Summary
Although several therapeutic approaches are available for wound and burn treatment and much progress has been made in this area, room for improvement still exists, driven by the urgent need of better strategies to accelerate wound healing and recovery, mostly for cases of severe burned patients. Bacterial cellulose (BC) is a biopolymer produced by bacteria with several advantages over vegetal cellulose, such as purity, high porosity, permeability to liquid and gases, elevated water uptake capacity and mechanical robustness. Besides its biocompatibility, BC can be modified in order to acquire antibacterial response and possible local drug delivery features. Due to its intrinsic versatility, BC is the perfect example of a biotechnological response to a clinical problem. In this review, we assess the BC main features and emphasis is given to a specific biomedical application: wound dressings. The production process and the physical–chemical properties that entitle this material to be used as wound dressing namely for burn healing are highlighted. An overview of the most common BC composites and their enhanced properties, in particular physical and biological, is provided, including the different production processes. A particular focus is given to the biochemistry and genetic manipulation of BC. A summary of the current marketed BC‐based wound dressing products is presented, and finally, future perspectives for the usage of BC as wound dressing are foreseen.
Introduction
Cellulose is the most abundant naturally occurring polymer obtained from renewable sources. It consists of a linear homopolysaccharide composed by β‐d‐glucopyranose units linked by β‐1,4 glycosidic bonds (Cannon and Anderson, 1991). The most commercially exploited natural source of cellulose is wood, due to its high availability that meets the demands of the paper industry (Klemm et al., 2005). However, a variety of plants also contains large amounts of cellulose, such as hemp, flax or cotton (Fernandes et al., 2017). In addition to these sources, cellulose can be produced, among others, by seaweed, fungi and some species of bacteria, being the most noteworthy the non‐pathogenic bacteria of the genus Komagateibacter, such as K. xylinus, former Acetobacter and Gluconacetobacter (Brown, 1886). Several strains of K. xylinus produce extracellular cellulose forming a biofilm of varying thickness with the purpose of maintaining a high oxygenation of the colonies near the surface, which serves as a protective barrier against drying, natural enemies and radiation.
Besides being biodegradable, non‐toxic and biocompatible, one of the major advantages of bacterial cellulose (BC) over vegetal cellulose is its unique native purity that allows for its direct utilization. It is chemically equivalent to plant cellulose, but it is free of by‐products such as lignin, pectin, hemicellulose and other constituents of lignocellulosic materials (Klemm et al., 2001; Rahman and Netravali, 2016). It is obtained by fermentation and only contains microbial cells, nutrients and other secondary metabolites that can be easily removed, yielding highly pure cellulose. Although BC molecular formula is similar to plant cellulose, BC mechanical and physical outstanding properties emerge from its unique 3D structure that differs significantly from that of vegetal source, as BC aggregates to form long fibrils of 1.5 nm width, providing higher surface area, elasticity, resistance and flexibility. BC presents a unique reticulate network of thin fibres with a diameter more than 100 times smaller than that of plant‐derived fibres (Klemm et al., 2005).
BC has many intrinsic characteristics that make it an ideal scaffold for protecting injured tissues through wound dressings, especially for burn wounds, tissue regeneration and as temporary skin substitutes. Some of advantageous features of BC for these particular applications are the fact that it is non‐toxic, non‐carcinogenic and biocompatible, and it has the capacity to retain moisture, absorb exudates from the injured tissue and accelerate granulation (Li et al., 2015; Khalid et al., 2017).
The largest organ in the human body is the skin. Three layers, the epidermis, dermis and the fat layer, also known as hypodermis, compose the skin. The epidermis is the external layer of the skin, having the critical function of maintaining homeostasis of the body internal environment and at the same time protecting the body from the external environment and from potential pathogenic bacteria. The dermis is where all the blood vessels, nerves, hair follicles, oil and sweat glands are located. In its native state, skin is dry and acidic in nature (pH between 4 and 6.8) being keratinocytes the skin cells responsible for producing skin lipids and to maintain hydration levels. Altered skin integrity may be due to systemic factors, such as the nutritional status of the individual, vascular disease, heart conditions and diabetes, among others, or to extrinsic episodes such as accidents, immobility, pressure and surgical procedures. When an individual suffers severe damage to large areas of skin, such as burns, he is exposed to decreased local function, dehydration and infections that can result in loss of limb and sometimes death. The wound healing process comprises a complex series of biological processes aiming to restore the skin barrier function (prevent dehydration and bacterial infection). However, skin regeneration and wound healing can be slow and lead to chronic inflammation, especially in burn patients with additional systemic impairments (Rowan et al., 2015).
In 2016, the American Burn Association reported 486 000 burn‐related injuries receiving medical treatment in the United States, including 40 000 hospitalizations (American Burn Association, 2016). Although the survival rate has considerably increased to 96.8%, severe burn injuries are difficult to manage, requiring extended hospital stays and expensive treatment choices (American Burn Association, 2016). The current standard of care for closing burn wounds and preventing wound sepsis includes early surgical excision of the damaged necrotic tissue followed by complete coverage of the exposed area (Rowan et al., 2015).
Another relevant skin condition for wound dressing application is cancer lesions, such as basal‐cell carcinoma or skin lesions related to chemotherapy and radiation therapy. Larger lesions are usually vascularized and necrotized, being responsible for high amounts of drainage.
For all these skin lesion conditions, the perfect wound dressing must maintain the moisture of the wound, allow for oxygen exchange, adsorb wound exudate, accelerate re‐epithelialization for wound closure, reduce pain and healing time, and prevent infection. However, also additional and specific treatments adapted to the needs of each individual lesion are needed. All these requirements demand for a creative, integrative and flexible dressing application.
In health‐related applications, natural‐derived polymers present several advantages when compared with synthetic ones, such as biocompatibility, biodegradability and/or biological activity, as most of them are present in the structural tissues of living organisms. Currently worldwide, burn wound and skin graft donor site treatments vary widely, and a large number of different wound dressing materials are available for their treatment (Voineskos et al., 2009). BC features, such as high porosity, high water retention capacity, high mechanical strength in the wet state, low density, biocompatibility, non‐toxicity and biodegradability, make BC an outstanding material that is suitable for technological applications, particularly in the fields of biomedicine and pharmacology (Hu et al., 2014).
BC has already been used quite successfully in wound healing applications, proving that it could become a high‐value product in the field of biotechnology (Klemm et al., 2001; Czaja et al., 2006). In fact, biomedical devices have gained a significant amount of attention because of an increased interest in tissue‐engineered products for both wound care and the regeneration of damaged or diseased organs (Czaja et al., 2007). The use of BC as a wound dressing scaffold material saw light during the early 1980s (Fontana et al., 1990) and has been constantly improved.
Cellulose biosynthesis in bacteria is a multistep process involving individual genes and an operon called bcsABCD (bacterial cellulose synthesis), first identified in K. xylinus (Umeda et al., 1999), encoding proteins and enzymes that associate the linear polymerization of glucose with the formation of a 3D structure of cellulose (Ross et al., 1991). Although bcsA and bcsB are essential genes, the maximum yield of BC production is only achieved with the expression of the entire operon, in which composition and structural organization are highly diverse among species (Karnezis et al., 2003; Perez‐Mendoza et al., 2015).
The biosynthetic pathway of the cellulose exopolysaccharide begins with the isomerization of a glucose 6‐phosphate molecule into glucose 1‐phosphate. This intermediary reacts with UTP, forming uridine‐5′‐diphosphate‐alfa‐D‐glucose (UDP‐glucose), that is polymerized into linear ‐ 1,4 glucan chains in a reaction catalysed by cellulose synthase A that is activated by cyclic‐di‐GMP, into linear ‐ 1,4 glucan chains. The newly formed cellulose chains are then secreted across the cell wall through 50–80 extrusion pores, aligned along the cell long axis as illustrated in Fig. 1 (Kimura et al., 2001; Krasteva et al., 2017).
Figure 1.
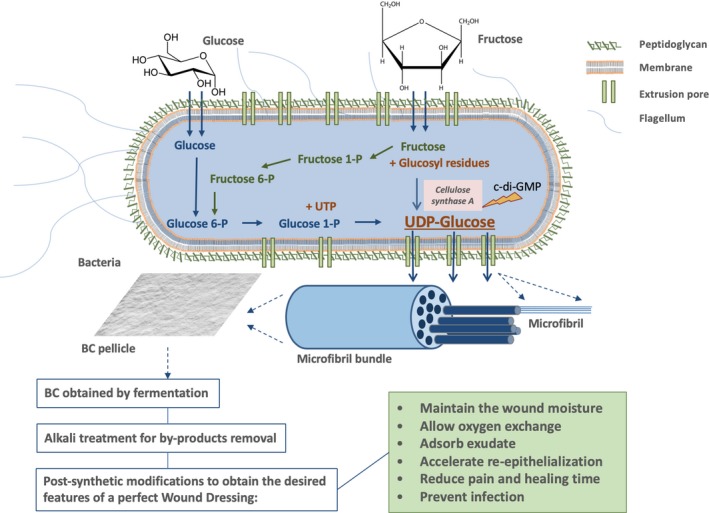
Schematic depiction of the steps involved in the production of a BC‐based wound dressing, from the molecular mechanism of UDP‐glucose biosynthesis in bacteria to the BC post‐synthetic modifications performed, highlighting the three‐dimensional structure formed by the secreted chains of glucose and the features desired to be present in a wound dressing‐based material.
After completing this first degree of organization – polymer formation – the linear chains are assembled into nanofibres of 10–15 polymer chains and are subsequently arranged into microfibrils (100 times smaller than those of plant cellulose) and then into microfibril bundles. The grouping of such bundles results in cellulose ribbons of 3–4 nm thickness and 70–80 wideness (Fig. 1), creating a 3D network that is stabilized through hydrogen bonds that establish intra‐ and interchemical links between the sheets of cellulose, forming a thick and gelatinous membrane characterized by a high mechanical strength (Fig. 2A) combined with a high malleability that allows it to perfectly mould to the wounded area (Fig. 2B) (Chawla et al., 2009).
Figure 2.
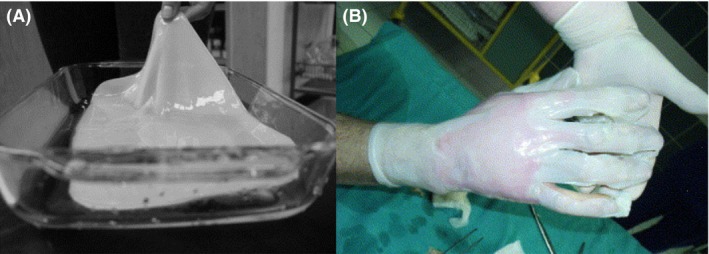
Bacterial cellulose.
A. Hydrated BC membrane with high mechanical strength.
B. BC wound dressing applied on a wounded hand. The BC's physical properties permits an excellent moulding to the wounded area (image courtesy of Center of Burn Healing, Siemianowice Slaskie, Poland), Reprinted from Biomaterials, Vol 27 (2), W. Czaja, A. Krystynowicz, S. Bielecki, R.M. Brown Jr., Microbial cellulose — the natural power to heal wounds, Pages No. 145–151, Copyright (2006), with permission from Elsevier.
Intrinsic features of bacterial cellulose
Mechanical properties of bacterial cellulose
The structural arrangement of the BC fibres confers mechanical properties that differentiate BC from plant‐derived cellulose, including a higher degree of crystallinity (84–89%) (Czaja et al., 2004) and absence of other contaminating polymers, allowing for a simple process of purification (Sani and Dahman, 2009). Also, the numerous hydroxyl groups that are available in glucose allow to establish interactions with over 90% of water molecules, which results in a high capacity of water retention (Gelin et al., 2007).
Despite the natural features of the BC polymer, the physical properties of BC strongly depend on the manufacturing and processing conditions. For instance, for dried samples of BC, it was found that typically, BC presents a tensile strength of around 240 MPa, a Young modulus of around 10 GPa and a maximum strain in the order of 3%, although it is estimated that the modulus of a single filament of BC can be as high as 114 GPa (Fernandes et al., 2013). The presented values show some dispersion between studies, for example, it is obtained for dried samples of BC a tensile strength of 400 MPa and an elongation at break of 10 % (Klemm et al., 2005). On hydrated BC samples (98% water content) of dry‐fabricated BC biofilm, the mechanical properties were in the order of: tensile strength of 380 kPa, maximum strain of 21% and a water vapour transmission rate of 2900 g m−2 day−1 (Fernandes et al., 2017). Also, for these samples the compression modulus was determined and found to be approximately 0.06 MPa (Klemm et al., 2001). Regarding the morphological properties on hydrated samples, it was found that BC had a specific surface area of approximately 60 m2 g−1, a specific pore volume of around 0.2 cm3 g−1 and an average pore diameter of around 13 nm (Qiao et al., 2015).
The above‐described characteristics of BC, together with the biocompatibility, non‐toxicity, cost‐effectiveness, formability, softness and the fact that the synthesis of BC can be highly adjusted for the optimization of these features and to incorporate secondary components, such as antibiotics into its pores, prove the existence of the fundamental properties ideal for wound dressing applications. In this sense, rheological characterization appears as a valuable tool to the design and optimization of BC materials, allowing to determine their response when subjected to a shear stress (Rebelo et al., 2018) and access, for instance, the time‐dependant rheological behaviour in such systems (Gao et al., 2016; Basu et al., 2017) or the elastic character of BC in function of its water content (Rebelo et al., 2018).
Bacterial cellulose water holding/release capacity
Some of the main features required for wound dressings, in particular for burn treatment, are the water content and water‐retaining properties, in order to maintain the wound hydrated, as well as to be able to absorb large amounts of exudates.
BC has a complex molecular structure, with water molecules bonded through hydrogen bonds. The BC fibres are composed of linear chains of glucan units linked through β‐1,4 glycosidic bonds. The glucan chains are linked through inter‐ and intramolecular hydrogen bonds, allowing BC to be mechanically robust while maintaining elasticity. The free water (unbonded) that is able to penetrate and to exit the BC molecular structure is responsible for the maintenance of the hydration level that is crucial for the wound dressing application (Fig. 3A).
Figure 3.
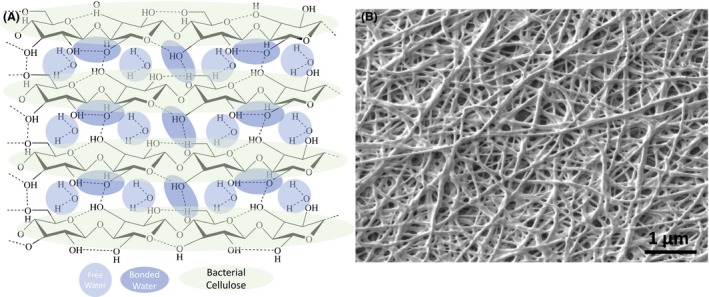
Bacterial cellulose.
A. Molecular structure of hydrated BC.
B. Typical microscopic BC fibre film morphology.
Proper moisture control usually increases the healing rates, shields the wound from infections, diminishes pain and reduces the global healthcare expenses (Agarwal et al., 2011). Furthermore, water absorption and holding capacities allow to charge liquid drugs and bioactive compounds on the wound dressing material (Shah et al., 2013). The capability to maintain humidity also avoids the dehydration of the wound dressing and so prevents it from attaching to the wound, thus defending the tissue from exposure and diminishing the pain throughout the dressing exchange (Ovington, 2007). A typical SEM image of a non‐woven BC fibre structure is presented in Fig. 3B.
The presence of exudates is known to cause separation of the tissue layers of the wound, which makes the healing process slower. Therefore, exudates should be eliminated from the wound (Hedlund, 2007) and good drainage capacity emerges as a decisive criterion in the application of dressings. However, it is necessary to ensure that the wound maintains the necessary hydration for the healing process to occur in the best manner, requiring the dressing to balance the absorption and release of liquid (Davidson, 2015). With this aim, the properties of the dressing with respect to the ability to retain water and the ability to release water have been the subject of several studies to characterize new BC systems that can be applied to wound dressings. The water holding capacity (WHC) and water release rate (WRR) values appear as quantitative physical parameters for this evaluation, which are strongly dependent on the physicochemical and on the structural characteristics of the BC system. In particular, the available surface area and pore size distribution (Ul‐Islam et al., 2012), as well as the presence of hydrophilic additives in the BC system, are known to introduce significant changes in WHC and WRR values. Another quality index for wound dressings is the water vapour transmission rate (WVTR). An excessively high WVTR accelerates the dehydration and scabbing of a wound, whereas an excessively low WVTR causes wound fluids to accumulate, impedes healing and raises the risk of bacterial contamination. A desirable WVTR is 2500 –3000 g m−2 day−1 (Paul and Sharma, 2004; Li et al., 2014a).
BC water holding capacity ranges from 60 to 700 times of its dry weight, depending on the synthesis conditions. In typical statically cultured pellicles, BC represents approximately 1% of the total weight, with the rest being water (Yamanaka et al., 1989; Okiyama et al., 1992). A possible explanation for such high hydrophilicity level relates to the fact that the assembly of the cellulose ribbons occurs extracellularly, in the liquid medium, and numerous micelles are then formed trapping large amounts of liquid. Moreover, the hydrophilicity of the BC pellicle results in part from the wide internal surface area of the interstitial space of the wet pellicle. In fact, upon drying, BC exhibits poor rehydration due to high crystallinity, restricting its applications as a dressing material (Huang et al., 2010). In order to improve this point, different strategies have been implemented, altering the BC structure with the aim of increasing its water holding/release capacity.
Bacterial cellulose structure – pore size and fibre morphology
The BC WHC relates directly with the available pore volume and surface area. A more compact BC structure, presenting denser fibril arrangements with reduced pore volume and surface area (Wang et al., 2012), is associated with a decrease in the WHC values. In such fibril structures, the available space and number of trapping sites to capture water molecules are diminished. On the other hand, a more compact BC structure is associated with lower WRR values. The denser microfibrils result in a greater amount of water retained in the system due to the formation of hydrogen bonds and a smaller amount of free bulk water (Gelin et al., 2007), which prevents water evaporation. Knowing these counteracting effects associated with the BC structure on the ability to hold and to release water, the possibility to access and adjust the BC structural parameters during the biosynthesis process or by post‐synthetic modifications has been the focus of several studies during the last decade. Alteration of the fermentation settings, such as culture growth conditions, culture media components and the presence of specific additives in the BC synthetic process, causes changes in the structural properties, namely in the crystallinity and in the fibre morphology (Sulaeva et al., 2015).
Investigations on the dependence of BC water holding and retention ability on structural characteristics showed that a reduction in pore size and surface area, obtained through BC modification, resulted in a decrease in WHC and in an increase in WRR (Ul‐Islam et al., 2012). In this study, the BC structure was modified by the addition of a single sugar‐linked glucuronic acid‐based oligosaccharide (SSGO) in the culture media and also via post‐synthetic treatment with inorganic montmorillonite clay. This direct relationship between WHC and pore size was also demonstrated in other works. A more compact BC structure with reduced porosity was obtained via a synthetic procedure in the presence of hydroxypropyl methylcellulose (HPMC), showing a reduced WHC value (Huang et al., 2011). On the contrary, BC structures with enhanced porosity presented a greater WHC value. BC synthesized in the presence of carboxymethylcellulose (CMC) in the culture media revealed a network with broader ribbons, due to the adhesion of CMC into the surface of BC fibrils, with greater WHC (Chen et al., 2016). An increased pore size distribution was also found in a BC structure with loose fibril arrangement (Grande et al., 2009), which corresponded to a greater WHC value (Seifert et al., 2004; Yu et al., 2011).
Higher porosity and therefore increased water holding/release ability can also be reached by post‐synthetic modifications of BC. A highly porous BC structure capable to absorb at least seven times more water than pure BC resulted from the use of foaming agents (Yin et al., 2012). Paximada et al. observed that a short ultrasonic pre‐treatment (1 min) applied to BC aqueous suspensions induced fibrils’ breakdown, which resulted in the increase in WHC, along with the viscosity and the solid‐like character of the samples (Paximada et al., 2016).
Mechanical and electrochemical properties were studied for BC under different water contents (100%, 80% and 50%), for which a progressive stiffening and increasing resistance with lower capacitance were observed after partial dehydration. A theoretical model for predicting BC water loss was developed and applied, which allows an understanding of the structural changes presented by post‐dried BC (Rebelo et al., 2018).
Bacterial cellulose structure – synthesis components
In general, pore size reduction can be obtained by the addition of a secondary component into the BC fibre network, causing pore filling. Yet, WHC and WRR can be influenced by the nature of the secondary component itself.
The presence of highly hydrophilic chitosan promoted the absorption of larger amounts of water in BC/chitosan composites, when compared with native BC, even though with a reduced porosity. WHC was increased due to the capability of chitosan molecules to establish hydrogen bonds at the same time with BC fibrils and with water molecules. Simultaneously, WRR was increased, caused by the reduced porosity. These results highlight the importance of additive choice when directing the characteristics of BC towards the desired dressing need (Ul‐Islam et al., 2012).
The progressively greater water absorption capacity of BC/chitosan films with increasing chitosan content was early described by Phisalaphong and Jatupaiboon (2008); Phisalaphong et al. (2008). The chitosan molar mass was also observed to influence WHC, where a greater WHC was associated with a higher molar weight. A more compact structure with smaller pore size was described for BC/chitosan composites by Lin et al. (2013). WHC and WRR presented no significant difference when comparing modified samples with pure BC, although the dressings obtained from modified BC provided a suitable moisture environment for wounds with low‐ and mid‐range amounts of exudate.
The influence of other highly hydrophilic compounds on WHC and WRR was also reported. The incorporation of alginate in the BC structure gave rise to a nanoporous structure and to a decrease in pore diameter, but an increase in water uptake ability was described (Phisalaphong et al., 2008). The increased WHC observed in such BC/alginate films was justified by Chiaoprakobkij et al., who associated the disruption of the hydrogen bonds between cellulose fibres to the mixture with the other component. The greater capability to water uptake observed in dried sponges was considered to better support exudate adsorption (Chiaoprakobkij et al., 2011).
Aloe vera gel also appeared as an advantageous component to be included in wound dressings. When introduced to the BC structure during the biosynthesis process at a gel content of less than 30%, aloe vera gel increased the WHC by about 1.5‐fold compared to the non‐modified material. Improvement of water vapour permeability was also observed in addition of BC/aloe vera gel (Saibuatong and Phisalaphong, 2010).
Recently, Chang and Chen (2016) characterized the physical properties of HOBC/chitosan/alginate films for wound dressing. The WVTR of BC films prepared using gel solutions of 98.0% and 98.5% water content was 2865 and 3034 g m−2 day−1, respectively, and was close to the ideal dressing. However, the lower fluidity of a gel solution with 98.0% water content was not favourable for moulding. The gel solution with 98.5% water content exhibited the most desirable mechanical properties, hydrophilicity and WVTR.
The composites BC/hydrophilic additive presented similar properties to the systems BC/chitosan, BC/alginate and BC/aloe vera gel, showing the possibility to access the adjustment of WCH and WRR during composite preparation and the production of materials with efficient dressing characteristics.
Other possible systems considered hydrophilic synthetic polymers to produce BC composites for wound dressing applications. For example, BC modified with glycerine, used as a plasticizer, was observed to promote an excellent skin moisturizing effect. Good biocompatibility and enhanced malleability suggested the use of this material in dry wound treatment, such as those appearing due to psoriasis and atopic dermatitis (Almeida et al., 2014).
The liquid absorption characteristics of BC may be considerably altered by adding hydrophilic synthetic polymers, prepared as anion exchange membranes, such as poly‐AEM. The BC/poly‐AEM composites presented increased swelling capacity, from 100% up to 6200%, in comparison with the non‐modified BC and with the composite material respectively. Such swelling performance resulted from the conjugation of the hydrophilic nature of the synthetic component together with its capacity to prevent the collapse of the BC structure during the drying process (Figueiredo et al., 2015).
BC/acrylic hydrogels also presented great improvement in the swelling ratio of up to 4000–6000%. These results were observed in in vivo experiments, in which the use of BC composites confirmed promotion of burn healing with enhanced epithelialization and fibroblast proliferation (Mohamad et al., 2014). Such BC composites were considered as a promising material for burn dressing.
Bacterial cellulose composites
A composite is a material that combines at least two distinct materials, with a clear interface between them, acquiring complementary properties of its constituents. Typically, the aim of producing composite materials is to provide the base material with properties (from the reinforcement) that it did not possess by itself, and that are required for a specific application. In most cases, these properties could not be achieved with the isolated base component. The reinforcing phase, which may consist of fibres (Lazarini et al., 2016), particles (Galateanu et al., 2015), sheets (layers) (Foong et al., 2018), interpenetrating networks (Lin et al., 2014) or cells, is dispersed in the so‐called matrix or continuous phase (usually the one presenting a higher percentage). The properties of the composites depend on both the nature of the materials employed and the degree of bonding between them through the interface. All type of materials can be used to produce composites ranging from polymers to ceramics or metals.
BC has drawn attention in applications such as a wound dressing material, due to its intrinsic properties, namely its hydrophilicity, very high purity, porosity, biocompatibility and, since it also exhibits a network structure, controlled drug release capability that can be availed. In order to improve its efficiency as a wound dressing material or to provide it with tailored properties or functionalities, strategies have relied on the exploitation of its natural properties and improvement as in the case of tensile strength, biocompatibility and water uptake, among others. Nevertheless, BC alone does not present several desirable characteristics, such as antibacterial activity or anti‐inflammatory properties. In this way, in addition to the improvement of the natural features, new properties have been introduced into BC, mainly through the development of BC composites (Fig. 4). In Table 1, a summary of examples of BC composites is presented in which the reinforcement meets the wound dressing function that was intended to be enhanced.
Figure 4.
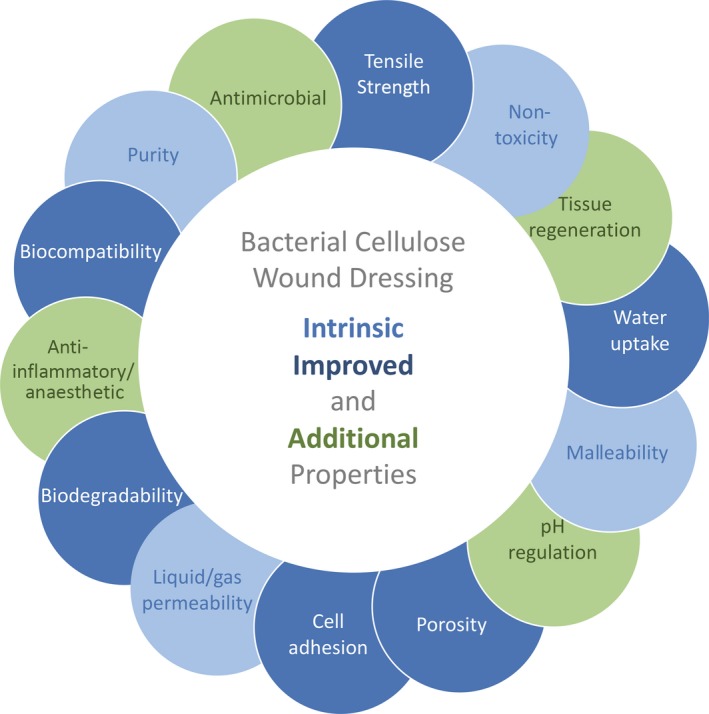
Main improvements of bacterial cellulose for wound dressing applications. Light blue – intrinsic properties of BC that present advantages for wound dressing applications. Dark blue – intrinsic properties of BC that suffered improvements. Green – additional properties that were introduced into BC for wound healing improvement.
Table 1.
Examples of BC composites and the respective improved properties
| BC reinforcement | Improved Function | References |
|---|---|---|
| Poly(vinyl alcohol) (PVA) | Enhancement of the mechanical performances | Castro et al. (2015) and Qiao et al. (2015) |
| Dehydrogenative polymer of coniferyl alcohol (DHP) | Improving the antibacterial activity | Zmejkoski et al. (2018) |
| Silver nanoparticles | Improving the antibacterial activity | Volova et al. (2018), Tabaii and Emtiazi (2018), Shao et al. (2016a,2016b), Wen et al. (2015), Wu et al. (2018) and Pal et al. (2017) |
| Chitosan and alginate | Higher elongation, rehydration, swelling ratios and water vapour transmission | Chang and Chen (2016) |
| Hyaluronan | Improving the thermal stability, lower total surface area and pore volume, weight loss and elongation at break | Li et al. (2014a,2014b, 2015) |
| Acrylic acid | Promoting faster wound healing, enhanced epithelialization and accelerated fibroblast proliferation | Mohamad et al. (2014a,b) |
| Zinc oxide (ZnO) | Improving the antibacterial activity | Janpetch et al. (2016) |
| Arginine | Promoting proliferation, migration and expression of collagen‐I of fibroblasts and endothelial cells | Qiao et al. (2018) |
| Antibiotics | Improving the antibacterial activity | Volova et al. (2018), Shao et al. (2016a,2016b), Lazarini et al. (2016) |
| Magnetic nanoparticles (magnetite) | Improving the efficiency of chronic wounds healing | Galateanu et al. (2015) |
| Agarose | Improving the mechanical properties and water uptake | Awadhiya et al. (2017) |
| Titanium dioxide (TiO2) | Promoting healing and tissue regeneration | Khalid et al. (2017) and Khan et al. (2015) |
| C60 nanoparticles | Improving skin cancer wound therapy | Chu et al. (2018) |
| Poly(lactic acid) (PLA) | Improving the mechanical properties | Foong et al. (2018) |
| BC bilayer with different fibre densities | Improving the controlled release of different antibiotics to treat skin infections. | Lazarini et al. (2016) |
| Gold nanoparticles | Improving the antibacterial efficiency | Li et al. (2017a,2017b) |
| Montmorillonites and silver nanoparticles | Improving the antibacterial efficiency | Li et al. (2017a,2017b) |
| Sodium alginate (SA) | Improving the mechanical properties | Lin et al. (2014) |
| Graphene oxide/silver nanohybrid | Improving the antibacterial efficiency | Mohammadnejad et al. (2018) |
| Plasticizers (PEG and Glycerol) | Improving the physicochemical properties | Sun et al. (2018) |
| Hydrolysed gelatin peptide | Improving the physicochemical properties | Lin et al. (2015a,2015b) |
| Chitosan | Improving the physicochemical and antibacterial properties | Savitskaya et al. (2017), Zhang et al. (2016) |
Physical properties’ optimization
In order to be used as a wound dressing material, the robustness of the BC films is a key issue that has driven efforts to improve their mechanical properties. Qiao et al. (2015) were able to produce a regular and uniformly distributed porous structure with enhanced mechanical properties, through the interaction of BC nanofibres with the PVA polymeric molecules, forming physical cross‐linked composite hydrogels. Chitosan (Ch), N‐deacetylated derivate of chitin, is a natural polysaccharide exhibiting exceptional physicochemical properties such as vapour permeability, antibacterial activity, biocompatibility and outstanding film‐forming capability. When chemically decomposed, chitosan releases N‐acetyl‐β‐d‐glucosamine instigating fibroblast proliferation and controlled collagen deposition, resulting in a quicker wound healing. Nevertheless, films produced from pure chitosan lack on mechanical robustness (brittle) and the cost of chitosan is relatively high, limiting its application.
Chang and Chen (2016) produced a chitosan and alginate BC composite, after treating the BC with hydrogen peroxide, that not only exhibited the desirable mechanical properties but also presented rehydration properties which enabled the usage of these composites as wound dressing materials for exudate absorption and the eventual controlled drug release. Another chitosan composite was proposed by Savitskaya et al. that they modify the BC by immobilization of chitosan, resulting in a composite material containing glucosamine and N‐acetylglucosamine units integrated into the cellulose chain. These composites presented challenging properties such as good mechanical properties, high moisture‐retaining properties and high antibacterial activity against gram‐negative and gram‐positive bacteria. These qualities make BC/Ch composite a candidate not only to be used as a wound dressing material but also for tissue engineering (Savitskaya et al., 2017).
Mohamad et al. developed and characterized BC/acrylic acid hydrogels with the purpose of improving the BC wound healing potential. These composites presented a macroporous network structure having high swelling ratio and high water vapour transmission rate which are important properties in terms of absorbing exudates and providing hydration for healing in particular burn wounds (Mohamad et al., 2014). By dissolving different amounts of magnetite nanoparticles throughout the biosynthesis process, BC composites were produced straight from the BC culture medium. It was proven that the existence of magnetite nanoparticles during the biosynthesis process does not disturbs it. Using this technique, and producing wound dressings from BC/magnetite composites, it was possible to improve the physical, chemical, morphological and biological properties of pure BC (Galateanu et al., 2015). Besides its antibacterial activity, silver nanoparticle composites proved to increase the physical properties of pure BC. The silver nanoparticles can be synthetized inside the porous three‐dimensional BC structure; this network is then irradiated with UV light so that the silver nanoparticles are photochemically deposited onto the BC hydrogel. The silver nanoparticles stay chemically bonded to the cellulose fibre surfaces, presenting a narrow size distribution along the BC. Since the composite pellicles are conserved in a moist environment, the wound healing is more efficient (Pal et al., 2017). Agarose is a biodegradable polymer with limited mechanical robustness and excessive water uptake. These properties limit its usage as wound dressing but when used in a composite with for instance BC, very challenging properties can be achieved. BC/agarose composites proved to present good mechanical and swelling properties, thermal stability and biodegradability, making them suitable as wound dressing materials (Awadhiya et al., 2017). Poly(lactic acid) (PLA) has been suggested as coating material (concentrations below 10%). This layer exhibits low moisture uptake, prolonged swelling simulated body fluid, high tear and burst indices. Foong et al. demonstrated that incorporating 8% of PLA on BC makes the composite more suitable to use as a wound dressing with antimicrobial properties. Using a BC/PLA composite, they were able to improve the mechanical properties, maintaining a reasonable wetting time. Also, they observe a preferable surface morphology on a microscopic level (the PLA coating changed into a more fibrous and porous morphology) with a low moisture uptake and prolonged swelling behaviour in simulated environment (Foong et al., 2018).
Alginate is a biomaterial that was already applied in several biomedical applications due to its profitable properties, such as biocompatibility and ease of gelation. Lin et al. presented a BC/sodium alginate (SA) composite having an interpenetrating polymer network structure. This composite presented outstanding swelling ratios, tensile modulus, tensile strength and elongation when compared with pure BC. This study confirmed that the interpenetrating structure radically changes the swelling and mechanical properties of the composite and enables it as a promising candidate for biomedical applications as wound dressings and skin tissue engineering (Lin et al., 2014). It is known that dried BC possesses poor gas permeability and water absorption. In order to improve dry BC physicochemical properties, Sun et al. performed comparative studies using two biocompatible plasticizers with different molecular weight and hydroxyl content, glycerol (G) and polyethylene glycol (PEG). They demonstrated that glycerol and PEG did not only cover the BC microfibres but also expanded the free space among the fibres, creating a highly porous structure. The toughness of the composites was efficiently increased when compared with pure BC, and the water absorption/retention capabilities of the BC composites were considerably higher than dry BC. Furthermore, the highly porous structure formed with the plasticized dry BC composites perfected its water vapour transmission. The plasticized dry BC composites also exhibited excellent resistance against bacteria (Sun et al., 2018).
Biological functions’ optimization
Although having outstanding physical and chemical properties as scaffolds for wound dressing applications, BC native characteristics are not enough to meet the current needs in the dressing material market. Nowadays, it is expected from a dressing material that it has a functional contribution in the healing process. The major complications that frequently arise include the contamination with opportunistic pathogens and subsequent development of infection and inflammation and also the development of tumours that contribute to the development of chronic wounds (Fonder et al., 2008).
The rapid emergence of antibiotic resistance among a high number of bacterial pathogenic species (World Health Organization, 2015) poses an additional problem for patients with chronic or severe burn wounds that are frequently and recurrently hospitalized. The scenario of an open wound is beneficial for nosocomial agents, usually multi‐drug‐resistant strains, that rise due to selective pressure characteristic of healthcare facilities. Bacterial infections are in fact the most common clinical complication, usually associated with skin conditions and play a pivotal role in treatment failure or delay of the healing process, causing patient distress and financial burden. Colonization with bacteria is especially critical in burn wounds since these patients usually have compromised immune systems and a wide disruption of the skin barrier (Calum et al., 2009).
Though BC provides a physical barrier that reduces bacterial penetration into the tissues, in its native form it does not present antimicrobial properties per se (Czaja et al., 2006). To improve its efficiency as a therapeutic agent for treatment or for prophylactic purposes, modifications have been introduced to BC structure or specific compounds were added, to confer diverse biological activities, such as antimicrobial or anti‐inflammatory, to BC wound dressings.
Different approaches have recently been adopted to develop topical functionalized wound dressings with altered composition. Compounds that were described to have been incorporated into BC, at stage of development or preclinical tests, include not only small molecules but also macromolecules and complex polymers. Three main compound‐loading strategies have been used so far, post‐synthesis loading by saturation, by chemical modification of the purified BC structure or through genetic engineering approaches. The choice of the incorporation strategy depends on the physicochemical characteristics of the active compound, such as molecular size, solubility, stability and working concentration, on the type of BC network, like native wet, semidried or freeze‐dried, and also on the bacterial strain used as producer. Importantly, the functionalization method will influence the time‐release rate of the compound.
Lignin‐derived compounds can be used as antibacterial reinforcement in BC composites in order to improve the antibacterial action of the wound dressings. Zmejkoski et al. (2018) used a lignin model polymer (dehydrogenative polymer of coniferyl alcohol) to produce BC composite hydrogels presenting a decrease in the pore number and size and, due to its antimicrobial action, a faster skin repair and decrease of pain in patients. Also, in recent years, due to the emergent threat of bacterial resistance to antibiotics, alternatives such as silver nanoparticle composites have drawn scientific attention. Several types of silver nanoparticles can be employed such as silver sulfadiazine (Shao et al., 2016b) or for instance silver nitrate (Tabaii and Emtiazi, 2018; Wu et al., 2018). These composites displayed excellent antibacterial performances for the most common human pathogens maintaining a good biocompatibility (Wu et al., 2018). The BC/silver nanoparticle composites are proven to be non‐toxic and exhibited good biocompatibility on peripheral blood mononuclear cells due to the controlled silver ion release (Tabaii and Emtiazi, 2018). These composites, containing silver nanoparticles, are transparent, allowing uninterrupted visualization of the wound without having to remove the dressing (Tabaii and Emtiazi, 2018). A comparative study performed on rat models demonstrated that the wound treated with pure BC containing silver nanoparticles presented greater healing rate when compared with BC, proving that these composites are very promising as wound dressing for burns (Wen et al., 2015). Volova et al. (2018) tested for the same purpose the usage of silver nanoparticles and antibiotics (amikacin and ceftriaxone), achieving a strong inhibitory effect on pathogens, without hindering the growth of epidermal cells. Other composites have been tested incorporating antibiotics, such as tetracyclines for improvement of the antibacterial activity (Shao et al., 2016a). It was stated by Lazarini et al. that BC produced in all culture media displays an intrinsic composite formed by a double layer (with different fibre densities) and three‐dimensional fibre network achieved in only one step. This 3D network structure of the bilayer with high‐density fibre entangling, produced in sugarcane molasses medium, is responsible for the greatest holding capacity and sustained release of the antibiotics such as ceftriaxone, used in the case of Staphylococcus aureus bacterial strains (Lazarini et al., 2016). Gold nanoparticle BC composites were said to present better efficacy than most of the antibiotics against gram‐negative bacteria, while preserving outstanding physicochemical properties such as water uptake capacity, high mechanical strain and biocompatibility. The broad antibacterial spectrum of these composites along with the desirable moisture retention and the good mechanical properties enables them as an excellent material for wound dressing (Li et al., 2017b). TiO2 nanoparticles are known for their super‐hydrophilic, chemical stability and biocompatibility (Fujishima et al., 2000). In vivo wound healing efficacy of BC/TiO2 composites was assessed in a burn wound model by measurements of wound area, per cent contraction and histopathology. The results showed that BC/TiO2 composites acquired an outstanding healing potential presenting faster re‐epithelialization degree as well as enhanced wound contraction capability (Khalid et al., 2017). In addition to antibacterial properties, Khan et al. (2015) demonstrated that the BC/TiO2 composites exhibit remarkable cell adhesion and proliferation capabilities with animal fibroblast cells without displaying any toxic effects. Janpetch et al. studied BC/zinc oxide composites for antimicrobial activity enhancement. Zinc oxide (ZnO) is known as an inorganic antibacterial agent and BC proven to be an excellent upholding template for the coordination of ZnO. They demonstrated that the ZnO content in these composites is determinant to improve the disinfection capabilities of BC (Janpetch et al., 2016). Qiao et al. studied BC/arginine composites after oxidizing the BC with a novel technique. High oxidation degree of BC increases the amount of aldehyde, which reduces the cell biocompatibility of BC. Using this new method to oxidize BC and producing arginine composites, they increased the roughness and surface energy of BC and were able to stimulate the propagation, migration and expression of collagen‐I of fibroblasts and endothelial cells (Qiao et al., 2018). Using photodynamic therapy, BC/C60 can be used as wound dressings for skin cancer treatment. The C60 particles, homogeneously distributed in the 3D BC network, proved to possess a high capability to generate reactive oxygen species under light exposure and so inhibit the growth of several bacteria. BC/C60 composites presented low cytotoxicity in the dark; however, they demonstrated substantial cancer cell destruction when exposed to visible light (Chu et al., 2018). Li et al. presented smaller‐sized silver nanoparticles evenly immobilized in montmorillonites, which gave rise to BC composites demonstrating high antimicrobial activity. Besides owing the desirable mechanical and hydrophilic properties, these composites revealed low silver release. Even though the silver release ratio was short, the small particle size of the silver nanoparticles allowed them to more effectively penetrate the bacterial cells and possess high electrostatic affinity to interrelate with the cell membrane to obstruct bacterial growing (Li et al., 2017a). Graphene oxide–silver nanohybrid was used to confer BC antibacterial activity by producing a BC/graphene oxide–silver nanohybrid composite. Mohammadnejad et al. (2018) demonstrated that the presence of graphene oxide–silver nanohybrid increased the mechanical strength and antibacterial activity of BC.
Compound loading by immersion
Directly related to its structural properties, as its microporous structure, large surface area and moisture retention capacity, BC is able to absorb and retain large amounts of active compounds. In the same way, these features of BC allow for the slow release of the compounds into the affected tissue and thus a more prolonged effect.
The loading of purified BC by submersion and saturation is the most frequent choice of compound incorporation, since the procedure is of simple implementation, although time‐consuming. The most reported strategy consists of soaking dried or semidried BC in solutions of the active compound. Through this method has been described the functionalization of BC with antiseptic compounds, such as octenidine, povidone‐iodine (PI) and polyhexanide (PHMB) (Table 2) (Moritz et al., 2014; Wiegand et al., 2015; Alkhatib et al., 2017), and the release of all these compounds relied on diffusion and swelling. Octenidine loading did not affect the tensile strength of the BC matrix that presented a biphasic release profile, as the release rate was faster during the first 8 h and subsequently decreased up to 96 h. Due to its high molar mass, functionalization with PI introduced structural changes in the BC matrix that increased its compressive strength while incorporation of PHMB did not alter the tensile properties of BC. In accordance, PI showed a slower release process in comparison with PHMB. All antiseptic composites showed high biocompatibility in human keratinocytes but different antimicrobial activity against S. aureus, being PI the less active compound.
Table 2.
Examples of incorporated biologically active agents in BC for wound dressings
| Incorporated agent | Incorporation strategy | Therapeutic purpose | References | |
|---|---|---|---|---|
| Antibiotics | ||||
| Fusidic acid | Small molecule | Adsorption by immersion | Antimicrobial activity | Liyaskina et al. (2017) |
| Tetracycline | Small molecule | Adsorption by immersion | Antimicrobial activity | Shao et al. (2016a,2016b) |
| Amoxicillin | Small molecule | Chemical cross‐linking | Antimicrobial activity | Ye et al. (2018) |
| Erythromycin | Small molecule | Adsorption by immersion | Antimicrobial activity | Zywicka et al. (2018) |
| Non‐steroidal anti‐inflammatory drug | ||||
| Diclofenac | Small molecule | Adsorption by immersion | Pain and inflammation relief | Silva et al. (2014) |
| Ibuprofen | Pain and inflammation relief | Trovatti et al. (2012) | ||
| Local anaesthetic | ||||
| Lidocaine | Small molecule | Adsorption by immersion | Pain relief | Trovatti et al. (2012) |
| Cationic antimicrobial agents | ||||
| Octenidine dihydrochloride | Small molecule | Adsorption by immersion | Antimicrobial activity | Moritz et al. (2014) |
| Incorporation through poloxamers micelles | Alkhatib et al. (2017) | |||
| Povidone‐iodine | Small molecule | Adsorption by immersion | Antimicrobial activity | Wiegand et al. (2015) |
| Polyhexanide (PHMB) | Macromolecule | Adsorption by immersion | Antimicrobial activity | Wiegand et al. (2015) |
| Benzalkonium chloride | Small molecule | Adsorption by immersion | Antimicrobial activity | Mohite et al. (2016) |
| Peptides or proteins | ||||
| Laccase | Adsorption by immersion (two‐step method) | Antimicrobial activity | Sampaio et al. (2016) | |
| Silk sericin | Adsorption by immersion | Re‐epithelialization (increases collagen production) | Napavichayanun et al. (2015) | |
| Lysozyme | Macromolecules | Adsorption to phosphorylated BC | Antimicrobial activity | Oshima et al. (2011) |
| ε‐poly‐l‐Lysine (antimicrobial peptide) | Covalent conjugation by carbodiimide chemistry | Antimicrobial activity | Fursatz et al. (2018) | |
| Cells | ||||
| Mesenchymal stem cells | Bobis et al., 2006) | |||
| Adipose mesenchymal stem cells | Souza et al. (2014) | |||
| Rabbit bone marrow mesenchymal stem cells | Cell seeding | Promote tissue regeneration | Silva et al. (2018) | |
| Human epidermal keratinocytes | Loh et al. (2018) | |||
| Dermal fibroblasts | Loh et al. (2018) | |||
| Other | ||||
| Berberine Isoquinoline alkaloid | Small molecule | Adsorption by immersion under boiling | Antibacterial, anti‐inflammatory, antitumour | Huang et al. (2013) |
| Quaternary ammonium compounds | Small molecule | Adsorption by immersion | Antimicrobial activity | Zywicka et al. (2018) |
| Arginine | Small molecule | Grafting to oxidized BC | Re‐epithelialization (increases collagen production) | Qiao et al. (2018) |
Antibiotics have also been loaded into BC by immersion as the case of tetracycline, a short‐acting broad‐spectrum drug that inhibits bacterial growth by inhibiting translation. Loading was performed during 24 h with gentle stirring which resulted in a denser BC network structure and, after an initial burst release, the BC composite displayed a steady release of tetracycline (Shao et al., 2016a). Recently, saturation of BC with fusidic acid, a steroid antibiotic usually used for topic applications through a cream or eyedrops, was performed by simple immersion of the BC films for 1–24 h, but the release rate was not monitored. The resulting membrane was shown to be active against S. aureus (Liyaskina et al., 2017). The BC/antibiotic composite membranes presented excellent biocompatibility and effective antibacterial activity against gram‐negative and gram‐positive species.
Benzalkonium chloride, an antimicrobial cationic surfactant effective against gram‐positive bacteria, widely used in commercial wound dressings, was tested for BC loading by overnight soaking. The drug‐loading capacity increased with the drug concentration, and the release rate was described to be of 90% of the drug within the first 24 h. The cytotoxicity was tested on human peripheral blood mononuclear cells with 90% cell viability, which allows its application as a regenerative biomaterial (Mohite et al., 2016).
Some variations of the loading process have been reported, such as vortexing of wet BC for protein loading. In comparison with conventional methods, vortexing for a 10‐min period resulted in the same protein loading level than submersion for 24 h. While protein distribution and stability were unaltered, vortex loading resulted in a much slower protein release, directly related to a denser BC matrix and reduced capacity of water holding (Muller et al., 2014). Another variation was performed by boiling the purified BC membrane in a solution of berberine, an isoquinoline alkaloid extracted from Chinese medicinal herbs with several therapeutic activities such as antimicrobial, anti‐inflammatory and antitumour, among others. Although the authors pretended to use BC membranes as controlled release systems for ingestion and survival to gastric fluids, release studies and transdermal assays showed that BC significantly extends berberine release time and the results were transposed to skin delivery applications. The lowest release rate observed for BC/berberine composite was for acidic conditions, such as the skin, in simulated gastric fluid or in H2SO4 solution, the highest rate was in simulated intestinal fluid, and an intermediate rate was found in alkaline conditions. This behaviour was found to be directly related to the pore size of the BC matrix, as the pore size decreased after treatment with NaOH due to swelling of the BC fibres, hindering the diffusion of the drug from the pores of BC. This study showed that besides the factors already described in the literature to influence drug release from BC, the external environment also plays a major role and must be considered. Interestingly, solid‐state NMR assays revealed an interaction between berberine and the structure of BC (Huang et al., 2013).
Hyaluronan, a glycosaminoglycan present in the synovial fluid that enwraps joints, cartilage and tissues, allows the binding of a large number of water molecules, improving tissue hydration. Also, its rheological properties increase fluid viscosity, providing tissue resistance to mechanical damage. Hyaluronan is known for its curative characteristics, associated with pro‐angiogenic and anti‐apoptotic properties, endorsing the recovery of wound skin tissue and decreasing scar formation (Li et al., 2015). Depending on its molecular size, it can have anti‐inflammatory and immunosuppressive effects – high molecular weight hyaluronan – or have pro‐inflammatory action – low molecular weight hyaluronan (Litwiniuk et al., 2016). In fact, during the skin repair process, a rapid increase in hyaluronan is associated with tissue swelling, epithelial and mesenchymal cell migration and proliferation, and induction of cytokine signalling. Hyaluronan extending from cell surface into structures called cables can trap leucocytes and platelets and change their functions, modulating inflammation. Li et al. studied the physical properties of BC composites containing high molecular weight hyaluronan as stimulus on the healing process. These BC composites, obtained through a solution impregnation method, presented enhanced properties in terms of thermal stability, lower total surface area and pore volume, weight loss and elongation at break (Li et al., 2014a).
Lidocaine, used as local anaesthetic, and ibuprofen, a common use non‐steroidal anti‐inflammatory drug, were chosen as model hydrophilic and hydrophobic compounds, respectively, for the development of topical BC drug delivery systems. While a lidocaine aqueous solution was used to soak previously drained BC membranes, in the case of ibuprofen, the water of the BC membrane was previously replaced by ethanol. Subsequently, these pre‐treated membranes were immersed in an alcoholic ibuprofen solution. Diffusion assays showed a lower release rate for lidocaine but a 3 times higher release rate for ibuprofen, than in commercial dressings (Trovatti et al., 2012). More recently, the loading of diclofenac, a non‐steroidal anti‐inflammatory compound, frequently used to relieve pain and inflammation in short‐term clinical situations, was performed by immersion of drained BC membranes in a diclofenac solution with 5% glycerol as plasticizer. The BC‐diclofenac membranes presented a 6 times higher swelling behaviour and a release rate much slower than commercial gels, suggesting BC membranes as an advantageous transdermal delivery system for diclofenac (Silva et al., 2014).
Quaternary ammonium compounds (QACs) are low molecular weight biocides that include a positive charge and a hydrophobic segment. They present high cell membrane penetration capacity, low toxicity and antibacterial activity, dependent on the chain length of the alkyl chain. Recently, a QAC was synthesized by coupling reaction between the C18 long‐chain unsaturated fatty acid, the dimer dilinoleic acid (DLA), tyrosine and positively charged ethylenediamine (Umeda et al., 1999). The resulting compound [EDA]‐[DLA‐Tyr] was loaded by immersion for 24 h on BC membranes and showed antimicrobial activity against S. aureus and S. epidermidis, both opportunistic pathogens highly associated with skin and wound infections, particularly in hospital settings such as surgical or indwelling device‐associated wounds.
Regarding enzyme immobilization, the choice of a suitable carrier is mandatory, since the procedure cannot impair the activity of the enzyme. Although most carriers need to be activated before immobilization, resulting in low efficiencies, BC membranes can be loaded by simple soaking, not affecting the enzyme correct folding. The incorporation, by immersion, of proteins into BC membranes for the development of delivery systems has been analysed using model proteins, such as serum albumin. The loading was optimized by using never‐dried, pre‐swelled BC membranes, which was due to alterations of the fibre network during the freeze‐drying process, and the biological activity of the proteins was maintained during the loading and release steps (Muller et al., 2013). Another variation strategy to the method of protein loading was assayed for lipase model protein, a strategy designated as repeated absorption, a two‐step method involving repeated drying and absorption and activation with glutaraldehyde‐reticulating agent. The limit solution was forced into dried BC during absorption and the enzyme immobilization efficiency was higher than 90%, for the different two‐step absorption methods tested. The immobilized lipase retained 60% of its native activity after 15 repeated usages, suggesting that the two‐step immobilization method of enzymes is suitable for industrial applications (Wu et al., 2016). The immobilization of an enzyme with a therapeutic application was performed for laccase, due to its antibacterial activity. The loading of the enzyme was done by immersion of BC membranes, and the specific activity of the immobilized enzyme did not differ much from that of the free enzyme. The entrapment process maintained some flexibility degree and even improved access to the substrate, resulting in high antimicrobial activity for gram‐positive bacteria and a cytotoxicity level acceptable for wound dressing applications (Sampaio et al., 2016).
Also, conjugated strategies have added more than one different activity to the BC matrix, through adsorption loading approaches. One recent example involved the incorporation by immersion of two components into BC, silk sericin to enhance collagen type I production, which is critical for re‐epithelialization and the antiseptic PHMB. The interactions between these two components were analysed, and it was observed that silk sericin needed to be loaded before PHMB to maintain PHMB antimicrobial activity against all tested bacteria (Bacillus subtilis, S. aureus, methicillin‐resistant S. aureus, Escherichia coli, Acinetobacter baumannii and Pseudomonas aeruginosa) (Napavichayanun et al., 2015, 2016). These type of approaches open new paths for the future incorporation of more than one valence into the same wound dressing.
Compound loading by modification
Although more rare than the immersion techniques, other strategies have been adopted to load active compounds into BC. Despite being more complex, expensive and time‐consuming, they can present advantages such as controlled release and increased activity. Chemical modifications of the BC composition, allowing for the immobilization of compounds or proteins, have enhanced the interaction between the two components. BC presents a large amount of exposed hydroxyl groups that can be functionalized through different approaches.
Different adsorption strategies have been tested for proteins, namely lysozyme and as model proteins, haemoglobin, myoglobin and albumin. The BC modifications that resulted in promising adsorption results open new routes for BC functionalization with a wide range of enzymatic activities of therapeutic interest in wound healing. In a phosphorylation approach, BC was phosphorylated with phosphoric acid in the presence of N,N‐dimethylformamide (DMF) and urea at various degrees. The adsorption capacity for lysozyme increased with the percentage of BC phosphorylation and was much higher than that of plant cellulose (PC), since the specific surface area of phosphorylated BC is much higher than that of phosphorylated PC. In fact, the adsorption capacity of small molecules was similar for both types of phosphorylated cellulose, since these can more easily access internal adsorption sites (Oshima et al., 2008, 2011). In the surface carboxymethylation approach, the hydroxyl groups of BC suffered chemical substitution by treatment with NaOH followed by addition of ethanol and chloroacetic acid. The adsorption of albumin to carboxymethylated BC occurred at pH values below its isoelectric point by electrostatic interaction and increased with the degree of substitution (Lin et al., 2015a).
In another modification strategy, quaternary ammonium groups were introduced into BC as an adsorption approach for proteins. This strategy did not produce alterations in the microfibrous structure, and the modified BC showed selectivity for proteins over other compounds and higher adsorption capacity than PC with the same modification. The model protein haemoglobin was adsorbed on the quaternary ammonium BC under pH conditions lower than its isoelectric point, via electrostatic interactions (Niidei et al., 2010).
Recently, controlled release studies were performed to develop a long‐term dermal wound dressing. While octenidine had previously been shown to be stable, releasable and biologically active for over 6 months storage, the drug release time window was approximately 96 h. The new approach involved the modification of BC by incorporation of poloxamers as micelles and gels and resulted in prolonged retention time of octenidine up to 1 week together with upgraded mechanical and antimicrobial properties (Alkhatib et al., 2017).
The association of amoxicillin, a β‐lactam antibiotic, with BC sponges was performed by a cross‐linking coupling strategy. The BC was pre‐treated with 3‐aminopropyltriethoxysilane (APTES) in order to graft aminoalkylsilane groups through Si‐O‐C bonds. The amoxicillin was also modified at the carboxylic reactive group by treatment with the carbodiimide cross‐linker EDC/NHS (1‐ethyl‐3‐(3‐dimethylaminopropyl)carbodiimide hydrochloride). The NHS‐activated ester groups of amoxicillin are then able to react with the terminal NH2 groups of BC, resulting in covalent links between amoxicillin and BC. The BC/amoxicillin graft increased the antimicrobial activity against S. aureus, E. coli and Candida albicans and showed good cytocompatibility (Ye et al., 2018).
Functionalization of BC was performed for ε‐poly‐l‐Lysine (ε‐PLL), an antimicrobial peptide with broad‐spectrum antimicrobial activity that belongs to the first line of the innate immune system of many organisms. Besides being non‐toxic, water‐soluble and biodegradable, its mechanism of action, disruption of the bacterial cell membrane, diminishes the hypotheses of resistance emergence. Low molecular weight ε‐PLL (~4–5 kDa) was functionalized into BC following two different strategies. In the first, ε‐PLL was covalently conjugated through carbodiimide chemistry to previously carboxymethyl‐functionalized BC membranes. In the second, ε‐PLL was directly cross‐linked with the BC structure using carbodiimide chemistry. Both strategies resulted in membranes with unaltered cytocompatibility to human fibroblasts and with capacity to inhibit growth of S. epidermidis on contact. The functionalization with ε‐PLL had no significant effects on the nanofibrous structure and mechanical properties of BC (Fursatz et al., 2018).
Charged BC derivatives, carboxylated and aminated forms, were obtained by 2,2,6,6‐tetramethylpiperidine‐1‐oxyl radical (TEMPO)‐catalysed oxidation reaction and by the epichlorohydrin‐mediated amination reaction. These BC derivatives showed interesting properties for drug delivery via ionic conjugation, since both the cationic and anionic forms showed increased water retention capacity in a pH‐responsive way (Spaic et al., 2014). Similarly, a recent BC composite was developed using oxidized BC of microporous structure that provides a higher contact area. Arginine was grafted into the oxidized BC and, besides showing enhanced biocompatibility, also promoted collagen synthesis (Qiao et al., 2018).
Several types of BC/metal nanocomposites were successfully developed and showed high levels of antibacterial activity. Berndt et al. used an incorporation approach of silver nanoparticles in BC by a stepwise modification of a method previously used for two‐dimensional cellulose films and now applied to a 3D structure. Usual methods use AgNO3 in combination with strong reducing agents, and the relatively large particle agglomerates formed are immobilized by physical interactions and not by chemical bonds. In this work, a mild chemical, dimethyl sulfoxide (DMSO) was used as a reducing agent to activate the BC membranes that were subsequently immersed in a solution of 1,4‐diaminobutane and finally in a solution of DMSO, sodium acetate and AgNO3. Appended amine groups operated as anchoring centres for the chemical immobilization of the AgNPs. The BC/AgNP chemical linkage showed increased retention time maintaining strong antimicrobial activity against E. coli, even for low amounts of AgNPs (Berndt et al., 2013).
More recently, modification of BC by TEMPO‐mediated oxidation was performed with TEMPO/NaClO/NaBr system to obtain anionic C6 carboxylate groups. The modified BC was then incorporated with silver nanoparticles (AgNP) by ion exchange in AgNO3 solution. The BC/AgNP membranes showed low cytotoxicity for a NIH3T3 fibroblast cell line (cell viability of 95.2 ± 3.0% after 48 h) and antibacterial activities of 100% and 99.2% against E. coli and S. aureus respectively (Wu et al., 2018).
Incorporation of cells into bacterial cellulose
One of the most recent strategies to improve a BC wound dressing for the effective treatment of skin injuries is the incorporation of mesenchymal stem cells (MSC) in the matrix. MSC are adult pluripotent cells that can differentiate into a minimum of two cellular types (Bobis et al., 2006). These cells are expected to integrate into the host tissue and promote the regeneration of the damaged tissue.
Several studies were performed in which MSC, multipotent cells that can differentiate into numerous cell types, including bone, cartilage, muscle and fat cells, were added to BC membranes. MSC have a great capacity to self‐renew, while maintaining its multipotency, an essential feature to improve the process of wound healing and inducing re‐epithelialization of the wound. In one example, adipose MSC (AMSC) were obtained from human adipose tissue liposuction and incorporated into BC membranes. The BC/AMSC membranes were then tested in rats with induced burns. The results showed that the AMSC differentiated into adipocytes and osteocytes with a high regenerative potential (Souza et al., 2014). Another example used rabbit bone marrow MSC (BM‐MSC) associated with BC (BC/BM‐MSC). The BM‐MSC were observed, by scanning electron microscopy (SEM), to have fully integrated within the BC matrix and to have the ability to differentiate into more than one mesenchymal lineage (chondrogenic, osteogenic or adipogenic) once integrated into the matrix, yielding membranes with good biocompatibility results (Silva et al., 2018). Besides stem cells, other types of cells were added to BC membranes; human epidermal keratinocytes and dermal fibroblasts (DF) were incorporated in a BC/acrylic acid (AA) hydrogel exhibiting wound healing ability in vitro and in in vivo models (Fig. 5). The results showed that the EK and DF cells can be transferred to the wound and accelerated the wound healing process (Loh et al., 2018).
Figure 5.
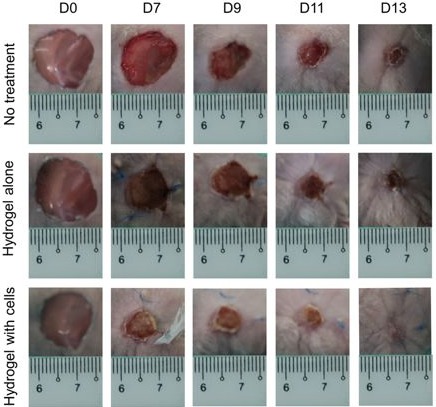
Healing process of wound excised (15 mm diameter) on a rat. Index D 0 stands for the initial state, D 7 for the 7th day, D 9 for the 9th day, D 11 for the 11th day and D 13 for the 13th day. On the first row (No treatment), control wounds of the untreated group. On the second row (hydrogel alone) test, wounds of the group treated with a BC wound dressing. On the third row (hydrogel with cells) test, wounds of the group treated with a BC/cells wound dressing. Reprinted from Scientific Reports, Vol 8, 2875, E.Y.X. Loh, N. Mohamad, M.B. Fauzi, M.H. Ng, S.F. Ng, M.C.I.M. Amin, Development of a bacterial cellulose‐based hydrogel cell carrier containing keratinocytes and fibroblasts for full‐thickness wound healing, Copyright (2018), with permission under a Creative Commons Attribution 4.0 International License.
The incorporation of AMSC and BM‐MSC cells in the BC membranes was performed by simple seeding. The ED and DF were also seeded on the sterilized BC/AA hydrogel pre‐soaked in the culture medium overnight.
Genetic manipulation of bacterial cellulose
Genetic engineering of the BC producing bacteria has been explored with the aim to optimize the intrinsic properties of BC and the cost‐effectiveness of the production process. Strain improvement has been performed through the transfer of BC‐related genetic determinants to a previously prepared ‘cell factory’ organism, resulting in the heterologous expression of genes, or through the genetic reprogramming of the natural BC producers.
Examples of genetic improvement of BC include the transfer of genes cmc, ccp, cesAB, cesC, cesD and bgl from K. xylinus to Synechococcus sp., resulting in an increase in BC production, especially upon low salinity conditions (Zhao et al., 2015). Also, the simultaneous expression of the bcsABCD operon and its upstream genes, cmcax and ccpAx, was performed in E. coli (Buldum et al., 2018). The cmcax genes encode for a BC‐hydrolysing enzyme, and ccpAx encodes for a protein related to the extrusion of the cellulose fibres (Wong et al., 1990) (Saxena et al., 1994) (Sunagawa et al., 2013). BC biosynthesis was detected earlier in the fermentation process and presented denser fibres than with K. hansenii. The heterologous expression of the bcsD gene from K. xylinus in a BC producer E. coli strain was shown to improve the crystallinity of the BC without altering the yield (Sajadi et al., 2017).
Regarding heterologous expression in the natural producer, the Vitreoscilla haemoglobin‐encoding gene vgb was expressed in K. xylinus improving BC production yield (Liu et al., 2018a). A chemical and physical random mutagenesis strategy was applied to K. hansenii and the mutants selected for presented low accumulation of organic acids, which is directly related to a higher BC production (Shigematsu et al., 2005). The accumulation of organic acids, by‐products of fermentation, competes with BC for carbon source utilization reducing its synthesis (Li et al., 2016).
To overcome the poor degradability of BC in vivo, K. xylinus was engineered to incorporate genes from Candida albicans to synthesize N‐acetyl‐glucosamine (GlcNAc) during BC synthesis, generating a modified cellulose that contains both glucose and GlcNAc. This altered BC structure showed susceptibility to lysozyme, a peptidoglycan hydrolytic enzyme that is abundantly produced in human secretions and by macrophages and polymorphonuclear neutrophils. This BC modification allows the development of wound dressings that can be degraded by the patient system, an advantage especially for burn wounds (Yadav et al., 2010).
The BC producer K. xylinus was also transformed with the curdlan synthase gene to produce biocomposites of cellulose and curdlan, an extracellular polysaccharide widely used in biomedical applications due to its low toxicity and non‐ionic gelation properties. This allowed the production of a pellicle of BC/curdlan, altering the pellicle's morphology and eliminating its pores without modifying the crystalline structure of BC (Fang et al., 2015).
A recent study used a sRNA interference system to control the native cellulose production path of the natural BC producer K. rhaeticus. The major achievements were the shut‐off of the constitutive BC production in order to prevent defective mutants to arise, a common phenomenon in well‐aerated conditions. Additionally, expression vectors were constructed to functionalize BC with specific proteins, by fusing the genes encoding the proteins of interest to short cellulose binding domains (Florea et al., 2016).
Biocompatibility of bacterial cellulose
All the accepted definitions of biocompatibility rely on the capacity of a given material to meet its therapeutic functions once implanted in an animal host without triggering a local or systemic adverse reaction. A biocompatible material must meet several requirements that include tests related to cytotoxicity, sensitization, genotoxicity and carcinogenicity, among others. Usually, the first stage to pass is the low induction of an inflammatory response. BC has grown as a promising biomaterial for wound dressings, a role that requires filling the criteria of biocompatibility. BC holds relatively high scores of biocompatibility, a characteristic attributed to its nanofibrillar structure and to its purity, that allow the host cells to adhere and to proliferate.
Several studies, already reviewed, were conducted in vitro and in vivo, addressing the biocompatibility of BC in different forms (pellicles, membranes, and discs) and subjected to different treatments (NaOH and radiation), using cell lines or animal models within a time lapse that ranged from 1 week to 1 year (Sulaeva et al., 2015). Regarding the recent literature, the studies addressing BC biocompatibility increased in numbers and included a wide range of techniques. However, studies conducted in humans remain rare. One example was the study of Almeida and co‐workers that showed that BC used in the form of patches for 24 h did not promote skin irritation (Almeida et al., 2014). Also, BC tested for the treatment of chronic varicose ulcers of lower limbs for 120 days showed a decrease in the depth of the ulcer suggesting that BC induced tissue remodelling without associated toxicity (Cavalcanti et al., 2017).
Despite the low number of human trials, biocompatibility has been assayed using animal models and cell lines. A common technique to determine cytotoxicity is the MTT assay for the assessment of the cell metabolic activity, regarded as a measurement of cell viability and consecutively biocompatibility. The NADH‐dependent oxidoreductases that reduce the tetrazolium dye MTT 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide to formazan are in a direct proportion to the number of viable cells (Berridge et al., 2005). This was used to assess the cytotoxicity of rabbit bone marrow mesenchymal stem cells (BM‐MSC) associated with BC in macrophages and showed a non‐toxic effect with 94% of cellular viability. In the same study, cytotoxicity was also assessed through the measurement of nitric oxide, produced by macrophages to eliminate pathogens, as an inflammatory response mediator, inhibiting or inducing inflammation. The colorimetric read of the NO released in the presence of the BC showed a non‐cytotoxic concentration (Silva et al., 2018).
Although toxicity determination can be reliably provided by indirect colorimetric methods, the effects of BC and of BC composites must be confirmed using cell lines and living animals. Chitosan is a promising agent for incorporation into BC because, once degraded by lysozyme, it releases mono‐ and oligosaccharides that stimulate angiogenesis (formation of new blood vessels) and tissue regeneration (Ishihara et al., 2006). In the context of regenerative medicine, a BC/chitosan composite, developed to treat hernias, was screened for biocompatibility using rats that were implanted with the BC/chitosan mesh. Histopathological examination of the organs and examination of the surrounding tissues searched for changes in the tissues and for the number and positioning of inflammatory cells. The same study also used rabbits to determine acute dermal irritation upon multiple dressing exchanges per day and to determine the intradermal reactivity through intracutaneous injections. No inflammation at the implant site was observed through histopathological analysis neither acute irritation nor allergic reactions. On the contrary, a higher degree of fibroplasia (the growth of fibrous tissue) was observed (Piasecka‐Zelga et al., 2018).
Although BC is recognized to have good biocompatibility with the different cells of the skin tissue, the adhesion of cells to BC in its native structure is not optimal. One factor that influences the response of the cells is the porosity of the mesh that can prevent the cell migration into the biomaterial. Usage of porogens, such as paraffin wax, to control the size of the pores was shown to lead muscle cells to be able to attach themselves inside the pores (Backdahl et al., 2008). In a recent study, the authors synthesized BC with the nanoporous structure altered to a microporous one. Gelatin microspheres were used both as porogens and as surface modification agents, eliminating the cytotoxic effects of other porogens and increasing its biocompatibility. Gelatin, a product of collagen hydrolysis, has been used for surface modification of polymeric scaffolds to mimic the composition of collagen in order to increase cell adhesion. The biocompatibility of the BC/gelatin with a microporous structure was determined in vitro using the HaCaT cell line as a keratinocyte model. Cells were able to proliferate and differentiate in the matrix, a required behaviour for tissue regeneration applications. Similar results were obtained in vivo through histological analysis of C57BL/6 (H‐2Kb) mice that suffered removal of a section of the dorsal flank skin. The process of re‐epithelialization was thus shown to occur in the BC/gelatin scaffold‐treated group with an increased healing effect and fewer inflammatory cells, compared with the control group (Khan et al., 2018).
Another study evaluated the biocompatibility of a hydrogel containing a mixture of carboxylated cellulose nanofibres (CNF) with aminated silver nanoparticles (Ag‐NH2 NPs) and gelatin (G). In vitro biocompatibility tests were performed with neonatal human dermal fibroblasts (NHDF) as a model of infected wounds. The viability of NHDF even increased due to the acceleration of the proliferation of the cells, induced by the matrix. In vivo wound healing testing was done on Kunming mice, and CNF/G/Ag showed a survival rate of 83%. However, the blood clotting tests showed good haemostatic properties that slightly decreased with the increase in Ag nanoparticles (Liu et al., 2018b).
Similarly, a recent biocomposite based on oxidized BC with microporous structure and in situ grafted with arginine that promotes collagen synthesis also showed enhanced biocompatibility (Qiao et al. 2018). The cytotoxicity of a BC wound dressing of TOBCP/AgNP, a BC pellicle that was modified by 2,2,6,6‐tetramethylpiperidine‐1‐oxyl radical (TEMPO)‐mediated oxidation and to which silver nanoparticles were incorporated for the antimicrobial activity of silver, was determined on a NIH3T3 fibroblast cell line, and the pellicle extracts presented a cell viability of 95.2 ± 3.0% after 48 h of incubation (Wu et al., 2018).
Mice models were used for the assessment of biocompatibility of BC with different pH values. An acidic pH at the wound site is described to promote faster wound healing, helping the proliferation of fibroblasts (Schreml et al., 2010). In this work, full‐thickness wounds were inflicted in the dorsal body surface of rats that subsequently received treatment with BC of different pH values (acidic, neutral and alkaline) and the evolution of the wound was monitored. The BC with acidic pH showed the best wound healing efficiency (Pourali et al., 2018).
The points mentioned address the efforts applied to this date towards the optimization of BC as a superior wound dressing agent. Such improvements were performed at all phases of BC production resorting to different strategies for the achievement of a higher performance as summarized in Fig. 6. However, these efforts still do not meet all the challenges that need to be addressed in a near future.
Figure 6.
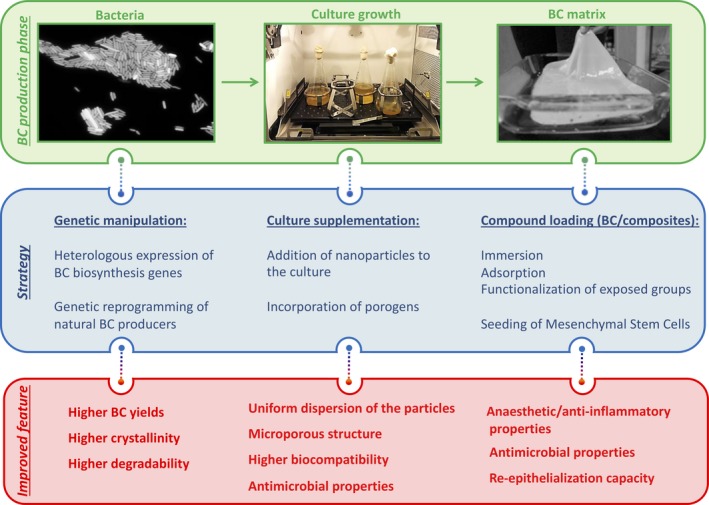
Schematic summary of the processes involved in the bacterial cellulose production (upper panel), the strategies followed for its upgrading (middle panel) and the improvements obtained in each step (lower panel). The BC matrix image was reprinted from Biomaterials, Vol 27 (2), W. Czaja, A. Krystynowicz, S. Bielecki, R.M. Brown Jr., Microbial cellulose – the natural power to heal wounds, Pages No. 145–151, Copyright (2006), with permission from Elsevier.
Marketed bacterial cellulose‐based wound dressing products
Although BC has intrinsic features that encourage its usage as wound dressing, in particular for burn wounds, its commercial dissemination is not exhaustively exploited. Even after all the studies that demonstrate that modifying pure BC in order to enhance its properties for a specific function achieving, for instance, better healing rates or antibacterial properties of the dressing, there are not many commercial products that are BC‐based, for wound dressing applications. The manufacturers are mainly located in the United States of America, Brazil and Poland. In the field of medicinal drug delivery systems, only polyhexamethylene biguanide (PHMB)‐supplemented BC (Suprasorb X+ PHMB) commercialized by the Activa Healthcare, L&R Company, has been marketed as wound dressing (Wild et al., 2012). A company branded Biofill offers several products that are BC‐based for wound dressing application such as Biofill to be used as temporary skin (substitute for ulcers and burns) allowing pain relief, reduced infection and faster healing; BioProcess intended for usage on ulcers and burns with antibacterial properties and increased healing rate; and Gengiflex to be used as dental implants or grafting material, favouring the recovery of periodontal tissue, reducing inflammatory response and surgical steps (Picheth et al., 2017).
Several other companies present similar products such as Membracel commercialized by Vuelo Pharma to act as temporary skin substitute for ulcers, burns and lacerations allowing fast skin regeneration; xCell commercialized by Xylos Corporation to be used as wound dressing for venous ulcer wounds, promoting autolytic debridement, pain relief and accelerating granulation; Nanoderm and Nanoderm Ag from Axcelon Biopolymers Corp. that prevent infections due to their antimicrobial properties; Nanoskin produced by Innovatec intended to be used as substitute blood vessels; and linfatics, lesions of tegument, facial peeling, infectious dermolysis, abrasion of tattoos and chronic ulcers, allowing the release of gases while obstructing the entry of microorganisms (Czaja et al., 2007; Picheth et al., 2017). Following the research performed in Lodz University of Technology (Poland), Bowil Biotech started the production and commercialization of BC wound dressings using the product name Celmat. Recent clinical trials demonstrate the BC wound dressing efficiency in human burn wounds (Fig. 7) where commercial dressings were used on wounds after being cleaned with normal saline and any bullae or debris removed. Microbial cellulose (EpiProtect ® from S2Medical AB, Sweden) sheets were applied under aseptic conditions, and in 28 days, the wound was healed (Aboelnaga et al., 2018).
Figure 7.
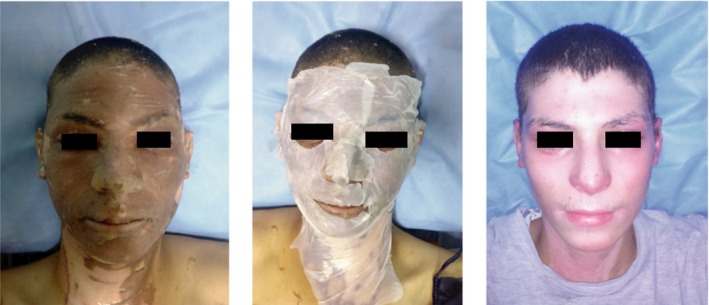
Time evolution of a facial burn wound during 28 days. On the left, before cleaning; on the middle, with BC sheets applied; and on the right, healed wound after the 28 days. Reprinted from Burns Vol 44, Ahmed Aboelnaga, Moustafa Elmasrya, Osama A. Adly, Mohamed A. Elbadawy, Ashraf H. Abbas, Islam Abdelrahman, Omar Salah, Ingrid Steinvall, Microbial cellulose dressing compared with silver sulphadiazine for the treatment of partial thickness burns: A prospective, randomized, clinical trial, Pages No. 1982–1988, Copyright (2018), with permission from Elsevier.
Future perspectives
The potential of BC usage in different industrial fields was recently evaluated revealing the emerging applications of BC‐based technology and anticipating a growing BC market in the order of 15% (Digital Journal, 2018).
BC is presently being used to develop bio‐based, commercial 3D printing materials, as an alternative to chemical products, such as resins, synthetic thickeners, strengtheners and plastics. Another advantage of 3D‐printed BC is the possibility to create adjustable dressings.
3D‐printable bioactivated BC/alginate hydrogel can offer a platform for the development of biomedical devices such as wearable sensors and drug‐releasing materials, allowing to monitor the condition of patients’ wounds while in the hospital (Leppiniemi et al., 2017). For example, the incorporation of specific sensors into the cellulosic material that glow with a different intensity in reaction to changes in the wound's pH level can be accessed using a UV lamp without removing the dressing. This procedure allows the healing process to continue unimpeded and can be easily followed at home by the patient. Another approach used silver ink to print the measurement electrodes onto a BC/polyurethane film allowing temperature reading into a computer and, in theory, to access instantly patient's wound condition. This film is assembled with the 3D‐printed wound care gel, with active ingredients of alginate, glycerol and BC, to promote the dress healing treatment. This 3D nanocellulose dress strategy enables the growth of healthy skin cells around a wound, creating a solution where a protein attaches to a 3D‐printed adhesive bandage favouring the cell growth – in this way, the healed area around the wound will stay malleable, instead of growing scab tissue.
The evolution to a personalized medicine approach when treating patients with chronic wounds, such as the case in diabetes, is inevitable, since management of skin tissue impairment is more than just managing a wound. A comprehensive assessment of the individual's health, nutrition, comorbidities and activity levels are key features to formulate an adequate treatment strategy. In this regard, the possibilities to design and to control specific drug release and to monitor the wound healing process in real time, without interference, are a challenging goal and appear to be within reach.
In conclusion, BC may be considered as a potential builder for nano‐based materials such as composites, films, foams and gels presenting distinctive properties. Such materials appear as promising alternatives to petroleum‐based ones, with the advantage of being environmentally friendly and recyclable. BC presents mechanical and physical outstanding properties that emerge from its unique 3D structure. BC is also biodegradable, non‐toxic and biocompatible, and is produced with a unique native purity, which allows for its direct utilization. BC‐based materials have been explored in a diversity of possible applications for biomedical purposes, in particular to develop improved dressing materials for severe wound healing. Novel scientific works, developing BC‐based materials for biomedical applications, have disclosed the potential of these materials, and in spite of further studies on in vivo biocompatibility, a promising future for BC materials is already revealed.
Conflict of interest
None declared.
Acknowledgements
This work is funded by FEDER funds through the COMPETE 2020 Program, and National Funds through FCT – Portuguese Foundation for Science and Technology under the projects: POCI‐01‐0145‐FEDER‐007688 (Reference UID/CTM/50025/2013), UID/Multi/04378/2019 (Unidade de Ciências Biomoleculares Aplicadas – UCIBIO) and co‐financed by FEDER funds through PT2020 Partnership Agreement (POCI‐01‐0145‐FEDER‐07728); PTDC/FIS‐NAN/0117/2014; and PTDC/BIA‐MIC/31645/2017.
Microbial Biotechnology (2019) 12(4), 586–610
Funding Information
This work is funded by FEDER funds through the COMPETE 2020 Program, and National Funds through FCT – Portuguese Foundation for Science and Technology under the projects: POCI‐01‐0145‐FEDER‐007688 (Reference UID/CTM/50025/2013), UID/Multi/04378/2019 (Unidade de Ciências Biomoleculares Aplicadas – UCIBIO) and co‐financed by FEDER funds through PT2020 Partnership Agreement (POCI‐01‐0145‐FEDER‐07728); PTDC/FIS‐NAN/0117/2014; and PTDC/BIA‐MIC/31645/2017.
Contributor Information
Pedro L. Almeida, Email: palmeida@adf.isel.pt.
Rita G. Sobral, Email: rgs@fct.unl.pt.
References
- Aboelnaga, A. , Elmasry, M. , Adly, O.A. , Elbadawy, M.A. , Abbas, A.H. , Abdelrahman, I. , et al (2018) Microbial cellulose dressing compared with silver sulphadiazine for the treatment of partial thickness burns: A prospective, randomised, clinical trial. Burns 44: 1982–1988. [DOI] [PubMed] [Google Scholar]
- Agarwal, A. , McAnulty, J.F. , Schurr, M.J. , Murphy, C.J. , and Abbott, N.L. (2011) Polymeric materials for chronic wound and burn dressings In Advanced Wound Repair Therapies. Farrar D. (ed). Amsterdam, The Netherlands: Elsevier Inc, pp. 186–208. [Google Scholar]
- Alkhatib, Y. , Dewaldt, M. , Moritz, S. , Nitzsche, R. , Kralisch, D. , and Fischer, D. (2017) Controlled extended octenidine release from a bacterial nanocellulose/Poloxamer hybrid system. Eur J Pharm Biopharm 112: 164–176. [DOI] [PubMed] [Google Scholar]
- Almeida, I.F. , Pereira, T. , Silva, N.H. , Gomes, F.P. , Silvestre, A.J. , Freire, C.S. , et al (2014) Bacterial cellulose membranes as drug delivery systems: an in vivo skin compatibility study. Eur J Pharm Biopharm 86: 332–336. [DOI] [PubMed] [Google Scholar]
- American Burn Association . (2016) Burn incidence and treatment in the United States: 2016. URL http://www.ameriburn.org/resources_factsheet.php.
- Awadhiya, A. , Kumar, D. , Rathore, K. , Fatma, B. , and Verma, V. (2017) Synthesis and characterization of agarose‐bacterial cellulose biodegradable composites. Polym Bull 74: 2887–2903. [Google Scholar]
- Backdahl, H. , Esguerra, M. , Delbro, D. , Risberg, B. , and Gatenholm, P. (2008) Engineering microporosity in bacterial cellulose scaffolds. J Tissue Eng Regen Med 2: 320–330. [DOI] [PubMed] [Google Scholar]
- Basu, P. , Saha, N. , Bandyopadhyay, S. , and Saha, P. (2017) Rheological performance of bacterial cellulose based nonmineralized and mineralized hydrogel scaffolds. AIP Conference Proceedings 1843: 050008. [Google Scholar]
- Berndt, S. , Wesarg, F. , Wiegand, C. , Kralisch, D. , and Muller, F.A. (2013) Antimicrobial porous hybrids consisting of bacterial nanocellulose and silver nanoparticles. Cellulose 20: 771–783. [Google Scholar]
- Berridge, M.V. , Herst, P.M. , and Tan, A.S. (2005) Tetrazolium dyes as tools in cell biology: new insights into their cellular reduction. Biotechnol Annu Rev 11: 127–152. [DOI] [PubMed] [Google Scholar]
- Bobis, S. , Jarocha, D. , and Majka, M. (2006) Mesenchymal stem cells: characteristics and clinical applications. Folia Histochem Cytobiol 44: 215–230. [PubMed] [Google Scholar]
- Brown, A.J. (1886) XIX.‐The chemical action of pure cultivations of bacterium aceti. J Chem Soc Trans 49: 172–187. [Google Scholar]
- Buldum, G. , Bismarck, A. , and Mantalaris, A. (2018) Recombinant biosynthesis of bacterial cellulose in genetically modified Escherichia coli . Bioprocess Biosyst Eng 41: 265–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calum, H. , Moser, C. , Jensen, P.O. , Christophersen, L. , Maling, D.S. , van Gennip, M. , et al (2009) Thermal injury induces impaired function in polymorphonuclear neutrophil granulocytes and reduced control of burn wound infection. Clin Exp Immunol 156: 102–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon, R.E. , and Anderson, S.M. (1991) Biogenesis of bacterial cellulose. Crit Rev Microbiol 17: 435–447. [DOI] [PubMed] [Google Scholar]
- Castro, C. , Zuluaga, R. , Rojas, O.J. , Filpponen, I. , Orelma, H. , Londono, M. , et al (2015) Highly percolated poly(vinyl alcohol) and bacterial nanocellulose synthesized in situ by physical‐crosslinking: exploiting polymer synergies for biomedical nanocomposites. RSC Adv 5: 90742–90749. [Google Scholar]
- Cavalcanti, L.M. , Pinto, F.C.M. , Oliveira, G.M. , Lima, S.V.C. , Aguiar, J.L.A. , and Lins, E.M. (2017) Efficacy of bacterial cellulose membrane for the treatment of lower limbs chronic varicose ulcers: a randomized and controlled trial. Rev Col Bras Cir 44: 72–80. [DOI] [PubMed] [Google Scholar]
- Chang, W.S. , and Chen, H.H. (2016) Physical properties of bacterial cellulose composites for wound dressings. Food Hydrocolloids 53: 75–83. [Google Scholar]
- Chawla, P.R. , Bajaj, I.B. , Survase, S.A. , and Singhal, R.S. (2009) Microbial cellulose: fermentative production and applications. Food Technol Biotechnol 47: 107–124. [Google Scholar]
- Chen, C.T. , Zhang, T. , Dai, B.B. , Zhang, H. , Chen, X. , Yang, J.Z. , et al (2016) Rapid fabrication of composite hydrogel microfibers for weavable and sustainable antibacterial applications. ACS Sustain Chem Eng 4: 6534–6542. [Google Scholar]
- Chiaoprakobkij, N. , Sanchavanakit, N. , Subbalekha, K. , Pavasant, P. , and Phisalaphong, M. (2011) Characterization and biocompatibility of bacterial cellulose/alginate composite sponges with human keratinocytes and gingival fibroblasts. Carbohydr Polym 85: 548–553. [Google Scholar]
- Chu, M.L. , Gao, H.C. , Liu, S. , Wang, L. , Jia, Y.G. , Gao, M. , et al (2018) Functionalization of composite bacterial cellulose with C‐60 nanoparticles for wound dressing and cancer therapy. RSC Adv 8: 18197–18203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czaja, W. , Romanovicz, D. , and Malcolm Brown, R. (2004) Structural investigations of microbial cellulose produced in stationary and agitated culture. Cellulose 11: 403–11. [Google Scholar]
- Czaja, W. , Krystynowicz, A. , Bielecki, S. , and Brown, R.M. (2006) Microbial cellulose – the natural power to heal wounds. Biomaterials 27: 145–151. [DOI] [PubMed] [Google Scholar]
- Czaja, W.K. , Young, D.J. , Kawecki, M. , and Brown, R.M. (2007) The future prospects of microbial cellulose in biomedical applications. Biomacromolecules 8: 586–12. [DOI] [PubMed] [Google Scholar]
- Davidson, J.R. (2015) Current concepts in wound management and wound healing products. Vet Clin North Am Small Anim Pract 45: 537–564. [DOI] [PubMed] [Google Scholar]
- Digital Journal . (2018) Global microbial and bacterial cellulose market will grow at a CAGR 14.8% and reach USD 570 Million by 2023, from USD 250 Million in 2017. URL http://www.digitaljournal.com/pr/3877005.
- Fang, J. , Kawano, S. , Tajima, K. , and Kondo, T. (2015) In vivo curdlan/cellulose bionanocomposite synthesis by genetically modified Gluconacetobacter xylinus . Biomacromolecules 16: 3154–3160. [DOI] [PubMed] [Google Scholar]
- Fernandes, S.N. , Geng, Y. , Vignolini, S. , Glover, B.J. , Trindade, A.C. , Canejo, J.P. , et al (2013) Structural color and iridescence in transparent sheared cellulosic films. Macromol Chem Phys 214: 25–32. [Google Scholar]
- Fernandes, S.N. , Almeida, P.L. , Monge, N. , Aguirre, L.E. , Reis, D. , de Oliveira, C.L.P. , et al (2017) Mind the microgap in iridescent cellulose nanocrystal films. Adv Mater 29: 1603560. [DOI] [PubMed] [Google Scholar]
- Figueiredo, A.R.P. , Figueiredo, A.G.P.R. , Silva, N.H.C.S. , Barros‐Timmons, A. , Almeida, A. , Silvestre, A.J.D. , et al (2015) Antimicrobial bacterial cellulose nanocomposites prepared by in situ polymerization of 2‐aminoethyl methacrylate. Carbohydr Polym 123: 443–453. [DOI] [PubMed] [Google Scholar]
- Florea, M. , Hagemann, H. , Santosa, G. , Abbott, J. , Micklem, C.N. , Spencer‐Milnes, X. , et al (2016) Engineering control of bacterial cellulose production using a genetic toolkit and a new cellulose‐producing strain. Proc Natl Acad Sci USA 113: E3431–E3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonder, M.A. , Lazarus, G.S. , Cowan, D.A. , Aronson‐Cook, B. , Kohli, A.R. , and Mamelak, A.J. (2008) Treating the chronic wound: a practical approach to the care of nonhealing wounds and wound care dressings. J Am Acad Dermatol 58: 185–206. [DOI] [PubMed] [Google Scholar]
- Fontana, J.D. , de Souza, A.M. , Fontana, C.K. , Torriani, I.L. , Moreschi, J.C. , Gallotti, B.J. , et al (1990) Acetobacter cellulose pellicle as a temporary skin substitute. Appl Biochem Biotechnol 24–25: 253–264. [DOI] [PubMed] [Google Scholar]
- Foong, C.Y. , Hamzah, M.S.A. , Abd Razak, S.I. , Saidin, S. , and Nayan, N.H.M. (2018) Influence of poly(lactic acid) layer on the physical and antibacterial properties of dry bacterial cellulose sheet for potential acute wound healing materials. Fibers Polym 19: 263–271. [Google Scholar]
- Fujishima, A. , Rao, T.N. , and Tryk, D.A. (2000) Titanium dioxide photocatalysis. J Photochem Photobiol, C 1: 586–21. [Google Scholar]
- Fursatz, M. , Skog, M. , Sivler, P. , Palm, E. , Aronsson, C. , Skallberg, A. , et al (2018) Functionalization of bacterial cellulose wound dressings with the antimicrobial peptide epsilon‐poly‐L‐Lysine. Biomed Mater 13: 025014. [DOI] [PubMed] [Google Scholar]
- Galateanu, B. , Bunea, M.C. , Stanescu, P. , Vasile, E. , Casarica, A. , Iovu, H. , et al (2015) In vitro studies of bacterial cellulose and magnetic nanoparticles smart nanocomposites for efficient chronic wounds healing. Stem Cells Int 10: 195096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, X. , Shi, Z.J. , Kusmierczyk, P. , Liu, C.Q. , Yang, G. , Sevostianov, I. , and Silberschmidt, V.V. (2016) Time‐dependent rheological behaviour of bacterial cellulose hydrogel. Mat Sci Eng C‐Mater Biol Appl 58: 153–159. [DOI] [PubMed] [Google Scholar]
- Gelin, K. , Bodin, A. , Gatenholm, P. , Mihranyan, A. , Edwards, K. , and Strømme, M. (2007) Characterization of water in bacterial cellulose using dielectric spectroscopy and electron microscopy. Polymer 48: 7623–7631. [Google Scholar]
- Grande, C.J. , Torres, F.G. , Gomez, C.M. , and Bano, M.C. (2009) Nanocomposites of bacterial cellulose/hydroxyapatite for biomedical applications. Acta Biomater 5: 1605–1615. [DOI] [PubMed] [Google Scholar]
- Hedlund, C.S. (2007) Surgery of the oral cavity and oropharynx In Small Animal Surgery. Fossum T.W. (ed). Maryland Heights, MO: Mosby Elsevier, pp. 339–371. [Google Scholar]
- Hu, Y. , Catchmark, J.M. , Zhu, Y.J. , Abidi, N. , Zhou, X. , Wang, J.H. , and Liang, N.Y. (2014) Engineering of porous bacterial cellulose toward human fibroblasts ingrowth for tissue engineering. J Mater Res 29: 2682–2693. [Google Scholar]
- Huang, H.C. , Chen, L.C. , Lin, S.B. , Hsu, C.P. , and Chen, H.H. (2010) In situ modification of bacterial cellulose network structure by adding interfering substances during fermentation. Bioresour Technol 101: 6084–6091. [DOI] [PubMed] [Google Scholar]
- Huang, H.C. , Chen, L.C. , Lin, S.B. , and Chen, H.H. (2011) Nano‐biomaterials application: in situ modification of bacterial cellulose structure by adding HPMC during fermentation. Carbohydr Polym 83: 979–987. [Google Scholar]
- Huang, L. , Chen, X. , Nguyen, T.X. , Tang, H. , Zhang, L. , and Yang, G. (2013) Nano‐cellulose 3D‐networks as controlled‐release drug carriers. J Mater Chem B 1: 2976–2984. [DOI] [PubMed] [Google Scholar]
- Ishihara, M. , Obara, K. , Nakamura, S. , Fujita, M. , Masuoka, K. , Kanatani, Y. , et al (2006) Chitosan hydrogel as a drug delivery carrier to control angiogenesis. J Artif Organs 9: 8–16. [DOI] [PubMed] [Google Scholar]
- Janpetch, N. , Saito, N. , and Rujiravanit, R. (2016) Fabrication of bacterial cellulose‐ZnO composite via solution plasma process for antibacterial applications. Carbohydr Polym 148: 335–344. [DOI] [PubMed] [Google Scholar]
- Karnezis, T. , Epa, V.C. , Stone, B.A. , and Stanisich, V.A. (2003) Topological characterization of an inner membrane (1–>3)‐beta‐D‐glucan (curdlan) synthase from Agrobacterium sp. strain ATCC31749. Glycobiology 13: 693–706. [DOI] [PubMed] [Google Scholar]
- Khalid, A. , Ullah, H. , Ul‐Islam, M. , Khan, R. , Khan, S. , Ahmad, F. , et al (2017) Bacterial cellulose‐TiO2 nanocomposites promote healing and tissue regeneration in burn mice model. RSC Adv 7: 47662–47668. [Google Scholar]
- Khan, S. , Ul‐Islam, M. , Khattak, W.A. , Ullah, M.W. , and Park, J.K. (2015) Bacterial cellulose‐titanium dioxide nanocomposites: nanostructural characteristics, antibacterial mechanism, and biocompatibility. Cellulose 22: 565–579. [Google Scholar]
- Khan, S. , Ul‐Islam, M. , Ikram, M. , Islam, S.U. , Ullah, M.W. , Israr, M. , et al (2018) Preparation and structural characterization of surface modified microporous bacterial cellulose scaffolds: a potential material for skin regeneration applications in vitro and in vivo. Int J Biol Macromol 117: 1200–1210. [DOI] [PubMed] [Google Scholar]
- Kimura, S. , Chen, H.P. , Saxena, I.M. , Brown, R.M. Jr , and Itoh, T. (2001) Localization of c‐di‐GMP‐binding protein with the linear terminal complexes of Acetobacter xylinum . J Bacteriol 183: 5668–5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemm, D. , Schumann, D. , Udhardt, U. , and Marsch, S. (2001) Bacterial synthesized cellulose ‐ artificial blood vessels for microsurgery. Prog Polym Sci 26: 1561–1603. [Google Scholar]
- Klemm, D. , Heublein, B. , Fink, H.P. , and Bohn, A. (2005) Cellulose: fascinating biopolymer and sustainable raw material. Angew Chem Int Ed Engl 44: 3358–3393. [DOI] [PubMed] [Google Scholar]
- Krasteva, P.V. , Bernal‐Bayard, J. , Travier, L. , Martin, F.A. , Kaminski, P.A. , Karimova, G. , et al (2017) Insights into the structure and assembly of a bacterial cellulose secretion system. Nat Commun 8: 2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarini, S.C. , de Aquino, R. , Amaral, A.C. , Corbi, F.C.A. , Corbi, P.P. , Barud, H.S. , and Lustri, W.R. (2016) Characterization of bilayer bacterial cellulose membranes with different fiber densities: a promising system for controlled release of the antibiotic ceftriaxone. Cellulose 23: 737–748. [Google Scholar]
- Leppiniemi, J. , Lahtinen, P. , Paajanen, A. , Mahlberg, R. , Metsa‐Kortelainen, S. , Pinornaa, T. , et al (2017) 3D‐printable bioactivated nanocellulose‐alginate hydrogels. ACS Appl Mater Interf 9: 21959–21970. [DOI] [PubMed] [Google Scholar]
- Li, X. , Li, B. , Ma, J. , Wang, X. , and Zhang, S. (2014a) Development of a silk fibroin/HTCC/ PVA sponge for chronic wound dressing. J Bioactive Compatible Polym 29: 398–411. [Google Scholar]
- Li, Y. , Qing, S. , Zhou, J.H. , and Yang, G. (2014b) Evaluation of bacterial cellulose/hyaluronan nanocomposite biomaterials. Carbohydr Polym 103: 496–501. [DOI] [PubMed] [Google Scholar]
- Li, Y. , Jiang, H. , Zheng, W.F. , Gong, N.Y. , Chen, L.L. , Jiang, X.Y. , and Yang, G. (2015) Bacterial cellulose‐hyaluronan nanocomposite biomaterials as wound dressings for severe skin injury repair. J Mater Chem B 3: 3498–3507. [DOI] [PubMed] [Google Scholar]
- Li, Y. , Tian, J. , Tian, H. , Chen, X. , Ping, W. , Tian, C. , and Lei, H. (2016) Mutation‐based selection and analysis of Komagataeibacter hansenii HDM1‐3 for improvement in bacterial cellulose production. J Appl Microbiol 121: 1323–1334. [DOI] [PubMed] [Google Scholar]
- Li, Y.T. , Lin, S.B. , Chen, L.C. , and Chen, H.H. (2017a) Antimicrobial activity and controlled release of nanosilvers in bacterial cellulose composites films incorporated with montmorillonites. Cellulose 24: 4871–4883. [Google Scholar]
- Li, Y. , Tian, Y. , Zheng, W.S. , Feng, Y. , Huang, R. , Shao, J.X. , et al (2017b) Composites of bacterial cellulose and small molecule‐decorated gold nanoparticles for treating Gram‐Negative bacteria‐infected wounds. Small 13: 10. [DOI] [PubMed] [Google Scholar]
- Lin, W.C. , Lien, C.C. , Yeh, H.J. , Yu, C.M. , and Hsu, S.H. (2013) Bacterial cellulose and bacterial cellulose–chitosan membranes for wound dressing applications. Carbohydr Polym 94: 603–611. [DOI] [PubMed] [Google Scholar]
- Lin, Q.H. , Zheng, Y.D. , Ren, L.L. , Wu, J. , Wang, H. , An, J.X. , and Fan, W. (2014) Preparation and characteristic of a sodium alginate/carboxymethylated bacterial cellulose composite with a crosslinking semi‐interpenetrating network. J Appl Polym Sci 131: 9. [Google Scholar]
- Lin, Q.H. , Zheng, Y.D. , Wang, G.J. , Shi, X.N. , Zhang, T. , Yu, J. , and Sun, J. (2015a) Protein adsorption behaviors of carboxymethylated bacterial cellulose membranes. Int J Biol Macromol 73: 264–269. [DOI] [PubMed] [Google Scholar]
- Lin, S.B. , Chen, C.C. , Chen, L.C. , and Chen, H.H. (2015b) The bioactive composite film prepared from bacterial cellulose and modified by hydrolyzed gelatin peptide. J Biomater Appl 29: 1428–1438. [DOI] [PubMed] [Google Scholar]
- Litwiniuk, M. , Krejner, A. , Speyrer, M.S. , Gauto, A.R. , and Grzela, T. (2016) Hyaluronic acid in inflammation and tissue regeneration. Wounds 28: 78–88. [PubMed] [Google Scholar]
- Liu, M. , Li, S. , Xie, Y. , Jia, S. , Hou, Y. , Zou, Y. , and Zhong, C. (2018a) Enhanced bacterial cellulose production by Gluconacetobacter xylinus via expression of Vitreoscilla hemoglobin and oxygen tension regulation. Appl Microbiol Biotechnol 102: 1155–1165. [DOI] [PubMed] [Google Scholar]
- Liu, R. , Dai, L. , Si, C. , and Zeng, Z. (2018b) Antibacterial and hemostatic hydrogel via nanocomposite from cellulose nanofibers. Carbohydr Polym 195: 63–70. [DOI] [PubMed] [Google Scholar]
- Liyaskina, E. , Revin, V. , Paramonova, E. , Nazarkina, M. , Pestov, N. , Revina, N. , and Kolesnikova, S. (2017) Nanomaterials from bacterial cellulose for antimicrobial wound dressing. J Phys Conf Ser 784: 012034. [Google Scholar]
- Loh, E.Y.X. , Mohamad, N. , Fauzi, M.B. , Ng, M.H. , Ng, S.F. , and Mohd Amin, M.C.I. (2018) Development of a bacterial cellulose‐based hydrogel cell carrier containing keratinocytes and fibroblasts for full‐thickness wound healing. Sci Rep 8: 2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamad, N. , Mohd Amin, M.C. , Pandey, M. , Ahmad, N. , and Rajab, N.F. (2014) Bacterial cellulose/acrylic acid hydrogel synthesized via electron beam irradiation: accelerated burn wound healing in an animal model. Carbohydr Polym 114: 312–320. [DOI] [PubMed] [Google Scholar]
- Mohammadnejad, J. , Yazdian, F. , Omidi, M. , Rostami, A.D. , Rasekh, B. , and Fathinia, A. (2018) Graphene oxide/silver nanohybrid: Optimization, antibacterial activity and its impregnation on bacterial cellulose as a potential wound dressing based on GO‐Ag nanocomposite‐coated BC. Eng Life Sci 18: 298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohite, B.V. , Suryawanshi, R.K. , and Patil, S.V. (2016) Study on the drug loading and release potential of bacterial cellulose. Cellulose Chem Technol 50: 219–223. [Google Scholar]
- Moritz, S. , Wiegand, C. , Wesarg, F. , Hessler, N. , Muller, F.A. , Kralisch, D. , et al (2014) Active wound dressings based on bacterial nanocellulose as drug delivery system for octenidine. Int J Pharm 471: 45–55. [DOI] [PubMed] [Google Scholar]
- Muller, A. , Ni, Z. , Hessler, N. , Wesarg, F. , Muller, F.A. , Kralisch, D. , and Fischer, D. (2013) The biopolymer bacterial nanocellulose as drug delivery system: investigation of drug loading and release using the model protein albumin. J Pharm Sci 102: 579–592. [DOI] [PubMed] [Google Scholar]
- Muller, A. , Wesarg, F. , Hessler, N. , Muller, F.A. , Kralisch, D. , and Fischer, D. (2014) Loading of bacterial nanocellulose hydrogels with proteins using a high‐speed technique. Carbohydr Polym 106: 410–413. [DOI] [PubMed] [Google Scholar]
- Napavichayanun, S. , Amornsudthiwat, P. , Pienpinijtham, P. , and Aramwit, P. (2015) Interaction and effectiveness of antimicrobials along with healing‐promoting agents in a novel biocellulose wound dressing. Mater Sci Eng C Mater Biol Appl 55: 95–104. [DOI] [PubMed] [Google Scholar]
- Napavichayanun, S. , Yamdech, R. , and Aramwit, P. (2016) The safety and efficacy of bacterial nanocellulose wound dressing incorporating sericin and polyhexamethylene biguanide: in vitro, in vivo and clinical studies. Arch Dermatol Res 308: 123–132. [DOI] [PubMed] [Google Scholar]
- Niidei, T. , Shiraki, H. , Oshima, T. , Baba, Y. , Kamiya, N. , and Gotoi, M. (2010) Quaternary ammonium bacterial cellulose for adsorption of proteins. Solvent Extract Res Develop Japan 17: 73–81. [Google Scholar]
- Okiyama, A. , Shirae, H. , Kano, H. , and Yamanaka, S. (1992) Bacterial cellulose. I. Two‐stage fermentation process for cellulose production by Acetobacter aceti . Food Hydrocolloids 6: 471–477. [Google Scholar]
- Oshima, T. , Kondo, K. , Ohto, K. , Inoue, K. , and Baba, Y. (2008) Preparation of phosphorylated bacterial cellulose as an adsorbent for metal ions. React Funct Polym 68: 376–383. [Google Scholar]
- Oshima, T. , Taguchi, S. , Ohe, K. , and Baba, Y. (2011) Phosphorylated bacterial cellulose for adsorption of proteins. Carbohydr Polym 83: 953–958. [Google Scholar]
- Ovington, L.G. (2007) Advances in wound dressings. Clin Dermatol 25: 33–38. [DOI] [PubMed] [Google Scholar]
- Pal, S. , Nisi, R. , Stoppa, M. , and Licciulli, A. (2017) Silver‐functionalized bacterial cellulose as antibacterial membrane for wound‐healing applications. ACS Omega 2: 3632–3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul, W. , and Sharma, C.P. (2004) Chitosan and alginate wound dressings: a short review. Trends Biomater Artif Organs 18: 18–23. [Google Scholar]
- Paximada, P. , Dimitrakopoulou, E.A. , Tsouko, E. , Koutinas, A.A. , Fasseas, C. , and Mandala, I.G. (2016) Structural modification of bacterial cellulose fibrils under ultrasonic irradiation. Carbohydr Polym 150: 5–12. [DOI] [PubMed] [Google Scholar]
- Perez‐Mendoza, D. , Rodriguez‐Carvajal, M.A. , Romero‐Jimenez, L. , Farias Gde, A. , Lloret, J. , Gallegos, M.T. , and Sanjuan, J. (2015) Novel mixed‐linkage beta‐glucan activated by c‐di‐GMP in Sinorhizobium meliloti. Proc Natl Acad Sci USA 112: E757–E765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phisalaphong, M. , and Jatupaiboon, N. (2008) Biosynthesis and characterization of bacteria cellulose–chitosan film. Carbohydr Polym 74: 482–488. [Google Scholar]
- Phisalaphong, M. , Suwanmajo, T. , and Tammarate, P. (2008) Synthesis and characterization of bacterial cellulose/alginate blend membranes. J Appl Polym Sci 107: 3419–3424. [Google Scholar]
- Piasecka‐Zelga, J. , Zelga, P. , Szulc, J. , Wietecha, J. , and Ciechanska, D. (2018) An in vivo biocompatibility study of surgical meshes made from bacterial cellulose modified with chitosan. Int J Biol Macromol 116: 1119–1127. [DOI] [PubMed] [Google Scholar]
- Picheth, G.F. , Pirich, C.L. , Sierakowski, M.R. , Woehl, M.A. , Sakakibara, C.N. , de Souza, C.F. , et al (2017) Bacterial cellulose in biomedical applications: a review. Int J Biol Macromol 104: 97–106. [DOI] [PubMed] [Google Scholar]
- Pourali, P. , Razavianzadeh, N. , Khojasteh, L. , and Yahyaei, B. (2018) Assessment of the cutaneous wound healing efficiency of acidic, neutral and alkaline bacterial cellulose membrane in rat. J Mater Sci Mater Med 29: 90. [DOI] [PubMed] [Google Scholar]
- Qiao, K. , Zheng, Y.D. , Guo, S.L. , Tan, J. , Chen, X.H. , Li, J.Q. , et al (2015) Hydrophilic nanofiber of bacterial cellulose guided the changes in the micro‐structure and mechanical properties of nf‐BC/PVA composites hydrogels. Compos Sci Technol 118: 47–54. [Google Scholar]
- Qiao, H. , Guo, T. , Zheng, Y. , Zhao, L. , Sun, Y. , Liu, Y. , and Xie, Y. (2018) A novel microporous oxidized bacterial cellulose/arginine composite and its effect on behavior of fibroblast/endothelial cell. Carbohydr Polym 184: 323–332. [DOI] [PubMed] [Google Scholar]
- Rahman, M.M. , and Netravali, A.N. (2016) Aligned bacterial cellulose arrays as “Green” nanofibers for composite materials. ACS Macro Lett 5: 1070–1074. [DOI] [PubMed] [Google Scholar]
- Rebelo, R. , Ana, A.J.A. , Chen, X. , Liu, C. , Yang, G. , and Liu, Y. (2018) Dehydration of bacterial cellulose and the water content effects on its viscoelastic and electrochemical properties. Sci Technol Adv Mater 19: 203–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross, P. , Mayer, R. , and Benziman, M. (1991) Cellulose biosynthesis and function in bacteria. Microbiol Rev 55: 35–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowan, M.P. , Cancio, L.C. , Elster, E.A. , Burmeister, D.M. , Rose, L.F. , Natesan, S. , et al (2015) Burn wound healing and treatment: review and advancements. Crit Care 19: 243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saibuatong, O.A. , and Phisalaphong, M. (2010) Novo aloe vera—bacterial cellulose composite film from biosynthesis. Carbohydr Polym 79: 455–460. [Google Scholar]
- Sajadi, E. , Babaipour, V. , Deldar, A.A. , Yakhchali, B. , and Fatemi, S.S. (2017) Enhancement of crystallinity of cellulose produced by Escherichia coli through heterologous expression of bcsD gene from Gluconacetobacter xylinus. Biotechnol Lett 39: 1395–1401. [DOI] [PubMed] [Google Scholar]
- Sampaio, L.M. , Padrao, J. , Faria, J. , Silva, J.P. , Silva, C.J. , Dourado, F. , and Zille, A. (2016) Laccase immobilization on bacterial nanocellulose membranes: antimicrobial, kinetic and stability properties. Carbohydr Polym 145: 586–12. [DOI] [PubMed] [Google Scholar]
- Sani, A. , and Dahman, Y. (2009) Improvements in the production of bacterial synthesized biocellulose nanofibres using different culture methods. J Chem Technol Biotechnol 85: 151–164. [Google Scholar]
- Savitskaya, I.S. , Kistaubayeva, A.S. , Digel, I.E. , and Shokatayeva, D.H. (2017) Physicochemical and antibacterial properties of composite films based on bacterial cellulose and chitosan for wound dressing materials. Eurasian Chem Technol J 19: 255–264. [Google Scholar]
- Saxena, I.M. , Kudlicka, K. , Okuda, K. , and Brown, R.M. (1994) Characterization of genes in the cellulose‐synthesizing operon (acs operon) of Acetobacter xylinum: implications for cellulose crystallization. J Bacteriol 176: 5735–5752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreml, S. , Szeimies, R.M. , Karrer, S. , Heinlin, J. , Landthaler, M. , and Babilas, P. (2010) The impact of the pH value on skin integrity and cutaneous wound healing. J Eur Acad Dermatol Venereol 24: 373–378. [DOI] [PubMed] [Google Scholar]
- Seifert, M. , Hesse, S. , Kabrelian, V. , and Klemm, D. (2004) Controlling the water content of never dried and reswollen bacterial cellulose by the addition of water‐soluble polymers to the culture medium. J Polym Sci A Polym Chem 42: 463–470. [Google Scholar]
- Shah, N. , Ul‐Islam, M. , Khattak, W.A. , and Park, J.K. (2013) Overview of bacterial cellulose composites: a multipurpose advanced material. Carbohydr Polym 98: 1585–1598. [DOI] [PubMed] [Google Scholar]
- Shao, W. , Liu, H. , Wang, S.X. , Wu, J.M. , Huang, M. , Min, H.H. , and Liu, X.F. (2016a) Controlled release and antibacterial activity of tetracycline hydrochloride‐loaded bacterial cellulose composite membranes. Carbohydr Polym 145: 114–120. [DOI] [PubMed] [Google Scholar]
- Shao, W. , Liu, H. , Wu, J.M. , Wang, S.X. , Liu, X.F. , Huang, M. , and Xu, P. (2016b) Preparation, antibacterial activity and pH‐responsive release behavior of silver sulfadiazine loaded bacterial cellulose for wound dressing applications. J Taiwan Inst Chem Eng 63: 404–410. [Google Scholar]
- Shigematsu, T. , Takamine, K. , Kitazato, M. , Morita, T. , Naritomi, T. , Morimura, S. , and Kida, K. (2005) Cellulose production from glucose using a glucose dehydrogenase gene (gdh)‐deficient mutant of Gluconacetobacter xylinus and its use for bioconversion of sweet potato pulp. J Biosci Bioeng 99: 415–422. [DOI] [PubMed] [Google Scholar]
- Silva, N.H. , Rodrigues, A.F. , Almeida, I.F. , Costa, P.C. , Rosado, C. , Neto, C.P. , et al (2014) Bacterial cellulose membranes as transdermal delivery systems for diclofenac: in vitro dissolution and permeation studies. Carbohydr Polym 106: 264–269. [DOI] [PubMed] [Google Scholar]
- Silva, M.A. , Leite, Y.K.C. , de Carvalho, C.E.S. , Feitosa, M.L.T. , Alves, M.M.M. , Carvalho, F.A.A. , et al (2018) Behavior and biocompatibility of rabbit bone marrow mesenchymal stem cells with bacterial cellulose membrane. PeerJ 6: e4656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza, C.M. , Mesquita, L.A. , Souza, D. , Irioda, A.C. , Francisco, J.C. , Souza, C.F. , et al (2014) Regeneration of skin tissue promoted by mesenchymal stem cells seeded in nanostructured membrane. Transplant Proc 46: 1882–1886. [DOI] [PubMed] [Google Scholar]
- Spaic, M. , Small, D.P. , Cook, J.R. , and Wan, W.K. (2014) Characterization of anionic and cationic functionalized bacterial cellulose nanofibres for controlled release applications. Cellulose 21: 1529–1540. [Google Scholar]
- Sulaeva, I. , Henniges, U. , Rosenau, T. , and Potthast, A. (2015) Bacterial cellulose as a material for wound treatment: properties and modifications ‐ A review. Biotechnol Adv 33: 1547–1571. [DOI] [PubMed] [Google Scholar]
- Sun, Y. , Meng, C. , Zheng, Y. , Xie, Y. , He, W. , Wang, Y. , et al (2018) The effects of two biocompatible plasticizers on the performance of dry bacterial cellulose membrane: a comparative study. Cellulose 25: 5893–908. [Google Scholar]
- Sunagawa, N. , Fujiwara, T. , Yoda, T. , Kawano, S. , Satoh, Y. , Yao, M. , et al (2013) Cellulose complementing factor (Ccp) is a new member of the cellulose synthase complex (terminal complex) in Acetobacter xylinum . J Biosci Bioeng 115: 607–612. [DOI] [PubMed] [Google Scholar]
- Tabaii, M.J. , and Emtiazi, G. (2018) Transparent nontoxic antibacterial wound dressing based on silver nano particle/bacterial cellulose nano composite synthesized in the presence of tripolyphosphate. J Drug Delivery Sci Technol 44: 244–253. [Google Scholar]
- Trovatti, E. , Freire, C.S. , Pinto, P.C. , Almeida, I.F. , Costa, P. , Silvestre, A.J. , et al (2012) Bacterial cellulose membranes applied in topical and transdermal delivery of lidocaine hydrochloride and ibuprofen: in vitro diffusion studies. Int J Pharm 435: 83–87. [DOI] [PubMed] [Google Scholar]
- Ul‐Islam, M. , Khan, T. , and Park, J.K. (2012) Water holding and release properties of bacterial cellulose obtained by in situ and ex situ modification. Carbohydr Polym 88: 596–603. [Google Scholar]
- Umeda, Y. , Hirano, A. , Ishibashi, M. , Akiyama, H. , Onizuka, T. , Ikeuchi, M. , and Inoue, Y. (1999) Cloning of cellulose synthase genes from Acetobacter xylinum JCM 7664: implication of a novel set of cellulose synthase genes. DNA Res 6: 109–115. [DOI] [PubMed] [Google Scholar]
- Voineskos, S.H. , Ayeni, O.A. , McKnight, L. , and Thoma, A. (2009) Systematic review of skin graft donor‐site dressings. Plast Reconstr Surg 124: 298–306. [DOI] [PubMed] [Google Scholar]
- Volova, T. G. , Shumilova, A. A. , Shidlovskiy, I. P. , Nikolaeva, E. D. , Sukovatiy, A. G. , Vasiliev, A. D. , and Shishatskaya, E. I. (2018) Antibacterial properties of films of cellulose composites with silver nanoparticles and antibiotics. Polym Test 65: 54–68. [Google Scholar]
- Wang, J. , Wan, Y.Z. , Luo, H.L. , Gao, C. , and Huang, Y. (2012) Immobilization of gelatin on bacterial cellulose nanofibers surface via crosslinking technique. Mater Sci Eng C 32: 536–541. [Google Scholar]
- Wen, X.X. , Zheng, Y.D. , Wu, J. , Yue, L.N. , Wang, C. , Luan, J.B. , et al (2015) In vitro and in vivo investigation of bacterial cellulose dressing containing uniform silver sulfadiazine nanoparticles for burn wound healing. Progr Nat Sci Mater Int 25: 197–203. [Google Scholar]
- Wiegand, C. , Moritz, S. , Hessler, N. , Kralisch, D. , Wesarg, F. , Muller, F.A. , et al (2015) Antimicrobial functionalization of bacterial nanocellulose by loading with polihexanide and povidone‐iodine. J Mater Sci Mater Med 26: 245. [DOI] [PubMed] [Google Scholar]
- Wild, T. , Bruckner, M. , Payrich, M. , Schwarz, C. , Eberlein, T. , and Andriessen, A. (2012) Eradication of methicillin‐resistant Staphylococcus aureus in pressure ulcers comparing a polyhexanide‐containing cellulose dressing with polyhexanide swabs in a prospective randomized study. Adv Skin Wound Care 25: 17–22. [DOI] [PubMed] [Google Scholar]
- Wong, H.C. , Fear, A.L. , Calhoon, R.D. , Eichinger, G.H. , Mayer, R. , Amikam, D. , et al (1990) Genetic organization of the cellulose synthase operon in Acetobacter xylinum . Proc Natl Acad Sci USA 87: 8130–8134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2015) Global Action Plan on Antimicrobial Resistance. Geneva, Switzerland: WHO Press, pp. 586–28. [Google Scholar]
- Wu, S.‐C. , Sian‐Ming, W. , and Feng‐Min, S. (2016) Novel process for immobilizing an enzyme on a bacterial cellulose membrane through repeated absorption. J Chem Technol Biotechnol 92: 109–114. [Google Scholar]
- Wu, C.‐N. , Fuh, S.‐C. , Lin, S.‐P. , Lin, Y.‐Y. , Chen, H.‐Y. , Liu, J.‐M. , and Cheng, K.‐C. (2018) TEMPO‐oxidized bacterial cellulose pellicle with silver nanoparticles for wound dressing. Biomacromolecules 19: 544–554. [DOI] [PubMed] [Google Scholar]
- Yadav, V. , Paniliatis, B.J. , Shi, H. , Lee, K. , Cebe, P. , and Kaplan, D.L. (2010) Novel in vivo‐degradable cellulose‐chitin copolymer from metabolically engineered Gluconacetobacter xylinus . Appl Environ Microbiol 76: 6257–6265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka, S. , Watanabe, K. , Kitamura, N. , Iguchi, M. , Mitsuhashi, S. , Nishi, Y. , et al (1989) The structure and mechanical properties of sheets prepared from bacterial cellulose. J Mater Sci 24: 3141–3145. [Google Scholar]
- Ye, S. , Jiang, L. , Wu, J.M. , Su, C. , Huang, C.B. , Liu, X.F. , and Shao, W. (2018) Flexible amoxicillin‐grafted bacterial cellulose sponges for wound dressing: vitro and in vivo evaluation. ACS Appl Mater Interf 10: 5862–5870. [DOI] [PubMed] [Google Scholar]
- Yin, N. , Chen, S. , Li, Z. , Ouyang, Y. , Hu, W. , Tang, L. , et al (2012) Porous bacterial cellulose prepared by a facile surfactant‐assisted foaming method in azodicarbonamide‐NaOH aqueous solution. Mater Lett 81: 131–134. [Google Scholar]
- Yu, J.W. , Liu, X.L. , Liu, C.S. , and Dong, P. (2011) Biosynthesis of carboxymethylated bacterial cellulose composite for wound dressing. Mater Sci Forum 685: 322–326. [Google Scholar]
- Zhang, P. , Chen, L. , Zhang, Q.S. , and Hong, F.F. (2016) Using in situ dynamic cultures to rapidly biofabricate fabric‐reinforced composites of chitosan/bacterial nanocellulose for antibacterial wound dressings. Front Microbiol 7: 260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, C. , Li, Z. , Li, T. , Zhang, Y. , Bryant, D.A. , and Zhao, J. (2015) High‐yield production of extracellular type‐I cellulose by the cyanobacterium Synechococcus sp. PCC 7002. Cell Discov 1: 15004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zmejkoski, D. , Spasojević, D. , Orlovska, I. , Kozyrovska, N. , Soković, M. , Glamočlija, J. , et al (2018) Bacterial cellulose‐lignin composite hydrogel as a promising agent in chronic wound healing. Int J Biol Macromol 118: 494–503. [DOI] [PubMed] [Google Scholar]
- Zywicka, A. , Fijalkowski, K. , Junka, A.F. , Grzesiak, J. , and El Fray, M. (2018) Modification of bacterial cellulose with quaternary ammonium compounds based on fatty acids and amino acids and the effect on antimicrobial activity. Biomacromolecules 19: 1528–1538. [DOI] [PubMed] [Google Scholar]


