One. 2016;11(1):e0146443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Niso G, et al. HERMES: towards an integrated toolbox to characterize functional and effective brain connectivity. Neuroinformatics. 2013;11(4):405–434. [DOI] [PubMed] [Google Scholar]
- 41. Krone L, et al. Top-down control of arousal and sleep: fundamentals and clinical implications. Sleep Med Rev. 2017;31:17–24. [DOI] [PubMed] [Google Scholar]
- 42. Jones BE. Arousal systems. Front Biosci. 2003;8:s438–s451. [DOI] [PubMed] [Google Scholar]
- 43. Morairty SR, et al. A role for cortical nNOS/NK1 neurons in coupling homeostatic sleep drive to EEG slow wave activity. Proc Natl Acad Sci U S A. 2013;110(50):20272–20277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Massimini M, et al. The sleep slow oscillation as a traveling wave. J Neurosci. 2004;24(31):6862–6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Steriade M. The corticothalamic system in sleep. Front Biosci. 2003;8:d878–d899. [DOI] [PubMed] [Google Scholar]
- 46. Montez T, et al. Synchronization likelihood with explicit time-frequency priors. Neuroimage. 2006;33(4):1117–1125. [DOI] [PubMed] [Google Scholar]
- 47. Steriade M, et al. Natural waking and sleep states: a view from inside neocortical neurons. J Neurophysiol. 2001;85(5):1969–1985. [DOI] [PubMed] [Google Scholar]