Abstract
Background:
Frequently, a country will procure a single vaccine vial size, but the question remains whether tailoring the use of different size vaccine vial presentations based on populations or location characteristics within a single country could provide additional benefits, such as reducing open vial wastage (OVW) or reducing missed vaccination opportunities.
Methods:
Using the Highly Extensible Resource for Modeling Supply Chains (HERMES) software, we built a simulation model of the Zambia routine vaccine supply chain. At baseline, we distributed 10-dose Measles-Rubella (MR) vials to all locations, and then distributed 5-dose and 1-dose MR vials to (1) all locations, (2) rural districts, (3) rural health facilities, (4) outreach sites, and (5) locations with average MR session sizes <5 and <10 children. We ran sensitivity on each scenario using MR vial opening thresholds of 0% and 50%, i.e. a healthcare worker opens an MR vaccine for any number of children (0%) or if at least half will be used (50%).
Result:
Replacing 10-dose MR with 5-dose MR vials everywhere led to the largest reduction in MR OVW, saving 573,892 doses (103,161 doses with the 50% vial opening threshold) and improving MR availability by 1% (9%). This scenario, however, increased cold chain utilization and led to a 1% decrease in availability of other vaccines. Tailoring 5-dose MR vials to rural health facilities or based on average session size reduced cold transport constraints, increased total vaccine availability (+1%) and reduced total cost per dose administered (−$0.01) compared to baseline.
Conclusions:
In Zambia, tailoring 5-dose MR vials to rural health facilities or by average session size results in the highest total vaccine availability compared to all other scenarios (regardless of OVT policy) by reducing open vial wastage without increasing cold chain utilization.
Keywords: Measles-rubella, Supply chain logistics, Tailoring, Vial size
1. Introduction
Frequently, a single vaccine vial size will be procured for an entire country, but the question remains whether tailoring the use of different size vaccine vial presentations based on populations or location characteristics within a single country could improve the vaccine supply chain system. Previous studies have shown how vaccine vial size can significantly affect vaccine availability and other measures of vaccine delivery, such as open vial wastage (OVW) and the costs of supply chain logistics and vaccines [1-5]. While ordering a single type of vaccine presentation for an entire country may seem to simplify ordering, procurement and management, tailoring vaccine vial sizes – particularly lyophilized vaccines like measles-rubella (MR) which must be discarded within six hours of opening – by location, population, or average session size may yield additional gains, such as reducing cold chain utilization, decreasing OVW, reducing costs or increasing vaccine availability.
Using the Highly Extensible Resource for Modeling Supply Chains (HERMES) software, we developed a discrete-event simulation model of the Zambia Expanded Programme on Immunization (EPI) vaccine supply chain to quantify the impact of tailoring measles-rubella (MR) vaccine vial size on supply chain performance and costs within the country. We compared the impact of utilizing uniform vial sizes (10-dose, 5-dose and 1-dose MR) throughout the country to strategies where vial sizes were tailored by district (rural and urban), health facility (rural and urban), location type (fixed and outreach), and average session size (10 children and 5 children).
2. Methods
2.1. Hermes
Our team used the HERMES software platform to create a model of the EPI vaccine supply chain in Zambia. As described in previous publications [1,6-8], a HERMES-generated model includes virtual representations of each storage and vaccination location, storage device (e.g. refrigerators and freezers), mode of transportation (e.g. trucks, motorbikes, public transit), personnel and vaccine in the supply chain.
2.2. Zambia vaccine supply chain and data sources
Fig. 1 shows the structure and flow of the Zambia vaccine supply chain and Table 1 includes a list of all vaccines in the Zambia EPI, in addition to the 5-dose and 1-dose MR vaccines introduced in the model. The HERMES team worked with John Snow, Inc. (JSI) and the Center for Infectious Disease Research in Zambia (CIDRZ) via the Dose Per Container Partnership (DPCP). CIDRZ provided data on each mode of transport and the characteristics of routes between locations; the number and type of cold chain equipment at each location; the population reached by each vaccinating location; each personnel, including personnel costs and the percentage of time dedicated to EPI activities; and information on policies for the frequency and size of deliveries between locations and the frequency of vaccination sessions at each location. JSI provided information on healthcare worker behavior related to opening a vial and data on the frequency, size, and distance of outreach sessions.
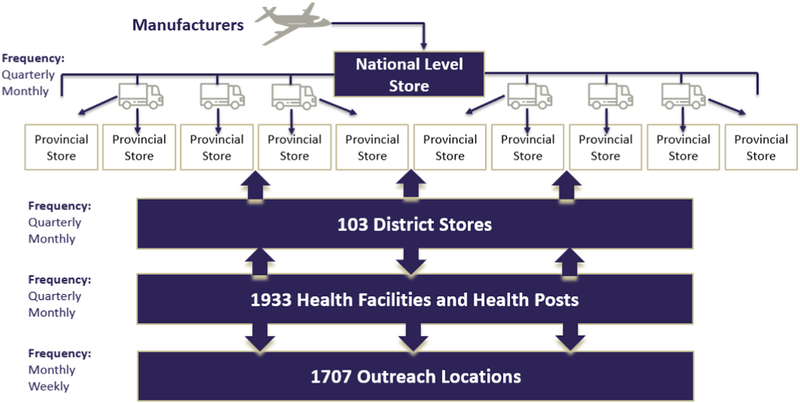
Fig. 1.
Zambia EPI Vaccine Supply Chain. The Zambia EPI supply chain consists of 4 fixed levels (National, Provincial, District, and Health Facility/Post) and a number of outreach locations. The figure above identifies the number of locations modeled at each level, and the frequency and direction of delivery between each level.
Table 1.
EPI vaccine characteristics.
| Presentation | Number of doses per vial |
Total volume per dose (cm3), incl. secondary packaging and diluent |
Total price per vial ($2017) |
Doses per person |
Age groups vaccinated |
Source |
|---|---|---|---|---|---|---|
| Measles-Rubella (MR) 10-dose | 10 | 5.2 | $6.25 | 2 | 9, 18 Months | [15-17] |
| Measles-Rubella (MR) 5-dose | 5 | 9.7 | $4.10 | [15,16] | ||
| Measles-Rubella (MR) 1-dose | 1 | 26.1 | $2.25 | [15,16] | ||
| Bacille Calmette-Guerin (BCG) | 20 | 2.26 | $2.81 | 1 | Birth | [16-18] |
| Pentavalent (Penta) | 1 | 14.06 | $1.06 | 3 | 6,10,14 weeks | [16-18] |
| Tetanus-Diphtheria (Td) | 10 | 3 | $1.10 | 4 | Pregnant Women | [16-18] |
| Oral polio vaccine (OPV) | 20 | 1 | $2.60 | 3 | 6,10,14 weeks | [16-18] |
| Pneumococcal conjugate vaccine (PCV-10) | 2 | 4.8 | $7.00 | 3 | 6,10,14 weeks | [16-18] |
| Rotavirus vaccine (Rota) | 1 | 17.1 | $2.32 | 2 | 6,10 weeks | [16-18] |
2.3. Locations and transport
The 2016 Zambia EPI vaccine supply chain is comprised of one national store, 10 provincial stores, 103 district stores, 1933 health facilities/posts, and 1707 outreach sites. As illustrated in Fig. 1, vaccines are first delivered from the national store to each of the provincial stores directly on quarterly basis. The district stores then collect vaccines from the provincial stores quarterly or monthly. Health facilities then collect vaccines from the district stores every two weeks, monthly, or quarterly; in some cases, district stores deliver vaccines to the health facilities, rather than having the health facilities retrieve the vaccines (represented in Fig. 1 as both up and down arrows). The average transport times for each route of the supply chain were collected and provided by CIDRZ.
Each route between the national and district levels use cold boxes. Between districts and health facilities, we assumed that approximately 1/3 (658 routes) used cold boxes to pick up or retrieve vaccines, while approximately 2/3 (1275 routes) used two small vaccine carriers, based on data collected from a subset of all health facilities. Each health facility that conducts outreach then delivers vaccines to its respective outreach site during each vaccination session using available modes of transport (e.g. trucks, motorbikes, or bicycles) and vaccine carriers or cold boxes.
2.4. Cold storage
Among fixed vaccination locations, 1830 of 2047 reported the availability of at least one working stationary cold storage device. The national and provincial stores have large cold rooms, while the district stores and health facilities use various sizes and types of refrigerators and freezers. Across the modeled health facilities, 217 did not report any stationary cold chain equipment. These locations were assumed to use a cold box for stationary storage and retrieve vaccines weekly from the district store using two small vaccine carriers, based on data collected by JSI from a subset of health facilities without existing cold storage.
2.5. Population demand
The number of vaccines ordered for each location is determined by the population demand, which were provided for each health facility by the CIDRZ. Health facilities order enough vaccines to meet average monthly demand, including an allowance for expected OVW as well as enough to maintain a 25% buffer. The populations to be vaccinated include pregnant women, newborns, and infants (grouped by age: 1–11 months and 12–24 months). For each vaccinating location, collected data included an estimate of the total population that would be reached by that location. We applied a 3% growth rate to the data collected in 2016 to estimate the 2017 population [9]. To estimate the number of pregnant women and newborns to be vaccinated at each location, we applied the crude birth rate per 1000 people to the total population served [10]. We then applied the neonatal mortality rate per 1000 live births and the infant mortality rate per 1000 live births to the number of newborns to estimate the number of neonates and infants served, respectively, by each location [11,12]. For health facilities with missing population data, we assumed a population between 1100 and 1500 people based on knowledge of clinic location and population sizes of similar facilities. The total 2017 Zambian population modeled was 16,612,395.
2.6. Vaccination sessions
The frequency of vaccination sessions varies between locations. Data on the frequency of vaccination sessions was provided by CIDRZ for each vaccinating location. For fixed health facilities, sessions ranged from daily to monthly, while outreach sessions ranged from weekly to monthly. During each MR vaccination session, the country policy for Zambia is to open an MR vial for any number of children. However, as healthcare workers reportedly wait for at least five children before opening a 10-dose vial [13], we simulated two scenarios for each tailoring policy – opening an MR vial for any number of children and opening an MR vial if at least half of the vial would be used.
Only health facilities with a reported stationary cold storage device were able to conduct outreach. Outreach sessions were assumed to reach 55% of a health facility catchment population, based on data collected by JSI and CIDRZ for a subset of health facilities. 50% of outreach sessions were assumed to occur within 10 KMs of the health facility while the other 50% occurred between 10 and 40 KMs from the health facility. Additionally, 50% of outreach sessions were assumed to include two personnel who were paid a standard per diem of 75 ZMW (appx. $7.50) per session, while the other 50% included one personnel.
2.7. Scenarios
The current immunization schedule in Zambia includes a 10-dose Measles-Rubella (MR) vaccine distributed to all locations. Using this as the baseline, we then test the effects of:
Replacing 10-dose MR vials with 5-dose and 1-dose MR vials at all immunization locations
Replacing 10-dose MR vaccine vials with 5-dose and 1-dose MR vials in rural districts
Replacing 10-dose MR vials with 5-dose and 1-dose MR vials at rural health facilities and associated outreach locations
Replacing 10-dose MR vials with 5-dose and 1-dose MR vials at all outreach locations only
Distributing 10-dose MR to vaccinating locations with average MR session sizes greater than 5 children and 10 children, and 5-dose MR to locations with average session sizes greater than 5 children and 10 children
We defined districts as rural if 70% or more of a district population met the Zambian census data definition of rural [14]. Health facilities were defined as rural if the catchment population was 10,000 or fewer and the facility was 5 KMs or more from the district store.
Average session size is roughly estimated by dividing the population demand at a given facility by the number of sessions per year. To better simulate reality, HERMES uses a stochastic model of consumer arrival for vaccination. For each session, HERMES calculates the actual session size by randomly drawing from a Poisson distribution, whose mean parameter is the average session size.
In each scenario, reported outcomes include open vial wastage, vaccine availability (i.e. the number of successful immunizations administered to patients as a percentage of immunizations needed), average peak transport utilization (i.e. the maximum percentage of available transport capacity needed to complete any shipment, averaged across all routes), logistics costs and total costs.
3. Results
All results are the average of 24 stochastic simulations, with the standard deviation for each scenario equaling less than 1%. Data presented in parentheses are the results when considering an open vial threshold of 50%. Tables 2 and 3 include all results for MR availability, MR doses administered, and MR doses wasted for each scenario.
Table 2.
Measles-Rubella (MR) availability, doses administered, and doses wasted across each tailoring scenario of 10-dose and 5-dose MR vaccines.
| Scenario | Location | 10-dose MR vaccine availability |
10-dose MR total doses administered |
10-dose MR doses wasted |
10-dose MR open vial wastage |
5-dose MR vaccine availability |
5-dose MR - total doses administered |
5-dose MR doses wasted |
5-dose MR open vial wastage |
Total MR vaccine availability |
Total MR doses administered |
Total MR doses wasted |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10 dose MR all locations | Total | 77% (62%) | 937,512 (758,288) | 955,517 (194,728) | 50% (20%) | - | - | - | - | 77% (62%) | 937,512 (758,288) | 955,517 (194,728) |
| Rural districts | 92% (72%) | 672,475 (513,762) | 732,623 (147,776) | 53% (22%) | - | - | - | - | - | 672,475 (513,762) | 732,623 (147,776) | |
| Urban districts | 57% (52%) | 265,037 (244,526) | 222,894 (46,952) | 40% (16%) | - | - | - | - | - | 265,037 (244,526) | 222,894 (46,952) | |
| Rural HFs | 97% (62%) | 344,534 (219,159) | 454,293 (87,350) | 62% (28%) | 344,534 (219,159) | 454,293 (87,350) | ||||||
| Urban HFs | 70% (64%) | 592,978 (539,129) | 501,223 (107,370) | 40% (16%) | 592,978 (539,129) | 501,223 (107,370) | ||||||
| 5 dose MR all locations | Total | - | - | - | - | 78% (71%) | 945,569 (859,530) | 381,625 (91,567) | 29% (10%) | 78% (71%) | 945,569 (859,530) | 381,625 (91,567) |
| Rural districts | 93% (82%) | 676,540 (599,500) | 309,620 (71,706) | 31% (11%) | 676,540 (599,500) | 309,620 (71,706) | ||||||
| Urban districts | - | - | - | - | 58% (56%) | 269,029 (260,030) | 72,005 (19,861) | 21% (7%) | 269,029 (260,030) | 72,005 (19,861) | ||
| Rural HFs | - | - | - | - | 98% (80%) | 345,859 (282,567) | 216,526 (45,229) | 38% (14%) | 345,859 (282,567) | 216,526 (45,229) | ||
| Urban HFs | - | - | - | - | 71% (68%) | 599,710 (576,963) | 165,099 (46,338) | 21% (7%) | - | 599,710 (576,963) | 165,099 (46,338) | |
| 5 dose MR rural districts; 10 dose urban districts | Total | 53% (49%) | 254,333 (234,892) | 172,727 (45,965) | 40% (16%) | 93% (82%) | 686,745 (609,387) | 309,933 (72,125) | 31% (11%) | 77% (69%) | 941,078 (844,279) | 482,660 (118090) |
| Rural districts | 0 | 0 | 93% (82%) | 686,745 (609,387) | 309,933 (72,125) | 31% (11%) | 686,745 (609,387) | 309,933 (72125) | ||||
| Urban districts | 53% (49%) | 254,333 (234,892) | 172,727 (45,965) | 40% (16%) | 0% (0%) | 0 | 0 | 254,333 (234,892) | 172,727 (45965) | |||
| Rural HFs | 97% (65%) | 56,824 (37,867) | 56,059 (17,928) | 58% (31%) | 98% (79%) | 290,199 (235,667) | 184,447 (37,498) | 39% (14%) | 347,023 (273,534) | 240,506 (55426) | ||
| Urban HFs | 51% (51%) | 197,509 (197,025) | 116,668 (29,803) | 31% (13%) | 89% (84%) | 396,546 (373,720) | 125,486 (34,632) | 24% (8%) | - | 594,055 (570,745) | 242,154 (64435) | |
| 5 dose MR rural HFs;10 dose urban HFs | Total | 69% (62%) | 591,216 (535,067) | 397,312 (107,053) | 40% (17%) | 97% (80%) | 350,023 (286,941) | 216,821 (45,371) | 38% (14%) | 77% (67%) | 941,239 (822,008) | 614,133 (152424) |
| Rural districts | 89% (77%) | 392,956 (340,500) | 281,400 (77,394) | 44% (19%) | 98% (79%) | 290,291 (235,578) | 185,134 (37,477) | 39% | 683,247 (576,078) | 466,534 (114871) | ||
| Urban districts | 48% (47%) | 198,260 (194,567) | 115,912 (29,693) | 31% (13%) | 96% (83%) | 59,732 (51,363) | 31,687 (7,889) | 35% | 257,992 (245,930) | 147,599 (37582) | ||
| Rural HFs | 0 | 0 | 97% (80%) | 350,023 (286,941) | 216,821 (45,371) | 38% (14%) | 350,023 (286,941) | 216,821 (45371) | ||||
| Urban HFs | 69% (62%) | 591,216 (535,067) | 397,312 (107,053) | 40% (17%) | 0% (0%) | 0 | 0 | 591,216 (535,067) | 397,312 (107053) | |||
| 5 dose MR outreach;10 dose fixed | Total | 78% (54%) | 442,769 (306,108) | 649,429 (78,877) | 59% (20%) | 77% (73%) | 496,702 (473,725) | 128,615 (41,032) | 20% (8%) | 77% (64%) | 939,471 (779,833) | 778,044 (119909) |
| Rural districts | 93% (58%) | 320,245 (199,566) | 512,021 (57,887) | 63% (22%) | 92% (87%) | 353,566 (333,897) | 100,399 (31,973) | 22% (9%) | 673,811 (533,463) | 612,420 (89860) | ||
| Urban districts | 57% (50%) | 122,524 (106,542) | 137,408 (20,990) | 47% (16%) | 57% (55%) | 143,136 (139,828) | 28,216 (9,064) | 16% (6%) | 265,660 (246,370) | 165,624 (30054) | ||
| Rural HFs | 98% (47%) | 162,277 (76,896) | 323,293 (27,430) | 70% (26%) | 96% (88%) | 181,496 (165,920) | 68,519 (22,047) | 27% (12%) | 343,773 (242,816) | 391,812 (49477) | ||
| Urban HFs | 71% (58%) | 280,492 (229,212) | 326,136 (51,448) | 49% (18%) | 70% (69%) | 315,206 (307,805) | 60,096 (18,990) | 16% (6%) | - | 595,698 (537,017) | 386,232 (70438) | |
| 5 dose MR to average session sizes < 5 | Total | 71% (62%) | 656,869 (567,547) | 526,405 (128061) | 44% (18%) | 95% (80%) | 290,677 (246,532) | 163,656 (35631) | 36% (13%) | 77% (66%) | 947,546 (814079) | 690,061 (163692) |
| Rural districts | 90% (74%) | 433,290 (352,117) | 421,260 (91526) | 49% (21%) | 95% (81%) | 253,677 (216,391) | 138,566 (29998) | 35% (12%) | - | 686,967 (568508) | 559,826 (121524) | |
| Urban districts | 51% (49%) | 223,579 (215,429) | 105,145 (36535) | 32% (15%) | 95% (77%) | 37,000 (30,140) | 25,091 (5633) | 40% (16%) | - | 260,579 (245569) | 130,236 (42168) | |
| Rural HFs | 97% (66%) | 143,206 (98,034) | 207,921 (37049) | 59% (27%) | 97% (79%) | 206,030 (168,235) | 132,886 (27775) | 39% (14%) | - | 349,236 (266269) | 340,807 (64824) | |
| Urban HFs | 66% (61%) | 513,662 (469,513) | 318,485 (91012) | 38% (16%) | 91% (84%) | 84,648 (78,296) | 30,770 (7856) | 27% (9%) | - | 598,310 (547809) | 349,255 (98868) | |
| 5 dose MR to average session sizes 10 | Total | 64% (56%) | 437,652 (382,715) | 328,939 (75816) | 43% (17%) | 94% (82%) | 510,870 (447,765) | 247,366 (56794) | 33% (11%) | 77% (68%) | 948,522 (830480) | 576,305 (132610) |
| Rural districts | 90% (72%) | 265,406 (212957) | 262,516 (53577) | 50 (20%) | 94 (82%) | 422,543 (368542) | 205,729 (46125) | 33% (11%) | 687,949 (581499) | 468,245 (99702) | ||
| Urban districts | 45% (44%) | 172,246 (169757) | 66,423 (22239) | 28 (12%) | 91(82%) | 88,327 (79223) | 41,638 (10669) | 32% (12%) | - | 260,573 (248980) | 108,061 (32908) | |
| Rural HFs | 97% (56%) | 63,181 (36203) | 115,466 (15319) | 65 (30%) | 97 (80%) | 286,764 (237374) | 172,183 (36455) | 38% (13%) | - | 349,945 (273577) | 287,649 (51774) | |
| Urban HFs | 61% (56%) | 374,471 (346511) | 213,474 (60497) | 36 (15%) | 90 (84%) | 224,107 (210392) | 75,184 (20399) | 25% (9%) | 598,578 (556903) | 288,658 (80896) |
MR = measles-rubella vaccine; HF = health facility.
Results presented in parentheses are when an MR vial is opened if at least half of the doses are used.
Table 3.
Measles-Rubella (MR) availability, doses administered, and doses wasted across each tailoring scenario of 10-dose and 1-dose MR vaccines.
| Scenario | Location | 10-dose MR vaccine availability |
10-dose MR total doses administered |
10-dose MR doses wasted |
10-dose MR open vial wastage |
1-dose MR vaccine availability |
1-dose MR - total doses administered |
1-dose MR doses wasted |
1-dose MR open vial wastage |
Total MR vaccine availability |
Total MR doses administered |
Total MR doses wasted |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10 dose MR all locations | Total | 77% (62%) | 937,512 (758,288) | 955,517 (194,728) | 50% (20%) | - | - | - | - | 77% (62%) | 937,512 (758,288) | 955,517 (194,728) |
| Rural districts | 92% (72%) | 672,475 (513,762) | 732,623 (147,776) | 53% (22%) | - | - | - | - | - | 672,475 (513,762) | 732,623 (147,776) | |
| Urban districts | 57% (52%) | 265,037 (244,526) | 222,894 (46,952) | 40% (16%) | - | - | - | - | - | 265,037 (244,526) | 222,894 (46,952) | |
| Rural HFs | 97% (62%) | 344,534 (219,159) | 454,293 (87,350) | 62% (28%) | - | - | - | - | - | 344,534 (219,159) | 454,293 (87,350) | |
| Urban HFs | 70% (64%) | 592,978 (539,129) | 501,223 (107,370) | 40% (16%) | - | - | - | - | - | 592,978 (539,129) | 501,223 (107,370) | |
| 1 dose MR all locations | Total | - | - | - | - | 74% | 904,616 | - | - | 74% (74%) | 904,616 | 0 |
| Rural districts | 90% | 667,293 | 667,293 | 0 | ||||||||
| Urban districts | - | - | - | - | 50% | 237,324 | - | - | - | 237,324 | 0 | |
| Rural HFs | - | - | - | - | 96% | 346,138 | - | - | - | 346,138 | 0 | |
| Urban HFs | - | - | - | - | 65% | 558,479 | - | - | - | 558,479 | 0 | |
| 1 dose MR rural districts; 10 dose urban districts | Total | 53% (49%) | 254,524 (234,381) | 172,379 (46,149) | 40% (16%) | 90% | 666,113 | - | - | 75% (74%) | 920,637 (900494) | 172,379 (46,149) |
| Rural districts | - | 0 | 0 | - | 90% | 666,113 | - | - | - | 666,113 (666,113) | 0 | |
| Urban DISTRICTS | 53% (49%) | 254,524 (234,381) | 172,379 (46,149) | 40% (16%) | 0% | 0 | - | - | - | 254,524 (234,381) | 172,379 (46,149) | |
| Rural HFs | 96% (65%) | 59,689 (40,343) | 83,597 (16,538) | 58% (29%) | 96% | 285,573 | - | - | - | 345,262 (325,916) | 83,597 (16,538) | |
| Urban HFs | 47% (47%) | 194,835 (194,038) | 88,782 (29,603) | 31% (13%) | 86% | 380,541 | - | - | - | 575,376 (574,579) | 88,782 (29,603) | |
| 1 dose MR rural HFs; 10 dose urban HFs | Total | 69% (62%) | 588,404 (535,820) | 394,377 (106,840) | 40% (17%) | 97% | 348,190 | - | - | 77% (72%) | 936,594 (884,010) | 394,377 (106,840) |
| Rural districts | 88%(77%) | 390,538 (340,635) | 304,374 (77,376) | 44% (19%) | 97% | 288,865 | - | - | - | 679,403 (629,500) | 304,374 (77,376) | |
| Urban districts | 48% (47%) | 197,866 (195,184) | 90,003 (29,456) | 31% (13%) | 96% | 59,325 | - | - | - | 257,191 (254,509) | 90,003 (29,456) | |
| Rural HFs | - | 0 | 0 | - | 97% | 348,190 | - | - | - | 348,190 (348,190) | 0 | |
| Urban HFs | 69% (62%) | 588,404 (535,820) | 394,377 (106,840) | 40% (17%) | - | 0 | - | - | - | 588,404 (535,820) | 394,377 (106,840) | |
| 1 dose MR outreach; 10 dose fixed | Total | 75% (52%) | 426,144 (297,562) | 629,135 (77,288) | 60% (21%) | 75% | 486,112 | - | - | 75% (64%) | 912,256 (783,674) | 629,135 (77,288) |
| Rural districts | 90% (57%) | 315,838 (200,442) | 527,645 (57,828) | 63% (22%) | 91% | 354,742 | - | - | - | 670,580 (555,184) | 527,645 (57,828) | |
| Urban districts | 50% (44%) | 110,306 (97,120) | 101,490 (19,460) | 48% (17%) | 51% | 131,369 | - | - | - | 241,675 (228,489) | 101,490 (19,460) | |
| Rural HFs | 96% (46%) | 161,556 (78,258) | 375,926 (27,573) | 70% (26%) | 96% | 184,700 | - | - | - | 346,256 (262,958) | 375,926 (27,573) | |
| Urban HFs | 66% (55%) | 264,588 (219,303) | 253,210 (49,707) | 49% (18%) | 66% | 301,411 | - | - | - | 565,999 (520,714) | 253,210 (49,707) |
MR = measles-rubella vaccine; HF = health facility.
Results presented in parentheses are when an MR vial is opened if at least half of the doses are used.
3.1. Replacing 10-dose MR with 5-dose and 1-dose MR at all vaccinating locations
Replacing 10-dose MR vials with 5-dose MR vials throughout the entire Zambian immunization supply chain resulted in a decrease in MR open vial wastage (OVW) from 50% to 29% (20% to 10% when requiring a vial opening threshold of 50% for MR). Total vaccine availability remained at 80% (79%); MR vaccine availability improved slightly by 1% (9%), but the availability of other antigens decreased slightly by <1% in both scenarios. The decrease in availability of non-MR vaccines was due primarily to the increase in average peak transport utilization from 101.6% to 102.7% (98–100.2%) which accompanied the increase in MR vaccine volume procured. High average peak transport utilization (e.g. greater than 100%) means that, even though some routes will have enough available transport space, on average across all routes, there is less transport storage space available than is needed, and some of the vaccines will not be shipped. As a proxy for coverage, the number of MR doses administered increased in both scenarios yet was greater when applying the practice of a vial opening threshold (from 758,288 to 859,530 doses). At a price-per-dose of $0.82, using 5-dose vials everywhere led to a decrease in the total cost per dose administered of all vaccines from $2.84 to $2.82 ($2.88–$2.83), due primarily to a decrease in total vaccine procurement costs of $212,951 ($121,586). Total vaccine procurement costs decreased as fewer doses of both MR vaccine and non-MR vaccines were procured.
Replacing 10-dose MR vials with 1-dose MR vials resulted in a decrease in MR OVW to 0% both when MR was opened for any number of children and when MR was opened if at least half of the doses would be used. Total vaccine availability, however, decreased to 76%, including a decrease both in the availability of MR and non-MR vaccines. The average peak transport utilization increased to 130.6% while storage utilization at the national level increased to 99%. At a price-per-dose of $2.25, using 1-dose vials everywhere led to an increase in total cost per dose administered to $3.01. Although vaccine procurement costs decreased by $94,505, this was accompanied by a sharp decrease in total doses administered (7,440,831 to 7,004,296) due to transport constraints, which drove up the total cost per dose administered.
3.2. Replacing 10-dose MR with 5-dose and 1-dose MR at rural districts
Replacing 10-dose MR vials with 5-dose MR vials at vaccinating locations in rural districts (n = 2813) resulted in a decrease in MR OVW from 50% to 34% (20–12% when requiring a vial opening threshold for MR). Total vaccine availability remained at 80% (79%) – MR vaccine availability increased by <1% (+7%) while the availability of other vaccines decreased by <1% in both scenarios. Using 5-dose MR in rural districts increased average peak transport utilization to 102.2% (99.7%), which had minimal impact on the flow of other vaccines. At a price-per-dose of $0.82 for 5-dose vials, vaccine procurement costs increased by $168,056 ($85,155) leading to an increase in the total cost per dose administered to $2.87 without the vial opening threshold, and a decrease in total cost per dose administered with the threshold from $2.88 to $2.86.
Replacing 10-dose MR vials with 1-dose MR vials in rural districts resulted in a decrease in MR open vial wastage from 50% to 16% (20–5%). Total vaccine availability decreased from 80% to 78% (79–78%). Without the vial opening threshold, both MR and non-MR availability decreased from baseline. However, with the vial opening threshold, MR availability improved (+7%) while the availability of non-MR vaccines decreased. As a proxy for coverage, the number of MR doses administered decreased (937,512–920,637) yet increased with the practice of a vial opening threshold (758,288–900,728). The average peak transport utilization increased to 123.6% (123.1%) while storage utilization at the national level also increased to 97% in both scenarios. At a price-per-dose of $2.25, using 1-dose vials in rural districts led to an increase in total cost per dose administered to $2.97 ($2.96).
3.3. Replacing 10-dose MR with 5-dose and 1-dose MR at rural health facilities
Replacing 10-dose MR vials with 5-dose MR vials at all rural health facilities (n = 2262) resulted in a decrease in MR open vial wastage from 50% to 39% (20–16% when requiring a vial opening threshold for MR). Total vaccine availability increased slightly to 81% (79%) – MR vaccine availability increased by <1% (5%) while the availability of other vaccines improved slightly by <1% in both scenarios. Using 5-dose MR in rural districts increased average peak transport utilization only slightly to 101.7% (99.3%), which allowed for the continued flow of non-MR vaccines. At a price-per-dose of $0.82 for 5-dose vials, vaccine procurement costs increased by $98,755 ($54,393) leading to a $0.01 increase in the total cost per dose administered to $2.85 without the vial opening threshold, and a decrease in total cost per dose administered with the threshold from $2.88 to $2.86.
Replacing 10-dose MR vials with 1-dose MR vials at rural health facilities resulted in a decrease in MR open vial wastage of from 50% to 30% (20–11%). Total vaccine availability remained at 80% and 79%. While the average peak transport utilization increased to 117.5% (116.9%), storage utilization at the national level remained below 90%, which together resulted in only a slight decrease in the flow of vaccines. As such, the availability of MR changed by <1% (+10%), while the availability of all other vaccines decreased by <1% in both scenarios. At a price-per-dose of $2.25, using 1-dose vials in rural health facilities led to an increase in total cost per dose administered to $2.88 ($2.90) due to increased vaccine procurement costs. Even though fewer MR doses were ordered, the larger price of the 1-dose MR vaccine led to a net increase in total vaccine procurement costs.
3.4. Replacing 10-dose MR with 5-dose and 1-dose MR at all outreach locations
Replacing 10-dose MR vials with 5-dose MR vials at all outreach locations (n = 1933) resulted in a decrease in MR open vial wastage from 50% to 45% (20–13%). Total vaccine availability remained the same at 80% (79%) – MR vaccine availability increased by <1% (+2%) while the availability of other vaccines remained the same. Using 5-dose MR in rural districts increased average peak transport utilization only slightly to 102.1% (99.0%). At a price-per-dose of $0.82 for 5-dose vials, the total cost per dose administered increased to $2.85 without the vial opening threshold, and decreased from $2.88 to $2.87 with the threshold.
Replacing 10-dose MR vials with 1-dose MR vials at outreach locations resulted in a decrease in MR open vial wastage of from 50% to 41% (20–9%). Total vaccine availability decreased to 78% (76%), due to a decrease in both MR availability and non-MR availability. At a price-per-dose of $2.25, using 1-dose vials in rural health facilities led to an increase in total cost per dose administered to $2.97 ($2.99) due to increased vaccine procurement costs and a reduction in total doses administered because of cold chain limitations.
3.5. Precise tailoring of 10-dose MR and 5-dose MR based on average session size
Replacing 10-dose MR vials with 5-dose MR vials at all vaccinating locations with session sizes of <5 (n = 1,836) decreased MR vial wastage from 50% to 42% (20–17%). Total vaccine availability improved slightly to 81% (79%) as both MR availability and non-MR availability improved. Compared to distributing 5-dose vials to rural health facilities, distributing to all locations with average session sizes <5 decreased average peak transport utilization from 101.6% (98%) to 101.4% (98.8%), allowing slightly more vaccines to flow through the system. At $0.82 per dose, vaccine costs changed from $2.84 to $2.85 ($2.88–$2.87) depending on the vial opening threshold.
Replacing 10-dose MR vials with 5-dose MR vials at all vaccinating locations with session sizes of <10 (n = 2635) decreased MR open vial wastage from 50% to 38% (20–14%). Total vaccine availability improved slightly to 81% (79%) as both MR availability and non-MR availability improved. Similar to using 5-dose vials at rural health facilities, distributing to all locations with average session sizes <10 led to a slight increase in average peak transport utilization from 101.6% (98%) to 102% (99.5%). At $0.82 per dose, vaccine costs changed from $2.84 to $2.85 ($2.88–$2.86) depending on the vial opening threshold.
4. Discussion
While using a single vaccine presentation for an entire country may simplify the ordering process, tailoring vaccines to certain locations and populations may provide additional benefit, depending on several factors including the vaccine cost- and volume-per-dose, vial opening practices, and cold chain constraints. As a decision-maker, identifying the primary outcomes of interest (e.g. reducing OVW, reducing costs, or improving vaccine availability or timely immunization coverage) as well as the country context (policy versus practice of vial opening threshold, current constraints on cold chain, greater reach through outreach or fixed immunization sites) can guide which vial ordering policy may be the best fit.
In Zambia, the practice of opening a vial only if at least half will be used could directly impact the vial ordering policy and increases the burden on the healthcare worker to make this decision on whether to ask a child to return and risk them not returning or open a vial and risk having high wastage. Under these conditions, any scenario using 5-dose MR vials improves the total number of doses administered compared to using 10-dose MR vials everywhere. Delivering 5-dose MR to all locations, rural districts, rural health facilities, and locations with average session sizes <5 or <10 children result in an improved number of vaccine doses administered. Delivering 5-dose MR vials to all locations primarily improves MR doses administered, while delivering 5-dose MR vials to rural health facilities and locations with average session sizes <5 or <10 maintains the flow of all vaccines while improving MR availability slightly less. Further, tailoring to rural health facilities or by session size reduces the total cost per dose administered by $0.01–$0.02.
When tailoring 5-dose MR vaccines to rural health facilities and by session sizes <5, vial sizes more appropriately match the expected number of children per session, reducing open vial wastage and resulting in a smaller volume of MR vaccine needing to be procured. This leads to a decrease in existing cold transportation constraints, which allows additional MR and non-MR vaccines to flow through the system.
These results indicate that a tailoring policy in Zambia centered on delivering 5-dose vials to rural health facilities or by average session size may provide benefits compared to using 10-dose or 5-dose MR vials everywhere, both when MR is opened for any number of children or if a vial opening threshold of 50% is in effect. In each of these scenarios, total and MR vaccine availability can improve while providing slight reductions in the total cost per dose administered.
However, having multiple vial sizes segmented by location or session size adds a layer of effort for supply chain managers as well as healthcare workers to identify the vial size to use. In Zambia, where data on the frequency of vaccination sessions and expected demand at each location were available, determining the average expected demand per session – and subsequently choosing between 10-dose and 5-dose MR – is possible. Without these data, countries may be able to more easily implement tailoring policies by a rural/urban designation. Additionally, the use of both 5-dose and 10-dose MR presentations may complicate efforts to conduct vaccination campaigns. Campaigns, which vaccinate larger volumes of people than routine sessions, can benefit from a greater number of doses per vial (e.g. 10-dose MR). Supply chain managers considering conducting MR campaigns will need to plan for either enough quantity of 10-dose MR or enough cold chain space for transporting larger-volume 5-dose vials.
The tailoring policies which benefit the Zambia routine vaccine supply chain may not hold in countries with more constrained cold chain systems, different average session sizes, or a different breakdown of urban and rural health facilities. Tailoring policies addressing other country contexts should attempt to more closely match vial size with average session size, while considering existing cold chain constraints.
5. Limitations
A computational model is a representation of reality that makes some simplifying assumptions and does not capture all possible factors or outcomes. One limitation of the model is that data gaps exist. To overcome missing data in the model, we generated assumptions based on available data and expert opinion. A second limitation of the HERMES model is that the results are not translated into clinical effects. The model shows changes in vaccine availability, but additional transmission models are needed to translate these vaccine supply chain changes into any impact on clinical outcomes, such as an increase or decrease in disease burden. A third limitation of the model is that results are not translated directly into vaccine coverage. Population demand is estimated based on the target population; the model does not restrict access to health facilities based on factors like accessibility, demand, or transport costs, and, as such, does not output estimated coverage.
6. Conclusions
When deciding to wholly replace or tailor vaccine presentations within a country, multiple factors need to be considered, including the size and cost per dose, vial opening practices, average session sizes, and existing cold chain constraints. In Zambia, tailoring MR vials at the health facility level or by average session size may provide some benefit compared to using 5-dose MR vials everywhere (e.g. reduce the total cost per dose administered, improve MR availability, and maintain the flow of all vaccines), while improving total vaccine availability and OVW compared to delivering 10-dose MR everywhere. Under certain conditions, using 1-dose MR vials in certain locations may provide an additional benefit. Additional evidence is needed to understand the feasibility of multiple vial sizes in a single system and the trade-offs on any implementation of this potential change.
Acknowledgments
This work was supported by the Bill and Melinda Gates Foundation, the Agency for Healthcare Research and Quality (AHRQ) via grant R01HS023317, USAID System and Complexity Monitoring, Evaluation, Research, and Learning (System and Complexity MERL) via AID-OAA-A-15-00064, National Institute of General Medical Sciences Modeling Infectious Disease Agent Study (MIDAS) Informatics Services Group grant 1U24GM110707, and the Global Obesity Prevention Center (GOPC) at Johns Hopkins and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the Office of the Director, National Institutes of Health (OD) under award number U54HD070725. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
References
- [1].Assi T-M, Brown ST, Djibo A, et al. Impact of changing the measles vaccine vial size on Niger’s vaccine supply chain: a computational model. BMC Publ Health 2011;11(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Haidari LA, Wahl B, Brown ST, et al. One size does not fit all: the impact of primary vaccine container size on vaccine distribution and delivery. Vaccine 2015;33(28):3242–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lee BY, Assi T-M, Rookkapan K, et al. Replacing the measles ten-dose vaccine presentation with the single-dose presentation in Thailand. Vaccine 2011;29 (21):3811–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lee BY, Norman BA, Assi T-M, et al. Single versus multi-dose vaccine vials: an economic computational model. Vaccine 2010;28(32):5292–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Heaton A, Krudwig K, Lorenson T, Burgess C, Cunningham A, Steinglass R. Doses per vaccine vial container: an understated and underestimated driver of performance that needs more evidence. Vaccine 2017;35(17):2272–8. [DOI] [PubMed] [Google Scholar]
- [6].Brown ST, Schreiber B, Cakouros BE, et al. The benefits of redesigning Benin’s vaccine supply chain. Vaccine 2014;32(32):4097–103. [DOI] [PubMed] [Google Scholar]
- [7].Lee BY, Haidari LA, Prosser W, et al. Re-designing the Mozambique vaccine supply chain to improve access to vaccines. Vaccine 2016;34(41):4998–5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lee BY, Wedlock PT, Haidari LA, et al. Economic impact of thermostable vaccines. Vaccine 2017;35(23):3135–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].World Bank. World Bank National Accounts: Population Growth; 2017. https://data.worldbank.org/indicator/SP.POP.GROW.
- [10].World Bank Bank. World Bank National Accounts: Crude Birth Rate; 2017. [Google Scholar]
- [11].World Bank. World Bank National Accounts: Infant Mortality Rate; 2017. [Google Scholar]
- [12].World Bank. World Bank National Accounts: Neonatal Mortality Rate; 2017. [Google Scholar]
- [13].DPCP. Snapshot vaccination in Zambia: Healthworker experiences using 5-dose MR. Vaccine 2018. [Google Scholar]
- [14].CS Office. Zambia 2010 census of population and housing; population summary report. 2012:1. [Google Scholar]
- [15].PAHO Revolving Fund. Expanded program of immunization vaccine prices for year 2017. Washington, DC: PAHO Revolving Fund; 2017. [Google Scholar]
- [16].World Health Organization. WHO prequalified vaccines; 2017. https://extranet.who.int/gavi/PQ_Web/.
- [17].World Health Organization. WHO vaccine-preventable diseases: monitoring system; 2017. [Google Scholar]
- [18].UNICEF. Vaccine price data; 2017. https://www.unicef.org/supply/index_57476.html.