Abstract
Background
The aim of this study was to evaluate the diagnostic value of C-reactive protein (CRP) test in detecting neonatal septicemia.
Material/Methods
We searched the Cochrane Library, PubMed, Springer, MBASE, Elsevier Science Direct, and Medline databases up to March 2017. To collect relevant data on CRP testing in patients with neonatal septicemia, we performed a meta-analysis of positive likelihood ratio (LR), sensitivity, negative LR, specificity and diagnostic odds ratio (dOR) of CRP testing, using Stata 12.0 and Meta-DiSc 1.4 data analysis software.
Results
Ten studies including 1819 participants were considered in this study. We found that positive LR, sensitivity, negative LR, specificity, and dOR of the CRP test for neonatal septicemia were 5.63 (95% CI=2.86 to 11.09), 0.70 (95% CI=0.66 to 0.75), 0.36 (95% CI=0.21 to 0.60), 0.89 (95% CI=0.87 to 0.91), and 17.99 (95% CI=6.50 to 49.83), respectively. The AUC and Q* index of this meta-analysis were 0.90 and 0.83, respectively.
Conclusions
The area under the curve (AUC), negative LR, positive LR, Q* index, specificity, and dOR of the CRP test suggest that it is appropriate for detecting neonatal septicemia.
MeSH Keywords: C-Reactive Protein, Meta-Analysis, Neonatal Screening
Background
Neonatal sepsis is the leading cause of morbidity and mortality of children during the neonatal period worldwide [1–5]; therefore, early diagnosis and timely treatment are needed. However, lack of clinical specificity can delay correct diagnosis. It is therefore of great importance to detect reliable biomarkers for early diagnosis of neonatal septicemia [6]. In the intensive care unit, neonatal septicemia, which is mainly caused by drug-resistant bacteria, is not only life-threatening, but may also lead to long-term sequelae [7]. C-reactive protein (CRP) is a sensitive indicator of inflammation in humans; it activates the complement system and promotes granulocyte and macrophage phagocytosis, which is the most commonly used test for diagnosis of neonatal sepsis.
Whether the diagnostic value of the CRP test is appropriate for detecting neonatal septicemia is controversial [8–11]. The objective of this study was to assess the diagnostic accuracy of CRP as a single test for the early detection of neonatal sepsis. We performed a meta-analysis to assess the sensitivity, specificity, positive LR, negative LR, and diagnostic odds ratio (dOR) in patients tested by CRP.
Material and Methods
Source of material
We searched the electronic databases Cochrane Library, PubMed, Springer, MBASE, Elsevier Science Direct, and Medline (up to October 2017) using the following terms: “C-reactive protein” or “CRP” or “neonatal septicemia” or “neonatal sepsis” and “diagnosis” or “diagnostic” and “study” or “trial” or “research”, limiting the search to English-language articles.
Study selection
Inclusion criteria were: (1) The internal standard mainly pertains to investigations of patients with neonatal septicemia; (2) The diagnosis of neonatal septicemia was pathologically confirmed, (3) The CRP test used for diagnosis for neonatal septicemia was included in the report; and the effect size included positive LR, sensitivity, negative LR, specificity, and dOR. We excluded reviews, case reports, and duplicate studies.
Date extraction
A standardized reporting form was used to abstract the data from each study, including study year, year of the publication, country, cutoff value, case/control, detection of CRP, TP, and FP FN TN. Data were extracted independently by 2 investigators. The results were compared and disagreements were resolved by consensus.
Evaluation of quality
Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) criteria were evaluated to determine the quality of included studies [12,13]. The QUADAS identifies 4 key domains – “patient selection”, “index test”, “reference standard”, and “flow and timing” – which are combined to assess the risk of bias.
Meta-analysis methods
A summarized receiver operating characteristic curve (SROC) was used to represent the performance of the diagnostic test [14]. The SROC curve includes multiple points, and the cutoff points are determined by selecting the maximum point, which is the sum of the sensitivity and the specificity [15]. The area under the curve (AUC) and exponential Q* are potential useful summaries of the curve. Based on the exact analysis of the expression, the upper limit is derived and the lower limit of the Q* is based on the limit, which is defined by the sensitivity equal to the feature point: Q* is not equal to heterogeneity [14]. We measured the asymmetry of the funnel by the natural logarithmic scale of effect size, and we used Egger linear regression [16] to assess publication bias.
We performed statistical analysis using STATA software package v.13.0 (Stata Corporation, College Station, TX, USA). All P values are bilateral. P values less than 0.05 were considered to be statistically significant.
Results
Characteristics of eligible studies
Our literature search identified 588 papers in total. The flow chart of the literature screening is shown in Figure 1. After deleting irrelevant or duplicate papers, a total of 65 possible studies were found. After reading the abstracts, we excluded 38 articles (19 for the commentary; 11 for CRP testing; 8 did not report neonatal sepsis). The remaining 27 studies were assessed in full, and 17 of them were excluded (12 did not apply to CRP test and 5 were not available); therefore, we finally included 10 papers met our criteria.
Figure 1.
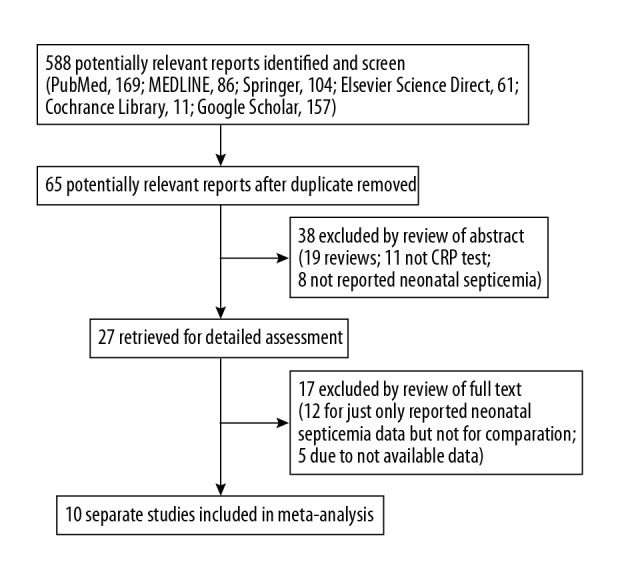
Flow diagram for selection of studies for the meta-analysis.
As is shown in Table 1, there were 10 studies [8–11,17–22] in which sample sizes were between 26 and 1002 and CRP threshold values were between 5.82 and 10 mg/L. Quality assessment is shown in Table 2. In combination with this meta-analysis, the accepted criterion standard for diagnosis of neonatal septicemia includes confirmation by blood culture, and thus entry 7 does not apply; CRP test results were interpreted by instruments, so entry 9 does not apply.
Table 1.
Characteristics of studies included in the meta-analysis.
| Study | Year of publication | Country | Cutoff value (mg/L) | Case/ control | Detection of CRP | CRP test | |||
|---|---|---|---|---|---|---|---|---|---|
| TP | FP | FN | TN | ||||||
| Sharma A, et al. [20] | 1993 | India | >6 | 10/16 | LA | 8 | 1 | 2 | 15 |
| Benitz WE, et al. [17] | 1998 | American | ≥10 | 20/982 | NA | 7 | 98 | 13 | 884 |
| Manucha V, et al. [19] | 2002 | India | >6 | 21/129 | LA | 16 | 27 | 5 | 102 |
| Vazzalwar R, et al. [21] | 2005 | American | >8 | 18/16 | NA | 14 | 6 | 4 | 10 |
| Schrama A, et al. [11] | 2008 | The Netherlands | >10 | 24/55 | NA | 22 | 1 | 2 | 54 |
| Boo NY, et al. [18] | 2008 | Malaysia | NA | 18/69 | NA | 10 | 7 | 8 | 62 |
| Zaki Mel-S, et al. [22] | 2009 | Egypt | >8 | 58/62 | PENIA | 50 | 2 | 8 | 60 |
| Celik IH, et al. [10] | 2010 | Turkey | >5.82 | 170/50 | ITM | 121 | 1 | 49 | 49 |
| Hotoura E, et al. [9] | 2011 | Greece | >10 | 25/50 | FNM | 16 | 11 | 9 | 39 |
| Choo YK, et al. [8] | 2012 | Korea | ≥10 | 12/14 | Standard sterile techniques | 1 | 2 | 11 | 12 |
TP – true positive; FP – false positive; FN – false negative; TN – true negative; NA – not available; PENIA – particle-enhanced nephelometric immunoassay; LA – latex agglutination; ITM – immune turbidimetric method; FNM – flow nephelometry method.
Table 2.
Quality assessment of the included articles.
| QUADAS list item | Reference number of the included studies | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| 1. Did the spectrum of patients represent the patients who will receive the test in practice? | + | 0 | + | 0 | + | + | 0 | + | + | + |
| 2. Were selection criteria clearly described? | + | + | + | + | + | + | + | + | + | + |
| 3. Is the reference standard likely to correctly classify the target condition? | + | + | + | + | + | + | + | + | + | + |
| 4. Is the period between the reference standard and index test short enough to be reasonably sure that the target condition did not change between the 2 tests? | + | 0 | + | + | + | + | + | + | 0 | + |
| 5. Did the entire sample or a random selection of the sample receive verification using a reference standard of diagnosis? | + | + | + | + | + | + | + | + | + | + |
| 6. Did patients receive the same reference standard regardless of index test result? | + | + | + | + | + | + | + | + | + | + |
| 7. Was the reference standard independent of the index test (i.e., index test did not form part of the reference standard)? | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 8. Was execution of the index test described in sufficient detail to permit replication of the test? | + | + | + | + | + | + | + | + | + | + |
| 9. Was execution of the reference standard described in sufficient detail to permit its replication? | + | + | + | 0 | + | + | + | + | + | + |
| 10. Were index test results interpreted without knowledge of results of the reference standard? | 0 | 0 | + | + | 0 | + | + | + | + | + |
| 11. Were reference standard results interpreted without knowledge of results of the index test? | 0 | + | + | 0 | 0 | + | + | + | + | + |
| 12. Were the same clinical data available when test results were interpreted as would be available when the test is used in practice? | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 13. Were uninterruptable/intermediate test results reported? | 0 | 0 | + | + | + | 0 | + | 0 | + | + |
| 14. Were withdrawals from the study explained? | + | + | + | + | + | + | + | + | + | + |
Analysis of diagnostic threshold
The cutoff values used in the included studies (see Table 1, column 4) cause differences in sensitivity and specificity, called threshold effects. A premise of our study is that there is no threshold effect in the combination of sensitivity, specificity, positive likelihood ratio, negative likelihood ratio, and other indicators. The first step in the meta-analysis of diagnostic tests is to explore the threshold effect and other heterogeneity sources. Using Meta-disc software, the Spearman correlation coefficient for the study was −0.418 and the p value was 0.229, suggesting that there was no threshold effect in this study.
Overall effects of diagnostic parameters of CRP test for neonatal septicemia in the meta-analysis
The overall results of our meta-analysis of neonatal septicemia with CRP is summarized in Table 3. A total of 10 studies were included in the study, including 1819 participants. We used the random-effects model (Q2=12.98, I2=84.6%, P<0.01) to combined the data on true positive (TP), false positive (FP), false negative (FN), and true negative (TN). The overall estimates of the meta-analysis showed that the CRP test may be appropriate for detecting neonatal septicemia among patients, in which sensitivity, specificity, positive LR, negative LR, and dOR were 0.70 (95% CI=0.66 to 0.75), 0.89 (95% CI=0.87 to 0.91), 5.63 (95% CI=2.86 to 11.09), 0.36 (95% CI=0.21 to 0.60), and 17.99 (95% CI=6.50 to 49.83), respectively. The Q* index and AUC were 0.83 and 0.90, respectively (Figure 2).
Table 3.
The indexes of neonatal septicemia diagnosed by CRP test.
| Parameter | Test of association | Test of heterogeneity | Model | Egger’s test for publication bias | ||||
|---|---|---|---|---|---|---|---|---|
| Estimates | 95% CI | Q | P value | I2 (%) | t | P value | ||
| Overall | – | – | 12.98 | <0.01 | 84.6 | Random | −1.87 | 0.10 |
| Sensitivity | 0.70 | 0.66 to 0.75 | 49.47 | <0.01 | 81.8 | – | – | – |
| Specificity | 0.89 | 0.87 to 0.91 | 43.03 | <0.01 | 79.1 | – | – | – |
| Positive LR | 5.63 | 2.86 to 11.09 | 49.54 | <0.01 | 81.8 | Random | – | – |
| Negative LR | 0.36 | 0.21 to 0.60 | 100.94 | <0.01 | 91.1 | Random | – | – |
| dOR | 17.99 | 6.50 to 49.83 | 41.98 | <0.01 | 78.6 | Random | – | – |
LR – likelihood ratio; dOR – diagnostic odds ratio.
Figure 2.
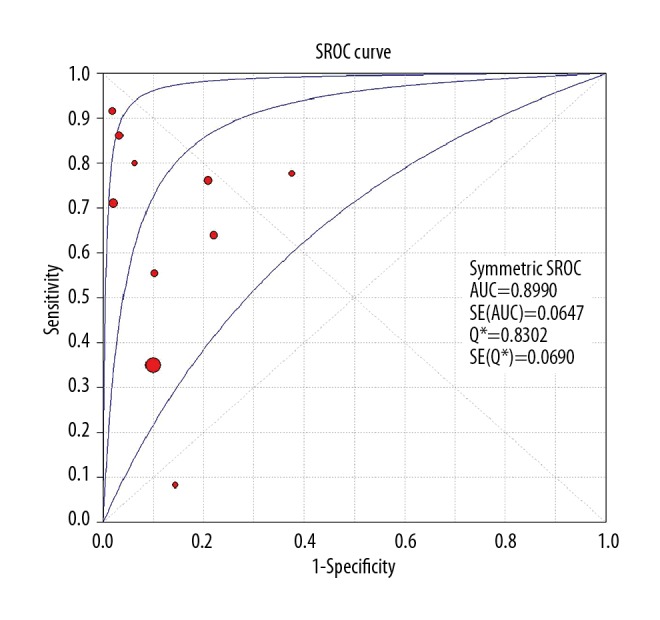
The summary receiver operating characteristic (SROC) curve of CRP test.
Publication bias
Egger’s test was performed to assess the publication bias of our study, showing there was no publication (t=−1.87, P>0.05).
Discussion
This meta-analysis evaluated the diagnostic value of the CRP test in detecting neonatal septicemia. After a comprehensive analysis of 10 papers, we found that sensitivity, specificity, positive LR, negative LR, and dOR of the CRP test for neonatal septicemia were 0.70 (95% CI=0.66 to 0.75), 0.89 (95% CI=0.87 to 0.91), 5.63 (95% CI=2.86 to 11.09), 0.36 (95% CI=0.21 to 0.60), and 17.99 (95% CI=6.50 to 49.83), respectively, which suggest that the CRP test has a good diagnostic value for neonatal sepsis.
Neonatal sepsis, which is defined as a 30-day infection after birth, remains an important clinical syndrome and is characterized by symptomatic systemic illness. Disease progression in neonates is rapid and mortality and morbidity rates are high [10]. Clinically inoculation of premature neonates with sepsis and late-onset neonatal sepsis were divided into 3 days and 4–28 days after birth [23]. Due to diagnostic procedures, early identification of neonatal sepsis is still a global problem [8]. Studies have shown that serum procalcitonin (PCT) has high specificity and sensitivity in diagnosing early neonatal sepsis, but the cost is high.
Under normal circumstances, serum CRP levels are very low; the body of a person infected by bacteria due to WBC and other inflammatory cells releases endogenous neurotransmitters to stimulate liver cells. Synthesis of CRP occurs within 4–6 h and peaks at 36~50 h, so the inflammatory process generally begins 6–12 h after the detection of CRP [24]. Neonatal sepsis, in which bacteria invade the blood, release toxins, and stimulates systemic inflammatory response, can lead to elevated CRP.
There are several limitations of this study that should be discussed. Neonatal sepsis was not divided into early-onset and late-onset in this study. In early-onset neonatal sepsis, bacteria are derived from the intrauterine and postpartum periods, and these pathogens are concentrated. For example, in Australia, 80% of sepsis occurs within 48 h of birth and is mostly caused by B streptococcus (GBS) and gram-negative bacteria, so the time is defined at 48 h in order to guide the clinical selection of antibiotics and predict prognosis. In addition, significant between-study heterogeneities were detected in our meta-analysis, and this may have affected our results. Heterogeneity is one of the main problems of the meta-analysis method [25], mainly due to misleading outcomes due to non-uniform data. In addition, the studies we included had differences in populations, including healthy newborns, premature children, low-birth-weight children, high-risk factors, neonatal hemolysis, intracranial hemorrhage, and wet lungs, and these differences may have affected our results. Different CRP detection methods, neonatal gestational age, and other factors may also have affected the results of this study.
Conclusions
The CRP test appears to be appropriate for use in detecting neonatal septicemia. The CRP test can help diagnose neonatal septicemia to guide rational drug use in clinical practice.
Acknowledgments
We would like to thank all respondents of the study and all the people who gave assistance in this study.
Footnotes
Source of support: Departmental sources
Conflict of interest
None.
References
- 1.Ullah O, Khan A, Ambreen A, et al. Antibiotic sensitivity pattern of bacterial isolates of neonatal septicemia in Peshawar, Pakistan. Arch Iran Med. 2016;19:866–69. doi: 10.1002/sim.4099. [DOI] [PubMed] [Google Scholar]
- 2.Shivanna V, Sunkappa SR, Venkatesha D. The rising trend of coagulase-negative staphylococci in neonatal septicemia. Indian J Pathol Microbiol. 2016;59:510–12. doi: 10.4103/0377-4929.191806. [DOI] [PubMed] [Google Scholar]
- 3.Muley VA, Ghadage DP, Bhore AV. Bacteriological profile of neonatal septicemia in a tertiary care hospital from Western India. J Glob Infect Dis. 2015;7:75–77. doi: 10.4103/0974-777X.154444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang ZL, Yu JL. [Recent progress in the diagnosis of neonatal septicemia]. Zhongguo Dang Dai Er Ke Za Zhi. 2013;15:236–41. [in Chinese] [PubMed] [Google Scholar]
- 5.Jyothi P, Basavaraj MC, Basavaraj PV. Bacteriological profile of neonatal septicemia and antibiotic susceptibility pattern of the isolates. J Nat Sci Biol Med. 2013;4:306–9. doi: 10.4103/0976-9668.116981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu YQ, Shen J, Zhou QL, et al. Interleukin-6 and interleukin-8 in diagnosing neonatal septicemia. J Biol Regul Homeost Agents. 2016;30:1107–13. [PubMed] [Google Scholar]
- 7.Joseph PV, Haridas AK. Neonatal osteomyelitis with pathologic fracture of mandible following MRSA septicemia: Management and three year follow up. J Maxillofac Oral Surg. 2015;14:77–80. doi: 10.1007/s12663-011-0312-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choo YK, Cho HS, Seo IB, Lee HS. Comparison of the accuracy of neutrophil CD64 and C-reactive protein as a single test for the early detection of neonatal sepsis. Korean J Pediatr. 2012;55:11–17. doi: 10.3345/kjp.2012.55.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hotoura E, Giapros V, Kostoula A, et al. Tracking changes of lymphocyte subsets and pre-inflammatory mediators in full-term neonates with suspected or documented infection. Scand J Immunol. 2011;73:250–55. doi: 10.1111/j.1365-3083.2010.02499.x. [DOI] [PubMed] [Google Scholar]
- 10.Celik IH, Demirel FG, Uras N, et al. What are the cut-off levels for IL-6 and CRP in neonatal sepsis? J Clin Lab Anal. 2010;24:407–12. doi: 10.1002/jcla.20420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schrama AJ, de Beaufort AJ, Poorthuis BJ, et al. Secretory phospholipase A(2) in newborn infants with sepsis. J Perinatol. 2008;28:291–96. doi: 10.1038/sj.jp.7211929. [DOI] [PubMed] [Google Scholar]
- 12.Bradley LA, Palomaki GE, Gutman S, et al. Comparative effectiveness review: Prostate cancer antigen 3 testing for the diagnosis and management of prostate cancer. J Urol. 2013;190:389–98. doi: 10.1016/j.juro.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 13.Yu T, Meng N, Chi D, et al. Diagnostic value of dynamic contrast-enhanced magnetic resonance imaging in detecting residual or recurrent prostate cancer after radical prostatectomy: A pooled analysis of 12 individual studies. Cell Biochem Biophys. 2015;72:687–94. doi: 10.1007/s12013-015-0519-6. [DOI] [PubMed] [Google Scholar]
- 14.Walter SD. Properties of the summary receiver operating characteristic (SROC) curve for diagnostic test data. Stat Med. 2002;21:1237–56. doi: 10.1002/sim.1099. [DOI] [PubMed] [Google Scholar]
- 15.Altman DG, Bland JM. Diagnostic tests 3: Receiver operating characteristic plots. BMJ. 1994;309:188. doi: 10.1136/bmj.309.6948.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benitz WE, Han MY, Madan A, Ramachandra P. Serial serum C-reactive protein levels in the diagnosis of neonatal infection. Pediatrics. 1998;102:E41. doi: 10.1542/peds.102.4.e41. [DOI] [PubMed] [Google Scholar]
- 18.Boo NY, Nor Azlina AA, Rohana J. Usefulness of a semi-quantitative procalcitonin test kit for early diagnosis of neonatal sepsis. Singapore Med J. 2008;49:204–8. [PubMed] [Google Scholar]
- 19.Manucha V, Rusia U, Sikka M, et al. Utility of haematological parameters and C-reactive protein in the detection of neonatal sepsis. J Paediatr Child Health. 2002;38:459–64. doi: 10.1046/j.1440-1754.2002.00018.x. [DOI] [PubMed] [Google Scholar]
- 20.Sharma A, Kutty CV, Sabharwal U, et al. Evaluation of sepsis screen for diagnosis of neonatal septicemia. Indian J Pediatr. 1993;60:559–63. doi: 10.1007/BF02751434. [DOI] [PubMed] [Google Scholar]
- 21.Vazzalwar R, Pina-Rodrigues E, Puppala BL, et al. Procalcitonin as a screening test for late-onset sepsis in preterm very low birth weight infants. J Perinatol. 2005;25:397–402. doi: 10.1038/sj.jp.7211296. [DOI] [PubMed] [Google Scholar]
- 22.Zaki Mel S, el-Sayed H. Evaluation of microbiologic and hematologic parameters and E-selectin as early predictors for outcome of neonatal sepsis. Arch Pathol Lab Med. 2009;133:1291–96. doi: 10.5858/133.8.1291. [DOI] [PubMed] [Google Scholar]
- 23.Ansari S, Nepal HP, Gautam R, et al. Neonatal septicemia in nepal: Early-onset versus late-onset. Int J Pediatr. 2015;2015 doi: 10.1155/2015/379806. 379806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McWilliam S, Riordan A. How to use C-reactive protein. Arch Dis Child Educ Pract Ed. 2010;95:55–58. doi: 10.1136/adc.2009.174367. [DOI] [PubMed] [Google Scholar]
- 25.Moreno SG, Sutton AJ, Thompson JR, et al. A generalized weighting regression-derived meta-analysis estimator robust to small-study effects and heterogeneity. Stat Med. 2012;31:1407–17. doi: 10.1002/sim.4488. [DOI] [PubMed] [Google Scholar]


