Clinical testing detects only a fraction of carbapenem-resistant enterobacteriaceae (CRE) carriers, an estimated 1 of every 9 carriers1–3, and therefore may underestimate true CRE burden. Since targeted interventions to prevent spread are applied only to known cases, the unseen part of the “iceberg” of CRE carriers can exacerbate pathogen spread through lack of applied contact precautions and other infection prevention strategies. We previously demonstrated that extensive patient sharing occurs among healthcare facilities in a region4, 5, which means the extent of regional spread could be vastly underestimated. In other words, clinical testing reveals only the “tip of the iceberg” and the entire iceberg could be much greater than anticipated. Here we estimate the size of the iceberg in a large US metropolitan area.
We used our previously described platform, the Regional Healthcare Ecosystem Analyst (RHEA)6, 7, to generate an agent-based model of all adult inpatient facilities in Orange County, California (28 acute care facilities, 74 nursing homes), the patients moving among them, and surrounding communities. We used this model to simulate the transmission and spread of CRE (previously described).8, 9
On simulation day 0, we introduced CRE to reach observed target prevalence values of 10% in long-term acute care facilities (LTACHs)10 and 3% in nursing homes in year 5, and current prevalence trends in hospitals (based upon epidemiologic surveys conducted in year 4 of emergence2). We assumed that CRE carriers had a 1.8-fold readmission risk within a year of discharge11, and that 30% of carriers had persistent lifetime carriage12, 13, while the remaining 70% experienced a spontaneous loss (sigmoidal with 45% loss at 12 months14 so 70% of all carriers remained colonized at 1 year).
In each simulation run (50,000 trajectories), healthcare facilities detected only those CRE carriers incidentally identified from clinical cultures (1 in 9 carriers1–3), regardless of having a clinical infection. In acute care facilities, known carriers (i.e., newly detected or historically known) were placed on contact precautions that reduced transmission by 40%, which accounted for both intervention efficacy and staff compliance based on existing studies15–19. Since acute care facilities often track CRE status, known carriers remained on contact precautions if readmitted to the same facility. We assumed inter-facility communication of a patients’ CRE status on direct transfer was 50%.20 Nursing homes, which typically reserve contact precautions for overt infections, placed 10% of known carriers on contact precautions for 10 days. The number of daily CRE carriers on contact precautions represents each facility’s enacted response to the cumulative knowledge of obtained carrier status from cultures, maintained historical status, and inter-facility communication. Thus, contact precautions were infrequently applied because only 1 in 9 carriers were detected/known and this information was transferred in 50% of cases.
Figure 1a–b contrasts the percentage of facilities where ≥1 CRE carrier (detected and undetected) occurs with those that initiated contact precautions in response to ≥1 known carrier. The gap between these two groups begins around day 30 and grows significantly to year 4 when 85 (83.3%) facilities had cared for ≥1 CRE carrier, yet only 5 (4.9%) had placed any CRE carrier under contact precautions. Most facilities were unaware of CRE’s presence and spread for many years.
Figure 1.
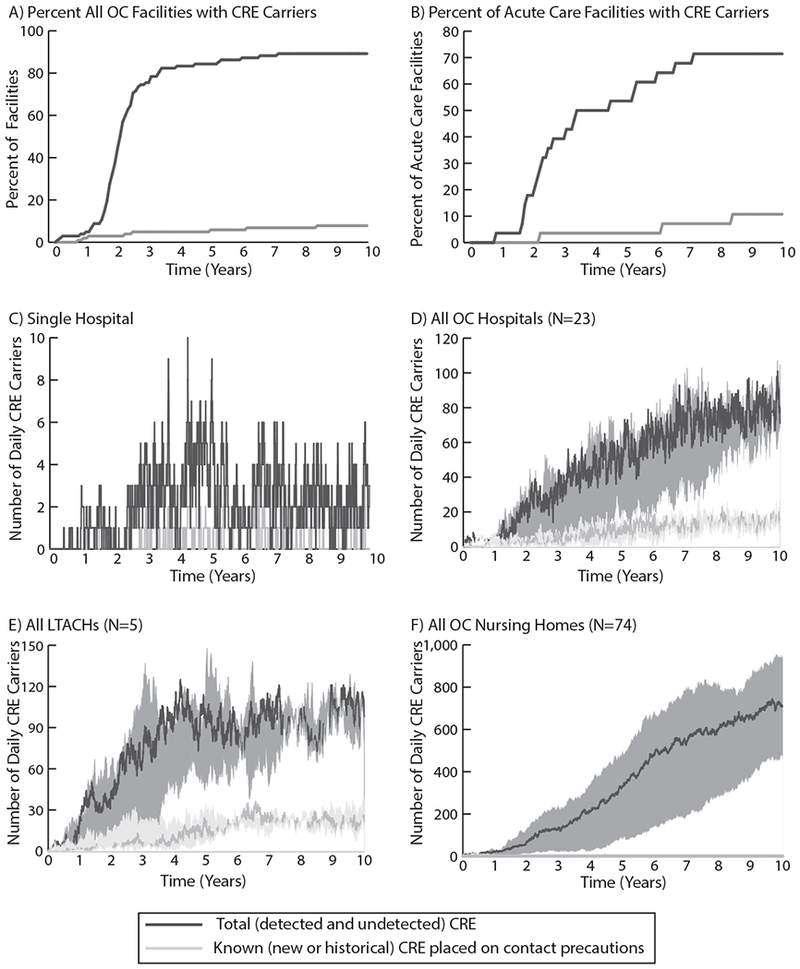
A) Number of all Orange County, California healthcare facilities (102 total; 23 hospitals, 5 LTACHs, and 74 nursing homes) that have at least one known (new or historical) CRE carrier under contact precautions compared to the number that have any CRE carriers (detected or undetected) over time; B) Number of OC acute care facilities (hospitals and LTACHs) that have at least one known CRE carrier on contact precautions compared to the number that have any CRE carriers (detected or undetected) over time; C) Number of known CRE carriers on contact precautions and total CRE carriers on any given day in an example OC acute care hospital; D) Number of known CRE carriers and total CRE carriers each day in all OC hospitals over time (median and range of simulated trajectories); E) Number of known CRE carriers and total CRE carriers each day in all OC LTACHs over time (median and range of simulated trajectories); F) Number of known CRE carriers and total CRE carriers each day in all OC nursing homes over time (median and range of simulated trajectories). Note differences in scale across panels.
Figure 1c–f highlights the substantial gap in perceived versus actual carriers when solely relying on clinical cultures to signal the need for contact precautions. Figure 1c shows a single hospital’s daily CRE carriers. Figure 1d–f show the number of carriers in hospitals, LTACHs, and nursing homes. The bands depict the range in the estimated number of daily carriers across simulations. On any day, there was a multi-fold underestimation of CRE carriers in facilities based on perceived burden from detected cases alone. In fact, even after 10 years, 70.4-86.4% of daily CRE carriers in individual hospitals remained undetected. This gap was further magnified in nursing homes because few carriers are known and even fewer are placed under contact precautions for colonization despite recommendations21. On an average day, nursing homes had <1 CRE carrier on contact precautions (Figure 1f).
The difference between total and known carriers represents the number of unknown carriers contributing to transmission. By year 5, clinical cultures identified approximately 19 new carriers a month countywide, when 102 transmission events actually occurred per month. This increased to 30 detected carriers versus 159 actual transmissions per month by year 10. There is likely a difference in CRE concern when a facility perceives 0-3 cases per day versus the full 5-15-fold burden. Countywide, 0.5-11% of total carriers were known at any given point in time, while 89-99.5% remained unknown. In the absence of effective facility-wide strategies for addressing unknown CRE, transmission will continue unabated. We quantified the rising daily difference between perceived spread based on clinical tests and actual spread. Not only are the number of cases substantially underestimated but many facilities may be completely unaware that CRE carriers are present and actively transmitting. However, all models are simplifications and have limitations, our model was tailored to one county’s healthcare facilities and their detailed patient-sharing data; therefore, these results may not be generalizable to other regions.
Knowing only the “tip of the iceberg” can significantly impair the ability to prevent and contain CRE spread. Extensive transmission is enhanced by complex patient sharing across healthcare facilities in a region.4, 5 This phenomenon makes it particularly important for infection prevention and public health programs to respond to the entire iceberg and mitigate its growth over time.
Acknowledgements
This manuscript was supported by the Agency for Healthcare Research and Quality (AHRQ) via grant R01HS023317, CDC SHEPheRD Task Order 2015-05, the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Office of Behavioral and Social Sciences Research (OBSSR) and the Global Obesity Prevention Center (GOPC) via grant U54HD070725 and NICHD via U01HD086861. Computing resources were provided by the University of Pittsburgh Center for Research Computing. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
JAM has received grants and personal fees from Achaogen, Allergang, Cempra, Expert Stewardship, Melinta, Menarini, Science 37, Theravance, and Thermo Fisher Scientific for the conduct of other studies.
Footnotes
Conflict of Interests
BYL, SMB, KFW, DSK, CC, and LEM, have nothing to disclose.
References
- 1.Pisney LM, Barron MA, Kassner E, Havens D, Madinger NE. Carbapenem-resistant enterobacteriaceae rectal screening during an outbreak of New Delhi metallo-B-lactamase producing Klebsiella pneumoniae at an acute care hospital. Infect Control Hosp Epidemiol. 2014;35(4):434–6. [DOI] [PubMed] [Google Scholar]
- 2.Gohil S, Singh RD, Gombosev A, et al. , editors. Emergence of Carbapenemase Resistant Enterobactereaceae (CRE) in Orange County, CA and Support for Regional Strategies to Limit Spread. ID Week: A Joint Meeting of the Infectious Disease Society of America, Society for Healthcare Epidemiology of America, HIV Medicine Association, and the Pediatric Infectious Disease Society; 2014. October 8-12; Philadelphia, PA. [Google Scholar]
- 3.Hayden MK, Lin MY, Lolans K, et al. Prevention of colonization and infection by Klebsiella pneumoniae carbapenemase-producing enterobacteriaceae in long-term acute-care hospitals. Clin Infect Dis. 2015;60(8):1153–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee BY, McGlone SM, Song Y, et al. Social network analysis of patient sharing among hospitals in Orange County, California. Am J Public Health. 2011;101:707–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee BY, Song Y, Bartsch SM, et al. Long-term care facilities: important participants of the acute care facility social network? PLoS One. 2011;6(12):e29342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee BY, McGlone SM, Wong KF, et al. Modeling the spread of methicillin-resistant Staphylococcus aureus (MRSA) outbreaks throughout the hospitals in Orange County, California. Infect Control Hosp Epidemiol. 2011;32(6):562–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee BY, Wong KF, Bartsch SM, et al. The Regional Healthcare Ecosystem Analyst (RHEA): simulation modeling tool to assist infectious disease control in a health system. J Am Med Inform Assoc. 2013;20(e1):e139–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Slayton RB, Toth D, Lee BY, et al. Vital Signs: Estimated effects of coordinated action to reduce antibiotic-resistant infections in health care facilities - United States. MMWR Morb Mortal Wkly Rep. 2015;64(30):826–31. [PMC free article] [PubMed] [Google Scholar]
- 9.Lee BY, Bartsch SM, Wong KF, et al. The potential trajectory of carbapenem-resistant Enterobacteriaceae, an emerging threat to health-care facilities, and the impact of the Centers for Disease Control and Prevention toolkit. Am J Epidemiol. 2016;183(5):471–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh RD, Jernigan JA, Slayton RB, et al. , editors. The CDC SHIELD Orange County Project – Baseline Multi Drug-Resistant Organism (MDRO) Prevalence in a Southern California Region. ID Week (6th Annual Joint Meeting of IDSA, SHEA, HIVMA, and PIDS); 2017. October 4-8; San Diego, CA. [Google Scholar]
- 11.Marquez P, Terashita D. Long-term acute care hospitals and carbapenem-resistant Enterobacteriaceae: a reservoir for transmission. Clin Infect Dis. 2013;57(9):1253–5. [DOI] [PubMed] [Google Scholar]
- 12.O’Fallon E, Gautam S, D’Agata EMC. Colonization with multidrug-resistant gram-negative bacteria; prolonged duration and frequent co-colonization. Clin Infect Dis. 2009;48(10):1375–81. [DOI] [PubMed] [Google Scholar]
- 13.Feldman N, Adler A, Molshatzki N, et al. Gastrointestinal colonization by KPC-producing Klebsiella pneumoniae following hospital discharge: duration of carriage and risk factors for persistent carriage. Clin Microbiol Infect. 2013;19(1E190-196). [DOI] [PubMed] [Google Scholar]
- 14.Bart Y, Paul M, Eluk O, Geffen Y, Rabino G, Hussein K. Risk Factors for Recurrence of Carbapenem-Resistant Enterobacteriaceae Carriage: Case-Control Study. Infect Control Hosp Epidemiol. 2015;36(8):936–41. [DOI] [PubMed] [Google Scholar]
- 15.Bearman GM, Marra AR, Sessler CN, et al. A controlled trial of universal gloving versus contact precautions for preventing the transmission of multidrug-resistant organisms. Am J Infect Control. 2007;35(10):650–5. [DOI] [PubMed] [Google Scholar]
- 16.Clock SA, Cohen B, Behta M, Ross B, Larson EL. Contact precautions for multidrug-resistant organisms: Current recommendations and actual practice. Am J Infect Control. 2010;38(2):105–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Golan Y, Doron S, Griffith J, et al. The impact of gown-use requirement on hand hygiene compliance. Clin Infect Dis. 2006;42(3):370–6. [DOI] [PubMed] [Google Scholar]
- 18.Weber DJ, Sickbert-Bennett EE, Brown VM, et al. Compliance with isolation precautions at a university hospital. Infect Control Hosp Epidemiol. 2007;28(3):358–61. [DOI] [PubMed] [Google Scholar]
- 19.Cromer AL, Hutsell SO, Latham SC, et al. Impact of implementing a method of feedback and accountability related to contact precautions compliance. Am J Infect Control. 2004;32(8):451–5. [DOI] [PubMed] [Google Scholar]
- 20.Kim J, Segreti J, Tomich A, Tongma C, Hayden MK, Lin MY. Surveillance and Inter-Facility Communication for Carbapenem-Resistant Enterobacteriaceae (CRE) (Abstract 594). SHEA Spring 2016 Conference; Atlanta, GA2016. [Google Scholar]
- 21.Centers for Disease Control and Prevention. Facility Guidance for Control of Carbapenem-resistant Enterobacteriaceae (CRE). November 2015 Update - CRE Toolkit In: National Center for Emerging and Zoonotic Infectious Diseases, Division of Healthcare Quality Promotion, editors. Atlanta, GA: Centers for Disease Control and Prevention; 2015. [Google Scholar]


