Abstract
Study Objectives
To present results from in vivo studies underlying the preclinical development of lemborexant (E2006), a novel dual orexin (hypocretin) receptor antagonist for sleep/wake regulation.
Methods
Rodent (wild-type rats and wild-type and orexin neuron-deficient [orexin/ataxin-3 Tg/+] mice) studies were performed to evaluate the effects of single-dose oral lemborexant (1–300 mg/kg) on orexin-induced increases in plasma adrenocorticotropic hormone (ACTH), locomotor activity, vigilance state measures (wakefulness, nonrapid eye movement [non-REM] sleep, rapid eye movement [REM] sleep), ethanol-induced anesthesia, and motor coordination, and the effects of multiple-dose oral lemborexant (30 mg/kg) on vigilance state measures. Active comparators were almorexant and zolpidem. Pharmacokinetics were assessed after single-dose lemborexant in mice and rats.
Results
Lemborexant prevented the orexin-promoted increase in ACTH in rats, therefore demonstrating inhibition of the orexin signaling pathway. Furthermore, lemborexant promoted sleep in wild-type mice and rats. Lemborexant promoted REM and non-REM sleep at an equal rate (there was no change in the REM sleep ratio). In contrast, zolpidem reduced REM sleep. The sleep-promoting effect of lemborexant was mediated via the orexin-peptide signaling pathway as demonstrated by a lack of sleep promotion in orexin neuron-deficient mice. Chronic dosing was not associated with a change in effect size or sleep architecture immediately postdosing. Lemborexant did not increase the sedative effects of ethanol or impair motor coordination, showing good safety margin in animals. Pharmacokinetic/pharmacodynamic data for mice and rats were well aligned.
Conclusions
These findings supported further clinical evaluation (ongoing at this time) of lemborexant as a potential candidate for treating insomnia and other sleep disorders.
Keywords: antagonist, dual orexin receptor antagonist, E2006, in vivo, insomnia, lemborexant, mouse, orexin, rat, sleep
Statement of Significance.
Traditional pharmacologic treatments for insomnia, such as benzodiazepines, non-benzodiazepine hypnotics, and sedating antidepressants, have varying effectiveness across differing clinical insomnia phenotypes and various safety concerns, which has led to investigation of potential treatments with alternative mechanisms of action. The orexin (hypocretin) signaling system is of interest because orexins play an important role in sleep/wake regulation by binding to orexin-1 and -2 receptors. Here, we summarize results from in vivo rodent studies underlying the preclinical evaluation of lemborexant, a novel dual orexin receptor antagonist for treating insomnia/other sleep disorders. We observed that lemborexant effectively promoted sleep without potentiating the sedative effects of ethanol or impairing motor coordination. These preclinical findings supported further clinical evaluation of lemborexant for treating insomnia/other sleep disorders.
Introduction
Insomnia is a prevalent sleep disorder that is associated with significant health and economic burdens [1, 2]; pharmaceutical treatment plays a key role in relieving these burdens. Currently, common treatments for insomnia include benzodiazepines, non-benzodiazepine hypnotics, and sedating antidepressants. Although these treatments are effective, various safety concerns [3] have highlighted the need for alternative treatment options that promote sleep via a different mechanism(s) of action.
One alternative mechanism of action is antagonism of the orexin (hypocretin)-mediated pathway. The hypothalamic neuropeptides, orexin-A (OXA) and orexin-B (OXB), have been demonstrated to activate postsynaptic G-protein-coupled orexin-1 and orexin-2 receptors (OX1R and OX2R) located in the central nervous system [4, 5]. The importance of orexins in sleep/wake regulation has been highlighted in animal studies, which have shown that deficits in orexin signaling are associated with a phenotype similar to the human sleep disorder narcolepsy [6–11] and that central infusion of OXA promotes wakefulness [12–14]. Further, human patients with narcolepsy type-I have been shown to have deficits in orexin signaling [15], which are due to degeneration of orexin neurons [16, 17]. These neurons, which comprise a relatively small population in the lateral hypothalamic area [18], project widely throughout the central nervous system, including to noradrenergic neurons in the locus coeruleus, serotonergic neurons in the dorsal and median Raphe nuclei, cholinergic neurons in the pedunculopontine/laterodorsal tegmental nuclei and basal forebrain, dopaminergic neurons in the ventral tegmental area, and histaminergic neurons in the tuberomammillary nucleus [6, 19–22]. All of these respective monoaminergic neurons express OX1R and/or OX2R [14, 23], and are recognized to be involved in sleep/wake control [24, 25]. Support for the latter comes from several elegant mouse optogenetic excitation studies, where activation of orexin neurons reduced the latency to wakefulness from both nonrapid eye movement (non-REM) and rapid eye movement (REM) sleep [26]. In contrast, hyperpolarization/inactivation of orexin neurons increased non-REM sleep [27, 28].
The activity of orexin neurons has been shown to be mediated by sleep- and wake-promoting neurons. Specifically, sleep-promoting GABAergic neurons from the ventrolateral preoptic area have been demonstrated to provide inhibitory input [29–31], whereas wake-promoting cholinergic neurons from the basal forebrain have been demonstrated to provide excitatory input [29, 32, 33]. These findings support the concept of orexin being a major controller/stabilizer of the “switch” between the mutually regulating states of sleep and wakefulness, with orexin stabilizing the switch in the “wake position,” in other words, stabilizing wakefulness [34]. Consistent with this proposed role in stabilizing wakefulness, in vivo single-unit recordings have shown that orexin neurons fire most rapidly during active wakefulness, slow down during quiet wakefulness, and are mostly inactive during REM and non-REM sleep [35–38]. Furthermore, recent fiber photometry studies in mice indicate that orexin neuron activity is causally linked to wakefulness [39] and directly influences the activity of wake-controlling neurons in the paraventricular thalamus [40]. Finally, OXA concentrations in the cerebrospinal fluid (CSF) of rats [41], monkeys [42], and humans [43] were found to exhibit circadian variation, increasing during the active phase and decreasing during the rest phase. This suggests that orexins are factors of vigilance control, which are in turn under circadian control.
In recent years, insomnia disorder has been viewed as less of an issue related to sleep and more of an issue related to excessive activity of wakefulness-related circuits [44]. Indeed, functional neuroimaging has shown that night time glucose metabolism is elevated in the brains of patients with insomnia, suggesting an inability of arousal mechanisms to decrease at the appropriate time [45]. Inhibiting the orexin-signaling pathway to dampen one of the major arousal-promoting circuits would therefore appear to be a reasonable pharmacological approach for treating insomnia. To this end, animal studies demonstrated that dual orexin receptor antagonists (DORA) and selective OX2R antagonists (selective orexin-2 receptor antagonist [2-SORA]) promoted sleep [39, 46–50], whereas selective OX1R antagonists (selective orexin-1 receptor antagonist [1-SORA]) had no obvious effect on sleep [39, 47]. This efficacy pattern is consistent with findings in orexin pathway knockout mice, which have shown that OX2R plays a pivotal role in the maintenance of wakefulness and suppression of non-REM and REM sleep [8, 14]. Conversely, OX1R is thought to play a role in REM sleep suppression, because, although OX1R knockout mice lack an overt sleep phenotype [18], OXA reduces REM sleep in OX2R knockout mice, but not in orexin receptor double knockout mice [14]. Further, OX2R knockout mice have milder REM sleep-related narcolepsy symptoms than preproorexin knockout mice, which are devoid of any functional input on both orexin receptors [8]. Unsurprisingly, several DORAs and 2-SORAs have been developed and evaluated as potential treatments for insomnia in humans [51]. To date, one DORA, suvorexant, has been approved for the treatment of insomnia [52].
Lemborexant (also known as E2006) is a DORA currently being evaluated in phase 3 clinical trials for the treatment of insomnia disorder and in phase 2 trials for irregular sleep/wake rhythm disorder in patients with Alzheimer’s dementia. Previous lemborexant publications have detailed the discovery [53], the in vitro and in silico characterization [54], and results from a phase 2 study in patients with insomnia [55]. Here, we describe the results from key preclinical studies underlying the in vivo characterization of lemborexant.
Materials and methods
Animals
Animal care and experimental procedures were performed in an animal facility accredited by the Health Science Center for Accreditation of Laboratory Animal Care and Use of the Japan Health Sciences Foundation. All protocols were approved by the Institutional Animal Care and Use Committee and carried out in accordance with the Animal Experimentation Regulations of Eisai Co., Ltd.
Male C57BL/6NCrlCrlj mice (hereafter referred to as wild-type mice) and male F344/DuCrlCrlj (F344) and Sprague Dawley rats were supplied by Charles River Laboratories (Yokohama, Japan). Orexin neuron-deficient mice (orexin/ataxin-3 Tg/+; C57BL/6N background) [7] were supplied by Prof. Takeshi Sakurai (University of Tsukuba, Japan) and propagated by breeding orexin/ataxin-3 Tg/+ males with C57BL/6NCrlCrlj females (Charles River Laboratories, Yokohama, Japan). All rodents were maintained under a 12-h light-dark cycle with food and water available ad libitum. All experiments were conducted during the light phase (Zeitgeber time 0:00 = lights on; Zeitgeber time 12:00 = lights off).
Experiments conducted only with wild-type mice were performed with breeder-supplied animals, while in experiments comparing orexin/ataxin-3 Tg/+ with wild-type mice, the corresponding wild-type (orexin/ataxin-3 +/+) littermates were used.
Chemical compounds
Lemborexant, almorexant, and zolpidem were synthesized in-house and suspended as free bases in the vehicle solution specified for each study. Doses for each compound in the mouse experiments were set based on the minimum necessary dose for sleep promotion in mice, and are therefore different for each compound.
Effect of single-dose lemborexant on the orexin-induced increase in ACTH in rats
Previous studies have demonstrated that OXA and OXB increase plasma corticosterone concentrations in rats [56], with the efficacy of OXB suggesting that OX2R is mediating this effect. Mediators located downstream of orexin signaling are thought to be corticotropin releasing factor [56] and neuropeptide Y [57]. As the release of corticosterone is triggered by ACTH, we developed an in vivo functional assay, in which plasma ACTH concentrations are increased by central application of OXB. In this assay (details ensue), we applied [Ala11, D-Leu15]-orexin B, which is far more selective for OX2R (>400-fold vs. OX1R) than natural OXB (>10-fold vs. OX1R) [58], to minimize potential cross-talk from OX1R. We carried out the study using rats because we have observed that vehicle-treated mice have intrinsically high ACTH levels that are not distinguishable from ACTH levels in [Ala11, D-Leu15]-orexin B-treated mice (data not shown). This is likely due to mice being more difficult to habituate to experimental conditions than rats. The ability of lemborexant to inhibit the [Ala11, D-Leu15]-orexin B-induced increase in plasma ACTH concentrations was determined. The optimum dose of [Ala11, D-Leu15]-orexin B was found to be 1 nmol/head (data not shown), which was associated with an approximate fourfold increase in plasma ACTH concentrations relative to vehicle treatment.
Male F344 rats (age: 5 weeks; body weight: 85.8–103.5 g) were implanted with infusion cannulae into the left lateral ventricle for intracerebroventricular (i.c.v.) injection. Four to five days after surgery, rats were habituated for oral administration (p.o.) and handling once before the study. Six to seven days after cannula implantation, rats received p.o. vehicle (5% [v/v] dimethyl sulfoxide, 9.5% [v/v] cremophor in saline; n = 10) or lemborexant (5 mL/kg suspended in vehicle) 1, 3, 10, or 30 mg/kg (n = 5, 6, 6, and 5, respectively). One hour later, vehicle control rats received 5 µL of phosphate buffered saline (PBS) or [Ala11, D-Leu15]-orexin B (1 nmol/head, 0.2 mmol/L in PBS, Tocris Bioscience, Japan) via i.c.v. injection (n = 5 each). All lemborexant pretreated rats received [Ala11, D-Leu15]-orexin B via i.c.v. injection. Fifteen minutes later, rats were decapitated and blood samples were collected with Na2EDTA (100 mg/mL, 100 µL). Blood samples were then centrifuged (1000 × g, 10 min at 4°C) and supernatant plasma was stored at −80°C for later measurement of ACTH and lemborexant concentrations. After decapitation, blue ink was injected i.c.v. to confirm the correct placement of cannulae. Coronal cross-sections were made near the cannulae; placement was judged (by an experienced observer) to be correct if blue ink was visible in the lateral ventricle(s). Data from 3 out of 32 rats with incorrect cannula placement were excluded from analysis.
Plasma ACTH concentrations were measured using an ACTH radioimmunoassay kit (ACTH IRMA “MITSUBISHI,” Mitsubishi Chemical Medience, Tokyo, Japan). Measurement of radioactivity and calculation of ACTH concentrations were conducted using a scintillation counter (ARC-1000M, Hitachi Aloka Medical, Ltd., Tokyo, Japan).
Plasma lemborexant concentrations were measured by liquid chromatography-tandem mass spectrometry (LC-MS/MS). Plasma samples were precipitated with four volumes of acetonitrile containing an internal standard (10 ng/mL imipramine). Following vortex mixing and centrifugation, the supernatant was filtered, and the resultant filtrate was injected into the LC-MS/MS. Lemborexant and the internal standard were identified based on their respective retention times and the mass units of the monitoring ions on the mass chromatograms. The calibration curve was obtained at a concentration range from 1 to 1,000 ng/mL by least-squares linear regression, with a weighting factor of 1/X2 on the ratio of the peak area of lemborexant to that of the internal standard against the nominal concentrations in the calibration standards. The limit of quantitation for lemborexant was 1 ng/mL. The unbound fraction of lemborexant (0.167) in F344 rat plasma was determined by equilibrium dialysis and the unbound plasma concentration was calculated.
Unbound plasma concentrations of lemborexant and corresponding inhibition of OXB-triggered increases in plasma ACTH were determined for each rat. Values were analyzed using Michaelis–Menten kinetic analysis, using the least-square fit method and fixing maximum inhibition to 100%.
Effect of single-dose lemborexant on spontaneous locomotor activity in wild-type and orexin neuron-deficient mice
Wild-type male mice (age: 9 weeks; body weight: 19.4–22.5 g) were dosed p.o. with vehicle (5% [v/v] dimethyl sulfoxide, 10% [v/v] cremophor in 10 mmol/L HCl; 10 mL/kg; n = 16) or lemborexant (30 [n = 8] or 100 mg/kg [n = 7]) at Zeitgeber time 3:40 or 5:30. One hour after dosing, mice were placed in an open field arena (VersaMax, AccuScan Instruments, Columbus, OH) and locomotor activity was automatically recorded as infrared light beam breaks as previously described [49]. For activity values, all horizontal and vertical infrared light beam break counts were summed over 1 h after the start of locomotor activity recording.
In a separate study, orexin neuron-deficient mice (age: 18–26 weeks; body weight: 27.9–36.0 g) were dosed p.o. with vehicle (as above; n = 8) or 100 mg/kg lemborexant (n = 8), the maximum dose tested in wild-type mice, at Zeitgeber time 3:40 or 5:30. Thirty minutes later, locomotor activity was recorded and summed over 1 h as described above.
Effect of single-dose lemborexant on vigilance state measures in wild-type and orexin neuron-deficient mice
Under deep ketamine/xylazine anesthesia, wild-type male mice (age: 10–11 weeks; body weight: 21.7–26.1 g) were implanted with transcranial supradural electroencephalography (EEG) and nuchal electromyography (EMG) electrodes as previously described [6, 49]. Briefly, four holes were drilled into the skull (relative to bregma: 1.1 mm rostral and 1.45 mm lateral on both sides; 3.5 mm caudal and 1.45 mm on both sides) and four gold-plated contacts of a six-contact board mount socket (#929975-01-36, 3M, Tokyo, Japan) were inserted to the dura mater and fixed with dental cement. The remaining two contacts were connected to teflon-coated stainless steel wires, gold-plated at the tips (EMG leads), which were placed intranuchally into pockets formed by blunt dissection of neck muscle left and right of midline. EEG signals were read from one side only (usually right) between rostral and caudal electrodes. EEG/EMG signals were collected via a connector attached to the board mount socket, which was connected to an amplifier via a rotating swivel, allowing the mouse to move freely while connected to the cable during the experiment. Mice were allowed to recover and to habituate to the recording room for 1 week while being housed in recording cages, and were then divided into body-weight matched groups and habituated to p.o. dosing with vehicle (10 mL/kg 0.5% [w/v] methylcellulose 400, WAKO, Osaka, Japan) at Zeitgeber time 3:30–4:10 for 2 consecutive days. The next day, mice were dosed p.o. with vehicle (n = 5), lemborexant (1 [n = 5] or 10 mg/kg [n = 4]), almorexant (10 [n = 5] or 100 mg/kg [n = 4]), or zolpidem (3 [n = 5] or 30 mg/kg [n = 4]) at Zeitgeber time 3:30–4:10. Note: almorexant, a DORA, and zolpidem, a widely used sleep drug, were used as active comparators for paradigm validation. EEG and EMG signals were continuously recorded for 7 h after dosing (sampling frequency 128 Hz), divided into 10-s epochs, and analyzed as previously described [49] according to standard rodent sleep criteria [59] using SleepSign software (v3, Kissei Comtec, Matsumoto, Japan). An experienced observer, blinded to treatment, subsequently provided visual confirmation of vigilance state judgement. Data were accumulated into 1-h bins for time course graphs and accumulated for the first 3 h after dosing for statistical analysis.
In a separate study, orexin neuron-deficient mice and wild-type control littermates (age: 12–13 weeks; body weight: 23.4–33.8 g) were implanted with EEG and EMG electrodes, as described above for wild-type mice. After approximately 2 months of recovery and housing in the recording room, mice were habituated to EEG/EMG-recording cages for 3 days before p.o. dosing with vehicle (10 mL/kg 0.5% [w/v] methylcellulose 400, WAKO) at Zeitgeber time 3:30–4:00 for three consecutive days under recording conditions. Preliminary EEG/EMG signal recordings and sleep time analyses were performed for p.o. dosing days 2 and 3. Mice with sufficient EEG/EMG signal quality (n = 7 per treatment group and genotype) were then randomly divided into treatment groups with minimal differences in average total sleep time (within 3 h after dosing). The next day, mice were dosed p.o. with vehicle or lemborexant (30 mg/kg) at Zeitgeber time 3:30–4:00. EEG/EMG signal recording (sampling frequency 128 Hz) was started shortly before dosing and was continued for 3 h and 45 min after dosing. Data were analyzed as described for the study in the previous paragraph.
Vigilance states assessed in both studies included wakefulness, non-REM sleep, and REM sleep. Representative traces for each of these vigilance states are shown in Supplementary Figure 1.
Effect of single-dose lemborexant on vigilance state measures in rats
Under deep sodium pentobarbital anesthesia, male Sprague Dawley rats (age: 7–10 weeks; body weight: 311–449 g) were intraperitoneally implanted with battery-driven, wireless telemetry devices (TL11M2-F40-EET, Data Sciences International, St Paul, MN) for EEG/EMG measurements. Two silver screws were fixed 2.0 mm left and right of lambda through the skull bone so as to touch the dura mater. EEG leads were knotted to the screws, while EMG leads were placed intranuchally into pockets formed by blunt dissection of neck muscle left and right of midline. After 9–11 days of recovery in home cages, rats were habituated to the recording room and p.o. dosing procedure for 2 days, while still being housed in the same home cages throughout the experiment. A total of 12 rats were then selected for the study and assigned to body-weight matched groups for five dosing and recording sessions. The sessions were conducted with 2–3 days of intermittent wash-out periods, with p.o. dosing taking place at Zeitgeber time 2:00–3:00 and subsequent recording of EEG/EMG signals for 4 h using a telemetry system (recording software: Dataquest A.R.T. Platinum v4.10; Data Sciences International, New Brighton, MN) for later off-line analysis (software: NeuroScore v1.1; Data Sciences International). Continuous recordings were divided into 10-s epochs and automatically analyzed via an algorithm that determined vigilance states. Automated analysis results were then verified and, if necessary, corrected by a trained observer blinded to treatment. No animal received the same test compound at the same dose twice. There were a total of 10 treatment groups in the study; vehicle (10 mL/kg 0.5% [w/v] methyl-cellulose 400 [n = 6]), lemborexant (3, 10, 30, 100, or 300 mg/kg [all n = 6]), and zolpidem (3, 10, 30, or 100 mg/kg [all n = 6]).
Vigilance states assessed included cumulative wakefulness, non-REM sleep, and REM sleep times for 2 h after dosing. Representative traces for each of these vigilance states are shown in Supplementary Figure 2.
Effect of chronic-dose lemborexant on vigilance state measures in rats
Male Sprague Dawley rats, fully habituated to experimental conditions from the previously described single-dose study, were used after a 5-day washout period. All rats were dosed p.o., once-daily at Zeitgeber time 2:00–3:00, with vehicle (0.5% [w/v] methylcellulose 400) on day 1–3, then vehicle (n = 2), lemborexant 30 mg/kg (n = 5), or zolpidem 100 mg/kg (n = 5) from day 4 to 24, and finally vehicle on days 25 and 26. EEG/EMG signals were recorded (as already described for the single-dose study) on days 1 and 2 (pretreatment), days 4, 7, 11, 14, 18, 21, and 24 (treatment), and days 25 and 26 (posttreatment) for nearly 3 hours. Lemborexant and zolpidem doses were chosen based on the maximum effects in the previous single-dose experiment.
Vigilance states assessed included wakefulness, non-REM sleep, and REM sleep for 2 h after dosing, using the same analysis procedure as described for the single-dose study. Sleep latency, defined as the time between dosing and the first occurrence of 1 min of uninterrupted sleep, was also assessed.
Effect of single-dose lemborexant on ethanol-induced anesthesia in wild-type mice
Wild-type male mice (age: 13 weeks; body weight: 21.8–28.1 g) were dosed p.o. with vehicle (10 mL/kg 0.5% [w/v] methylcellulose 400), lemborexant (1, 3, or 10 mg/kg), almorexant (30, 100, or 300 mg/kg), or zolpidem (3, 10, or 30 mg/kg) (all groups, n = 6) during the light phase. After 5 min, mice received intraperitoneal injections of 3.0 g/kg ethanol (20% [w/v] in saline). The almorexant and zolpidem doses were based on those used in a previous rat study, where almorexant up to 300 mg/kg did not show interaction with ethanol, but zolpidem from 10 mg/kg upwards showed interaction with ethanol [60]. Note that the dose of ethanol was chosen because it caused comparably short anesthesia with little data variation (Supplementary Figure 3). The time from ethanol injection to regaining of righting reflex was measured and taken as anesthesia duration, with 240 min as the cutoff time.
In a second ethanol study, wild-type male mice (age: 13 weeks; body weight: 23.2–27.7 g) were dosed p.o. with vehicle (10 mL/kg 0.5% [w/v] methylcellulose 400) or lemborexant (30, 100, or 300 mg/kg) (all groups, n = 6) during the light phase. The time from ethanol injection to regaining of righting reflex was recorded as already described.
Effect of single-dose lemborexant on motor coordination in wild-type mice
Motor coordination was assessed using a rotarod treadmill MK-660C (Muromachi Kikai, Tokyo, Japan). With the device on hold, five mice were placed on the central axle, facing the same direction away from the experimenter, without interacting with or seeing each other. The axle was then accelerated to 40 rpm within 180 s, at which point the test was terminated. The time from the start of rotation until a mouse fell was automatically recorded and taken as the latency to fall, with a cutoff at 180 s. Before the first test of the day, a prerun with mice not used for testing was carried out to scent the device. Between each test, the axle was wiped to remove urine and feces.
For this study, male wild-type mice (age: 14 weeks; body weight: 22.1–29.1 g), previously trained on the treadmill for 3 consecutive days with subsequent 14 days rest, were allocated to body-weight equivalent groups to receive single p.o. dosing of vehicle (10 mL/kg 0.5% [w/v] methylcellulose 400), lemborexant (30, 100, or 300 mg/kg), or zolpidem (100 mg/kg) (all groups, n = 11). The starting lemborexant dose (30 mg/kg) was selected as this is approximately threefold the sleep-promoting dose, while zolpidem 100 mg/kg has previously been reported to impair motor coordination in rats [60]. On the day before the study, mice were given 0.25 mL p.o. water and placed on the treadmill for a training run. Mice that fell before 120 s were placed back on the device. On the day of the study, mice were first tested on the treadmill at Zeitgeber time 2:00 (predosing). Treatments were then administered at Zeitgeber time 4:00 and motor coordination was assessed at Zeitgeber times 4:30, 6:00, 7:30, and 9:00. All five dosing groups were allocated through all five test compartments on the treadmill. Between tests, mice were returned to their home cages and allowed to rest, with free access to food and water.
Plasma and CSF concentrations of lemborexant after single dosing in rats
Male Sprague Dawley rats (age: 9 weeks; body weight: 337–355 g) were dosed p.o. with lemborexant 30 mg/kg in 0.5% (w/v) methylcellulose 400 (5 mL/kg) at Zeitgeber time 4:30–5:00. Two hours later, rats were anesthetized and plasma and CSF samples were obtained from the abdominal aorta and cisterna magna, respectively, for the measurement of lemborexant concentrations by LC-MS/MS.
Plasma concentrations of lemborexant after single dosing in mice
Male C57BL/6N mice (age: 14 weeks; body weight: 26.7–31.7 g) were dosed p.o. with lemborexant 10 or 300 mg/kg in 0.5% [w/v] methylcellulose 400 (10 mL/kg) at Zeitgeber time 3:00–9:15. At 0.25, 0.5, 1, 3, 5, 6, 18, and 24 h after dosing, mice were anesthetized and plasma samples were obtained from the abdominal aorta for measurement of lemborexant concentrations by LC-MS/MS.
Statistical analyses
Data are presented as the mean ± standard error of the mean (SEM) and were generally compared by t test (for comparisons involving two groups) or one-way analysis of variance (ANOVA) followed by Dunnett multiple comparison test (for comparisons involving three or more groups). Exceptions to one-way ANOVA were: the single-dose vigilance study in rats (mixed-effects model with treatment group and period modeled as fixed effects and animal as a random effect); the chronic-dose vigilance study in rats (repeated measures ANOVA); and the motor coordination study in mice (repeated measures analysis of covariance with predosing values as a covariate). For all studies, p <0.05 (two-sided) was considered to indicate statistical significance. Statistical analyses were performed using the SAS software package version 8.2 (SAS Institute Japan. Tokyo, Japan).
Results
Effect of single-dose lemborexant on orexin-induced increases in ACTH in rats
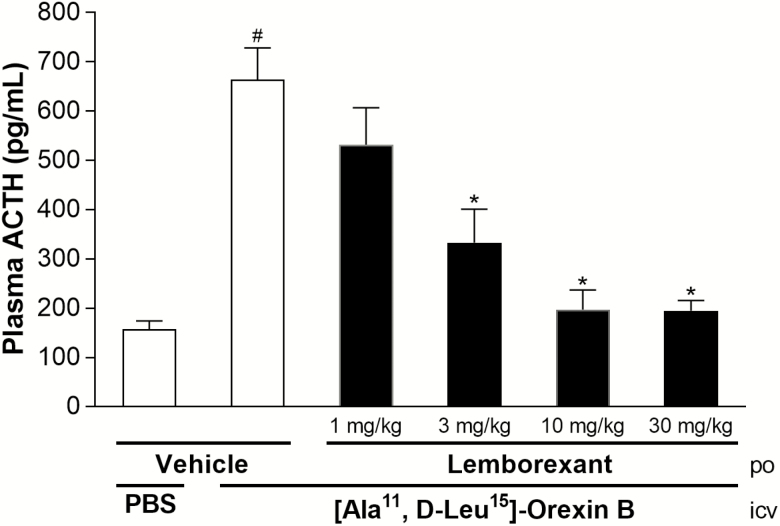
Rats centrally treated with [Ala11, D-Leu15]-orexin B had significantly higher plasma ACTH concentrations than rats treated with vehicle (Figure 1). Lemborexant exhibited dose-related inhibition of this increase in ACTH, with the level of inhibition being significant from 3 mg/kg p.o. and higher. ACTH concentrations that were equivalent to those in rats treated with vehicle occurred with lemborexant doses from 10 mg/kg p.o. and higher.
Figure 1.
Inhibitory effect of single-dose lemborexant on the plasma ACTH increase induced by centrally applied [Ala11, D-Leu15]-orexin B in rats. Rats received single-dose oral doses of vehicle or lemborexant (1, 3, 10, or 30 mg/kg) (all n = 5, except for 30 mg/kg n = 4) during the light phase. #p < 0.05 versus i.c.v. PBS control (t-test); *p < 0.05 versus vehicle/[Ala11, D-Leu15]-orexin B (one-way analysis of variance followed by Dunnett multiple comparison test).
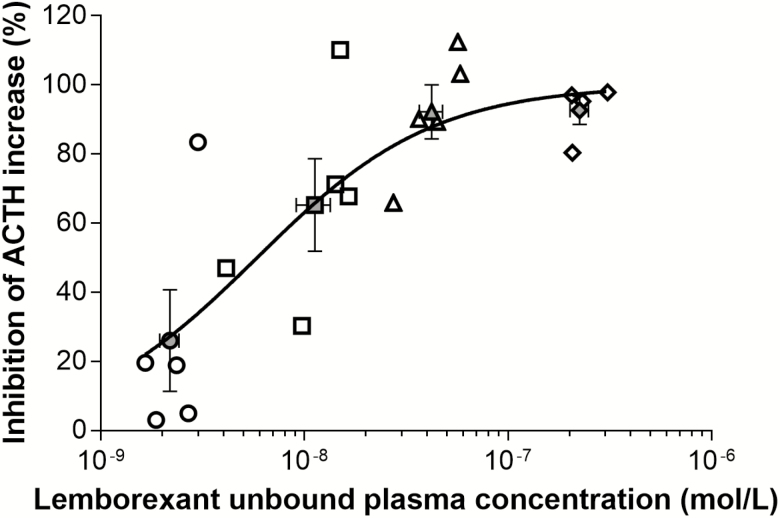
The unbound plasma lemborexant concentration achieving 50% inhibition (IC50) was (mean ± SEM) 5.9 ± 1.7 nmol/L (Figure 2).
Figure 2.
Relationship between unbound plasma concentrations of lemborexant and corresponding inhibition of [Ala11, D-Leu15]-orexin B-triggered increases in plasma ACTH concentrations in rats. Data for individual animals are depicted as white open symbols; cohort means are depicted as gray open symbols (mean ± standard error of the mean). Circles = 1 mg/kg lemborexant; squares = 3 mg/kg lemborexant; triangles = 10 mg/kg lemborexant; diamonds = 30 mg/kg lemborexant.
Effect of single-dose lemborexant on spontaneous locomotor activity in wild-type and orexin neuron-deficient mice
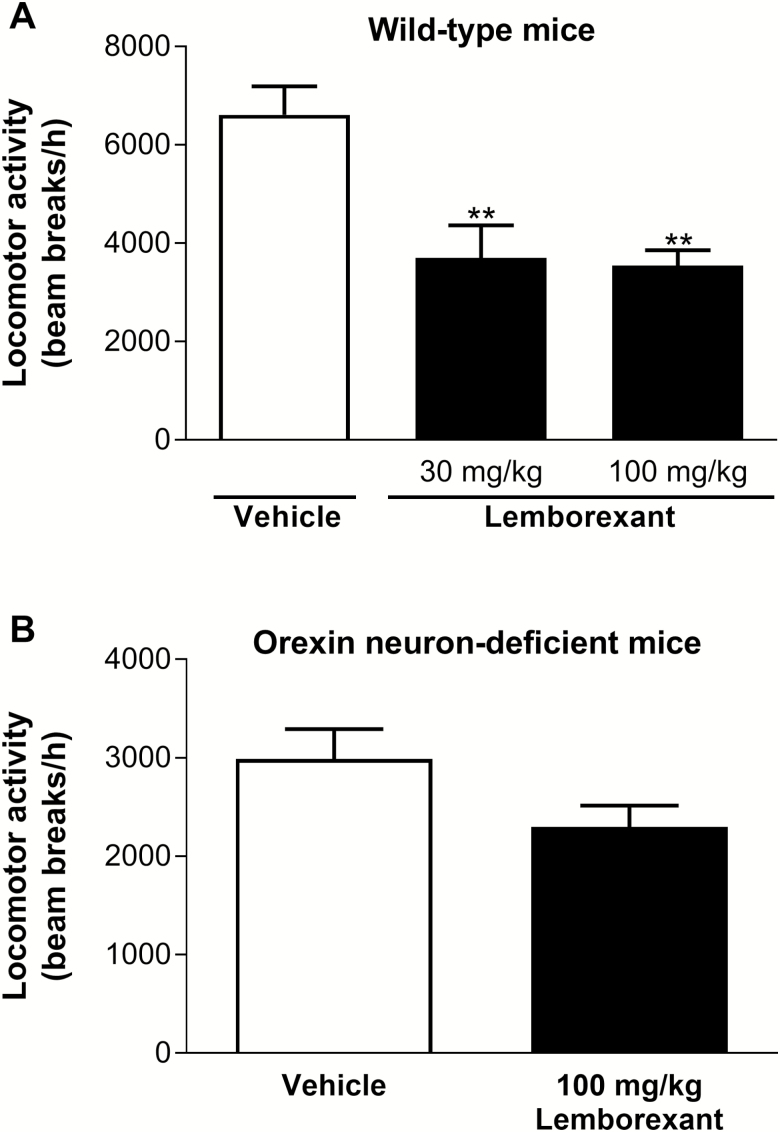
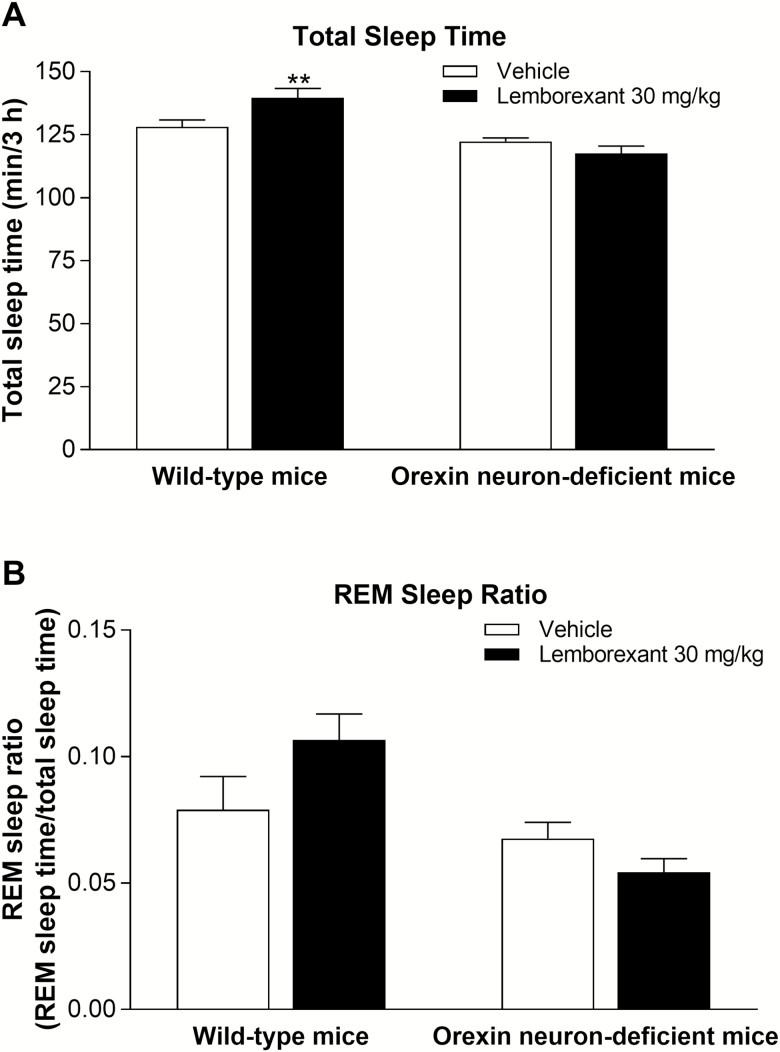
In wild-type mice, lemborexant significantly reduced spontaneous locomotor activity compared with vehicle at both p.o. doses tested (30 and 100 mg/kg) (Figure 3A). Conversely, in orexin neuron-deficient mice, which exhibited less activity than vehicle-treated wild-type mice, lemborexant (100 mg/kg p.o.) did not significantly reduce spontaneous locomotor activity compared with vehicle (Figure 3B).
Figure 3.
Effect of single-dose lemborexant on spontaneous locomotor activity in wild-type (A) and orexin neuron-deficient (B) mice. Wild-type mice received single oral doses of vehicle (n = 16), lemborexant (30 mg/kg [n = 8]), or lemborexant (100 mg/kg [n = 7]). Orexin neuron-deficient orexin/ataxin-3 Tg/+ mice received single oral doses of vehicle (n = 8) or lemborexant (100 mg/kg [n = 8]) at Zeitgeber time 3:40 or 5:30 (Zeitgeber time 0:00 = lights on; Zeitgeber time 12:00 = lights off). Data are mean ± standard error of the mean and represent all horizontal and vertical infrared light beam breaks within 1 h after introduction of the mouse to the open field arena. **p < 0.01 versus vehicle (wild-type mice: one-way analysis of variance followed by Dunnett multiple comparison test; orexin neuron-deficient mice: two-tailed unpaired t test).
Effect of single-dose of lemborexant on vigilance state measures in wild-type and orexin neuron-deficient mice
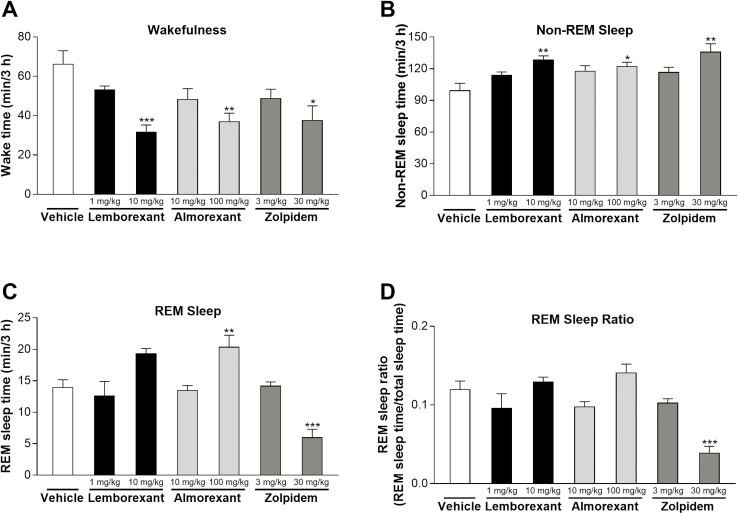
In wild-type mice, lemborexant (10 mg/kg p.o.) significantly reduced wakefulness and increased non-REM sleep compared with vehicle (Figure 4A and B). Lemborexant (10 mg/kg p.o.) also increased REM sleep compared with vehicle, although the magnitude of increase was not statistically significant (Figure 4C). The respective vigilance state time courses of lemborexant-treated mice are shown in Supplementary Figure 4. Like lemborexant, the active comparators almorexant (100 mg/kg p.o.) and zolpidem (30 mg/kg p.o.) significantly reduced wakefulness and increased non-REM sleep compared with vehicle (Figure 4A and B), thus validating our vigilance state measurement paradigm. Almorexant (100 mg/kg p.o.) also significantly increased REM sleep compared with vehicle, whereas zolpidem (30 mg/kg p.o.) significantly decreased REM sleep compared with vehicle (Figure 4C). Lemborexant (30 mg/kg p.o.) increased total sleep time in wild-type mice (Figure 5A); however, the REM sleep ratio (ratio of REM sleep time to total sleep time) did not change significantly (Figures 4D and 5B) following either lemborexant or almorexant application. This indicates that lemborexant and almorexant promoted both non-REM sleep and REM sleep, while zolpidem promoted non-REM sleep and decreased REM sleep. In orexin neuron-deficient mice, lemborexant (30 mg/kg p.o.) had no effect on total sleep time (Figure 5A) or the REM sleep ratio (Figure 5B) relative to vehicle.
Figure 4.
Effect of single-dose lemborexant on cumulative wakefulness time (A), non-REM sleep time (B), REM sleep time (C), and REM sleep ratio (D) over 3 h after dosing in wild-type mice. Mice received single oral doses of vehicle (n = 5), lemborexant (1 mg/kg, n = 5; 10 mg/kg, n = 4), almorexant (10 mg/kg, n = 5; 100 mg/kg, n = 4), or zolpidem (3 mg/kg, n = 5; 30 mg/kg, n = 4) at Zeitgeber time 3:30–4:10 (Zeitgeber time 0:00 = lights on; Zeitgeber time 12:00 = lights off). Data are mean ± standard error of the mean. *p < 0.05; **p < .01; ***p < 0.001 versus vehicle (one-way analysis followed by Dunnett multiple comparison test).
Figure 5.
Effect of single-dose lemborexant on cumulative total sleep time (A) and REM sleep ratio (B) over 3 h after dosing in wild-type and orexin neuron-deficient orexin/ataxin-3 Tg/+ mice. Mice received single oral doses of vehicle or lemborexant 30 mg/kg (both n = 7 per genotype) at Zeitgeber time 3:30–3:48 (Zeitgeber time 0:00 = lights on; Zeitgeber time 12:00 = lights off). Data are mean ± standard error of the mean. **p < 0.01 versus vehicle (t test).
Effect of single-dose lemborexant on vigilance state measures in rats
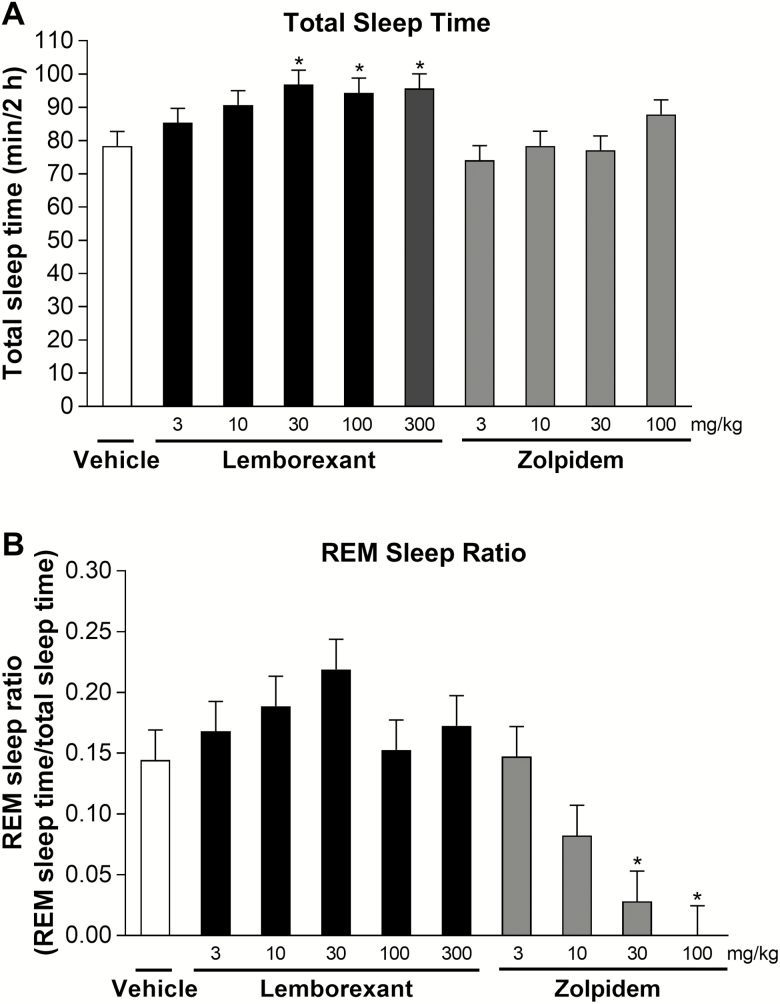
Lemborexant (3–30 mg/kg p.o.) increased total sleep time in a dose-related manner (Figure 6A), with the dose to achieve 50% of maximum effect being 4.4 mg/kg. Lemborexant significantly increased total sleep time compared with vehicle from doses of 30 mg/kg p.o. and higher. Zolpidem did not significantly increase total sleep time compared with vehicle at any dose up to 100 mg/kg p.o. Lemborexant had no significant influence on the REM sleep ratio at any dose tested, whereas zolpidem resulted in dose-related decreases in the REM sleep ratio that were significant compared with vehicle at 30 and 100 mg/kg (Figure 6B). No recordings, regardless of treatment, showed the narcolepsy-like symptom of sleep-onset REM sleep in the 2 h analyzed after dosing; every sleep episode started with non-REM sleep.
Figure 6.
Effect of single-dose lemborexant on total sleep time (A) and REM sleep ratio (B) in rats. Rats received single oral doses of vehicle, lemborexant (3, 10, 30, 100, or 300 mg/kg), or zolpidem (3, 10, 30, or 100 mg/kg) (all n = 6) at Zeitgeber time 2:00–3:00 (Zeitgeber time 0:00 = lights on; Zeitgeber time 12:00 = lights off). Data are mean ± standard error of the mean for the 2 h after dosing. *p < 0.05 versus vehicle (mixed effect model followed by Dunnett multiple comparison test).
Effect of chronic-dose lemborexant on vigilance state measures in rats
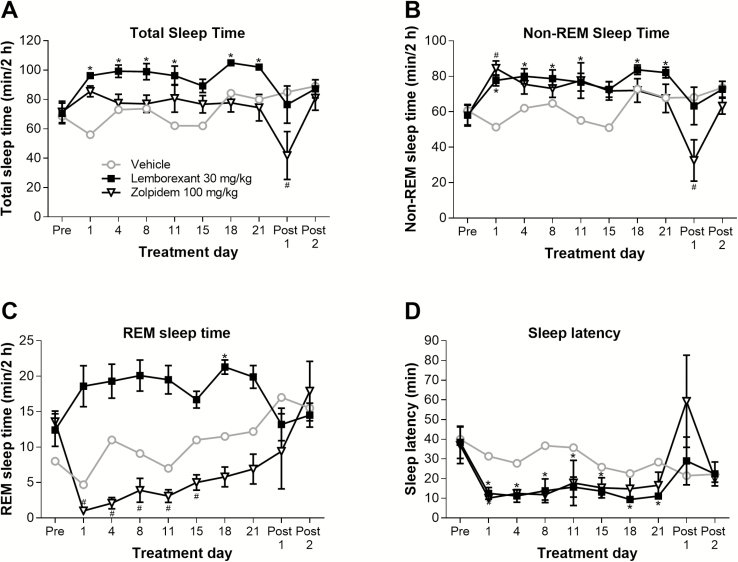
All results described in this paragraph were derived from the 2 h directly after dosing. Once-daily chronic dosing of lemborexant (30 mg/kg p.o.) significantly increased total and non-REM sleep time (relative to pretreatment) throughout the 21-day treatment period, except for day 15 (Figure 7A and B). Similarly, REM sleep time was numerically increased throughout the treatment period; however, the only statistically significant increase detected was at day 18 (Figure 7C). On cessation of treatment (posttreatment days 1 and 2), all sleep times in the lemborexant group returned to pretreatment values. However, chronic dosing of zolpidem (100 mg/kg p.o.) did not increase total sleep time (Figure 7A), significantly increased non-REM sleep time on day 1 only (Figure 7B), and significantly decreased REM sleep time from days 1 through 15 (Figure 7C). Zolpidem dosing was also associated with a significant decrease in total sleep time and non-REM sleep time (relative to pretreatment) on posttreatment day 1; however, total sleep time and non-REM sleep time recovered to pretreatment values on posttreatment day 2 (Figure 7A and B).
Figure 7.
Effect of chronic-dose lemborexant on total sleep time (A), non-REM sleep time (B), REM sleep time (C), and sleep latency (D) in rats. Rats received once-daily oral doses of vehicle (n = 2 per time point), lemborexant 30 mg/kg (n = 5 per time point), or zolpidem 100 mg/kg (n = 4–5 per time point) at Zeitgeber time 2:00–3:00 (Zeitgeber time 0:00 = lights on; Zeitgeber time 12:00 = lights off). Data are mean ± standard error of the mean. *p < 0.05 versus pretreatment value for lemborexant group; #p < 0.05 versus pretreatment value for zolpidem group (both repeated measures analysis of variance followed by Dunnett type multiple comparison test).
Lemborexant (30 mg/kg p.o.) significantly decreased sleep latency (relative to pretreatment) at all times throughout the 21-day treatment period (Figure 7D). Zolpidem (100 mg/kg p.o.) also seemed to decrease sleep latency throughout the treatment period; however, none of the decreases were statistically significant relative to pretreatment.
Chronic dosing with lemborexant (30 mg/kg p.o.) had no significant effect on the REM sleep ratio on any day (Supplementary Figure 5). In contrast, chronic dosing of zolpidem (100 mg/kg p.o.) significantly decreased the REM sleep ratio (relative to pretreatment) from days 1 through 15 (Supplementary Figure 5). There was a gradual increase in the REM sleep ratio with zolpidem from day 1 onwards, suggesting a tolerance effect; values on days 18 and 21 were not significantly different to the pretreatment value. On stopping treatment (posttreatment days 1 and 2), values in the zolpidem group returned to pretreatment values.
As with single dosing, no recordings, regardless of treatment, showed the narcolepsy-like symptom of sleep-onset REM sleep in the 2 h analyzed after dosing over the complete experimental period.
Effect of single-dose lemborexant on ethanol-induced anesthesia in wild-type mice
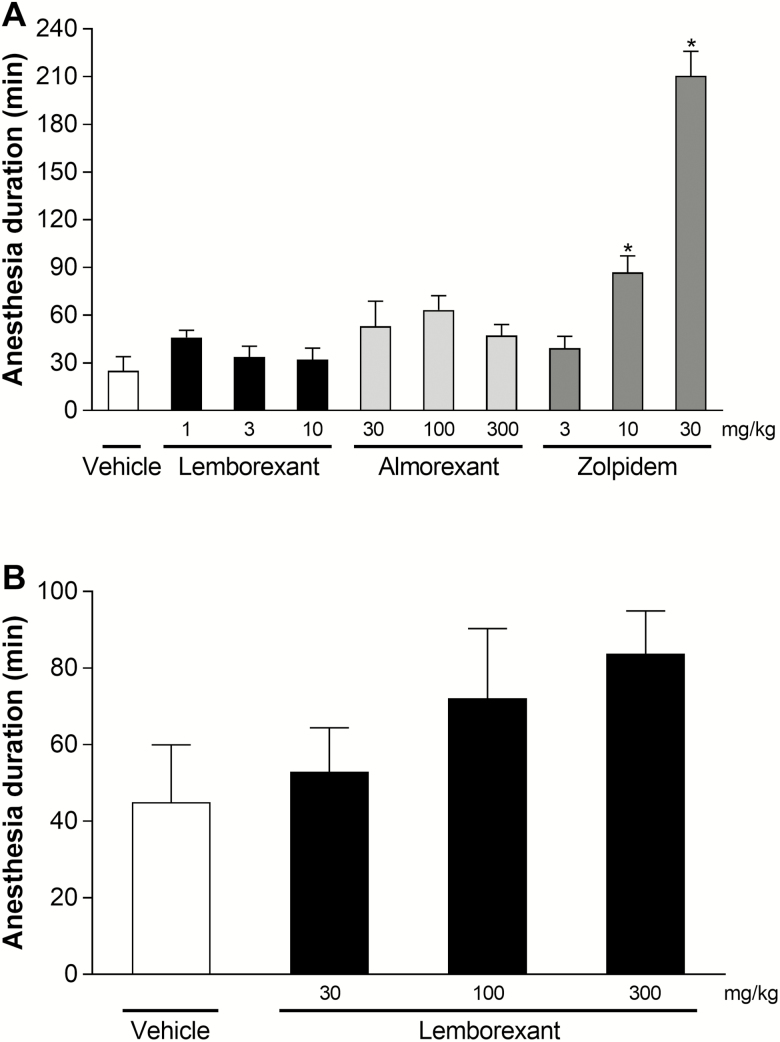
Lemborexant (10 mg/kg p.o.) and almorexant (100 mg/kg p.o.) both promoted sleep in mice (Figure 4), but had no significant effect on the duration of ethanol-induced anesthesia (Figure 8A). Likewise, a threefold higher dose of almorexant (300 mg/kg p.o.) had no significant effect on the duration of ethanol-induced anesthesia. In contrast, zolpidem doses that were one-third of or equal to the sleep-promoting dose of 30 mg/kg p.o. significantly increased the duration of ethanol-induced anesthesia compared with vehicle. Zolpidem as a positive control validates this paradigm.
Figure 8.
Effect of single-dose lemborexant on ethanol-induced anesthesia duration in wild-type mice. (A) Mice received single oral doses of vehicle, lemborexant (1, 3, or 10 mg/kg), almorexant (30, 100, or 300 mg/kg), or zolpidem (3, 10, or 30 mg/kg) (all n = 6) during the light phase. (B) Mice received single oral doses of vehicle or lemborexant (30, 100, or 300 mg/kg) (all n = 6) during the light phase. Data are mean ± standard error of the mean. *p < 0.05 versus vehicle (one-way analysis of variance followed by Dunnett multiple comparison test).
In a separate study, high doses of lemborexant (30, 100, and 300 mg/kg p.o.), up to 30-fold the sleep-promoting dose, had no significant effect on the duration of ethanol-induced anesthesia (Figure 8B).
Effect of single-dose lemborexant on motor coordination in wild-type mice
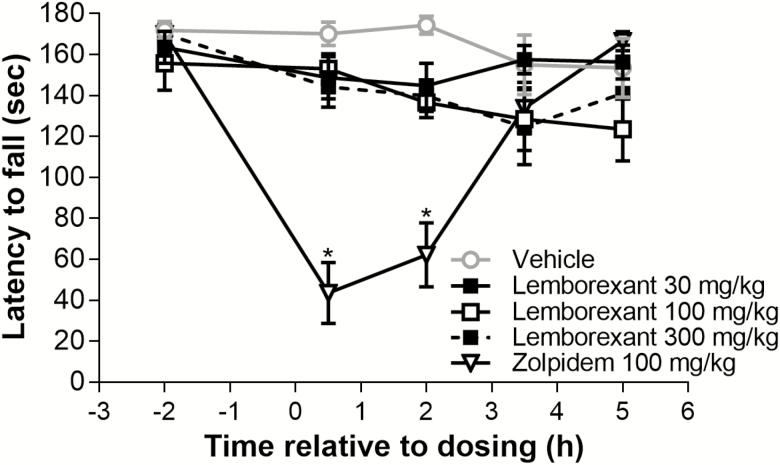
Lemborexant (30, 100, and 300 mg/kg p.o.), up to 30-fold the sleep-promoting dose, had no significant effect (relative to vehicle) on the latency to fall from the rotarod treadmill at any time point after dosing (Figure 9) for up to 5 h. In contrast, zolpidem (100 mg/kg p.o.), at threefold the sleep-promoting dose, significantly decreased the latency to fall (relative to vehicle) 0.5 and 2 h after dosing, with performance recovery at subsequent time points. Zolpidem as a positive control validates this paradigm.
Figure 9.
Effect of single-dose lemborexant on the latency to fall in wild-type mice. Mice received single oral doses of vehicle, lemborexant (30, 100, or 300 mg/kg), or zolpidem 100 mg/kg (all n = 11 per time point) at Zeitgeber time 4:00 (Zeitgeber time 0:00 = lights on; Zeitgeber time 12:00 = lights off) and had to balance on a rotating axle for a maximum of 180 s at Zeitgeber times 4:30, 6:00, 7.30, and 9:00. Data are mean ± standard error of the mean. There were no significant differences between the vehicle and lemborexant groups. *p < 0.05 versus vehicle (repeated measures analysis of covariance using Zeitgeber time 2:00 predosing values as a covariate).
Plasma and CSF concentrations of lemborexant after single dosing in rats
Plasma and CSF concentrations at 2 h after single dosing with lemborexant (30 mg/kg p.o.) were (mean ± SEM) 134 ± 44 and 9.5 ± 3.6 nmol/L, respectively.
Plasma concentrations of lemborexant after single dosing in mice
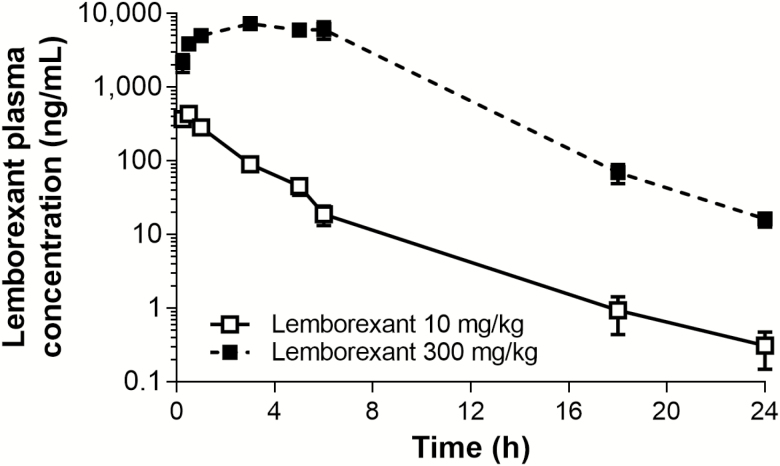
Figure 10 shows the plasma concentration–time profile of lemborexant in mice after dosing with the effective dose for sleep promotion (10 mg/kg p.o.) and the maximum dose in the ethanol-induced anesthesia and motor coordination safety paradigms (300 mg/kg p.o.). Table 1 shows the key pharmacokinetic parameters for both these doses of lemborexant. The time to peak concentration (tmax) was prolonged at 300 mg/kg compared with 10 mg/kg, presumably reflecting solubility-limited absorption due to the high concentration in the 300 mg/kg dosing solution. The tmax (0.5 h) after single oral dosing with lemborexant 10 mg/kg is within the analyzed time window of 2 h (sleep paradigm). Likewise, the tmax (3 h) after single oral dosing with lemborexant 300 mg/kg is within the analyzed time window of 4 h (ethanol interaction) and 5 h (motor coordination), respectively. We can therefore calculate multiples as safety margins between the sleep-promoting dose (10 mg/kg) and the maximum safety paradigm dose (300 mg/kg) regarding maximum concentration (16.8-fold) or exposure over 24 h (72.5-fold). Taking a conservative approach, we estimated the safety margin for mice in the ethanol interaction and motor coordination paradigms to be 16.8-fold or higher, because no negative effects were seen even at the highest tested dose of 300 mg/kg.
Figure 10.
Plasma concentration profile of lemborexant over time in mice. Mice received single oral doses of lemborexant (10 and 300 mg/kg; both n = 3 per time point) at Zeitgeber time 3:00–9:15 (Zeitgeber time 0:00 = lights on; Zeitgeber time 12:00 = lights off) and plasma samples were taken over 24 h. Data are mean ± standard error of the mean.
Table 1.
Lemborexant pharmacokinetic parameters in mice after dosing for sleep promotion (10 mg/kg, p.o.) and after maximum dosing in the ethanol-induced anesthesia and motor coordination safety paradigms (300 mg/kg, p.o.)
| Parameter | Lemborexant | |
|---|---|---|
| 10 mg/kg, p.o. | 300 mg/kg, p.o. | |
| C max (ng/mL) | 437 | 7,340 |
| t max (h) | 0.5 | 3 |
| AUC(0–24 h) (ng h mL−1) | 998 | 72,400 |
AUC(0–24 h), area under the concentration–time curve from 0 to 24 h; Cmax, maximum concentration; p.o., oral administration; tmax, time to peak concentration after dosing.
Discussion
Effect of single-dose lemborexant on orexin-induced increases in ACTH in rats
In the rat functional assay study, which is a functional in vivo test specific for the OX2R, we found that lemborexant inhibited the [Ala11, D-Leu15]-orexin B-induced increase in plasma ACTH concentration in a dose-related manner. Further, the unbound plasma IC50 of 5.9 nmol/L is in good agreement with our previously reported [54] receptor binding assay-derived IC50 of 2.6 nmol/L for human OX2R and the cell-based functional assay-derived inhibitory constant (Ki) values for human OX2R (0.61 nmol/L) and rat OX2R (0.66 nmol/L). Of note, no species difference for antagonists against human and rat orexin receptors has been reported [54]. Taken together with the previously reported [54] results from an off-target panel binding assay, where lemborexant up to 10 μmol/L did not show interaction with any of the corticotropin releasing factor or neuropeptide Y receptors tested, our findings indicate that lemborexant exerts a central physiological effect via antagonism of OX2R and does not engage a target located downstream of OX2R.
Effect of single-dose lemborexant on spontaneous locomotor activity in wild-type and orexin neuron-deficient mice
We found that lemborexant reduced spontaneous locomotor activity in wild-type, but not in orexin neuron-deficient mice. Interestingly, we did not see a clear dose–response relationship with lemborexant in wild-type mice; this likely reflects a ceiling effect due to the high doses used. Indeed, we previously reported a similar effect with regards to wakefulness reduction in mice [53].
In our study, orexin neuron-deficient mice had lower spontaneous locomotor activity at baseline (after vehicle treatment) compared with wild-type mice. This finding contrasts with those from two previous studies in which there was no notable difference in spontaneous locomotor activity and sleep/wake patterns between wild-type and orexin neuron-deficient orexin/ataxin-3 Tg mice in the light phase [7, 61]. However, the two previous studies were performed under fully habituated conditions, as opposed to shortly after mice were introduced to a novel open-field environment in our study. As we aimed to detect a wakefulness-decreasing effect on drug treatment in our paradigm, high locomotor activity due to exposure to an unknown environment was desirable. Nevertheless, our finding that orexin neuron deficiency leads to decreased locomotor activity on exposure to a novel environment warrants further investigation. Notably, on treatment with lemborexant, and unlike their wild-type counterparts that have a functioning orexin signaling pathway, orexin neuron-deficient mice did not show a significant reduction in locomotor activity. Taken together with the previously reported finding that central application of both OXA and OXB in rats increases spontaneous locomotor activity [62], our finding indicates that the decrease in spontaneous locomotor activity in wild-type mice after treatment with lemborexant is mediated via the orexin signaling pathway and encouraged us to proceed with sleep assessment studies.
Effect of single-dose lemborexant on vigilance state measures in wild-type and orexin neuron-deficient mice and in rats
Consistent with the reduction in spontaneous locomotor activity, we found that lemborexant also reduced wakefulness and promoted sleep in mice and rats. Both active comparators (almorexant and zolpidem) also promoted sleep, with zolpidem reducing REM sleep as expected [63, 64], thus validating our experimental paradigm. In mice, the sleep-promoting effect of lemborexant was observed in orexin neuron-containing wild-type mice in the form of a significant increase in total sleep time. Such an effect was not observed in orexin neuron-deficient orexin/ataxin-3 Tg mice. In addition to expressing OX1R and OX2R [65], these mice have been shown to have functional orexin receptors in studies where the administration of OXA reduced the narcolepsy symptom cataplexy [13] and where dosing with a non-peptide OX2R-selective agonist decreased the narcolepsy symptom sleep-onset REM sleep [66]. Our finding contrasts with those of a previous study in which the DORA almorexant promoted sleep in orexin/ataxin-3 Tg mice, albeit at a lower level than in wild-type mice, and exacerbated cataplexy [67]. This finding, termed “paradoxical” by the authors, was hypothesized to be a reflection of low-level residual orexinergic tone in these mice, which was functionally nullified by almorexant. The precise reason for the differential effects of almorexant and lemborexant in orexin/ataxin-3 Tg mice cannot be explained at this point; however, we do believe that the lack of an effect with lemborexant is reasonable given the mechanism of orexin receptor antagonism. Potential explanations include extensive separate breeding, rendering the orexin/ataxin-3 Tg lines incomparable, and/or differences in OX2R dissociation kinetics. Specifically, almorexant dissociates much more slowly from the OX2R than lemborexant (dissociation constant, 0.0022 and 0.0626 per minute; dissociation half-life, 309 and 11.1 min, for almorexant and lemborexant, respectively) [54].
As already reported [53], the CSF concentration of lemborexant in mice 3 h after oral administration of lemborexant (10 mg/kg) is 7.6 nmol/L, which is similar to the cell-based functional assay-derived Ki value on the mouse OX1R (8.3 nmol/L), and exceeds the Ki value on the mouse OX2R (0.64 nmol/L) [54]. Pharmacokinetic and pharmacodynamics data in mice appear to be in good agreement.
In rats, we found that the sleep-promoting dose of lemborexant (30 mg/kg) 2 h after dosing was associated with a CSF concentration (9.5 nmol/L) that exceeded the previously published [54] cell-based functional assay-derived Ki values for lemborexant for rat OX2R (0.66 nmol/L) and OX1R (7.7 nmol/L). Thus, there is good agreement between the rat pharmacokinetic and pharmacodynamic data.
Of note, lemborexant did not alter the REM sleep ratio, whereas zolpidem showed clear suppression of REM sleep at high doses. As DORAs block both orexin receptors, promotion of both REM and non-REM sleep is expected; our findings indicate that lemborexant has a proportional effect on both types of sleep. This notion is supported by our findings with the DORA almorexant, which, consistent with previous reports [64], also did not alter the REM sleep ratio.
There is some controversy in the literature regarding whether a DORA or a 2-SORA provides optimal sleep promotion. Preclinical data from studies in rats indicate that: (1) the 2-SORAs JNJ-10397049 [68] and JNJ-42847922 [50] promote non-REM sleep, but not REM sleep; (2) simultaneous administration of a 1-SORA might in part counteract the sleep promotion achieved by a 2-SORA [47]; and (3) this simultaneous administration might overpromote REM sleep relative to non-REM sleep to an extent that REM-related cataplexy symptoms like sleep-onset REM sleep may result [68]. However, to date, there is no evidence to suggest that REM sleep promotion by a sleep drug would be detrimental (assuming there is no overpromotion to the point of REM sleep-related abnormalities). Indeed, in the studies described herein, we saw no sleep-onset REM sleep episodes at any of the lemborexant or almorexant doses tested. Further, we observed no change in the REM sleep ratio relative to vehicle treatment, indicating, as already noted, that there was proportional promotion of non-REM and REM sleep by both DORAs, and no overpromotion of REM. Of note, in a separate study, and in contrast to findings with the DORA almorexant, neither the 1-SORA SB-334867 nor the 2-SORA EMPA significantly reduced the latency to non-REM sleep in rats [64].
Recent results from a phase 2 study of the 2-SORA seltorexant (MIN-202/JNJ-42847922) revealed that selective blockade of the OX2R in humans reduced sleep latency and increased sleep efficiency [69], findings which are in keeping with those reported for suvorexant [70] and lemborexant [55]. However, in contrast to preclinical data in rats, this 2-SORA clearly reduced latency to REM sleep, as well as increasing time spent in REM sleep, which is consistent with the previously discussed purported role of OX2R in REM sleep gating [8, 14] and with observations in phase 2 studies of suvorexant [70] and lemborexant [55]. Upcoming results from phase 3 clinical trials will provide important information concerning the DORA versus 2-SORA comparison regarding sleep promotion.
Effect of chronic-dose lemborexant on vigilance state measures in rats
As in the single dose study, we found that 21 days of treatment with lemborexant (30 mg/kg/day) had an immediate-onset sleep-promoting effect, evident as an increase in sleep time and a reduction in sleep latency, as assessed in the 2 h immediately after dosing. In regard to this immediate sleep-promoting effect, we did not see a change in effect size with chronic dosing, nor did we see any influence on sleep composition. In contrast, in the 2 h after dosing, zolpidem (100 mg/kg) caused tolerance, was associated with increased wakefulness when treatment stopped, and also changed sleep composition. These data suggest that, different from zolpidem, the immediate sleep-promoting efficacy of lemborexant may be sustained with extended treatment. The caveat of this experimental paradigm is that it does not allow conclusions on sleep/wake changes outside of the 2 h immediately after dosing.
Effect of single-dose lemborexant on ethanol-induced anesthesia and motor coordination in wild-type mice
Our single-dose studies in wild-type mice demonstrated that lemborexant, at doses up to 300 mg/kg, did not prolong ethanol-induced anesthesia or impair motor coordination. These safety findings are particularly noteworthy and contrast with those associated with zolpidem treatment, which prolonged ethanol-induced anesthesia and impaired motor coordination at doses equal or lower than needed for sleep promotion. The 16.8-fold difference between the maximum concentration values after oral dosing with the effective dose for sleep promotion (10 mg/kg) and the maximum dose in the safety paradigm assessments (300 mg/kg) indicates that lemborexant has a sufficient safety margin in mice.
Conclusion
In conclusion, the key results from the in vivo rodent studies described in this publication demonstrate that lemborexant affects sleep/wake regulation, enabling sleep, notably both REM and non-REM sleep equally, through the orexin peptide signaling pathway. Further, lemborexant did not potentiate the sedative effects of alcohol or impair motor coordination, showing a good safety margin in rodents. These and previously reported [53, 54] preclinical findings supported further [55] and ongoing clinical evaluation of lemborexant as a potential treatment for insomnia disorder.
Authors Contributions
All authors were involved in designing and conducting the studies, collecting and analyzing the data, interpreting the study results, drafting and critically revising the article, and approved of the final version of the article.
Funding
This research was supported by Eisai Co., Ltd. Medical writing assistance was provided by Luke Carey, PhD, of ProScribe—Envision Pharma Group, and was funded by Eisai Co., Ltd. ProScribe’s services complied with international guidelines for Good Publication Practice (GPP3).
Conflict of interest statement. CTB, MN, TU, and SA are employees of Eisai Co., Ltd. MS is employee of EA Pharma Co., Ltd. CTB, TU, and SA are shareholders of Eisai Co., Ltd.
Supplementary Material
Acknowledgments
We would like to express our gratitude to Prof. Dr Takeshi Sakurai (University of Tsukuba, Japan, at the time) for kindly providing orexin/ataxin-3 Tg/+ mice. We also thank Masahiro Bando for statistical analysis, and Margaret Moline and Nancy Bower for help with the article.
References
- 1. Ozminkowski RJ, et al. . The direct and indirect costs of untreated insomnia in adults in the United States. Sleep. 2007;30(3):263–273. [DOI] [PubMed] [Google Scholar]
- 2. Kessler RC, et al. . Insomnia and the performance of US workers: results from the America Insomnia Survey. Sleep. 2011;34(9):1161–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Equihua AC, et al. . Orexin receptor antagonists as therapeutic agents for insomnia. Front Pharmacol. 2013;4:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Inutsuka A, et al. . The physiological role of orexin/hypocretin neurons in the regulation of sleep/wakefulness and neuroendocrine functions. Front Endocrinol (Lausanne). 2013;4:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sakurai T, et al. . Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92(4):573–585. [DOI] [PubMed] [Google Scholar]
- 6. Chemelli RM, et al. . Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98(4):437–451. [DOI] [PubMed] [Google Scholar]
- 7. Hara J, et al. . Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron. 2001;30(2):345–354. [DOI] [PubMed] [Google Scholar]
- 8. Willie JT, et al. . Distinct narcolepsy syndromes in orexin receptor-2 and orexin null mice: molecular genetic dissection of non-REM and REM sleep regulatory processes. Neuron. 2003;38(5):715–730. [DOI] [PubMed] [Google Scholar]
- 9. Lin L, et al. . The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98(3):365–376. [DOI] [PubMed] [Google Scholar]
- 10. Beuckmann CT, et al. . Expression of a poly-glutamine-ataxin-3 transgene in orexin neurons induces narcolepsy-cataplexy in the rat. J Neurosci. 2004;24(18):4469–4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tabuchi S, et al. . Conditional ablation of orexin/hypocretin neurons: a new mouse model for the study of narcolepsy and orexin system function. J Neurosci. 2014;34(19):6495–6509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hagan JJ, et al. . Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proc Natl Acad Sci U S A. 1999;96(19):10911–10916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mieda M, et al. . Orexin peptides prevent cataplexy and improve wakefulness in an orexin neuron-ablated model of narcolepsy in mice. Proc Natl Acad Sci U S A. 2004;101(13):4649–4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mieda M, et al. . Differential roles of orexin receptor-1 and -2 in the regulation of non-REM and REM sleep. J Neurosci. 2011;31(17):6518–6526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ritchie C, et al. . Hypocretin ligand deficiency in narcolepsy: recent basic and clinical insights. Curr Neurol Neurosci Rep. 2010;10(3):180–189. [DOI] [PubMed] [Google Scholar]
- 16. Peyron C, et al. . A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med. 2000;6(9):991–997. [DOI] [PubMed] [Google Scholar]
- 17. Thannickal TC, et al. . Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27(3):469–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sakurai T. The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nat Rev Neurosci. 2007;8(3):171–181. [DOI] [PubMed] [Google Scholar]
- 19. Peyron C, et al. . Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18(23):9996–10015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Horvath TL, et al. . Hypocretin (orexin) activation and synaptic innervation of the locus coeruleus noradrenergic system. J Comp Neurol. 1999;415(2):145–159. [PubMed] [Google Scholar]
- 21. Yamanaka A, et al. . Orexins activate histaminergic neurons via the orexin 2 receptor. Biochem Biophys Res Commun. 2002;290(4):1237–1245. [DOI] [PubMed] [Google Scholar]
- 22. Fadel J, et al. . Anatomical substrates of orexin-dopamine interactions: lateral hypothalamic projections to the ventral tegmental area. Neuroscience. 2002;111(2):379–387. [DOI] [PubMed] [Google Scholar]
- 23. Marcus JN, et al. . Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435(1):6–25. [DOI] [PubMed] [Google Scholar]
- 24. Tyree SM, et al. . Hypocretin as a hub for arousal and motivation. Front Neurol. 2018;9:413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Eban-Rothschild A, et al. . Neuronal mechanisms for sleep/wake regulation and modulatory drive. Neuropsychopharmacology. 2018;43(5):937–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Adamantidis AR, et al. . Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450(7168):420–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tsunematsu T, et al. . Acute optogenetic silencing of orexin/hypocretin neurons induces slow-wave sleep in mice. J Neurosci. 2011;31(29):10529–10539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tsunematsu T, et al. . Long-lasting silencing of orexin/hypocretin neurons using archaerhodopsin induces slow-wave sleep in mice. Behav Brain Res. 2013;255:64–74. [DOI] [PubMed] [Google Scholar]
- 29. Sakurai T, et al. . Input of orexin/hypocretin neurons revealed by a genetically encoded tracer in mice. Neuron. 2005;46(2):297–308. [DOI] [PubMed] [Google Scholar]
- 30. Yoshida K, et al. . Afferents to the orexin neurons of the rat brain. J Comp Neurol. 2006;494(5):845–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Xie X, et al. . GABA(B) receptor-mediated modulation of hypocretin/orexin neurones in mouse hypothalamus. J Physiol. 2006;574(Pt 2):399–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li Y, et al. . Hypocretin/orexin excites hypocretin neurons via a local glutamate neuron—a potential mechanism for orchestrating the hypothalamic arousal system. Neuron. 2002;36(6):1169–1181. [DOI] [PubMed] [Google Scholar]
- 33. Yamanaka A, et al. . Regulation of orexin neurons by the monoaminergic and cholinergic systems. Biochem Biophys Res Commun. 2003;303(1):120–129. [DOI] [PubMed] [Google Scholar]
- 34. Saper CB, et al. . Sleep state switching. Neuron. 2010;68(6):1023–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mileykovskiy BY, et al. . Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron. 2005;46(5):787–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lee MG, et al. . Discharge of identified orexin/hypocretin neurons across the sleep-waking cycle. J Neurosci. 2005;25(28):6716–6720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Takahashi K, et al. . Neuronal activity of orexin and non-orexin waking-active neurons during wake-sleep states in the mouse. Neuroscience. 2008;153(3):860–870. [DOI] [PubMed] [Google Scholar]
- 38. Tyree SM, et al. . Optogenetic investigation of arousal circuits. Int J Mol Sci. 2017;18(8):E1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Li SB, et al. . Optical probing of orexin/hypocretin receptor antagonists. Sleep. 2018;41(10):zsy141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ren S, et al. . The paraventricular thalamus is a critical thalamic area for wakefulness. Science. 2018;362(6413):429–434. [DOI] [PubMed] [Google Scholar]
- 41. Yoshida Y, et al. . Fluctuation of extracellular hypocretin-1 (orexin A) levels in the rat in relation to the light-dark cycle and sleep-wake activities. Eur J Neurosci. 2001;14(7):1075–1081. [DOI] [PubMed] [Google Scholar]
- 42. Zeitzer JM, et al. . Circadian and homeostatic regulation of hypocretin in a primate model: implications for the consolidation of wakefulness. J Neurosci. 2003;23(8):3555–3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Salomon RM, et al. . Diurnal variation of cerebrospinal fluid hypocretin-1 (orexin-A) levels in control and depressed subjects. Biol Psychiatry. 2003;54(2):96–104. [DOI] [PubMed] [Google Scholar]
- 44. Kay DB, et al. . Hyperarousal and beyond: new insights to the pathophysiology of insomnia disorder through functional neuroimaging studies. Brain Sci. 2017; 7(3):E23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nofzinger EA, et al. . Functional neuroimaging evidence for hyperarousal in insomnia. Am J Psychiatry. 2004;161(11):2126–2128. [DOI] [PubMed] [Google Scholar]
- 46. Brisbare-Roch C, et al. . Promotion of sleep by targeting the orexin system in rats, dogs and humans. Nat Med. 2007;13(2):150–155. [DOI] [PubMed] [Google Scholar]
- 47. Dugovic C, et al. . Blockade of orexin-1 receptors attenuates orexin-2 receptor antagonism-induced sleep promotion in the rat. J Pharmacol Exp Ther. 2009;330(1):142–151. [DOI] [PubMed] [Google Scholar]
- 48. Winrow CJ, et al. . Promotion of sleep by suvorexant-a novel dual orexin receptor antagonist. J Neurogenet. 2011;25(1–2):52–61. [DOI] [PubMed] [Google Scholar]
- 49. Yoshida Y, et al. . Design, synthesis, and structure-activity relationships of a series of novel N-aryl-2-phenylcyclopropanecarboxamide that are potent and orally active orexin receptor antagonists. Bioorg Med Chem. 2014;22(21):6071–6088. [DOI] [PubMed] [Google Scholar]
- 50. Bonaventure P, et al. . Characterization of JNJ-42847922, a selective orexin-2 receptor antagonist, as a clinical candidate for the treatment of insomnia. J Pharmacol Exp Ther. 2015;354(3):471–482. [DOI] [PubMed] [Google Scholar]
- 51. Winrow CJ, et al. . Discovery and development of orexin receptor antagonists as therapeutics for insomnia. Br J Pharmacol. 2014;171(2):283–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sutton EL. Profile of suvorexant in the management of insomnia. Drug Des Devel Ther. 2015;9:6035–6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yoshida Y, et al. . Discovery of (1R,2S)-2-{[(2,4-Dimethylpyrimidin-5-yl)oxy]methyl}-2-(3-fluorophenyl)-N-(5-fluoropyridin-2-yl)cyclopropanecarboxamide (E2006): a potent and efficacious oral orexin receptor antagonist. J Med Chem. 2015;58(11):4648–4664. [DOI] [PubMed] [Google Scholar]
- 54. Beuckmann CT, et al. . In vitro and in silico characterization of lemborexant (E2006), a novel dual orexin receptor antagonist. J Pharmacol Exp Ther. 2017;362(2):287–295. [DOI] [PubMed] [Google Scholar]
- 55. Murphy P, et al. . Lemborexant, a dual orexin receptor antagonist (DORA) for the treatment of insomnia disorder: results from a Bayesian, adaptive, randomized, double-blind, placebo-controlled study. J Clin Sleep Med. 2017;13(11):1289–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Jászberényi M, et al. . Effects of orexins on the hypothalamic-pituitary-adrenal system. J Neuroendocrinol. 2000;12(12):1174–1178. [DOI] [PubMed] [Google Scholar]
- 57. Jászberényi M, et al. . The role of neuropeptide Y in orexin-induced hypothalamic-pituitary-adrenal activation. J Neuroendocrinol. 2001;13(5):438–441. [DOI] [PubMed] [Google Scholar]
- 58. Asahi S, et al. . Development of an orexin-2 receptor selective agonist, [Ala(11), D-Leu(15)]orexin-B. Bioorg Med Chem Lett. 2003;13(1):111–113. [DOI] [PubMed] [Google Scholar]
- 59. Radulovacki M, et al. . Adenosine analogs and sleep in rats. J Pharmacol Exp Ther. 1984;228(2):268–274. [PubMed] [Google Scholar]
- 60. Steiner MA, et al. . Differential effects of the dual orexin receptor antagonist almorexant and the GABAA-α1 receptor modulator zolpidem, alone or combined with ethanol, on motor performance in the rat. Neuropsychopharmacology. 2011;36(4):848–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sagawa Y, et al. . Wake-promoting effects of ONO-4127Na, a prostaglandin DP1 receptor antagonist, in hypocretin/orexin deficient narcoleptic mice. Neuropharmacology. 2016;110(Pt A):268–276. [DOI] [PubMed] [Google Scholar]
- 62. Matsuzaki I, et al. . Involvement of the serotonergic system in orexin-induced behavioral alterations in rats. Regul Pept. 2002;104(1–3):119–123. [DOI] [PubMed] [Google Scholar]
- 63. Renger JJ, et al. . Sub-chronic administration of zolpidem affects modifications to rat sleep architecture. Brain Res. 2004;1010(1–2):45–54. [DOI] [PubMed] [Google Scholar]
- 64. Morairty SR, et al. . Dual hypocretin receptor antagonism is more effective for sleep promotion than antagonism of either receptor alone. PLoS One. 2012;7(7):e39131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Mishima K, et al. . Hypocretin receptor expression in canine and murine narcolepsy models and in hypocretin-ligand deficient human narcolepsy. Sleep. 2008;31(8):1119–1126. [PMC free article] [PubMed] [Google Scholar]
- 66. Irukayama-Tomobe Y, et al. . Nonpeptide orexin type-2 receptor agonist ameliorates narcolepsy-cataplexy symptoms in mouse models. Proc Natl Acad Sci U S A. 2017;114(22):5731–5736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Black SW, et al. . Almorexant promotes sleep and exacerbates cataplexy in a murine model of narcolepsy. Sleep. 2013;36(3):325–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Dugovic C, et al. . Orexin-1 receptor blockade dysregulates REM sleep in the presence of orexin-2 receptor antagonism. Front Neurosci. 2014;8:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. De Boer P, et al. . A randomized phase 2 study to evaluate the orexin-2 receptor antagonist seltorexant in individuals with insomnia without psychiatric comorbidity. J Psychopharmacol. 2018;32(6):668–677. [DOI] [PubMed] [Google Scholar]
- 70. Herring WJ, et al. . Orexin receptor antagonism for treatment of insomnia: a randomized clinical trial of suvorexant. Neurology. 2012;79(23):2265–2274. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.