Summary
Biomass recalcitrance is still a main challenge for the production of biofuels and high‐value products. Here, an alternative Miscanthus pretreatment method by using lignin‐degrading bacteria was developed. Six efficient Miscanthus‐degrading bacteria were first cultured to produce laccase by using 0.5% Miscanthus biomass as carbon source. After 1–5 days of incubation, the maximum laccase activities induced by Miscanthus in the six strains were ranged from 103 to 8091 U l−1. Then, the crude enzymes were directly diluted by equal volumes of citrate buffer and added Miscanthus biomass to a solid concentration at 4% (w/v). The results showed that all bacterial pretreatments significantly decreased the lignin content, especially in the presence of two laccase mediators (ABTS and HBT). The lignin removal directly correlated with increases in total sugar and glucose yields after enzymatic hydrolysis. When ABTS was used as a mediator, the best lignin‐degrading bacteria (Pseudomonas sp. AS1) can remove up to 50.1% lignin of Miscanthus by obtaining 2.2‐fold glucose yield, compared with that of untreated biomass. Therefore, this study provided an effective Miscanthus pretreatment method by using lignin‐degrading bacteria, which may be potentially used in improving enzymatic hydrolysability of biomass.
Introduction
Various sources of lignocellulosic feedstocks have been regarded as emerging biofuels to partly replace the fossil fuels in the near future. However, their complicated cell wall structure makes the production of biofuels more difficult and costly due to the existence of biomass recalcitrance (Pauly and Keegstra, 2008). Among the three main polymeric components (cellulose, hemicellulose and lignin) constituting lignocellulosic biomass, lignin, which covers the cellulose and hemicellulose in various ways, is the most stable and complex polymer and acts as an obstacle in the enzymatic hydrolysis of cellulose (Mussatto et al., 2008; Lee et al., 2014). To make more cellulose and hemicellulose available before enzymatic hydrolysis, a pretreatment step is necessary to weaken and remove lignin (Lee et al., 2014). The lignin can be disrupted via physical, chemical and biological processes (Alvira et al., 2010). Various pretreatments methods through physical and chemical processes (Hu and Wen, 2008), alkaline and dilute acid (Si et al., 2015), organic solvent (Sun et al., 2000), steam explosion (Öhgren et al., 2007) and wet oxidation (Hendriks and Zeeman, 2009) are available to remove lignin from lignocellulosic biomass. However, high energy input, high costs of chemicals and increased environmental risk make these methods challenging to scale up (Millati et al., 2011). In addition, the lignin degraded by physical and chemical processes will release some inhibiting compounds, such as furfurals, 5‐hydroxymethyl furfurals and other volatile products, which inhibit the next stages of enzymatic hydrolysis and yeast fermentation (Hendriks and Zeeman, 2009; Camesasca et al., 2015). Organosolv pretreatment may cause severe damage like fire explosions in the absence of proper safety measures, although it can achieve a biomass fractionation to lignin, hemicellulosic sugars and a relatively pure cellulose fraction (Kumar and Sharma, 2017; Li et al., 2017). These are the disadvantages for large‐scale application.
Lignin removal via biological pretreatment has received increasing attention as an alternative to physical and chemical pretreatment for enhancing cellulose digestibility due to its environmental and economical benefits (Wan and Li, 2012; Sindhu et al., 2016). Among the various biological pretreatments adopted for lignin removal from lignocellulosic biomass, fungal pretreatments have been widely studied because fungi, especially white‐rot fungi, were thought to be excellent laccase and peroxidase producers (Wesenberg et al., 2003; Wan and Li, 2012). However, fungi usually grow slowly and require a long time to produce lignolytic enzymes like laccase, manganese peroxidase (MnP) and lignin peroxidase (LiP), which means that long pretreatment time is necessary for lignin removal by fungi (Leonowicz et al., 1999; Wan and Li, 2012). Moreover, large loss of cellulose and hemicellulose is another major weakness of fungal pretreatment, although remarkable enhancement of cellulose digestibility has been obtained from lignocellulosic biomass pretreated by white‐rot fungi (Wu et al., 2005; Lee et al., 2007).
A number of bacteria are able to produce various lignocellulolytic enzymes like endoglucanase (CMCase), xylanase and laccase (Bugg et al., 2011; Guo et al., 2017a). Of these bacteria, Bacillus sp., Pseudomonas sp., Streptomyces sp. and Aeromonas sp. strains have been reported to break down lignin (Bugg et al., 2011; Chang et al., 2014). Bacillus sp. CS‐1 isolated from forest soils was used to degrade up to 20% lignin in rice straw with remarkable laccase production (Chang et al., 2014). The laccase from Bacillus licheniformis showed strong oxidation capacity towards substrates 2,2‐azino‐bis (3‐ethylbenzothiazoline‐6‐sulphonic acid) (ABTS), syringaldazine, 2,6‐dimethoxyphenol and phenolic acids (Koschorreck et al., 2008). A soil bacterium, Pseudomonas putida, known as aromatic degrader, was capable of removing lignin from various lignocellulose biomasses accompanied by producing monocyclic phenolic products (Ahmad et al., 2010). However, the laccase production yields of bacteria are much lower than those of fungi and thus impeding their application in industry. In our previous study, it was found that the laccase production of bacteria can be significantly induced by lignocellulosic biomass, such as algae and Miscanthus. The high activity of laccase induced by Miscanthus may be due to the high components of total ester‐bound phenolics, which can stimulate the secretion of laccase (Guo et al., 2017b). In addition, the delignification efficiency of laccase can be largely improved by the laccase mediators, which can increase the oxidation of polymeric lignin by transferring hydrogen atoms (Baiocco et al., 2003; Yao and Ji, 2014).
Therefore, a two‐step pretreatment procedure was developed in this study. A low concentration Miscanthus biomass (0.5%, w/v) was first used to induce the production of laccase, and then, the crude enzymes of these bacteria induced by 0.5% Miscanthus biomass were directly mixed with equal volumes of citrate buffer and Miscanthus at solid concentration of 4% (w/v) to debilitate the lignin. The effects of two laccase mediators, 2,2‐azino‐bis(3‐ethylbenzothiazoline‐6‐sulphonic acid) (ABTS) and 1‐hydroxybenzotriazole (HBT), on the degradation of lignin were also determined in the process of pretreatment. Then, the pretreatment effect was evaluated by measuring the sugar released after enzymatic hydrolysis.
Results
Screening the Miscanthus‐degrading bacteria
Twelve bacterial strains isolated previously by Guo and colleagues (2017a,2017b), Maki and colleagues (2012) and Paudel and Qin (2015), including 7 Bacillus, 2 Pseudomonas, 1 Exiguobacterium, 1 Aeromonas and 1 Raoultella species (Table 1), were tested for the hydrolysis of biomass using Miscanthus as carbon source. All tested strains and positive strain C. xylanilytica showed a halo region in the plate after Gram's iodine staining, and nine strains (A0, A4, AS1, AS2B, GH2OS1, K1, X1, X4 and X8) showed bigger halo regions than that of C. xylanilytica (Fig 1). The hydrolytic potential of Miscanthus of each bacterial colony was further evaluated by calculating the square of the halo diameter: colony diameter ratio. The hydrolytic potential values of these strains ranged from 2.8 to 18.1, while only strains A4, AS1, AS2B, K1, X4 and X8 had higher hydrolytic potential than that of positive strain (hydrolytic potential value: 9.8) (Table 1). Therefore, these strains were selected for the next experiments.
Table 1.
Miscanthus biomass hydrolytic abilities of twelve different bacteria and two controls
| Isolates | Genus | Accession no. | Source | Halo diameter (D, cm) | Colony diameter (d, cm) | Hydrolytic potential (D/d)2 |
|---|---|---|---|---|---|---|
| A0 | Bacillus | KP974676 | Paudel and Qin (2015) | 3.25 ± 0.21b | 1.45 ± 0.09a | 5.02 |
| A4 | Bacillus | KX665584 | Guo and colleagues (2017b) | 3.43 ± 0.15ab | 0.83 ± 0.02ef | 17.1 |
| AS1 | Pseudomonas | HM063909 | Maki and colleagues (2012) | 2.85 ± 0.12c | 0.67 ± 0.05 g | 18.1 |
| AS2B | Exiguobacterium | HM134063 | Maki and colleagues (2012) | 3.55 ± 0.06a | 0.94 ± 0.06de | 12.5 |
| CDS1B | Aeromonas | FJ168772 | Maki and colleagues (2012) | 1.13 ± 0.1f | 0.67 ± 0.03 g | 2.8 |
| CTS1A | Bacillus | KF482855 | Maki and colleagues (2012) | 2.34 ± 0.10e | 1.10 ± 0.09c | 4.5 |
| GH2OS1 | Pseudomonas | JQ320089 | Maki and colleagues (2012) | 3.38 ± 0.10b | 1.32 ± 0.06b | 6.5 |
| K1 | Bacillus | KP987117 | Paudel and Qin (2015) | 3.35 ± 0.13b | 0.85 ± 0.04e | 15.5 |
| X1 | Raoultella | KY290273 | Guo and colleagues (2017a) | 3.30 ± 0.18b | 0.95 ± 0.07d | 10.0 |
| X4 | Bacillus subtilis | KY327801 | Guo and colleagues (2017a) | 3.57 ± 0.10a | 0.89 ± 0.04e | 16.1 |
| X8 | Bacillus | KY941135 | In this study | 3.33 ± 0.10b | 0.98 ± 0.05d | 11.5 |
| 6S1 | Bacillus | AB849115 | Maki and colleagues (2012) | 2.45 ± 0.26de | 0.78 ± 0.04f | 9.9 |
| Positive | C. xylanilytica | AY303668 | Rivas and colleagues (2004) | 2.50 ± 0.08d | 0.80 ± 0.02f | 9.8 |
| Negative | E.coli BL21 | – | – | – | 0.65 ± 0.03 g | – |
Values represent mean ± SDs (n = 3). Different letters indicate a significant difference at P < 0.05.
Figure 1.
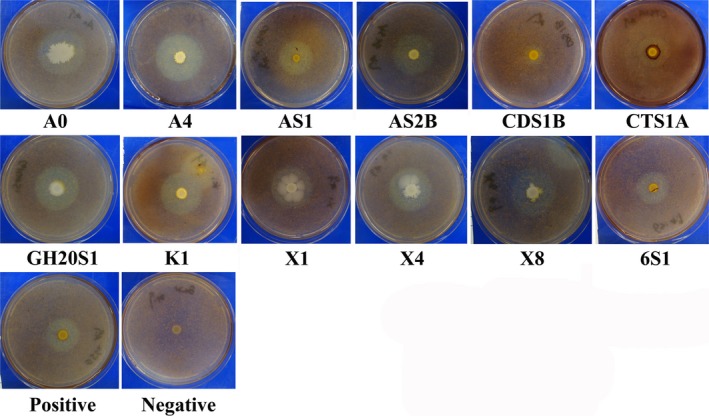
Visualization of the hydrolysis activities of Miscanthus biomass using twelve different bacteria strains and two control strains by staining with Gram's iodine solution. Positive: C. xylanilytica; Negative: E. coli.
Activities of laccase secreted by selected bacteria
The laccase production abilities were different among the six bacterial strains when Miscanthus was used as the sole carbon source (Fig. 2). Peak laccase activity was detected on the first day in strains AS1 (8091 U l−1) and K1 (1049 U l−1). Maximum activity occurred on the third day in strains AS2B (2365 U l−1) and X8 (103 U l−1), on the fourth day in stain X4 (646 U l−1), and on the fifth day in strain A4 (1122 U l−1) (Fig. 2).
Figure 2.
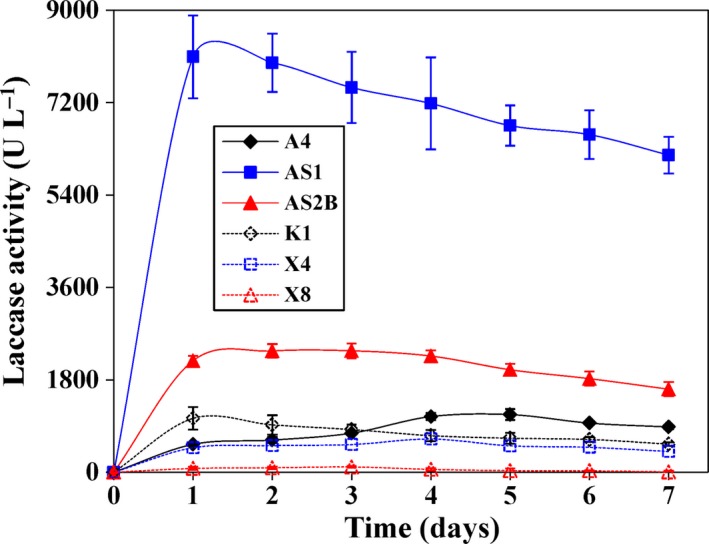
Laccase activities of different bacteria cultivated in the mineral salt medium containing 0.5% Miscanthus (w/v). Values represent mean ± SDs (n = 3).
Effects of bacteria pretreatment on delignification
The crude enzymes (Lac) produced by bacteria and two laccase mediators (Lac + ABTS and Lac + HBT) were tested in the enzymatic pretreatment of Miscanthus biomass. The crude enzymes autoclaved at 121°C for 25 min were used as a control (Inact). The native M. sacchariflorus used in this study mainly consisted of 32.9% cellulose, 22.5% hemicellulose and 27.9% lignin. After pretreatment of crude enzymes produced by the six bacteria, the cell wall composition of Miscanthus significantly differed with selective degradation of lignin, especially in the presence of two laccase meditators. Without laccase mediators, the lignin contents were 22.0, 19.5, 21.2, 16.1, 22.5 and 22.7% for Lac pretreatment of A4, AS1, AS2B, K1, X4 and X8 respectively. The most lignin was removed by Lac + HBT for all strains, and the lignin removal percentages were 47, 59.5, 42.7, 47.7, 52.7 and 41.2% for strains A4, AS1, AS2B, K1, X4 and X8 respectively. For Lac + ABTS pretreatment, strain AS1 degraded the most lignin of Miscanthus, with a lignin removal percentage of 50.9%, while the least lignin remove was found by X8 with the lignin removal percentage of 29.7%, compared with that of untreated Miscanthus (Table 2). Interestingly, the cellulose (Calculated as glucan) and hemicellulose (Calculated as xylan) contents were all significantly increased after pretreatment, compared with that of untreated and Inact‐treated Miscanthus (Table 2). The maximum cellulose content was obtained by Lac + HBT pretreatment of strain AS1, being 49.4% and 46.7% higher than those of untreated biomass and biomass treated by Inact respectively. The biomass pretreated by Lac + ABTS of X4 strain showed the highest hemicellulose content (30.1%) compared with that of untreated (22.5%) and Inact of X4 strain (23.5%). The almost no cellulose and hemicellulose loss were proved by the little sugar increase in the liquor after bacteria pretreatment (Table 2). Moreover, the addition of laccase mediators in all bacterial strains significantly changed the cellulose and lignin contents, while there were no significant effects to hemicellulose content compared with Lac pretreatment alone (Table 2 and Fig. 3). The Lac pretreatment alone included cellulose content ranging from 36.0% to 39.5%, hemicellulose from 25.6% to 30.1% and lignin from 16.1% to 22.7%, whereas using Lac + ABTS pretreatment, the ranges were 38.3–44.5%, 27.1–30.1% and 13.7–19.6%, respectively, and using Lac + HBT pretreatment, they were 39.3–49.3%, 21.1–28.9% and 11.3–16.4% respectively (Fig. 3A‐C).
Table 2.
Composition changes of treated Miscanthus from different bacteria
| Isolates | Methods | Glucan (%) | Xylan (%) | Lignin (%) | ||
|---|---|---|---|---|---|---|
| Liquor | Solid | Liquor | Solid | |||
| – | Untreated | – | 32.9 ± 1.7 | – | 22.5 ± 1.5 | 27.9 ± 0.7 |
| A4 | Inactive | 0.83 | 35.1 ± 4.4b | 0.24 | 24.2 ± 2.7a | 26.2 ± 1.1a |
| Lac | 1.08 | 38.1 ± 1.3ab | 0.37 | 27.4 ± 3.9a | 22.0 ± 2.6b | |
| Lac + ABTS | 1.00 | 40.2 ± 2.6a | 0.47 | 27.1 ± 1.2a | 15.1 ± 2.1c | |
| Lac + HBT | 1.04 | 41.1 ± 2.4a | 0.54 | 26.7 ± 1.2a | 14.8 ± 1.4c | |
| AS1 | Inactive | 0.80 | 33.6 ± 2.7b | 0.26 | 23.6 ± 1.0b | 26.1 ± 0.6a |
| Lac | 1.09 | 36.2 ± 1.1b | 0.47 | 25.6 ± 1.5b | 19.5 ± 1.1b | |
| Lac + ABTS | 1.07 | 44.5 ± 5.0a | 0.48 | 27.0 ± 1.0a | 13.7 ± 1.7c | |
| Lac + HBT | 1.02 | 49.3 ± 3.4a | 0.43 | 23.6 ± 1.3b | 11.3 ± 3.4c | |
| AS2B | Inactive | 0.88 | 32.5 ± 2.8b | 0.29 | 23.1 ± 2.6ab | 25.8 ± 0.8a |
| Lac | 1.19 | 36.0 ± 3.6b | 0.47 | 26.1 ± 1.9a | 21.2 ± 0.9b | |
| Lac + ABTS | 1.15 | 38.5 ± 3.0ab | 0.42 | 27.3 ± 3.2a | 18.9 ± 1.2bc | |
| Lac + HBT | 1.03 | 41.4 ± 5.3a | 0.49 | 21.1 ± 1.7b | 16.0 ± 2.3c | |
| K1 | Inactive | 0.85 | 33.5 ± 2.0b | 0.27 | 23.5 ± 1.8b | 26.4 ± 2.4a |
| Lac | 0.98 | 38.3 ± 1.0a | 0.51 | 30.1 ± 2.8a | 16.1 ± 1.5b | |
| Lac + ABTS | 1.12 | 40.8 ± 3.6a | 0.56 | 27.4 ± 1.9a | 15.5 ± 2.5b | |
| Lac + HBT | 1.17 | 41.5 ± 2.8a | 0.61 | 26.3 ± 3.7ab | 14.6 ± 3.5b | |
| X4 | Inactive | 0.85 | 32.9 ± 2.8c | 0.25 | 23.5 ± 1.6b | 26.2 ± 2.4a |
| Lac | 1.13 | 36.6 ± 2.6c | 0.38 | 25.7 ± 3.5a | 22.5 ± 2.7b | |
| Lac + ABTS | 1.04 | 43.3 ± 4.5b | 0.49 | 30.1 ± 1.9a | 15.7 ± 1.0c | |
| Lac + HBT | 1.01 | 48.0 ± 4.5a | 0.48 | 28.9 ± 2.0a | 13.2 ± 1.1c | |
| X8 | Inactive | 0.86 | 34.5 ± 2.4a | 0.24 | 25.9 ± 2.6a | 26.8 ± 1.8a |
| Lac | 1.02 | 39.5 ± 1.2a | 0.43 | 28.7 ± 1.9a | 22.7 ± 2.3b | |
| Lac + ABTS | 1.09 | 38.8 ± 1.7a | 0.45 | 28.3 ± 2.4a | 19.6 ± 2.2bc | |
| Lac + HBT | 1.10 | 39.3 ± 2.1a | 0.51 | 26.0 ± 3.8a | 16.4 ± 3.5c | |
Values represent mean ± SDs (n = 4). Different letters indicate a significant difference at P < 0.05.
Figure 3.

Variation of cell wall composition of Miscanthus after different bacteria pretreatment using ABTS or HBT as a mediator. (A) Cellulose content (n = 24); (B) hemicellulose content (n = 24); (C) lignin content (n = 24). ABTS: 2,2’‐Azino‐bis(3‐ethylbenzothiazoline‐6‐sulphonate), HBT: 1‐hydroxybenzotriazole. Different letters indicate a significant difference at P < 0.05.
Effects of bacteria pretreatment on enzymatic hydrolysis
To evaluate the effects of bacteria pretreatment on biomass digestibility, the sugar yield (hexoses and pentoses) released from pretreated Miscanthus after hydrolysing with a mixed cellulase cocktail (Celluclast 1.5L and Novozyme 188) was determined (Fig. 4). The hexoses released (% of cellulose), pentoses released (% of hemicellulose) and total sugar released (including hexoses and pentoses, % of dry weight) from untreated Miscanthus were 43.2, 40.3 and 25.4% respectively (Fig. 4). After enzymatic hydrolysis, the hexoses and total sugar released from pretreated Miscanthus by Lac, Lac + ABTS and Lac + HBT were all significantly increased (P < 0.05) in all strains, compared with that of untreated and corresponding pretreated Miscanthus by Inact. For each strain, the total sugar released from Lac + HBT was higher than that from Lac + ABTS and Lac. The highest total sugar yield was obtained by Lac + HBT of strain AS1 (54.2%), followed by Lac + HBT of strain X4 (53.1%) (Fig. 4).The hexose released from Lac + ABTS pretreated Miscanthus was higher than that of Lac + HBT and Lac in all strains except the strain A4, which obtained the peak hexoses yield by Lac + HBT (Fig. 4). The maximum hexoses were released by the Lac + ABTS of strain AS1 (87.0%; Fig. 4). For the release of pentoses, the maximum yields were harvested by Lac of strain X4 (59.5%), by Lac + ABTS of strain A4 (53.8%), by Lac + HBT of strains AS1 (48.8%), AS2B (78.7%), K1 (66.9%) and X8 (54.6%) respectively (Fig. 4).
Figure 4.

Biomass digestibility of Miscanthus after six different bacteria pretreatments. Total sugar yields (% of dry weight) released by mixed cellulase after pretreatment; Hexoses yields (% of cellulose) released by mixed cellulase after pretreatment; pentose yields (% of hemicellulose) released by mixed cellulase after pretreatment. Values represent mean ± SDs (n = 4). Different letters indicate a significant difference at P < 0.05.
Correlation of lignin removal and sugars release and mass balance
We analysed the correlation between lignin removal and sugar released from six bacteria strains with or without mediators pretreated Miscanthus (Fig. 5). Significantly, a positive linear correlation was observed with correlation coefficient (R 2) values of 0.9044 (P < 0.01) and 0.6286 (P < 0.01) for the lignin removal and total sugar and hexoses yield released after bacteria pretreatment (Fig. 5A and B). However, there was no positive correlation between lignin removal and pentoses released by bacteria pretreated Miscanthus (R 2 = 0.2581; P = 0.14; Fig. 5C).
Figure 5.
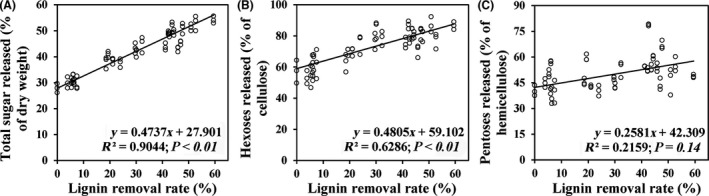
Correlation analysis between sugars released and lignin content after different bacteria pretreatment. (A) Total sugar released (n = 24); (B) hexoses released (n = 24); (C) pentoses released (n = 24).
To further evaluate the effects of bacteria pretreatment and track carbohydrate degradation during the pretreatment process, the results obtained from laccase production, delignification and enzymatic hydrolysis were used to make a detailed mass balance using Lac + ABTS of strain AS1 (Fig. 6). The maximum laccase production in AS1 strain was 1618 U g−1 dry biomass. After Lac + ABTS pretreatment of strain AS1, the solid recovery was 72.6%, and the glucan content was unchanged (P > 0.05) compared with that of raw material, while the xylan and lignin content decreased from 2.25 g to 1.96 g and 2.79 g to 1.10 g per 10 g dry biomass compared with that of raw material respectively. After enzymatic hydrolysis, the glucose released from Lac + ABTS pretreated Miscanthus was 2.2‐fold higher than that of untreated Miscanthus (Fig. 6). The total monosaccharide yield was 4.34 g per 10 g dry biomass, which was a 1.7‐fold increase compared with that of untreated Miscanthus after enzymatic hydrolysis (Fig. 6).
Figure 6.
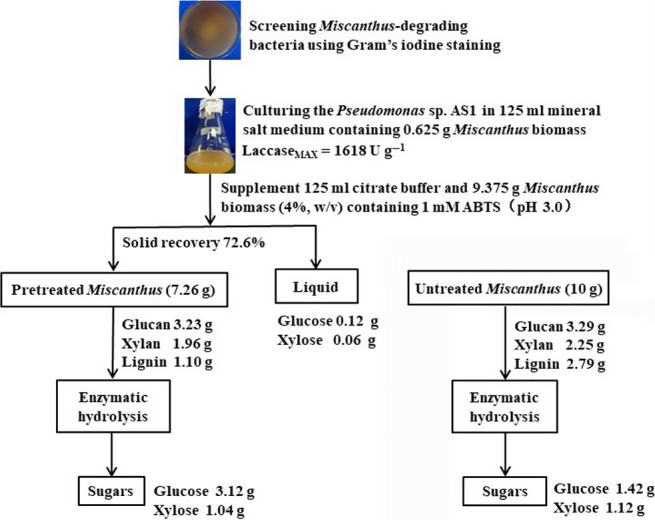
Flow chart of experiments investigated the effects of Pseudomonas sp. AS1 on laccase production, Miscanthus hydrolytic ability, lignin de‐polymerization and enzymatic hydrolysis in the presence of ABTS.
Discussion
Miscanthus biomass is a good candidate for laccase production
Twelve biomass‐degrading bacteria (seven Bacillus, two Pseudomonas, one Exiguobacterium, one Aeromonas and one Raoultella species) were isolated on plates using carboxymethyl cellulose as the sole carbon source by our previously laboratory members (Bugg et al., 2011; Maki et al., 2012; Paudel and Qin, 2015). It has been reported that the degradation ability was extremely different using different carbon sources in both fungi (Olsson et al., 2003; Sipos et al., 2010) and bacteria (Waghmare et al., 2014; Mohammadkazemi et al., 2015). In this study, the degradation abilities of the twelve bacteria to Miscanthus biomass were determined using the Gram's iodine method, while only six bacteria strains (A4, AS1, AS2B, K1, X4 and X8) showed higher hydrolytic potential than that of the industrial strain C. xylanilytica when Miscanthus was used as the sole carbon source. To further evaluate the characteristics of these six strains, laccase activities were monitored in the mineral salt medium containing 0.5% (w/v) Miscanthus. The results showed the strains A4, AS1, AS2B, K1, X4 and X8 can efficiently secrete laccase into the medium, with the significantly different among the bacteria strains. Previous studies have shown the production of bacterial lignocellulolytic enzymes differed markedly using different carbon sources even if the same strain was used (Mohammadkazemi et al., 2015; Guo et al., 2017a). The maximum laccase activities induced by wheat bran in strains A4, AS2B, K1 and X4 were 246.7, 67.0, 82.4 and 137.0 U l−1, respectively (Guo et al., 2017a), and they were 501, 1149, 586 and 378 U l−1 in the presence of Miscanthus respectively. The lignocellulolytic enzyme activity produced by Klebsiella sp. PRW‐1 was higher when grass power and sugarcane were used as carbon source compared with that of other agricultural wastes like sorghum husks, corn straw and paddy straw (Waghmare et al., 2014). The biodegradation and enzyme production of lignocellulosic biomass by fungi and bacteria mainly depended on the porosity of biomass materials and crystallinity of cellulosic fibre (Zhang et al., 2006; Kumar et al., 2009). A series of lignocellulosic enzymes were produced by microorganisms using lignocellulosic biomass as carbon source, and certain lignocellulosic biomasses possess abundant soluble carbohydrates and enzyme synthesis‐related inducers, which can efficiently increase the production of lignocellulosic enzymes (Rosales et al., 2005; Singhania et al., 2010). Moreover, our results showed that in the presence of Miscanthus, the Pseudomonas strain AS1 specifically produced a large amount of laccase, which has been regarded as the main lignin‐degrading enzymes in the process of delignification of biomass (Martinez et al., 2009; Pollegioni et al., 2015).
Delignification of Miscanthus and improved saccharification by bacterial pretreatment and laccase mediators
The polymers of lignin closely overlay the other polymers (mainly including cellulose and hemicellulose) in various ways in the lignocellulosics biomass. Lignin is very impervious and hard to degrade by enzymes or chemicals, and this is the main barrier to make cellulose and hemicellulose more susceptible to enzymatic hydrolysis in industrial processes (Brebu and Vasile, 2010). The degradation of lignin by using fungi has been well‐studied, especially by using white‐rot and brown‐rot fungi (Leonowicz et al., 1999; Guillén et al., 2005). But research about the degradation of lignin by using bacteria is scarce. In this study, six Miscanthus‐degrading bacteria were screened to degrade the lignin of Miscanthus biomass. The results showed that the crude enzymes produced by all the tested bacteria efficiently decreased the lignin content of Miscanthus, especially in the presence of two laccase mediators. The maximum lignin removal percentage (59.5%) was observed by the Pseudomonas sp. AS1 strain with the addition of 0.5% HBT (g g−1 dry biomass). Our previous study reported that the Pseudomonas sp. AS1 isolated from municipal waste showed good potential for black liquor decolourization and industrial degradation of lignocellulosic biomass (Maki et al., 2012). Moreover, the other four Bacillus sp. strains and one Exiguobacterium similarly reduced the content of lignin even if only their crude enzymes were used, which obtained the lignin removal percentage range from 18.6% to 42.3%. It has been reported that some of bacterial stains were able to degrade lignin via producing laccase and other lignin peroxidase (Bugg et al., 2011; Chang et al., 2014). The Bacillus sp. CS‐1 strain isolated from forest soils can degrade at least 61% alkali lignin within 48 h, and pretreatment using this strain followed by lactic acid bacteria removed 61.9% lignin of rice straw and enhanced cellulase performance (Chang et al., 2014). The Pseudomonas sp. LD002 and Bacillus sp. LD003 were isolated from beneath decomposing wood logs and exhibited good growth on lignin fractions and excellent dye‐decolourizing abilities (Bandounas et al., 2011).
The chemicals of ABTS and HBT have been regarded as the most efficient laccase mediators, which play as an electron carrier between laccase and oxidized substrate (Munk et al., 2015). In the current study, the average lignin contents of Miscanthus pretreated by six bacteria strains with two laccase mediators (ABTS and HBT) were 20.2% and 30.1% lower than that without laccase mediator. This was consistent with the higher lignin removal percentage obtained by laccase pretreatment in the presence of various laccase mediators (Rico et al., 2014; Rencoret et al., 2016). Furthermore, all bacteria pretreatment in the citrate buffer (pH 3.0) selectively degraded the lignin and improved the cellulose and hemicellulose contents of Miscanthus. The results were similar to that of pretreatment of feedstock with commercial laccase, which removed up to 50% of lignin and significantly increased the content of glucose and xylose in the presence of laccase mediators (Rico et al., 2014; Rencoret et al., 2016).
A previous study showed Miscanthus biomass saccharification largely depends on the effective lignin removal rather than the hemicellulose extraction under various alkali and acid pretreatments (Si et al., 2015). In this study, total sugar and hexoses released after enzymatic hydrolysis were significantly improved by all pretreatments and showed a positive correlation with the lignin removal percentage, which indicated that lignin ratio was the key factor that positively influenced Miscanthus digestibility after bacterial pretreatments. In addition, the maximum enzymatic digestibility (calculated as glucose released) from bacterial pretreatment was up to 87%, which was comparable with that of most fungal pretreatments, such as Trameteshirsute yj9 (73.99%; Sun et al., 2011) and Pycnoporus sp. SYBC‐13 (90%; Liu et al., 2013). Therefore, the bacterial pretreatment in this study might be more promising in the removal of lignin on Miscanthus compared with that of direct incubation biomass with fungi from the following three aspects: first, the laccase secreted by most of the tested bacteria in this study can rapidly reach the maximum after 1–3 days of incubation, which was easier to obtain crude laccases than most of fungi (Wesenberg et al., 2003; Liu et al., 2013). Second, even though the lower redox potential was found in most bacterial laccases (Bugg et al., 2011), the lignolytic enzyme activities induced by biomass in these bacteria were much higher than that in fungi. For example, the highest laccase activity of AS1 strain in this study was 1618 U g−1 dry biomass, while the maximum of it in reported fungi was 935.4 U g−1 dry biomass (Chang et al., 2012; Yang et al., 2012). Third, the process of lignin removal by fungal pretreatment was accompanied by large losses of cellulose and hemicellulose, which may be utilized as a carbon source to support fungal growth and metabolism (Wan and Li, 2010; Bugg et al., 2011).
Conclusions
This study showed that the enzymatic digestibility of Miscanthus can be improved by laccase‐secreted bacteria. Six efficient Miscanthus‐degrading bacteria secreted abundant laccase when Miscanthus was used as sole carbon source. The crude enzymes induced by Miscanthus markedly decreased the content of lignin, especially in the presence of two laccase mediators, with the lignin removal percentage of 29.7–59.5%. After enzymatic hydrolysis, the glucose released from bacterial pretreated Miscanthus was 1.3–2.2‐fold higher than that from untreated biomass. These results will provide a two‐step pretreatment method to further studies on the use of laccase‐producing bacteria for bioenergy production.
Experiment procedures
Strains and culture conditions
The following twelve lignocellulose‐degrading bacteria, which were isolated and identified previously by our laboratory members, were used in this study (Table 1). The detailed information of Bacillus sp. A0 and K1 was described by Paudel and Qin (2015), Bacillus sp. A4, Raoultella sp. X1 and Bacillus subtilis X4 by Guo and colleagues (2017a) and Pseudomonas sp. AS1, Exiguobacterium sp. AS2B, Aeromonas sp. CDS1B, Bacillus sp. CTS1A, Pseudomonas sp. GH2OS1 and Bacillus sp. 6S1 by Maki and colleagues (2012). The strain Bacillus sp. X8 was isolated from forest soil (Thunder Bay, Ontario, Canada) and identified as Bacillus sp. X8 in this study. Cellumonas xylanilytica and Escherichia coli BL21 were used as positive and negative controls respectively. The strains and their corresponding accession number and source are shown in Table 1. All the strains were stored at −70°C. Prior to the experiments, all the strains were activated in Luria–Bertani (LB) medium at 37°C, with agitation at 200 rpm for 12 h. The bacteria were then cultured on a large scale in mineral salt medium (0.1% NaNO3, 0.1% K2HPO4, 0.1% KCl, 0.05% MgSO4, 0.05% yeast extract and 0.3% peptone) containing 0.5% (w/v) Miscanthus sacchariflorus (M. sacchariflorus) biomass for 7 days at 37°C, with agitation at 200 rpm. The production of laccase was monitored every day, and the culture medium from different strains with the highest laccase activity was directly used for M. sacchariflorus pretreatment.
Screening and evaluation of Miscanthus‐degrading bacteria
To measure the hydrolysis activity of M. sacchariflorus by these bacteria strains, the strains were cultured on an agar plate using M. sacchariflorus as the sole carbon source according to our previous study (Guo et al., 2017a). Briefly, 5 μl of overnight‐grown each bacterial culture was dropped in an agar plate containing 0.1% NaNO3, 0.1% K2HPO4, 0.1% KCl, 0.05% MgSO4, 0.05% yeast extract, 0.3% peptone, 1.5% agar and 0.5% M. sacchariflorus biomass. All of the plates were incubated at 37°C for 48 h and flooded with Gram's iodine solution for 3–5 min. The diameters of the halo region (D) and bacterial colony (d) were then measured on a centimetre scale. The hydrolysis activity was calculated as (D/d)2, as described previously by Guo et al., 2017a.
Determination of enzyme activities
For determination of laccase activities, 1 ml of cultures of each strain was collected after 1, 2, 3, 4, 5, 6 and 7 days of incubation and centrifuged at 12 000 g for 3 min. The supernatants were used as crude enzymes for enzyme activities analysis. The activities of laccase were measured according to the description of Guo and colleagues (2017a). Briefly, 200 μl of reaction solution including 20 μl diluted crude enzyme and 20 μl of 20 mM ABTS in a 0.1 M citrate buffer (pH 3.0) was incubated at 40°C for 3 min. Then, the optical density (OD) was measured at 420 nm (ε 420 = 36 000 M−1 cm−1) using a Microplate Spectrophotometer (Epoch; Bio Tek Instruments, Winooski, VT, USA). One unit of laccase activity was defined as the amount of enzyme required to oxidize 1 μmol of substrate per min.
Inoculum preparation and pretreatment
The screened six Miscanthus‐degrading bacteria (A4, AS1, AS2B, K1, X4 and X8) were firstly cultured in 125 ml mineral salt medium containing 0.625 g (0.5%, w/v) M. sacchariflorus biomass to produce laccase at 37°C, with agitation at 200 rpm. At the day with the highest laccase activity in the culture medium, equal volumes of citrate buffer (pH 3.0) and another 9.375 g M. sacchariflorus biomass were added to the culture medium to make the final biomass ratio at 4.0% (w/v). The final pH was adjusted to 3.0 using 1M hydrochloric acid. Then, the mixture was saturated with oxygen by bubbling air for 20 min. Tetracycline (40 mg l−1) was added into the mixture to control the growth of bacteria. For the effects of laccase mediators (HBT and ABTS) on the delignification of Miscanthus biomass, 0.1 mM ABTS and 5% HBT (g g−1 dry weight biomass) were added to the culture. Bacterial culture autoclaved at 121°C for 25 min was used as a control. The pretreatment was performed at 37°C, with agitation at 200 rpm. After 96 h of pretreatment, the samples were washed with distilled water five times to remove the bacterial cells through a double‐layered muslin cloth with the meshes of 300. Then, solid residues were oven dried at 50°C until constant weight and used for cell wall composition analysis and subsequent enzymatic hydrolysis.
Plant cell wall composition analysis
The cellulose and hemicellulose contents were measured according to the method of Guo and colleagues (2017c). Briefly, the sample (0.1 g) was extracted twice with 1.5 ml hot water, once with 1.5 ml absolute ethanol and once with 1.5 ml acetone at 65°C for 30 min. Then, the sediment was air‐dried in a chemical hood for 2 days, and the dry samples were hydrolysed with 1 ml of 72% (w/w) sulphuric acid (H2SO4) at 30°C for 1 h. The hydrolysates were diluted to 3% sulphuric acid by adding deionized water and autoclaved at 121°C for 1 h. After cooling down, the mixture was filtered through a pre‐weighted glass microfilter. The solutions were used to determine the contents of hexose and pentose using the anthrone‐sulphuric acid method and orcinol‐hydrochloric acid method respectively. The filter and solids were dried at 105°C until constant weight and weighed, and then, the solids were ashed at 575°C for 3 h and weighted again. The Klason lignin contents were calculated as the weight of the dry solids minus that of the ash as a percentage of the weight of the initial dry weight of sample.
Determination of total hexoses and pentoses
The total hexose and pentose contents were determined according to the method of Xu and colleagues (2012), with minor modifications. For determination of total hexoses, 80 μl diluted sample was added to 160 μl of 0.2% (w/v) anthrone in concentrated H2SO4, mixed well and incubated in boiling water for 5 min. After cooling down, 200 μl of the mixture was removed to the 96‐well microtiter plate and the absorbance was determined at 620 nm using a microplate spectrophotometer (Epoch; Bio Tek Instruments). For determination of total pentoses, 75 μl diluted sample and 10 μl of 6% orcinol (0.6 g orcinol dissolved in 10 ml ethanol) were mixed with 150 μl of 0.1% (w/v) ferric chloride in concentrated HCl, mixed well and incubated in the boiling water for 20 min. After cooling down, 200 μl of the mixture was transferred to the 96‐well microtiter plate and the absorbance was determined at 660 nm using a microplate spectrophotometer (Epoch; Bio Tek Instruments). A range of D‐glucose (0–100 μg ml−1) and D‐xylose (0–40 μg ml−1) concentrations were used to plot the standard curves for hexoses and pentoses respectively.
Enzymatic hydrolysis
Enzymatic hydrolysis was performed according to NREL Laboratory Analytical Procedure LAP 009. The solid residues of pretreatment and untreated Miscanthus biomass were loaded at a solid concentration of 2.0% (w/v) in a 50 mM citrate buffer (pH 4.8) containing 0.005% (w/v) sodium azide. Cellulase (Celluclast 1.5L; Novozymes, Franklinton, NC, USA) from Trichoderma reesei and beta‐glucosidase (Novozyme 188; Novozymes, Bagsvaerd, Denmark) from Aspergillus niger were used for hydrolysis experiments. The enzyme loadings of Celluclast 1.5L and Novozyme 188 were 20 FPU g−1 glucan and 30 CBU g−1 glucan respectively. The hydrolysis experiment was conducted at 50°C and 150 rpm in a shaking incubator for 72 h. After enzymatic hydrolysis, the mixture was centrifuged at 12 000 g for 3 min and the supernatants were collected for measurement of hexose and pentose content. The sugars released were calculated using the following equation:
The value of 1.11 is the conversion factor of glucan to equivalent glucose. The value of 1.14 is the conversion factor of xylan to equivalent xylose. The total sugars were calculated as the total mass of glucose and xylose released treated and untreated dry biomass.
Statistical analysis
Correlation coefficients were calculated by performing spearman rank correlation analysis. All the experiments were repeated at least three times, and the results were shown as mean ± SDs. Differences between treatments were evaluated, and statistical significance was accepted at P < 0.05.
Conflict of interest
The authors declare no competing financial interest.
Acknowledgements
Authors would like to thank Andrew Linklater for assistance in improving the written English. This work was supported by the National Natural Science Foundation of China (21801143) to HPG, Natural Sciences and Engineering Research Council of Canada (grant number RGPIN‐2017‐05366) to WQ, General Research Project of Zhejiang Education Department, China, (Y201839299) to HPG, and the KC Wong Magna Fund of Ningbo University.
Microbial Biotechnology (2019) 12(4), 787–798
Funding information
The KC Wong Magna Fund of Ningbo University, (Grant / Award Number: ‘NA’) General Research Project of Zhejiang Education Department, (Grant / Award Number: ‘Y201839299’) The National Natural Science Foundation of China, (Grant / Award Number: ‘21801143’) Natural Sciences and Engineering Research Council of Canada, (Grant / Award Number: ‘RGPIN‐2017‐05366’).
Contributor Information
Haipeng Guo, Email: guohaipeng@nbu.edu.cn.
Wensheng Qin, Email: wqin@lakeheadu.ca.
References
- Ahmad, M. , Taylor, C.R. , Pink, D. , Burton, K. , Eastwood, D. , Bending, G.D. , and Bugg, T.D. (2010) Development of novel assays for lignin degradation: comparative analysis of bacterial and fungal lignin degraders. Mol BioSyst 6: 815–821. [DOI] [PubMed] [Google Scholar]
- Alvira, P. , Tomás‐Pejó, E. , Ballesteros, M. , and Negro, M. (2010) Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: a review. Bioresour Technol 101: 4851–4861. [DOI] [PubMed] [Google Scholar]
- Baiocco, P. , Barreca, A.M. , Fabbrini, M. , Galli, C. , and Gentili, P. (2003) Promoting laccase activity towards non‐phenolic substrates: a mechanistic investigation with some laccase–mediator systems. Org Biomol Chem 1: 191–197. [DOI] [PubMed] [Google Scholar]
- Bandounas, L. , Wierckx, N.J. , de Winde, J.H. , and Ruijssenaars, H.J. (2011) Isolation and characterization of novel bacterial strains exhibiting ligninolytic potential. BMC Biotechnol 11: 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brebu, M. , and Vasile, C. (2010) Thermal degradation of lignin—a review. Cell Chem Technol 44: 353. [Google Scholar]
- Bugg, T.D. , Ahmad, M. , Hardiman, E.M. , and Singh, R. (2011) The emerging role for bacteria in lignin degradation and bio‐product formation. Curr Opin Biotechnol 22: 394–400. [DOI] [PubMed] [Google Scholar]
- Camesasca, L. , Ramírez, M.B. , Guigou, M. , Ferrari, M.D. , and Lareo, C. (2015) Evaluation of dilute acid and alkaline pretreatments, enzymatic hydrolysis and fermentation of napiergrass for fuel ethanol production. Biomass Bioenerg 74: 193–201. [Google Scholar]
- Chang, A.J. , Fan, J. , and Wen, X. (2012) Screening of fungi capable of highly selective degradation of lignin in rice straw. Int Biodeterior Biodegrad 72: 26–30. [Google Scholar]
- Chang, Y.‐C. , Choi, D. , Takamizawa, K. , and Kikuchi, S. (2014) Isolation of Bacillus sp. strains capable of decomposing alkali lignin and their application in combination with lactic acid bacteria for enhancing cellulase performance. Bioresour Technol 152: 429–436. [DOI] [PubMed] [Google Scholar]
- Guillén, F. , Martínez, M.J. , Gutiérrez, A. , and Del Rio, J. (2005) Biodegradation of lignocellulosics: microbial, chemical, and enzymatic aspects of the fungal attack of lignin. Int Microbiol 8: 195–204. [PubMed] [Google Scholar]
- Guo, H. , Chen, H. , Fan, L. , Linklater, A. , Zheng, B. , Jiang, D. , and Qin, W. (2017a) Enzymes produced by biomass‐degrading bacteria can efficiently hydrolyze algal cell walls and facilitate lipid extraction. Renew Energ 109: 195–201. [Google Scholar]
- Guo, H. , Lin, C. , Wang, S. , Jiang, D. , Zheng, B. , Liu, Y. , and Qin, W. (2017b) Characterization of a novel laccase‐producing Bacillus sp. A4 and its application in Miscanthus degradation. BioResources 124: 776–4794. [Google Scholar]
- Guo, H. , Wu, Y. , Hong, C. , Chen, H. , Chen, X. , Zheng, B. , et al (2017c) Enhancing digestibility of Miscanthus using lignocellulolytic enzyme produced by Bacillus. Bioresour Technol 245: 1008–1015. [DOI] [PubMed] [Google Scholar]
- Hendriks, A. , and Zeeman, G. (2009) Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour Technol 100: 10–18. [DOI] [PubMed] [Google Scholar]
- Hu, Z. , and Wen, Z. (2008) Enhancing enzymatic digestibility of switchgrass by microwave‐assisted alkali pretreatment. Biochem Eng J 38: 369–378. [Google Scholar]
- Koschorreck, K. , Richter, S.M. , Ene, A.B. , Roduner, E. , Schmid, R.D. , and Urlacher, V.B. (2008) Cloning and characterization of a new laccase from Bacillus licheniformis catalyzing dimerization of phenolic acids. Appl Microbiol Biotechnol 79: 217–224. [DOI] [PubMed] [Google Scholar]
- Kumar, A.K. , and Sharma, S. (2017) Recent updates on different methods of pretreatment of lignocellulosic feedstocks: a review. Bioresour Bioprocess 4: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, P. , Barrett, D.M. , Delwiche, M.J. , and Stroeve, P. (2009) Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind Eng Chem Res 48: 3713–3729. [Google Scholar]
- Lee, J. , Gwak, K. , Park, J. , Park, M. , Choi, D. , Kwon, M. , and Choi, I. (2007) Biological pretreatment of softwood Pinus densiflora by three white rot fungi. J Microbiol 45: 485. [PubMed] [Google Scholar]
- Lee, H. , Hamid, S. , and Zain, S. (2014) Conversion of lignocellulosic biomass to nanocellulose: structure and chemical process. Sci World J 2014: 631013. 10.1155/2014/631013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonowicz, A. , Matuszewska, A. , Luterek, J. , Ziegenhagen, D. , Wojtaś‐Wasilewska, M. , Cho, N.‐S. , et al (1999) Biodegradation of lignin by white rot fungi. Fungal Genet Bio 27: 175–185. [DOI] [PubMed] [Google Scholar]
- Li, T. , Fang, Q. , Chen, H. , Qi, F. , Ou, X. , Zhao, X. , and Liu, D. (2017) Solvent‐based delignification and decrystallization of wheat straw for efficient enzymatic hydrolysis of cellulose and ethanol production with low cellulase loadings. RSC Adv 7: 10609–10617. [Google Scholar]
- Liu, J. , Cai, Y. , Liao, X. , Liaoa, X. , Huang, Q. , Hao, Z. , et al (2013) Purification and characterisation of a novel thermal stable laccase from Pycnoporus sp. SYBC‐L3 and its use in dye decolorisation. Biol Environ 113: 27–39. [Google Scholar]
- Maki, M.L. , Idrees, A. , Leung, K.T. , and Qin, W. (2012) Newly isolated and characterized bacteria with great application potential for decomposition of lignocellulosic biomass. J Mol Microbiol Biotechnol 22: 156–166. [DOI] [PubMed] [Google Scholar]
- Martinez, A.T. , Ruiz‐Dueñas, F.J. , Martínez, M.J. , del Río, J.C. , and Gutierrez, A. (2009) Enzymatic delignification of plant cell wall: from nature to mill. Curr Opin Biotechnol 20: 348–357. [DOI] [PubMed] [Google Scholar]
- Millati, R. , Syamsiah, S. , Niklasson, C. , Cahyanto, M.N. , Ludquist, K. , and Taherzadeh, M.J. (2011) Biological pretreatment of lignocelluloses with white‐rot fungi and its applications: a review. BioResource 6: 5224–5259. [Google Scholar]
- Mohammadkazemi, F. , Azin, M. , and Ashori, A. (2015) Production of bacterial cellulose using different carbon sources and culture media. Carbohydr Polym 117: 518–523. [DOI] [PubMed] [Google Scholar]
- Munk, L. , Sitarz, A.K. , Kalyani, D.C. , Mikkelsen, J.D. , and Meyer, A.S. (2015) Can laccases catalyze bond cleavage in lignin? Biotechnol Adv 33: 13–24. [DOI] [PubMed] [Google Scholar]
- Mussatto, S.I. , Fernandes, M. , Milagres, A.M. , and Roberto, I.C. (2008) Effect of hemicellulose and lignin on enzymatic hydrolysis of cellulose from brewer's spent grain. Enzyme Microbial Technol 43: 124–129. [Google Scholar]
- Öhgren, K. , Bura, R. , Saddler, J. , and Zacchi, G. (2007) Effect of hemicellulose and lignin removal on enzymatic hydrolysis of steam pretreated corn stover. Bioresour Technol 98: 2503–2510. [DOI] [PubMed] [Google Scholar]
- Olsson, L. , Christensen, T.M. , Hansen, K.P. , and Palmqvist, E.A. (2003) Influence of the carbon source on production of cellulases, hemicellulases and pectinases by Trichoderma reesei Rut C‐30. Enzyme Microbial Technol 33: 612–619. [Google Scholar]
- Paudel, Y.P. , and Qin, W. (2015) Characterization of novel cellulase‐producing bacteria isolated from rotting wood samples. Appl Biochem Biotechnol 177: 1186–1198. [DOI] [PubMed] [Google Scholar]
- Pauly, M. , and Keegstra, K. (2008) Cell‐wall carbohydrates and their modification as a resource for biofuels. Plant J 54: 559–568. [DOI] [PubMed] [Google Scholar]
- Pollegioni, L. , Tonin, F. , and Rosini, E. (2015) Lignin‐degrading enzymes. FEBS J 282: 1190–1213. [DOI] [PubMed] [Google Scholar]
- Rencoret, J. , Pereira, A. , José, C. , Martínez, A.T. , and Gutiérrez, A. (2016) Laccase‐mediator pretreatment of wheat straw degrades lignin and improves saccharification. BioEnerg Res 9: 917–930. [Google Scholar]
- Rico, A. , Rencoret, J. , del Río, J.C. , Martínez, A.T. , and Gutiérrez, A. (2014) Pretreatment with laccase and a phenolic mediator degrades lignin and enhances saccharification of Eucalyptus feedstock. Biotechnol Biofuel 7: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas, R. , Trujillo, M.E. , Mateos, P.F. , Martinez-Molina, E. , and Velazquez, E. (2004) Cellulomonas xylanilytica sp. nov., a cellulolytic and xylanolytic bacterium isolated from a decayed elm tree. Int J Syst Evol Microbiol 54: 533–536. [DOI] [PubMed] [Google Scholar]
- Rosales, E. , Couto, S.R.G. , and Sanromán, M.A. (2005) Reutilisation of food processing wastes for production of relevant metabolites: application to laccase production by Trametes hirsuta . J Food Eng 66: 419–423. [Google Scholar]
- Si, S. , Chen, Y. , Fan, C. , Hu, H. , Li, Y. , Huang, J. , et al (2015) Lignin extraction distinctively enhances biomass enzymatic saccharification in hemicelluloses‐rich Miscanthus species under various alkali and acid pretreatments. Bioresour Technol 183: 248–254. [DOI] [PubMed] [Google Scholar]
- Sindhu, R. , Binod, P. , and Pandey, A. (2016) Biological pretreatment of lignocellulosic biomass–An overview. Bioresour Technol 199: 76–82. [DOI] [PubMed] [Google Scholar]
- Singhania, R.R. , Sukumaran, R.K. , Patel, A.K. , Larroche, C. , and Pandey, A. (2010) Advancement and comparative profiles in the production technologies using solid‐state and submerged fermentation for microbial cellulases. Enzyme Microbial Technol 46: 541–549. [Google Scholar]
- Sipos, B. , Benkő, Z. , Dienes, D. , Réczey, K. , Viikari, L. , and Siika‐Aho, M. (2010) Characterisation of specific activities and hydrolytic properties of cell‐wall‐degrading enzymes produced by Trichoderma reesei Rut C30 on different carbon sources. Appl Biochem Biotechnol 161: 347–364. [DOI] [PubMed] [Google Scholar]
- Sun, R. , Tomkinson, J. , and Jones, G.L. (2000) Fractional characterization of ash‐AQ lignin by successive extraction with organic solvents from oil palm EFB fibre. Polym Degrad Stab 68: 111–119. [Google Scholar]
- Sun, F.‐H. , Li, J. , Yuan, Y.‐X. , Yan, Z.‐Y. , and Liu, X.‐F. (2011) Effect of biological pretreatment with Trametes hirsuta yj9 on enzymatic hydrolysis of corn stover. Int Biodeterior Biodegrad 65: 931–938. [Google Scholar]
- Waghmare, P. , Kshirsagar, S. , Saratale, R. , Govindwar, S. , and Saratale, G. (2014) Production and characterization of cellulolytic enzymes by isolated Klebsiella sp. PRW‐1 using agricultural waste biomass. Emir J Food Agr 26: 44. [Google Scholar]
- Wan, C. , and Li, Y. (2010) Microbial pretreatment of corn stover with Ceriporiopsis subvermispora for enzymatic hydrolysis and ethanol production. Bioresour Techno 101: 6398–6403. [DOI] [PubMed] [Google Scholar]
- Wan, C. , and Li, Y. (2012) Fungal pretreatment of lignocellulosic biomass. Biotechnol Adv 30: 1447–1457. [DOI] [PubMed] [Google Scholar]
- Wesenberg, D. , Kyriakides, I. , and Agathos, S.N. (2003) White‐rot fungi and their enzymes for the treatment of industrial dye effluents. Biotechnol Adv 22: 161–187. [DOI] [PubMed] [Google Scholar]
- Wu, J. , Xiao, Y.Z. , and Yu, H.Q. (2005) Degradation of lignin in pulp mill wastewaters by white‐rot fungi on biofilm. Bioresour Technol 96: 1357–1363. [DOI] [PubMed] [Google Scholar]
- Xu, N. , Zhang, W. , Ren, S. , Liu, F. , Zhao, C. , Liao, H. , et al (2012) Hemicelluloses negatively affect lignocellulose crystallinity for high biomass digestibility under NaOH and H2SO4 pretreatments in Miscanthus . Biotechnol Biofuel 5: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Y. , Zhou, J. , Lu, H. , Yuan, Y. , and Zhao, L. (2012) Isolation and characterization of Streptomyces spp. strains F‐6 and F‐7 capable of decomposing alkali lignin. Environ Technol 33: 2603–2609. [DOI] [PubMed] [Google Scholar]
- Yao, B. , and Ji, Y. (2014) Lignin biodegradation with laccase‐mediator systems. Frontier Energ Res 2: 12. [Google Scholar]
- Zhang, Y.‐H.P. , Himmel, M.E. , and Mielenz, J.R. (2006) Outlook for cellulase improvement: screening and selection strategies. Biotechnol Adv 24: 452–481. [DOI] [PubMed] [Google Scholar]


