Abstract
Background: The prevalence of vitamin D deficiency (VDD) is predicted to be high in patients with type 2 diabetes mellitus (T2DM), but the exact figure is not known in Jazan, Saudi Arabia. Emerging data suggests that VDD plays a role in glycemic control. The aim of this study was to measure the prevalence of VDD among T2DM patients and to investigate its association with patients’ characteristics and glycemic control in Jazan.
Methods: This is an analytical cross-sectional study which recruited 309 patients with T2DM randomly from primary health care centers in Jazan. Logistic regression analysis was conducted to determine the VDD predictors and to examine the association of VDD and glycemic control.
Results: The VDD prevalence was found to be 60.8% in patients with T2DM. Age, gender, diabetic retinopathy (DR), dyslipidemia, glycemic control, and obesity were significantly associated with VDD, and all except obesity were independent predictors of VDD. There was a significant negative correlation between 25-hydroxyvitamin D and HbA1c. VDD was a significant independent predictor of poor glycemic control after adjustment for hypertension, DR, diabetic neuropathy, type of diabetes medication, diabetes duration, and education level.
Conclusion: In this Saudi Arabian population, VDD is highly prevalent in people with T2DM and is associated with poor glycemic control. Health education targeting patients with T2DM and national strategies regarding vitamin D fortification are needed to prevent VDD in Saudi Arabia. Earlier VDD diagnosis by health care providers may help to improve the outcome for patients with T2DM. Establishing the causal association between VDD and glycemic control and clarifying the biological role of vitamin D in T2DM are important aims for future studies.
Keywords: diabetes mellitus, T2DM, vitamin D deficiency, VDD, glycemic control, diabetes complication
Introduction
Globally, diabetes mellitus (DM) is a highly prevalent chronic disease that places a significant burden on the patient’s life and health care costs.1 “Diabetes mellitus is a heterogeneous metabolic disorder characterized by the presence of hyperglycemia due to impairment of insulin secretion, defective insulin action or both”.2 T2DM is the most common type of DM, affecting 90–95% of all patients with DM.3 In the UK, the reported prevalence of T2DM is 5.26%.4 In Saudi Arabia, a recent review study reported that the prevalence of T2DM is much higher, around 33%, and it is expected to increase to 45.8% by 2030.5 Therefore, it is important to investigate factors that might affect T2DM, such as vitamin D deficiency (VDD), to help provide information to improve the quality of T2DM care.
Vitamin D (cholecalciferol) is acquired through ultraviolet irradiation of the skin and vitamin D intake via dietary sources.6 Serum 25-hydroxyvitamin D level is the main circulatory indicator of vitamin D status,7 and (VDD is defined as a serum level of 25-hydroxyvitamin D lower than 20 ng/mL.8 High prevalence of VDD has been reported worldwide,9 including Saudi Arabia, where it was recently estimated to affect approximately 60% of the general population.10 VDD plays a central role in several non-skeletal diseases, such as DM and cardiovascular disease.11
The co-occurrence of VDD and T2DM has been reported in several studies. For instance, in the UK, a cross-sectional study found that the prevalence of VDD tended to be higher in patients with diabetes than in healthy individuals (83% versus 70%).12 In Saudi Arabia, a case–control study found that 76.6% of the patients with T2DM had VDD and 58.1% of the healthy participants had VDD.13 Overall, VDD in patients with T2DM was found to range from 30% to 91.4% across the literature.12–17 To our knowledge, no study has been conducted to measure the prevalence of VDD among patients with T2DM in Jazan. Therefore, a suitably designed cross-sectional study in Jazan is needed to determine the prevalence of VDD in patients with T2DM.
Despite the growing evidence which suggests that there is a link between VDD and high prevalence of T2DM, few research studies have investigated the possible connection between VDD and microvascular complications.18 For instance, some studies have suggested that DR,19,20 DN,19,21 and diabetic nephropathy (DNP)18,21 are significantly associated with VDD. Conversely, other studies showed that there was no significant association between VDD and DR,18,21 DN,18 and DNP.19 Overall, there are conflicting findings in the current literature regarding the association between VDD and microvascular complications in T2DM.
Good glycemic control is important in diabetes management as it reduces the risk of diabetes complications.22 According to the American Diabetic Association, HbA1c <7% is the optimal glycemic control.22 It has been suggested that VDD is associated with glycemic control in T2DM.23 However, the role of VDD in glycemic control is not yet fully understood. Only a few research studies have investigated the link between VDD and glycemic control.24 A negative relationship between serum 25-hydroxyvitamin D levels and HbA1c in patients with T2DM has been observed in several populations, including patients in Saudi Arabia.24,25 Moreover, a low serum 25-hydroxyvitamin D level has been shown to be an independent predictor for HbA1c in patients with T2DM.18 In contrast, a cross-sectional study conducted in western India failed to show a significant association between serum 25-hydroxyvitamin D and HbA1c in patients with T2DM.14 To our knowledge, no study has investigated the association between VDD and glycemic control in patients with T2DM in Jazan.
Although the role of VDD in T2DM is not fully understood, there are a number of potential mechanisms that have been suggested to describe the role of vitamin D in DM, such as systemic inflammation via the formation and the effects of modulating cytokines, which lead to enhanced insulin sensitivity and promote beta-cell survival, and insulin resistance via inducing insulin receptor expression, thus increasing insulin sensitivity during glucose transportation, and pancreatic beta cell malfunction via a change of calcium flux, which can adversely affect beta cell function.26 Moreover, obesity could be a confounder as VDD is typically associated with obesity due to 25-hydroxyvitamin D storage in fat tissue, which leads to low serum 25-hydroxyvitamin D leading to both insulin resistance and insulin sensitivity in T2DM.26
This study aimed to study the prevalence of VDD and its association with microvascular complications and glycemic control in patients with T2DM in Jazan.
Materials and methods
Study design, area, and target population
An anaytical cross-sectional design was applied in this study, which was conducted in Jazan, southwestern Saudi Arabia.27 The population of Jazan is relatively homogeneous, with inhabitants sharing the same language, ethnicity, and religion.27 Every patient with T2DM in Jazan is registered in a primary health care center. Therefore, the study sample was recruited from primary healthcare centers in Jazan.
Sample size and sampling technique
The total calculated sample size was 380 participants. The sample size calculation was conducted using Epi-Info software (version 7.2.2.6 for Windows, Centres for Disease Control and Prevention (CDC), Atlanta, GA, USA) using the following parameters: CI, 95%; Alpha, 0.5; 76.6% expected frequency of VDD in cases of T2DM, which was obtained from a study carried out in southern Saudi Arabia.13 The computed sample size was 266, and then the rate of 30% was used to adjust for the expected non-response rate. The current study successfully recruited 309 out of the 380 participants using random sampling technique (Figure 1).
Figure 1.
Participant enrolment and the sampling technique used in the current study.
Abbreviation: T2DM; type two diabetes mellitus.
Inclusion and exclusion criteria
Patients with T2DM who were older than 18 years of age and those who signed informed consent were included. Individuals on vitamin D supplements and pregnant women were excluded.
Ethical considerations
Ethical approval for the current study was granted by the local ethics committee in Jazan (reference number: 1813). Informed consent was obtained by all participants.
Data collection
The data were gathered using structured face-to-face interviews with patients by a trained physician. During the interview, the physician will take sociodemographic and clinical data including age, gender, education level, work status, marital status, smoking status, height, weight, diabetes duration, type of diabetes medication, diabetes microvascular complications, and comorbidity). Blood samples were subsequently obtained from the participants to measure serum 25-hydroxyvitamin D and HbA1c levels.
Data processing and analysis
The data were coded with anonymous identification numbers in order to guarantee the privacy of the participants. A single data entry method was conducted by the principal investigator using the Statistical Package for the Social Sciences (version 24 for Windows, IBM Corp., Armonk, NY, USA). Body mass index (BMI) was categorized as underweight, normal, overweight, and obese according to BMI scores <18.5 kg/m2, from 18.50 to <24.9 kg/m2, from 25 to 29.9 kg/m2, and ≥30 kg/m2, respectively, Vitamin D status as deficient when serum 25-hydroxyvitamin is <20 ng/mL or non-deficient when serum 25-hydroxyvitamin is ≥20 ng/mL, glycemic control status as good glycemic control when HbA1c level is <7% or poor glycemic control when HbA1c level is ≥7%. The continuous variables were described by mean and SD and the categorical variables by percentages and frequencies. Binary logistic regression analyses were used to assess the predictors of VDD and the predictors of glycemic control. A P-value of <0.05 was set for the statistical significance level.
Results
Population characteristics
Table 1 presents the participants’ characteristics and their association with VDD. The results showed that the mean level of 25-hydroxyvitamin D was 19.4±6.7 ng/mL and the mean level of HbA1c was 8.7±1.8%. Furthermore, around half of the participants had had T2DM for more than 10 years. Regarding microvascular complications amongst participants, DR, DNP, and DN were evident, with 35.3%, 4.9%, and 10%, respectively. Oral antidiabetic medications were used in 46.6% of the participants, while 53.4% were being treated with oral antidiabetic agents plus insulin. The percentage of participants of normal, overweight, or obese was 18.5%, 38.5% , and 43%,respectively, with a higher obesity rate in females relative to males (69.2% versus 30.8%).
Table 1.
Sociodemographic and clinical characteristics of all participants, participants with VDD and participants without VDD
| Overall (n=309) | VDD, N (%)=188 (60.8%) |
95% CI, 55.3–66.1% |
No-VDD, N (%)=121 (39.2%) |
95% CI, 33.9–44.7% |
P | |
|---|---|---|---|---|---|---|
| Age, years (mean ± SD) | 58.9±12 | 54.2±11.8 | 52.9–55.4 | 58.4±12 | 57.1–59.7 | 0.003 |
| Gender | ||||||
| Male | 130 (42.1%) | 64 (49.2%) | 40.8–57.7% | 66 (50.8%) | 42.3–59.2% | |
| Female | 179 (57.9%) | 124 (69.3%) | 62.2–75.6% | 55 (30.7%) | 24.4–37.8% | <0.001 |
| Education | ||||||
| Educated | 174 (56.3%) | 106 (60.9%) | 53.5–67.9% | 68 (39.1%) | 32.1–46.5% | |
| Illiterate | 135 (43.7%) | 82 (60.7%) | 52.3–68.6% | 53 (39.3%) | 31.4–47.7% | 0.975 |
| Smoking status | ||||||
| Nonsmoker | 264 (85.4%) | 156 (59.1%) | 53.1–64.9% | 108 (40.9%) | 35.2–46.9% | |
| Smokers | 45 (14.6%) | 32 (71.1%) | 56.6–82.3% | 13 (28.9%) | 17.7–43.4% | 0.127 |
| HbA1c | ||||||
| DM controlled | 68 (22.0%) | 25 (36.8%) | 26.3–48.6% | 43 (63.2%) | 51.1–73.7% | |
| DM uncontrolled | 241 (78.0%) | 163 (67.6%) | 61.5–73.2% | 78 (32.4%) | 26.8–38.5% | <0.001 |
| Diabetes duration | ||||||
| ≤10 | 159 (51.5%) | 105 (66.0%) | 58.4–72.9% | 54 (34.0%) | 27.1–41.6% | |
| >10 | 150 (48.5%) | 83 (55.3%) | 47.34–63.1% | 67 (44.7%) | 36.9–52.7% | 0.054 |
| DR | ||||||
| Absent | 200 (64.7%) | 112 (56.0%) | 49.1–62.7% | 88 (44.0%) | 37.3–50.9% | |
| Present | 109 (35.3%) | 76 (69.7%) | 60.6–77.6% | 33 (30.3%) | 22.4–39.5% | 0.018 |
| DN | ||||||
| Absent | 278 (90.0%) | 174 (62.6%) | 56.8–68.1% | 104 (37.4%) | 31.9–43.2% | |
| Present | 31 (10.0%) | 14 (45.2%) | 29.2–62.2% | 17 (54.8%) | 37.8–70.9% | 0.059 |
| DNP | ||||||
| Absent | 294 (95.1%) | 180 (61.2%) | 55.5–66.6% | 114 (38.8%) | 33.4–44.5% | |
| Present | 15 (4.9%) | 8 (53.3%) | 30.1–75.2% | 7 (46.7%) | 24.8–69.9% | 0.541 |
| HTN | ||||||
| Absent | 103 (33.3%) | 58 (56.3%) | 46.7–65.5% | 45 (43.7%) | 34.5–53.3% | |
| Present | 206 (66.7%) | 130 (63.1%) | 56.3–69.4% | 76 (36.9%) | 30.6–43.7% | 0.249 |
| DLP | ||||||
| Absent | 208 (67.3%) | 118 (56.7%) | 49.9–63.3% | 90 (43.3%) | 36.7–50.1% | |
| Present | 101 (32.7%) | 70 (69.3%) | 59.7–77.5% | 31 (30.7%) | 22.5–40.3% | 0.034 |
| BMI | ||||||
| Non-obese | 176 (57%) | 98 (55.7%) | 48.3–62.8% | 78 (44.3%) | 37.2–51.7% | |
| Obese | 133 (43.0%) | 90 (67.7%) | 59.3–75.0% | 43 (32.3%) | 25.0–40.7% | 0.033 |
Notes: P, P-value <0.05 is the significance level, as tested by t test, chi-square and Fisher’s Exact, where appropriate. Significant P-values are shown in bold.
Abbreviations: VDD, vitamin D deficiency (serum 25-hydroxyvitamin D <20 ng/mL); BMI, body mass index; DR, diabetic retinopathy; DNP, diabetic nephropathy; DN, diabetic neuropathy; HTN, hypertension; DLP, dyslipidemia; N, sample size.
Prevalence of vitamin D deficiency and its association with the participants’ characteristics
The prevalence of VDD in patients with T2DM was 60.8% (95% CI 55.3–66.1%), whereas for non-VDD patients with T2DM it was 39.2% (95% CI 33.9–44.7%). Younger age was more significantly associated with VDD (54.2±11.8 years) than older age (58.4±12 years) (t(307)=3.03, P=0.003). Additionally, the number of females with VDD was significantly higher than the number of males (69.3 versus 49.2, X2(1)=12.70, P=<0.001). There were significantly higher proportions of VDD in patients with DR (69.7%) than in patients without DR (56%) (X2(1)=5.58, P=0.018). Patients with uncontrolled diabetes have significantly higher VDD rate (67.6%) than the patients with controlled diabetes (36.8%) (X2(1)=21.21, P<0.001). Patients with dyslipidemia were also associated with significantly higher VDD compared to those without dyslipidemia (69.3% and 56.7%, respectively, X2(1)=4.51, P=0.34). There was a significant difference in the proportion of VDD between obese (67.7%) compared with non-obese (55.7%) participants (X2(1)=4.57, P=0.033).
Table 2 shows the univariate and multivariate binary logistic regression analysis in order to identify the predictors of VDD in T2DM. At the 95% CI, the univariate logistic regression analysis showed that being younger, female, having DR, dyslipidemia, and uncontrolled diabetes were found to be statistically significant predictors of VDD among patients with T2DM in the study. The multivariate logistic regression analysis revealed that poor glycemic control was independently associated with VDD, with participants with poor glycemic control being 3.45 times more likely to be VDD than participants with good glycemic control. This was followed by the female gender, with females being 2.34 times more likely to be VDD than males in our sample. Dyslipidemia, DR, and age were found to be independent predictors of VDD, with age being the weakest predictor.
Table 2.
Logistic regression analysis of association between patient characteristics and VDD
| Variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR (CI 95%) | P | OR (CI 95%) | P | |
| Age (years) | 0.97 (0.95–0.99) | 0.003 | 0.96 (0.94–0.98) | <0.001 |
| Gender Male |
1 |
1 |
||
| Female | 2.33 (1.46–3.71) | <0.001 | 2.34 (1.38–3.94) | 0.002 |
| BMI Non-obese |
1 |
1 |
||
| Obese | 1.67 (1.04–2.66) | 0.033 | 1.32 (0.78–2.22) | 0.300 |
| DR Absent |
1 |
1 |
||
| Present | 1.81 (1.10–2.97) | 0.019 | 1.93 (1.07–3.47) | 0.028 |
| DLP Absent |
1 |
1 |
||
| Present | 1.72 (1.04–2.85) | 0.035 | 2.12 (1.20–3.74) | 0.010 |
| HbA1c Controlled |
1 |
1 |
||
| Uncontrolled | 3.60 (2.05–6.31) | <0.001 | 3.45 (1.85–6.46) | <0.001 |
Notes: P, P-value; P of <0.05 is the significance level. The variables with odds ratios of 1 are the reference categories. Significant P-values are shown in bold.
Abbreviations: BMI, body mass index; DR, diabetic retinopathy; DLP, dyslipidemia.
The association of vitamin D deficiency with glycemic control
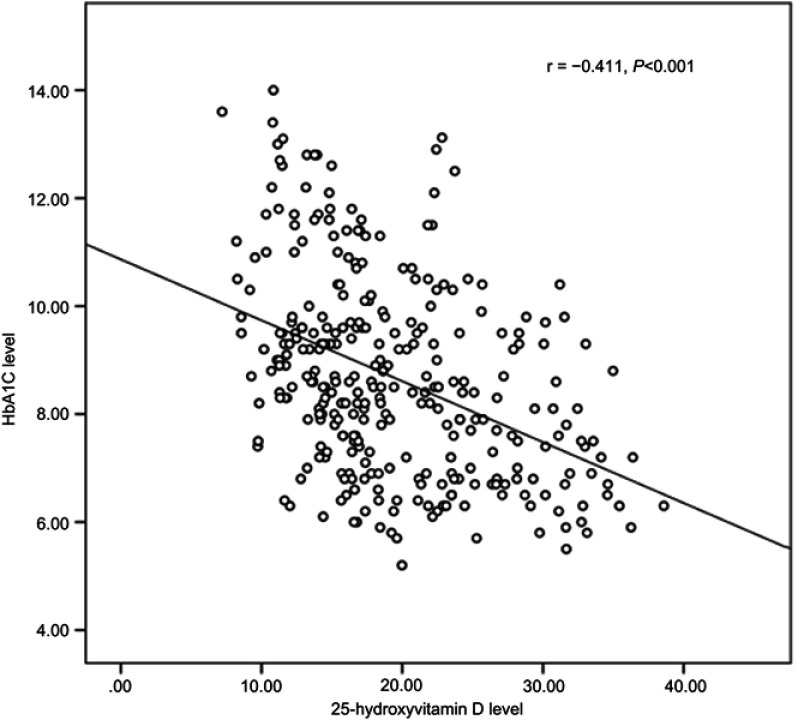
Figure 2 shows a medium significant negative correlation between HbA1c and 25-hydroxyvitamin D levels in patients with T2DM (r=−0.41, p<0.001), ie the lower Vitamin D level, the higher HbA1c. Moreover, the prevalence of poor glycemic control in patients with T2DM was 78% (95% CI: 73.1–82.3%). Table 3 shows that VDD, education (illiteracy level), a DM duration of >10 years, DR, DN, hypertension, and the type of diabetic medication (both oral and insulin) were significantly associated with glycemic control.
Figure 2.
Correlation between vitamin D and glycemic control in all study participants.
Table 3.
Participants’ characteristics and their association with glycemic control (poor vs good)
| Characteristics | Glycemic control (poor); N=241 (78%) | 95% CI 73.1–82.3% |
P |
|---|---|---|---|
| Age, years (mean ± SD) | 56.5±12 | 55–58 | 0.096 |
| Gender | |||
| Male | 95 (73.1%) | 64.9–80.0% | |
| Female | 146 (81.6%) | 75.2–86.6% | 0.075 |
| Education | |||
| Educated | 125 (71.8%) | 64.7–78.0% | |
| Illiterate | 116 (85.9%) | 79.1–90.8% | 0.003 |
| Diabetes duration | |||
| ≤10 | 114 (71.7%) | 64.3–78.1% | |
| >10 | 127 (84.7%) | 78.0–89.6% | 0.006 |
| DR | |||
| Absent | 139 (69.5%) | 62.8–75.5% | |
| Present | 102 (93.6%) | 87.3–96.9% | <0.001 |
| DN | |||
| Absent | 212 (76.3%) | 70.9–80.9% | |
| Present | 29 (93.5%) | 79.3–98.2% | 0.028 |
| DNP | |||
| Absent | 227 (77.2%) | 72.1–81.6% | |
| Present | 14 (93.3%) | 70.2–98.8% | 0.142 |
| HTN | |||
| Absent | 70 (68.0%) | 58.4–76.2% | |
| Present | 171 (83.0%) | 77.3–87.5% | 0.003 |
| DLP | |||
| Absent | 163 (78.4%) | 72.3–83.4% | |
| Present | 78 (77.2%) | 68.1–84.3% | 0.821 |
| Diabetes medication | |||
| Oral | 95 (66.0%) | 57.9–73.2% | |
| Oral + insulin | 146 (88.5%) | 82.7–92.5% | <0.001 |
| BMI | |||
| Non-obese | 131 (74.4%) | 67.5–80.3% | |
| Obese | 110 (82.7%) | 75.4–88.2% | 0.082 |
| Vitamin D status | |||
| Not deficient | 78 (64.5%) | 55.6–72.4% | |
| Deficient | 163 (86.7%) | 81.1–90.8% | <0.001 |
Notes: P, P-value; P of <0.05 is the significance level, as tested by t test, chi-square and Fisher’s Exact, where appropriate. Significant P-values are shown in bold. The data for the good glycemic control group can be calculated based on the overall values from Table 1.
Abbreviations: BMI, body mass index; DR, diabetic retinopathy; DNP, diabetic nephropathy; DN, diabetic neuropathy; DLP, dyslipidemia; HTN, hypertension; M, mean; N, sample size.
Table 4 illustrates the results of the univariate and multivariate binary logistic regression analyses used to examine the predictors of poor glycemic control. The univariate logistic regression analysis revealed with a 95% CI that VDD, illiteracy level, a DM duration of >10 years, DR, DN, hypertension, and taking both oral diabetic medication and insulin were significantly associated with poor glycemic control. The results of the multivariate binary logistic regression analysis showed a significant association between VDD and glycemic control after adjustment for the other covariates and that the odds of reporting poor glycemic control were 3.79 times higher in patients with T2DM and VDD than in patients with T2DM without VDD.
Table 4.
Logistic regression analysis between patient characteristics and glycemic control
| Variable | Univariate analysis |
Multivariate analysis | ||
|---|---|---|---|---|
| OR (CI 95%) | P | OR (CI 95%) | P | |
| Vitamin D status Not deficient |
1 |
1 |
||
| Deficient | 3.59 (2.05–6.31) | <0.001 | 3.79 (2.01–7.14) | <0.001 |
| Diabetes duration ≤10 year |
1 |
1 |
||
| >10 year | 2.18 (1.24–3.83) | 0.007 | 0.93 (0.43–2.01) | 0.859 |
| DR Absent |
1 |
1 |
||
| Present | 6.40 (2.81–14.56) | <0.001 | 2.12 (1.097–4.11) | 0.011 |
| DN Absent |
1 |
1 |
||
| Present | 4.53 (1.05–19.42) | 0.043 | 3.83 (0.79–18.48) | 0.094 |
| HTN Absent |
1 |
1 |
||
| Present | 2.30 (1.33–4.00) | 0.003 | 1.58 (0.85–2.94) | 0.151 |
| Diabetes medication Oral |
1 |
1 |
||
| Oral & insulin | 3.96 (2.20–7.15) | <0.001 | 2.42 (1.20–4.90) | 0.014 |
| Education Educated |
1 |
1 |
||
| Illiterate | 2.39 (1.33–4.30) | 0.004 | 2.12 (1.10–4.11) | 0.025 |
Notes: P, P-value; P of <0.05 is the significance level. Significant P-values are shown in bold. The variables with odds ratios of 1 are the reference categories.
Abbreviations: VDD, vitamin D deficiency; DR, diabetic retinopathy; DN, diabetic neuropathy; HTN, hypertension.
Discussion
The current study included a total of 309 patients with T2DM and it is found that the prevalence of VDD in patients with T2DM was 60.8% in Jazan. Moreover, the study found that younger age, female gender, DR, dyslipidemia, poor glycemic control, and BMI (obese) were significantly associated with VDD and that all of them except BMI were independent predictors of VDD. In addition, there was a significant negative correlation between 25-hydroxyvitamin D and HbA1c. Further analysis revealed that VDD was a significant independent predictor of poor glycemic control after adjustment with hypertension, DR, DN, type of diabetes medication, diabetes duration, and education level.
The current study found a high prevalence of VDD in patients with T2DM (60.8%). This result is slightly lower than that of a previous study conducted in southern Saudi Arabia, which found that 76.6% of patients with T2DM had VDD;13 however, there were certain limitations in this study which might have led to this higher rate, such as its case–control design and that the patients were recruited from tertiary hospitals.13 In line with our finding, previous studies have reported that the prevalence of VDD among patients with T2DM was around 60%.16,18,28 Several features of the current study sample may have influenced this high VDD prevalence. Firstly, most of the study sample were females. Furthermore, the female sex was independently significantly associated with VDD in our results, as shown by some29–32 but not all other studies.33,34 The traditional clothes might be a predisposing factor to VDD due to the reduction of sun exposure. Secondly, the majority of the current study’s participants were overweight or obese. The rate of obesity was high in our sample and obesity was associated with VDD. Similarly, several studies have found a link between the excess weight and VDD in T2DM30,31,35 but others have not.14,18 A possible explanation for this association is that vitamin D is sequestered in the adipose tissue,26 so patients with obesity report lower serum 25-hydroxyviatmin D levels. The current study found that there was a higher rate of obesity in females than in males, which could have led to the detected association between gender and VDD. However, when adjusted with other covariates, obesity loses its effects. It seems that obesity plays as a confounder in the relationship of VDD with T2DM. Thirdly, dyslipidemia was associated with VDD even after adjustment with other covariates, as shown previously.30 The higher rate of VDD in patients with T2DM in the current sample could be attributed to dyslipidemia. However, some studies have reported that there is no association between dyslipidemia and VDD among patients with T2DM.31,33 The current study suggests that a younger age is more highly associated with VDD, which is consistent with other published research.34 This finding could be explained by dietary behavior. A study conducted in Saudi Arabia found that healthy diet was adopted by older individuals and that the unhealthy diet was adopted by younger individuals.36 Although this is statistically significant, it may not have any clinical relevance since the odds ratio was close to 1. However, other studies have reported that there is no significant association between VDD and age.14,18,30,31,33,35
Our results showed that there was a marginal significance level (P=0.054) of the association between VDD and diabetes duration. This result is supported by a previous study which reported a significant association between vitamin D and diabetes duration.37 Conversely, other studies have found that diabetes duration was not associated with VDD.30,33 A possible explanation for our findings is that longer duration with diabetes is associated with diabetes complications,38 which, in turn, is associated with VDD.18
The current study found that there is a significant positive association between DR and VDD in patients with T2DM. Similarly, some studies reported that serum 25-hydroxyvitamin D levels were significantly lower in participants with DR than in those without DR in patients with T2DM.18,21 Conversely, other studies have found that VDD was not associated with DR in patients with T2DM.19,20 The current study findings suggest that DN and DNP are not associated with VDD in patients with T2DM. This is consistent with other research studies, which found that DN19,21 and DNP18,21 were not associated with VDD. Conversely, some research studies have suggested that VDD is significantly associated with DN18 and DNP.19
Good glycemic control is considered as one of the most important goals of T2DM management.22 The current study found that 25-hydroxyvitamin D levels were inversely correlated with glycemic control measured by HbA1c levels. This finding is consistent with a number of previous research studies.18,23,25 Additionally, the current study found that glycemic control is independently associated with VDD. Similarly, a cross-sectional study reported a significant positive association between VDD and poor glycemic control.39 Conversely, a cross-sectional study found that vitamin D is not associated with HbA1c.14 The exact mechanism by which VDD may affect glycemic control is not fully understood; however, potential pathways have been suggested which involve pancreatic beta-cell dysfunction, reduced insulin sensitivity, and inflammation.40 These proposed mechanisms are important focuses for future studies.
Strengths and limitations
The strengths of the current study were that it was a population-based and the participants were selected randomly. There was an 18.3% non-response or excluded rate; however, this was mitigated when the sample size was calculated by accounting for 30% non-response rate. The limitations of the current study were that sun exposure and vitamin D dietary intake were not assessed in the current study; however, patients on a vitamin D supplement were excluded. Moreover, Jazan’s population is homogeneous27 and it was assumed that they follow a similar pattern.
Recommendations and conclusion
The current study found VDD to be highly prevalent among patients with T2DM in Jazan, which is a major health issue. It also successfully identified an association for VDD in T2DM with glycemic control. As such, a number of measures are recommended in order to improve diabetes care. For instance, primary prevention, health education, and promotional campaigns targeted at patients with T2DM should be implemented to encourage a healthier lifestyle and to promote weight reduction to reduce levels of obesity, especially in females as the current study shows that they have a higher rate of VDD. The importance of moderate sun exposure should also be emphasized and the use of appropriate clothing. Moreover, the majority of the products fortified with vitamin D in Saudi Arabia contain less than the recommended levels.41 Therefore, national policies are needed to support vitamin D fortification, as VDD is common not only in T2DM patients, but also in the general population in Saudi Arabia.10 In relation to the secondary prevention of VDD, health care providers should raise the suspicion of VDD in T2DM patients and give vitamin D supplements judiciously to those affected. Further observational and experimental research with an appropriate design are needed to prove the causality of VDD in association with diabetes microvascular complications and also glycemic control and to elucidate the pathogenesis of vitamin D in T2DM.
Acknowledgments
We would like to thank the study participants for their participation and cooperation and to acknowledge the effort of Jazan Diabetes Centre for providing technical support.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.World Health Organization. Global Report on Diabetes. Geneva: World Health Organization; 2016. [Google Scholar]
- 2.Punthakee Z, Goldenberg R, Katz P. Definition, classification and diagnosis of diabetes, prediabetes and metabolic syndrome. Can J Diabetes. 2018;42:S10–5. doi: 10.1016/j.jcjd.2017.10.003 [DOI] [PubMed] [Google Scholar]
- 3.American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2018. Diabetes Care. 2018;41(Supplement 1):S13–27. [DOI] [PubMed] [Google Scholar]
- 4.Zghebi SS, Steinke DT, Carr MJ, Rutter MK, Emsley RA, Ashcroft DM. Examining trends in type 2 diabetes incidence, prevalence and mortality in the UK between 2004 and 2014. Diabetes Obes Metab. 2017;19(11):1537–1545. doi: 10.1111/dom.2017.19.issue-11 [DOI] [PubMed] [Google Scholar]
- 5.Meo SA. Prevalence and future prediction of type 2 diabetes Mellitus in the Kingdom of Saudi Arabia: a systematic review of published studies. J Pak Med Assoc. 2016;66(6):722–725. [PubMed] [Google Scholar]
- 6.Borel P, Caillaud D, Cano NJ. Vitamin D bioavailability: state of the art. Crit Rev Food Sci Nutr. 2015;55(9):1193–1205. doi: 10.1080/10408398.2012.688897 [DOI] [PubMed] [Google Scholar]
- 7.Deluca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004;80(6):1689S–96S. [DOI] [PubMed] [Google Scholar]
- 8.Simsek Y, Kucukler FK, Arduc A, Guler S. Is there an association between vitamin D level and microvascular complications of type 2 diabetes mellitus? West Indian Med J Open. 2015;2(3):138–141. [Google Scholar]
- 9.Griz LH, Bandeira F, Gabbay MA, Dib SA, Carvalho EF. Vitamin D and diabetes mellitus: an update 2013. Braz Arch Endocrinol Metab. 2014;58(1):1–8. [DOI] [PubMed] [Google Scholar]
- 10.Al-Alyani H, Al-Turki HA, Al-Essa ON, Alani FM, Sadat-Ali M. Vitamin D deficiency in Saudi Arabians: a reality or simply hype: a meta-analysis (2008–2015). J Family Community Med. 2018;25(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Papandreou D, Hamid Z-T-N. The role of vitamin D in diabetes and cardiovascular disease: an updated review of the literature. Dis Markers. 2015;2015:580474. doi: 10.1155/2015/105358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tahrani AA, Ball A, Shepherd L, Rahim A, Jones AF, Bates A. The prevalence of vitamin D abnormalities in South Asians with type 2 diabetes mellitus in the UK. Int J Clin Pract. 2010;64(3):351–355. doi: 10.1111/ijcp.2010.64.issue-3 [DOI] [PubMed] [Google Scholar]
- 13.Alhumaidi M, Agha A, Dewish M. Vitamin D deficiency in patients with type-2 diabetes mellitus in Southern Region of Saudi Arabia. Maedica. 2013;8(3):231–236. [PMC free article] [PubMed] [Google Scholar]
- 14.Sheth JJ, Shah A, Sheth FJ, et al. Does vitamin D play a significant role in type 2 diabetes? BMC Endocr Disord. 2015;15(1):5. doi: 10.1186/s12902-015-0003-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ozder A, Eker HH, Bilginc M. Status of vitamin D among Turkish adults with type 2 diabetes mellitus in primary health care. Acta Med Mediterr. 2015;31:229–236. [Google Scholar]
- 16.Saedisomeolia A, Taheri E, Djalali M, Moghadam AM, Qorbani M. Association between serum level of vitamin D and lipid profiles in type 2 diabetic patients in Iran. J Diabetes Metab Disord. 2014;13(1):7. doi: 10.1186/2251-6581-13-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alam MS, Kamrul-Hasan M, Kalam ST, Selim S, Akter F, Saifuddin M. Vitamin D status in newly diagnosed type 2 diabetes patients attending in a Tertiary Hospital of Bangladesh. Mymensingh Med J. 2018;27(2):362–368. [PubMed] [Google Scholar]
- 18.Ahmadieh H, Azar ST, Lakkis N, Arabi A. Hypovitaminosis D in patients with type 2 diabetes mellitus: a relation to disease control and complications. ISRN Endocrinol. 2013;2013:641098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Usluogullari CA, Balkan F, Caner S, et al. The relationship between microvascular complications and vitamin D deficiency in type 2 diabetes mellitus. BMC Endocr Disord. 2015;15(1):33. doi: 10.1186/s12902-015-0029-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reddy GB, Sivaprasad M, Shalini T, et al. Plasma vitamin D status in patients with type 2 diabetes with and without retinopathy. Nutrition. 2015;31(7–8):959–963. doi: 10.1016/j.nut.2015.01.012 [DOI] [PubMed] [Google Scholar]
- 21.Suzuki A, Kotake M, Ono Y, et al. Hypovitaminosis D in type 2 diabetes mellitus: association with microvascular complications and type of treatment. Endocr J. 2006;53(4):503–510. doi: 10.1507/endocrj.K06-001 [DOI] [PubMed] [Google Scholar]
- 22.American Diabetes Association. 6. Glycemic targets: standards of medical care in diabetes—2018. Diabetes Care. 2018;41((Supplement 1)):S55–64. [DOI] [PubMed] [Google Scholar]
- 23.Saif-Elnasr M, Ibrahim IM, Alkady MM. Role of vitamin D on glycemic control and oxidative stress in type 2 diabetes mellitus. J Res Med Sci. 2017;22(1):22. doi: 10.4103/jrms.JRMS_976_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Almetwazi MS, Noor AO, Almasri DM, et al. The association of vitamin D deficiency and glucose control among diabetic patients. Saudi Pharm J. 2017;25(8):1179–1183. doi: 10.1016/j.jsps.2016.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aldossari K, Aljowair AM, Alqahtani NT, Al-Shiprain MS, Al-Shathri MM, Alshehri DA. Association between low vitamin D level and glycemic control. Prim Care Diabetes. 2017;11(Supplement 1):e5. [Google Scholar]
- 26.Mezza T, Muscogiuri G, Sorice GP, et al. Vitamin D deficiency: a new risk factor for type 2 diabetes. Ann Nutr Metab. 2012;61(4):337–348. doi: 10.1159/000342771 [DOI] [PubMed] [Google Scholar]
- 27.Mahfouz MS, Rahim BE, Solan YM, Makeen AM, Alsanosy RM. Khat chewing habits in the population of the Jazan region, Saudi Arabia: prevalence and associated factors. PLoS One. 2015;10(8):e0134545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Di Cesar DJ, Ploutz-Snyder R, Weinstock RS, Moses AM. Vitamin D deficiency is more common in type 2 than in type 1 diabetes. Diabetes Care. 2006;29(1):174. doi: 10.2337/diacare.29.01.06.dc05-1876 [DOI] [PubMed] [Google Scholar]
- 29.Yu JR, Lee SA, Lee JG, et al. Serum vitamin D status and its relationship to metabolic parameters in patients with type 2 diabetes mellitus. Chonnam Med J. 2012;48(2):108–115. doi: 10.4068/cmj.2012.48.2.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rolim MC, Santos BM, Conceição G, Rocha PN. Relationship between vitamin D status, glycemic control and cardiovascular risk factors in Brazilians with type 2 diabetes mellitus. Diabetol Metab Syndr. 2016;8(1):77. doi: 10.1186/s13098-016-0188-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Y, Zhao CT, Zhen Z, Wong A, Tse HF, Yiu KH. Association of myocardial dysfunction with vitamin D deficiency in patients with type 2 diabetes mellitus. J Diabetes Complications. 2014;28(3):286–290. doi: 10.1016/j.jdiacomp.2014.01.003 [DOI] [PubMed] [Google Scholar]
- 32.Sipahi S, Acikgoz SB, Genc AB, Yildirim M, Solak Y, Tamer A. The association of vitamin D status and vitamin D replacement therapy with glycemic control, serum uric acid levels, and microalbuminuria in patients with type 2 diabetes and chronic kidney disease. Med Princ Pract. 2017;26(2):146–151. doi: 10.1159/000454952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alcubierre N, Castelblanco E, Martínez-Alonso M, et al. Vitamin D deficiency is associated with poorer satisfaction with diabetes-related treatment and quality of life in patients with type 2 diabetes: a cross-sectional study. Health Qual Life Outcomes. 2018;16(1):44. doi: 10.1186/s12955-018-0873-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zoppini G, Galletti A, Targher G, et al. Lower levels of 25-hydroxyvitamin D3 are associated with a higher prevalence of microvascular complications in patients with type 2 diabetes. BMJ Open Diabetes Res Care. 2015;3(1):e000058. doi: 10.1136/bmjdrc-2014-000058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Caretta N, de Kreutzenberg SV, Valente U, et al. Hypovitaminosis D is associated with erectile dysfunction in type 2 diabetes. Endocrine. 2016;53(3):831–838. doi: 10.1007/s12020-015-0851-z [DOI] [PubMed] [Google Scholar]
- 36.Moradi-Lakeh M, El Bcheraoui C, Afshin A, et al. Diet in Saudi Arabia: findings from a nationally representative survey. Public Health Nutr. 2017;20(6):1075–1081. doi: 10.1017/S1368980017002567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aksoy H, Akçay F, Kurtul N, Baykal O, Avci B. Serum 1,25 dihydroxy vitamin D (1,25(OH)2D3), 25 hydroxy vitamin D (25(OH)D) and parathormone levels in diabetic retinopathy. Clin Biochem. 2000;33(1):47–51. doi: 10.1016/S0009-9120(99)00085-5 [DOI] [PubMed] [Google Scholar]
- 38.Nanayakkara N, Ranasinha S, Gadowski A, et al. Age, age at diagnosis and diabetes duration are all associated with vascular complications in type 2 diabetes. J Diabetes Complications. 2018;32(3):279–290. doi: 10.1016/j.jdiacomp.2017.11.009 [DOI] [PubMed] [Google Scholar]
- 39.Iqbal K, Islam N, Mehboobali N, Asghar A, Iqbal MP. Association of vitamin D deficiency with poor glycaemic control in diabetic patients. J Pak Med Assoc. 2016;66(12):1562–1565. [PubMed] [Google Scholar]
- 40.Pittas AG, Lau J, Hu FB, Dawson-Hughes B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab. 2007;92(6):2017–2029. doi: 10.1210/jc.2007-0298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sadat-Ali M, Al Elq A, Al-Farhan M, Sadat NA. Fortification with vitamin D: comparative study in the Saudi Arabian and US markets. J Family Community Med. 2013;20(1):49. doi: 10.4103/2230-8229.108186 [DOI] [PMC free article] [PubMed] [Google Scholar]