Abstract
Study Objectives
To determine whether there is a consistent epiglottic pressure value that predicts respiratory arousal from sleep.
Methods
Thirty-one patients with obstructive sleep apnea underwent overnight polysomnography while instrumented with an epiglottic catheter to measure airway pressures. Nadir epiglottic pressures during respiration events (obstructive apneas/hypopneas) terminated with or without arousals were compared. The events were selected by two methods, (1) 20 events with/without arousals were randomly selected, and (2) Events were sampled in pairs (one terminated with arousal and one without arousal) to minimize the effect of sleep duration/stage on the measurement.
Results
A total of 1,317 respiratory events were analyzed. There was substantial variability in nadir epiglottic pressure within an individual and among different individuals. The average pressure of 20 randomly selected events with arousals was (−21.2 ± 11.2, ranged −6.68 to −63.34 cm H2O). The nadir epiglottic pressure during respiratory events in NREM stage 2 sleep terminated with arousals was more negative compared with those terminated without arousals using both sampling methods (−23.5 vs. −18.5 cm H2O, p = 0.007 and −20.3 vs. −16.3 cm H2O, p < 0.001).
Conclusions
There were very different levels of epiglottic pressures that preceded arousals within and among individuals. However, cortical arousals are associated with a level of more negative epiglottic pressure compared to events terminated without arousal, findings which support the concept of a respiratory arousal threshold.
Clinical Trial Registration
The study used existing data to study methodology (from clinical trial “The Impact of Arousal Threshold in Obstructive Sleep Apnea” https://clinicaltrials.gov/show/NCT02264353) and it is not a clinical trial.
Keywords: obstructive sleep apnea, arousal, respiratory arousal threshold, epiglottic pressure
Statement of Significance.
The arousal threshold level is thought to be important for obstructive sleep apnea pathogenesis. The concept of respiratory arousal threshold was challenged since no single “threshold” that predicted arousal was found in some previous studies. The study found very different levels of epiglottic pressures that preceded arousals within and among individuals. Although the values were overlapping between epiglottic pressures at the end of events that terminate with and without arousal, the respiratory events terminated with cortical arousals are found to be associated with a level of more negative epiglottic pressure compared with respiratory events terminated without arousals, findings which support the concept of a respiratory arousal threshold.
Introduction
The arousal threshold is an important concept defining the propensity of an individual to wake up from sleep [1, 2]. The respiratory arousal threshold is used in applied physiology to define the propensity of a specific breathing stimulus to wake an individual from sleep. Although multiple possible stimuli such as hypoxemia and hypercapnia might contribute to an arousal, classic studies including by Gleeson et al. suggest that the final common pathway is negative intrathoracic pressure [3]. In this prior study, sleeping subjects instrumented with esophageal catheters were stimulated by added inspiratory resistive load, hypoxemia, or hypercapnia. Arousals from nonrapid eye movement sleep (NREM) were found to be associated with a particular level of negative intrathoracic pressure in a given individual regardless of the initial stimulus [3].
The respiratory arousal threshold level is thought to be one of the physiological traits important for obstructive sleep apnea (OSA) pathogenesis [4, 5]. A low arousal threshold (i.e. easy to wake up) might predispose to repetitive apnea/hypopneas and sleep fragmentation, rather than stable sleep with flow limitation [2]. For example, increasing respiratory stimuli such as carbon dioxide or increasing negative pressure can recruit pharyngeal dilator muscles like the genioglossus, the major protruder of the tongue. Genioglossus activity is generally high during spontaneous periods of stable breathing in OSA. Thus, an individual with a tendency toward sleep apnea based on vulnerable upper airway mechanics might be protected by a high arousal threshold that allows for the accumulation of adequate respiratory stimuli to promote stable breathing. On the other hand, a patient with similar anatomy and muscles but a low arousal threshold would not have adequate activation of pharyngeal dilator muscle activity before arousing from sleep—leading to repetitive apnea. The respiratory arousal threshold has thus been considered a therapeutic target whereby a nonmyorelaxant hypnotic agent could raise the arousal threshold (harder to wake up) and allow stabilization of breathing pattern [6]. For example, we previously demonstrated that the sedative-hypnotic eszopiclone could raise the arousal threshold and reduce the AHI in those with a low arousal threshold [7]. Efforts to estimate the arousal threshold using clinically available data have also been reported [8] such that clinicians could potentially estimate what might be predisposing an individual to apnea and perhaps guide therapy for appropriately selected patients in an individualized manner. Conversely, subjects with a high arousal threshold who might be theoretically harmed by administration of a sedative with increased hypoxemia/hypercapnia might be identified.
However, more recent work has emphasized that many events are terminated without arousal [9, 10]. Also the concept of the respiratory arousal threshold was questioned by Xiao et al. who studied 17 subjects with esophageal pressure monitoring and found similar esophageal pressure/diaphragm electromyography during respiratory events terminated by cortical arousal and those terminated without a respiratory arousal [11]. Moreover, there was wide variability in the neural respiratory drive at arousal within subjects. Overall, this study challenged the concept of respiratory arousal threshold as potentially problematic in that (1) there was no single “threshold” that predicted arousal and (2) that this threshold might not be constant during the night.
Based on the work by Xiao et al., we analyzed and synthesized our own data to determine whether we could identify a consistent epiglottic pressure value that could reliably predict respiratory arousal from sleep. We further sought to test various definitions of a respiratory arousal threshold using survival analysis, time to awakening, and other exploratory analyses to determine whether this value was constant through the night. Ultimately, these analyses would help to guide further research by defining mechanisms underlying apnea in a reproducible manner which could be a therapeutic target in appropriately selected patients.
Methods
Study population
The study population consisted of 31 patients who participated in overnight polysomnography (PSG) at the University of California San Diego. Data from this study have previously been presented (e.g. demographic data); however, the current aims, analysis, and results are novel and have never previously been reported.
Patients were eligible for inclusion in the study if they were 18–70 years of age. All subjects had a history of OSA with an AHI greater than 5 events/h according to American Academy of Sleep Medicine (AASM) Chicago criteria. Exclusion criteria were the presence of pulmonary, cardiac, neurological, and other current severe medical or psychiatric diseases; currently using continuous positive airway pressure therapy; current smoking or consuming alcohol >3 oz per day.
Written informed consent was given before participation in the study, and the study protocol was approved by the Human Research Committee, University of California San Diego.
Study design
This was a retrospective analysis of patients who underwent single night PSGs while instrumented with an epiglottic catheter to measure airway pressures during upper airway narrowing/occlusion.
Polysomnography
Electroencephalograms, electrooculograms, and surface electromyograms were applied to score arousals, leg movements, and sleep stage. Abdominal and chest movements, pulse oximeter, oral and nasal flow were recorded to detect respiratory events. Participants were instructed to sleep supine as much as possible throughout the duration of the night. PSGs were analyzed by experienced technicians who were blinded to the ArTh measurement according to the scoring guidelines of AASM Chicago criteria. Arousal was defined as an abrupt shift in the EEG that lasted longer than 3 s.
Epiglottic pressure measurement
Epiglottic pressures were measured using previously reported methods [7–9, 12]. Briefly, subjects were anesthetized with topical lidocaine nasal spray and instrumented overnight with an epiglottic pressure catheter (model MCP-500; Millar, Houston, TX) 2–3 h before sleep. The catheter passed through the nose and was advanced 1–2 cm below the base of the tongue under direct visualization. The sampling frequency was 125 Hz for epiglottic pressure.
Epiglottic pressure during obstructive apneas and hypopneas in the supine position was further analyzed. The following parameters of the respiratory events were recorded:
1) For events terminated with an arousal, nadir epiglottic pressure immediately prior to cortical arousal was measured [8, 9] (Figure 1). If the nadir epiglottic pressure occurred in the middle part of the events/distant from the arousal (further than one breath circle), the nadir epiglottic pressure was not considered to be associated with the cortical arousal (and the event would not be used for sampling). To minimize the artifact from baseline drifting, the baseline (pre-event) epiglottic pressure was defined as an average of the beginning of inspiration and the beginning of expiration (usually close to atmosphere pressure). For the events terminated without an arousal, nadir epiglottic pressure during the event was measured. The flow-limited breaths or respiratory effort related arousals were not included in the measurement.
2) The interval from the first pressure swing to the breath with nadir pressure in the epiglotic pressure trace during the event, which we term event duration.
3) Change in epiglottic pressure during the event: The difference of epiglottic pressure between the first pressure swing and the breath with nadir epiglottic pressure during the event.
4) The rate of change in epiglotic pressure: The slope of epiglottic pressure change divided by the interval from the first pressure swing to the breath with nadir pressure.
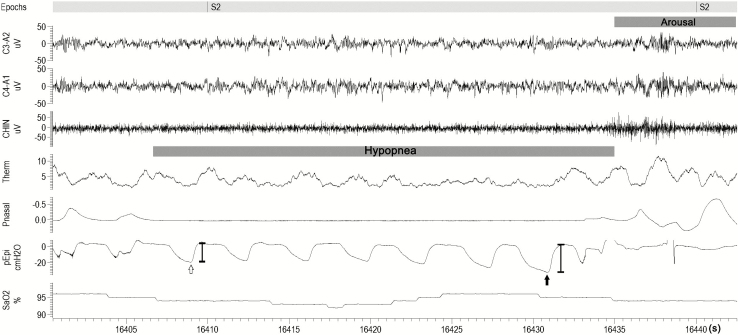
Figure 1.
An example of determining the arousal threshold during polysomnogram (PSG). C3–A2, C4–A1: electroencephalograms; CHIN, chin electromyogram; Pnasal, nasal pressure; SaO2, arterial blood oxygen saturation; Therm, thermistor; Pepi, epiglottic pressure trace. The respiratory arousal threshold was taken as the delta pressure swing immediately prior to arousal (PA, the solid arrow); the magnitude of the first pressure swing during the respiratory events in the epiglottic pressure trace was also taken (PT, the hollow arrow); The interval from the first pressure swing to the breath with nadir epiglottic pressure (the interval between the hollow arrow and the solid arrow).
Moreover, other parameters associated with respiratory events, including sleep stage, maximum relative and absolute nadir desaturation associated with the events were recorded as well.
Data analysis
Arousal threshold measurements (traditional method)
ArTh was defined as the average nadir epiglottic pressure immediately before cortical arousal from 20 (or as many as possible if less than 20) randomly selected apneas or hypopneas, as previously described [7, 8].
Nadir epiglottic pressure during events terminated without an arousal
Nadir epiglottic pressure was measured during 20 (or as many as possible) randomly selected apneas /hypopneas terminated without arousals. Since arousal thresholds have been reported to be affected by sleep stage, analyses were performed using samples from (1) N2 sleep only; (2) NREM sleep (combined N1, N2, and N3); and (3) all sleep stage when differences between events terminated with/without arousals were compared.
Nadir epiglottic pressure during respiratory events terminated with and without arousal using a paired sample method
The nadir negative pressures were reported to be higher (hard to wake up) in N3 sleep compared with N2; and higher in N2 compared with N1. The values were similar in N1 and REM sleep [2, 7, 13]. To minimize the effect of sleep times (sleep stage) on the measurement, the nadir epiglottic pressures during respiratory events were only sampled in pairs. Events were considered paired (one terminated with arousal and one without arousal) if they occurred in the same sleep stage, of the same type (hypopneas or apneas), within 3 min of one another, and with no scored wakefulness in between the two events. Twenty (or as many as available) paired events were measured in each overnight study.
Arousal threshold described using survival analysis
In lieu of a single threshold value, we used a Kaplan–Meier survival analysis to calculate the epiglottic pressure associated with a 25%, 50%, and 75% chance that cortical arousals would be triggered, as performed in prior animal studies [14]. The two reasons for using survival analysis to describe the individual respiratory arousal threshold. (1) The epiglottic pressures that preceded arousals within an individual may be variable according to some previous studies [11]. (2) To integrate the level of negative epiglottic pressure during respiratory events terminated without arousal. For each subject, nadir epiglottic pressures present during obstructive apneas/hypopneas with cortical arousals and without cortical arousals (20 randomly selected events or as many as possible) were used for the survival analysis.
Statistical analysis
Statistical analyses were performed using SPSS 22.0 (SPSS, Inc., Chicago, IL). Sleep parameters were compared using the Wilcoxon signed ranks test. The epiglottic pressure changes during respiratory events with or without arousals were compared using Mann–Whitney U test. Spearman’s correlation coefficient was used to identify significant associations. Statistical significance was set at P < 0.05.
Results
Characteristics of the subjects are listed in Table 1. Three of the subjects had mild sleep apnea (9.7%); three of them had moderate sleep apnea (9.7%) and 25 subjects had severe sleep apnea (80.6%).
Table 1.
Characteristics of the subjects (mean ± SD)
| Characteristics | All subjects (n = 31) |
|---|---|
| Age (years) | 51 ± 12 |
| Gender (male/female) | 20/11 |
| Race | 26 Caucasians, 2 African Americans, 3 Asians |
| Body mass index (kg/m2) | 30.7 ± 5.5 |
| Sleep efficiency (%) | 77.9 ± 14.0 |
| Apnea hypopnea index (events/h) | 49.7 ± 25.3 |
| NREM AHI in supine position (events/h) | 50.7 ± 26.7 |
| Fraction of events that were hypopnea (%) | 64.2±25.3 |
| Fraction of events that were obstructive apnea (%) | 31.1 ± 25.5 |
| Nadir oxygen saturation during sleep (%) | 79.5 ± 7.9 |
| Arousal index (events/h) | 46.9 ± 20.4 |
NREM = nonrapid eye movement sleep.
Variability of epiglottic pressure before event termination during sleep
A total of 1,317 respiratory events (411 obstructive apneas, 906 hypopneas) were analyzed (42.3 events per patient on average). Among those events, there were 443 events terminated without arousals (131 obstructive apneas and 312 hypopneas, 14.3 events per patient on average).
The average pressure of 20 randomly selected events with arousals (traditional method) was (−21.2 ± 11.2, ranged −6.68 to −63.34 cm H2O). As shown in Figure 2, there was substantial variability in nadir epiglottic pressure that precedes arousal from sleep within an individual and among different individuals. (The mean and coefficient of variation of epiglottic pressure that precedes arousal from sleep in each subject were listed in the Supplementary Material).
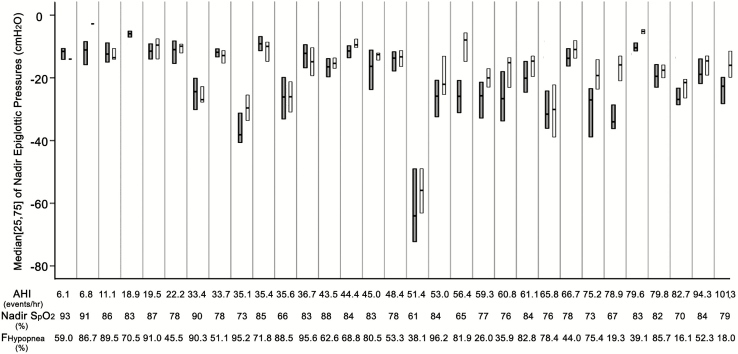
Figure 2.
Medians and quartiles of individual nadir epiglottic pressures that (1) precede arousals from sleep during respiratory events (dark gray bars) and (2) were reached during events terminated without arousals (white bars). The three reported surrogates of arousal threshold (AHI, nadir SpO2 and Fraction of events that were hypopneas) of each subject are listed below. In three subjects, there were few respiratory events terminated without cortical arousals. Thus the figure fails to show their medians and quartiles. The comparison of nadir epiglottic pressures during events terminated with/without arousals are demonstrated in the Results section and in the Supplementary Figure S1. Note the substantial overlap in the ranges of the epiglottic pressures for events that terminate with and without arousal (more individual data are listed in Supplementary Table S1). Note also the differences in pressures between individuals.
Nadir epiglottic pressure during respiratory events terminated with and without arousal
The nadir epiglottic pressure during apneas and hypopneas terminated with arousals was more negative compared with respiratory events terminated without arousals, (−18.5 [−26.2, −13.3] vs. −16.7 [−22.2, −13.5] cm H2O, Z = −4.076, p < 0.001, combined NREM and REM stages). Since arousal thresholds were reported to be affected by sleep stage, analysis was performed using samples from (1) N2 sleep only and (2) NREM sleep (combined N1, N2, and N3). For the events occurring during NREM sleep, the nadir epiglottic pressure during events with arousals was also more negative (−17 [−22.3, −13.18] vs. −19.95 [−27.175, −13.5], Z = −3.258, p = 0.001) and was also seen in N2 sleep (−18.5[−26.8, −15.4] vs. −23.5[−27.9, −15.1], Z = −2.701, p = 0.007). Subjects were excluded if they had insufficient respiratory events without arousals (≤5) sampled.
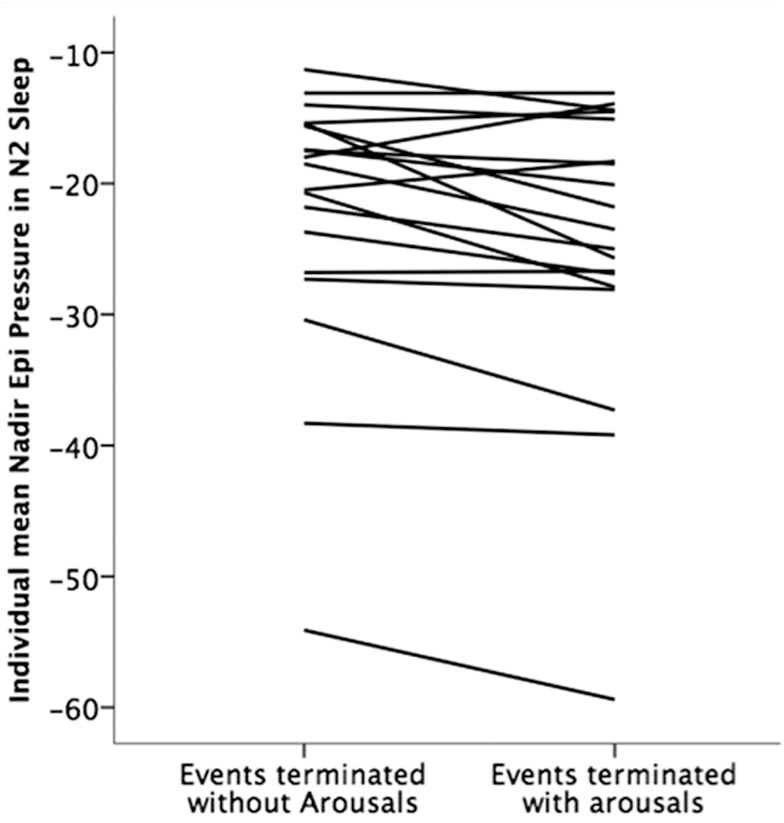
However, four patients had more negative nadir epiglottic pressures during apneas and hypopneas terminated without arousals than events with arousals (Figure 3). The value of nadir epiglottic pressure in respiratory events terminated with arousals had considerable overlap with those without arousal in all individuals, regardless of the sleep stage.
Figure 3.
Mean nadir epiglottic pressures during events terminated with and without arousals in each individual in NREM stage 2 sleep (n = 19, note that four patients had more negative mean nadir epiglottic pressures during apneas and hypopneas terminated without arousals than events with arousals).
Nadir epiglottic pressure during respiratory events terminated with and without arousal using a paired sample method
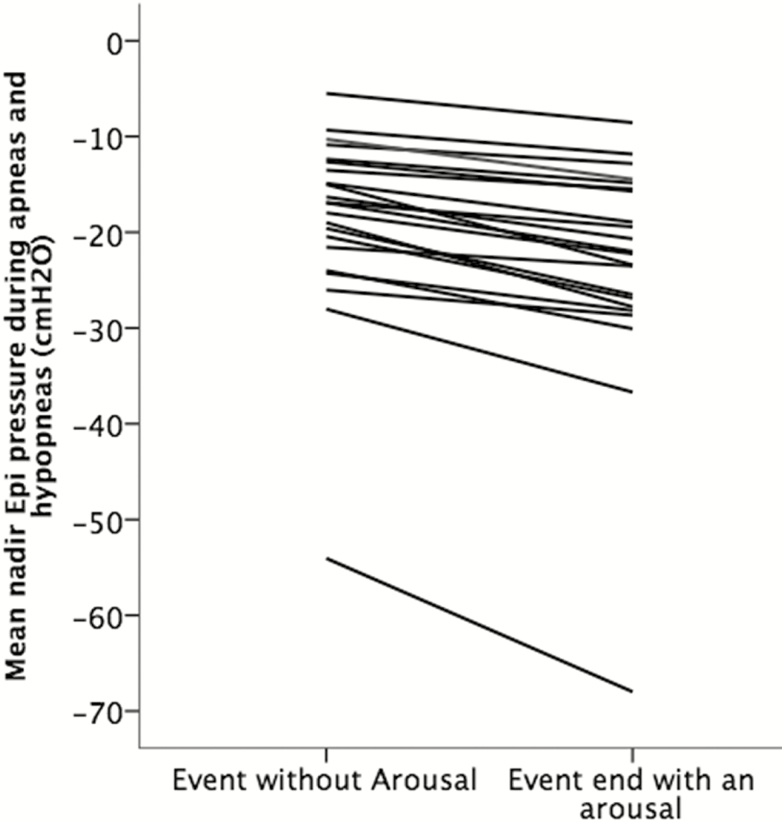
To minimize the effect of sleep times (or sleep stage) on the measurement, the nadir epiglottic pressure during respiratory events with and without cortical arousal was resampled in pairs (see Methods section). Figure 4 shows the mean nadir epiglottic pressures from paired events terminated with and without arousals in each individual. Note that in this analysis all subjects had more negative nadir epiglottic pressure during events that terminated with arousals compared with those events terminated without an arousal.
Figure 4.
Mean nadir epiglottic pressures during events terminated with and without arousals in each individuals (n = 22, subjects had less than five available pairs of events were excluded); Note that all subjects had more negative nadir epiglottic pressure during events that terminated with arousals.
Other event features that might contribute to arousal
Two hundred fifty-eight pairs of events from 27 subjects were compared using Wilcoxon signed-rank test. Results are shown in Table 2. Events with arousal had more negative epiglottic pressure compared with events without arousal. They were also greater in duration, had a more rapid increase of negative epiglottic pressure, and with more severe desaturation related to the events terminated without an arousal.
Table 2.
Comparison of the characteristics of epiglottis pressure during respiratory events terminated with and without cortical arousal
| Variable of interest | Events with arousal | Events without arousal | Z | P value |
|---|---|---|---|---|
| Interval from first breath to the last breath(s) | 17.51 [13.44, 24.20] | 16.15 [11.75,21.26] | −4.132 | 0.0000 |
| Change in Pepi during the event (cm H2O) | −9.76 [−15.16, −5.83] | −6.92 [−10.52, −3.85] | −7.381 | 0.0000 |
| The rate of change in epiglottic pressure (cm H2O/s) | 0.511 [0.312, 0.815] | 0.422 [0.245, 0.656] | −4.596 | 0.0000 |
| Negative epiglottic pressure in the first pressure swing in the event (cm H2O) | −10.84 [−15.31, −6.24] | −8.74 [−13.21, −5.14] | −5.203 | 0.0000 |
| Negative epiglottic pressure in the last breath (or the nadir negative pressure) in the event (cm H2O) | −20.3 [−29.17, −15.58] | −16.34 [−22.18, −12.23] | −11.884 | 0.0000 |
| Desaturation related to the event (percentage) | 4 [3, 6] | 4 [3, 5] | −2.794 | 0.0052 |
How the traditional arousal threshold corresponds to the negative epiglottic pressure at which 50% of arousals would occur using survival analysis
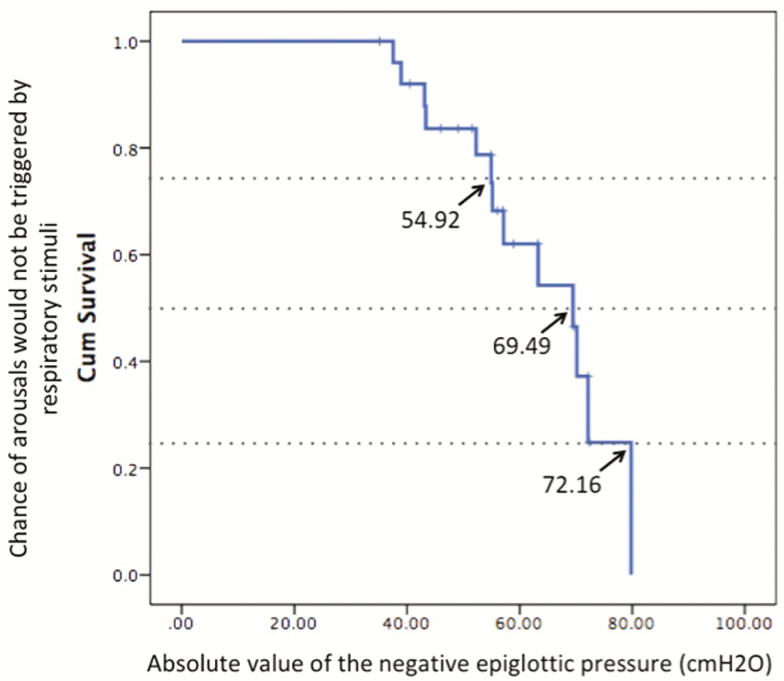
Figure 5 shows an example of defining the negative epiglottic pressure at which arousals would be triggered by respiratory stimuli using a Kaplan–Meier survival analysis in a subject.
Figure 5.
An example of defining the negative epiglottic pressure at which arousals would be triggered by respiratory stimuli using a Kaplan–Meier survival analysis in a subject with arousal threshold of −63.3 cm H2O (traditional way of measurement). At the negative epiglottic pressure of −54.9, −69.5, and −72.2 cm H2O, 25%, 50%, and 75% of the events would be terminated with arousals. (More data of individual traditional arousal threshold and the negative epiglottic pressure at which 25%/50%/75% of arousal would occur using survival analysis are listed in Supplementary Table S3)
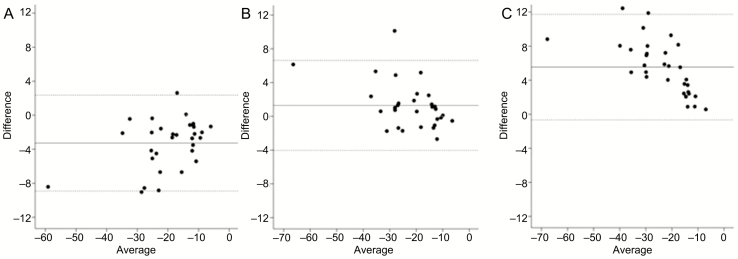
The mean negative epiglottic pressure at which arousal would occur (traditional description of arousal threshold) and the negative epiglottic pressure at which 25%/50%/75% of arousals would be triggered by respiratory stimuli in each individual were listed in Supplementary Material. Figure 6 shows the comparison of the individual traditional description of arousal threshold and the negative epiglottic pressure at which 25%/50%/75% of arousal would occur using survival analysis. The arousal threshold (as traditionally measured) has a significant correlation with the negative epiglottic pressure at which 50% of arousals would occur using survival analysis (r = 0.940, p = 0.000).
Figure 6.
The Bland–Altman difference plot for the individual traditional description of arousal threshold and using survival analysis. The Bland–Altman difference plot for the individual traditional description of arousal threshold and the negative epiglottic pressure at which 25% (A), 50% (B), 75% (C) of arousal would occur using survival analysis.
Discussion
Consistent with prior reports, we found that (1) a number of respiratory events can be terminated without cortical arousal, (2) that there were very different levels of epiglottic pressures that preceded arousals in different individuals, and (3) that even in a given individual there is overlap between epiglottic pressures at the end of events that terminate with and without arousal.
However, our new findings add to the existing literature in a number of important ways. First, we observed a level of more negative epiglottic pressure which triggered cortical arousal in the majority of our participants compared to events terminated without arousal, findings which support the validity of the concept of a respiratory arousal threshold. Second, in a careful paired analysis accounting for sleep stage, the epiglottic pressure was consistently lower (more negative) when an arousal occurred. Finally, our study supported that there is a “threshold range” rather than a single-value threshold that will always lead to arousal. Our study defined the negative epiglottic pressure at which arousals would be triggered by respiratory stimuli using a Kaplan–Meier survival analysis, which might be used as an alternative way to consider the arousal threshold, rather than as absolute limit beyond which no sleep is possible.
Role of arousals in the pathogenesis of obstructive sleep apnea
We have previously referred to arousal as a double edged sword. On one hand, arousal can be life saving by terminating respiratory events and avoiding severe hypoxemia and hypercapnia and their associated end-organ consequences. On the other hand, recurrent arousals lead to sleep fragmentation, daytime symptoms, autonomic activation, and impairment in memory consolidation [15]. Thus, agents to raise the arousal threshold could theoretically be beneficial for select patients by allowing the accumulation of respiratory stimuli to activate pharyngeal dilator muscles yielding stable breathing. By the same token, a hypnotic agent could in theory worsen hypoxemia and hypercapnia if arousal were delayed excessively. We have previously observed raising of the arousal threshold with hypnotic agents with associated improvement in the apnea hypopnea index [7]. These findings provide at least proof of concept regarding the notion of the physiology around the respiratory arousal threshold.
Is there a threshold for respiratory related cortical arousal?
In healthy young subjects, average arousal intensity was reported to be highly variable among healthy young adults and stable within individuals [16, 17]. As for respiratory-related cortical arousal, the mean of negative epiglottic or intrathoracic pressure (respiratory drive) has been used to describe individual arousal threshold by several authors; decisions regarding pharmacological interventions could then be individualized. The notion of the respiratory arousal threshold was challenged by Xiao et al. who observed similar esophageal pressure with respiratory events with or without cortical arousal in 17 subjects. The reasons for the discrepancy between studies are unclear, but may reflect variation in study population or perhaps subtle methodological differences, that is sampling methods.
Sampling is very important, since the possibility of arousal may vary with depth of sleep (even in the same sleep stage) and the event type. For example, respiratory events are more likely to resolve without arousals in deep sleep (i.e. N3) compared with a lighter stage of sleep (e.g. N1). Of note, the negative pressure swing at arousal was statistically greater during an apnea compared with a hypopnea [9]. The variability related to sampling may have been minimized by the paired sampling method in the study. When analyzed in this fashion, subjects had more negative nadir epiglottic pressure during events that terminated with arousals compared to those without arousal. Thus, our data provide some reassurance that the concept of the arousal threshold is sound although marked variability is notable as with most human physiology.
In our study, the difference between median epiglottic pressures at the end of events that terminate with/without arousal was significant, albeit small. Moreover, although the differences were small (0.9, 0.2, 2.2, and 4.4 cm H2O, respectively), more mean negative epiglottic pressure in events without arousals compared to events with arousals in four patients in N2 sleep. There may be two explanations for the lack of clear delineation between pressures that trigger versus do not trigger cortical arousal: (1) There is no “threshold” of respiratory drive which is responsible for triggering cortical arousals or (2) Considering the highly variable nature of the negative airway pressure that triggers arousal, we speculate that there is a “threshold range” rather than a single-level threshold. Moreover, it should be recognized that regardless of the presence or absence of cortical activity, all events were terminated by airway opening. Events without cortical activity upon termination have been conceptualized as terminating with a subcortical arousal, where pharyngeal dilators and autonomic activity alone are activated. Thus, the distinction between these event types may be a matter of degree, consistent with small differences in pressure stimuli that were observed to determine cortical arousal versus not within individuals.
To describe the “threshold range,” we have used a relatively novel technique of survival analyses which has been used in rodent studies [14] to assess arousal pathways but not previously in humans to our knowledge. Because arousals invariably occur from human sleep, the survival analyses allow quantification of probabilities rather than simply “yes” or “no.” Theoretically, if the respiratory drive is lower than the lower range there should not be arousal occurring. In contrast, within the “threshold range,” both events with/without arousals could be observed; if it exceeds the highest threshold, all the events will trigger arousals. Survival analyses may be a useful way to study various pharmacological agents in the future, and to compare their effects on important physiology. As seen in Supplementary Table S3, the traditionally measured and KM derived 50% survival values were generally close to one another, but the survival analysis may more usefully imply a range rather than a fixed value.
Limitations
We acknowledge a number of limitations. First, our sample size was modest. Due to the nature of our intensive human physiological studies, large sample sizes or Big Data approaches are not feasible. Moreover, although we wanted to analyze the characteristics of respiratory effort change in each sleep stage, only a limited number of patients had slow wave sleep with events with/without arousal sampled. Thus the analyses were not done due to limited samples. Second, we did not measure diaphragm EMG as was done in the Xiao study or the intensity of arousals was not scaled in this study. We deliberately tried to minimize instrumentation in the present study since, in theory, the equipment itself may influence arousal and sleep architecture. However, we acknowledge that the diaphragm EMG might provide additional valuable information toward such analyses. Third, we did not manipulate the arousal threshold pharmacologically as we have done in prior studies and examine the impact on these analyses.
Conclusions
In conclusion, there are very different levels of epiglottic pressures that preceded arousals within and among individuals. Although the values were overlapping between epiglottic pressures at the end of events that terminate with and without arousal, the respiratory events terminated with cortical arousals are found to be associated with a level of more negative epiglottic pressure compared with respiratory events terminated without arousals. These findings support the validity of the concept of a respiratory arousal threshold.
Funding
The study is supported by National Institutes of Health (R01 HL085188 and K24 HL132105).
Conflict of interest statement. Dr Yanru Li is co-investigator on the National Key R&D Program of China (no. 2017YFC0112500) and Priming Scientific Research Foundation for the senior researcher in Beijing Tongren Hospital, Capital Medical University (TRYY-KYJJ-2017-016). Dr. Orr was supported by NIH F32 HL131306. Dr Sands was supported by the American Heart Association (15SDG25890059), the National Health and Medical Research Council of Australia (NHMRC, Early Career Fellowship 1053201), the Menzies Foundation, the American Thoracic Society Foundation and was Co-Investigator on grants from the NHMRC (1064163) and NIH (R01 HL128658). Dr Malhotra is PI on NIH R01 HL085188, K24 HL132105, MPI on T32 HL134632 and co-investigator on R21 HL121794, R01 HL 119201, R01 HL081823. As an Officer of the American Thoracic Society, Dr Malhotra has relinquished all outside personal income since 2012. ResMed, Inc. provided a philanthropic donation to the UC San Diego in support of a sleep center. Yanru Li, Scott Sands, Jeremy Orr, Rachel Jen, and Atul Malhotra have no conflicts of interest to disclose. Robert L. Owens has received travel and honoraria from ResMed and Itamar Medical (both <$5,000). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Supplementary Material
Acknowledgment
Author Contributions: Yanru Li contributed to acquisition of data, analysis and interpretation of data, and drafting of the article; Jeremy Orr contributed to analysis and interpretation of the data; Pamela DeYoung and Erik Smales contributed to acquisition and scoring of the sleep studies; Scott Sands contributed to provide the code for arousal threshold analysis; Rachel Jen contributed to acquisition of data and revision of the article; Bradley Edwards and Robert L. Owens contributed to interpretation of the data, drafting of the article and revision of the article; Atul Malhotra contributed to study conception and design, data synthesis/interpretation, and revision of the article for important intellectual content.
Work Performed: Pulmonary, Critical Care and Sleep Division, University of California at San Diego, CA.
References
- 1. Berry RB, et al. Respiratory arousal from sleep: mechanisms and significance. Sleep. 1997;20(8):654–675. [DOI] [PubMed] [Google Scholar]
- 2. Eckert DJ, et al. Arousal from sleep: implications for obstructive sleep apnea pathogenesis and treatment. J Appl Physiol (1985). 2014;116(3):302–313. [DOI] [PubMed] [Google Scholar]
- 3. Gleeson K, et al. The influence of increasing ventilatory effort on arousal from sleep. Am Rev Respir Dis. 1990;142(2):295–300. [DOI] [PubMed] [Google Scholar]
- 4. Eckert DJ, et al. Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets. Am J Respir Crit Care Med. 2013;188(8):996–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sands SA, et al. Phenotyping pharyngeal pathophysiology using polysomnography in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2018;197(9):1187–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Eckert DJ, et al. Trazodone increases the respiratory arousal threshold in patients with obstructive sleep apnea and a low arousal threshold. Sleep. 2014;37(4):811–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Eckert DJ, et al. Eszopiclone increases the respiratory arousal threshold and lowers the apnoea/hypopnoea index in obstructive sleep apnoea patients with a low arousal threshold. Clin Sci (Lond). 2011;120(12):505–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Edwards BA, et al. Clinical predictors of the respiratory arousal threshold in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2014;190(11):1293–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jordan AS, et al. Termination of respiratory events with and without cortical arousal in obstructive sleep apnea. Am J Respir Crit Care Med. 2011;184(10):1183–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Younes M, et al. Genioglossus activity available via non-arousal mechanisms vs. that required for opening the airway in obstructive apnea patients. J Appl Physiol (1985). 2012;112(2):249–258. [DOI] [PubMed] [Google Scholar]
- 11. Xiao SC, et al. Neural respiratory drive and arousal in patients with obstructive sleep apnea hypopnea. Sleep. 2015;38(6):941–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Berry RB, et al. Effect of ethanol on the arousal response to airway occlusion during sleep in normal subjects. Am Rev Respir Dis. 1992;145(2 Pt 1):445–452. [DOI] [PubMed] [Google Scholar]
- 13. Ratnavadivel R, et al. Upper airway function and arousability to ventilatory challenge in slow wave versus stage 2 sleep in obstructive sleep apnoea. Thorax. 2010;65(2):107–112. [DOI] [PubMed] [Google Scholar]
- 14. Kaur S, et al. A genetically defined circuit for arousal from sleep during hypercapnia. Neuron. 2017;96(5):1153.e5–1167.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Younes M. Role of arousals in the pathogenesis of obstructive sleep apnea. Am J Respir Crit Care Med. 2004;169(5):623–633. [DOI] [PubMed] [Google Scholar]
- 16. Azarbarzin A, et al. Arousal responses during overnight polysomnography and their reproducibility in healthy young adults. Sleep. 2015;38(8):1313–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Amatoury J, et al. Arousal intensity is a distinct pathophysiological trait in obstructive sleep apnea. Sleep. 2016;39(12):2091–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.