Abstract
For nearly two decades, adaptive radiation therapy (ART) has been proposed as a method to account for changes in head and neck tumor and normal tissue to enhance therapeutic ratios. While technical advances in imaging, planning and delivery have allowed greater capacity for ART delivery, and a series of dosimetric explorations have consistently shown capacity for improvement, there remains a paucity of clinical trials demonstrating the utility of ART. Furthermore, while ad hoc implementation of head and neck ART is reported, systematic full-scale head and neck ART remains an as yet unreached reality. To some degree, this lack of scalability may be related to not only the complexity of ART, but also variability in the nomenclature and descriptions of what is encompassed by ART. Consequently, we present an overview of the history, current status, and recommendations for the future of ART, with an eye towards improving the clarity and description of head and neck ART for interested clinicians, noting practical considerations for implementation of an ART program or clinical trial. Process level considerations for ART are noted, reminding the reader that, paraphrasing Elbert Hubbard, “Art is not a thing, it is a way.”
“When an artist uses a conceptual form of art, it means that all of the planning and decisions are made beforehand and the execution is a perfunctory affair. The idea becomes a machine that makes the art.”
- Sol LeWitt (1)
The concept of “adaptive radiation therapy” (ART) has been widely praised, serially modeled in silico, and heavily discussed, but to date, at a practical level, remains rarely implemented in vivo outside the research setting. We aim to discuss the “state of the ART” in head and neck therapy, with an emphasis on specification of the intent with which ART is performed; the terms of ART used, or a disambiguation of the nomenclature; technical aspects considered at the implementation of ART; and, importantly, rigorous and standardized means of reporting.
The standard of care for locoregional organ-sparing therapy of squamous cell carcinoma in the head and neck is chemoradiotherapy with systemic administration of a platinum in combination with fractionated radiotherapy to a dose of 63–70 Gy. The effects of chemoradiotherapy for seven weeks of treatment for head and neck cancer are substantial. Patients suffer from general side- effects, such as weight loss and distress, as well as acute and late toxicities induced by chemotherapy, radiotherapy or a combination of the two. Important acute radiation induced toxicities include severe mucositis, dermatitis, xerostomia and the need for a feeding tube. Important late or chronic toxicities include xerostomia, dysphagia and fatigue, which have been shown to influence quality of life for years after treatment(2, 3). Although weight loss or reduction of nodular volume might be apparent by physical examination, anatomical changes that occur during treatment have been shown to result in unintended (or, at least, unmonitored) deviation from the initial planning geometry. Sometimes it deviates to such a degree that inadvertent clinical target volume (CTV) undercoverage and/or organ at risk (OAR) overdosage occurs(4–6), even when isocentric image-guided alignment is applied.
ART history
Modern head and neck adaptive radiotherapy (ART) can be conceptually traced to the seminal work by Yan et al.(7), who proposed a method for offline assessment of 3D-conformal RT head and neck cancer set-up error, and replanning when sufficient deviation from the planned dose would be deleterious to delivery of planned dose, using planar imaging in 3 cardinal axes with electronic portal imaging devices.
Shortly thereafter, in-room imaging improved with the introduction of cone-beam CT by Jaffrey et al.(8), and the rise of commercial devices such as the in-line megavoltage tomographic/tomotherapeutic imaging, as well as less popular, but formative in-room CT approaches (CT-on-rails) (9) allowed the capacity to monitor not only isocentric error, but also multi-point/multi-ROI displacement as well as morphometric alteration in soft tissues, allowing more accurate treatment delivery through image guided radiotherapy (IGRT). IGRT has provided insight in the magnitude of anatomical changes that occur during treatment. This was initially demonstrated by Barker et al., showing in-room-CT-derived quantitative assessment of tumor and parotid alteration via daily imaging(9). They demonstrated a nearly 70% reduction in GTV volume with a median mass displacement of >3mm at the end of radiation treatment in patients with head and neck cancers, as well as significant alterations in parotid volumes during static therapy. They thereby enabled Yan’s definition of ART, ‘to customize each patients’ treatment plan to patient-specific variation by evaluating and characterizing the systematic and random variations through image feedback and including them in adaptive planning’ (7).
The extent of the customization as proposed Yan (7), has changed over time. In early ART the main purpose was often to confirm set-up accuracy, perform serial plan dose delivery consistency with pre-therapy planning (i.e. verification), or to maintain treatment as planned at onset (i.e., without CTV modification). In other words: to keep both target and organ at risk (OAR) dose equal to that of the original treatment plan, by accounting for anatomical changes in the adaptive plan (10, 11). In this regimen, additional OAR sparing compared to the original plan was a de facto bonus, rather than an intended goal of adaptation.
Modern ART
Current practice, however, more often specifies OAR sparing as the leading purpose or most important benefit of ART(12, 13). Unfortunately, the existing clinical data on the effect of ART is sparse, as is clearly shown by review of the limited prospective data available (Table 1, adapted from Castelli et al.(14)).
Table 1.
Clinical benefits of ART in patients with head and neck cancer.
| Nb patients |
Replanning strategies |
Clinical endpoint |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Author (year) | ART | No ART | Tumor site | Total dose (Gy) | Nb | Timing | Follow-up (months) | Loco-regional control and survival | Acute toxicity | Late toxicity |
| Schwartz (2012) (10)a | 22 | 0 | OPC | 66–70 | 1 or 2 | 16th and 22th fr | 31 | 2-year LRC = 95% | G III mucosal = 100% G II xerostomia = 55% G III xerostomia = 5% |
Full preservation or functional recovery of speech and eating at 20 months |
| Kataria (2016) (66) | 36 | 0 | LAHNC | 70 | 1 | 54 Gy | 2-year DFS = 72% 2-year OS = 75% |
G II-III mucosal = 100% | G II xerostomia= 8% G II mucosal = 11% No G III |
|
| Yang (2013)a(67) | 86 | 43 | NPC | 70–76 | 1 or 2 | 15th and/or 25th fr | 29 | 2-year LRC 97.2% (ART) 82.2% (no-ART) p = .04 2-year OS 89.8% (ART) 82.2% (No-ART) p = .47 |
Improvements in quality of life with ART | |
| Chen (2014)(68) | 51 | 266 | LAHNC | 60b 70μ |
1 | 40 Gy (10–58Gy) | 30 | 2-year LRC 88% (ART) 79% (No-ART) p = .01 2-year OS 73% (ART) 79% (No-ART) p = .55 |
G III: 39% (ART) 30% (No-ART) p = .45 |
G III: 14% (ART) 19% (No-ART) p = .71 |
| Zhao (2011)(69) | 33 | 66 | NPC | 70 | 1 | 15th (+/−5) fr | 38 | 3-year LRFS 72.7% (ART) 68.1% (No-ART) p = .3 |
No difference except less xerostomia and mucosal with ART for N2 and N3 patients | |
prospective studies (non-randomized)
= Adjuvant RT
= Definitive RT
Nb pts: number of patients; LAHNC: locally advanced head and neck cancer; OPC: oropharynx cancer; NPC: nasopharyngeal carcinoma; Nb: number; ART: adaptive radiotherapy; Fr: fraction; LRC: loco-regional control; LRFS: loco-regional free survival; DFS: disease-free survival; OS: overall survival
The intuitive and cost-free benefit of sparing OAR during ART may be the underlying cause for the gap in literature, as its clinical implementation has preceded clinical trials, despite a frankly limited scope of direct Level I evidence. Consequently, in a recent survey by Krishnatry et al. of 32 institutions at the Tata Memorial Hospital Radiotherapy Practicum(15), 92% of respondents listed head and neck as a site of adaptive therapy implementation. Despite the benefits, ART is not without implementation costs. Krishnatry et al noted in their survey: “ Eighty-four per cent of the respondents were willing to increase the use of ART in practice and believed (strongly) that ART improves clinical outcomes (70%), productivity (66%) and the therapeutic ratio (88%). The most important hindrances were the lack of equipment (48%), training (36%) and tools/management support (26%).” In head and neck cancer, these barriers are often a function of the need for human-defined (or at least, approved) regions of interest via target volume segmentation, and the “equipment”, “tools”, and “education” needs of Krishnatry et al. likely refer to this as-yet-unmet need, To date, several automated/semi-automated segmentation approaches have been investigated leveraging CT and MRI for OAR (16–18) and PET for gross tumor volume (19). For example, using the combination of a deep learning neural network and a shape representation model, Tong et al. recently published a competitive algorithm that can delineate 9 OARs on a new scan under 10 seconds(20). Similarly, Cardenas et al. (21, 22)have shown that rapid CTV generation can be performed with machine learning approaches, obviating an often time-consuming step in target delineation. Although these results are promising, general consensus remains that automatic segmentation has yet to completely replace physician contouring, as manual checks and sometimes adjustments remain necessary(23); Voet et al.(24), for example, showed that a commercial-software-autosegmented CTV protocol delivered clinically meaningful undercoverage, which was not reflected decisively in similarity metric assessment, despite potential clinical risk if implemented without oversight. Despite this need for continued physician involvement, automated/semi-automated OAR segmentation has been shown to improve segmentation time, with performance metrics approaching human performance in selected cases in randomized blinded human performance trial(17). These time savings, ideally, pave the way for more facile clinical implementation of head and neck adaptive trials and protocols.
Depending on frequency, timing and ad-hoc or planned nature of ART, it is consistently regarded a recourse-heavy and time-consuming intervention. The technical and procedural efforts required have prevented full-scale implementation (at present, to our knowledge, no site uses adaptive planning for all head and neck definitive cases), and have instead led to a large amount of in-silico trials, aiming to assess the optimal time and frequency of adaptive replanning, as well as a robust way of patient selection for this tool (Table 2, excerpted from a systematic review by Castelli et al. (14)). However, subsequent clinical implementation of this data remains both rarely attempted and underreported. And although the in silico results convincingly show benefit for OAR when ART is utilized to spare them, currently no international guidelines exist on how or when to apply ART for head and neck cancer.
Table 2.
Dosimetric benefits of ART in patients with head and neck cancer (from Castelli et al.).
| Replanning strategies |
Dosimetric analysis |
Dosimetric benefit |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Author (year) | Nb patients | Nb | Timing | Time point to cumulate the dose | Method to cumulate The dose | Total dose for the comparison (Gy) | Parotid Gland (Dmean) | Spinal cord (Dmax) | Target Volume (PTV) |
| Capelle (2012) (70) | 20 | 1 | 3rd week | 2 | Average DVH | 66 | −0.6 Gy * | −0.6 Gy * | +0.5 Gy (D1%) * |
| Castelli (2015) (71) | 15 | 6 | Weekly | 7 | DIR | 70 | −3.8 Gy* | - | - |
| Dewan (2016) (72) | 30 | 1 | 40 Gy | 2 | DVH | 30 | IL −6 Gy*
CL −2.2 Gy |
−66Gy* | More uniform coverage Decrease V110% by 2* |
| Duma (2012) (73) | 11 | 1 | 16th (9th–21st) fr | 1 | DVH | 2 | No variation | −0.14 Gy | - |
| Jensen (2012) (4) | 15 | 2 to 4 | - IL-3.8% |
3 to 5 | DIR | 70 | CL: − 11.5%a | - | Improvement of coverage by 8% |
| Olteanu (2014) (74) | 10 | 2 | 8th and 18th fr | 3 | DIR | 70 | −6%a* | - | Higher minimum and lower maximum doses |
| Schwartz (2013) (10) | 22 | 1 or 2 | 16th and 22nd fr | 2 or 3 | DIR | 70 | −0.7Gy* | - | Increase coverage and dose homogeneity |
| Zhao (2011) (69) | 33 | 1 | 15th (+/−) fr | 2 | DVH | 37.5 Gy (20–50 Gy) | Decrease mean dose* | - | - |
p-value <0.05
median dose
- = not assessed; Nb pts = Number of patients; Nb = number; fr. = Fraction; DVH = dose volume histogram; DIR = deformable image registration; Dmean = mean dose; Dmax = maximum dose; D1=Dose received by 1% of the volume; V110%: Percentage of target volume receiving 110% of the dose prescribed (hot spot); IL= ipsilateral; CL= contralateral; PTV= planning target volume
Terms of ART: Toward a critical nomenclature of ART intent
A “term of art” indicates “a word or phrase that has a precise, specialized meaning within a particular field or profession”(25). Sadly, in many cases the lack of clear terminology and specification has served to obfuscate the application of ART, and certainly hampered clinical reproducibility. Chief among vagaries is the lack of a definitive nomenclature for plan intent. That is to say, if adaptive therapy can encompass dose escalation, OAR de-escalation, static plan verification, and shrinking volumes simultaneously, does ART have any intrinsic meaning at all?
The proliferation and increased use of the term “adaptive therapy” thus means that a plethora of approaches can fall under the umbrella of ART. To overcome this, we have sought to define a formalism for defining the relative planning intent of a given ART trial (Table 3), with the aim of specifying and categorizing future efforts in prospective ART approaches.
Table 3:
ART terminology
| Name | Technique | Tumor dose | OAR dose | Example study/trial |
|---|---|---|---|---|
| ARTex_aequo | Serial plan verification to ensure pretherapy plan parameters are stable. | = | = | Yan et al. (7). |
| ARTOAR |
Reduced OAR dose; pre-therapy CTV is conserved; |
= | ↓ | Schwartz et al.(10, 29); |
| ARTamplio | Increased dose to tumor; isotoxic (or lower) OAR dose | ↑ | = | ADMIRE (Al Mamgani et al.(75)) |
| ARTreduco | “Shrinking CTV” for on-treatment responders | = | ↓ | MR-ADAPTOR (Bahig et al. (28)) |
| ARTtotale | Increase dose to subvolume of initial CTV | ↑ | ↓ | UZ Gent DBPN trials (74, 76–80) |
OAR = organ at risk. CTV = clinical target volume; DBPN=Dose painting by numbers.
For example, despite the lack of international consensus or guidelines on various aspects of ART, there is a currently an on-going multi-center phase two clinical trial in which ART is an implemented treatment arm. The ARTFORCE study is an ongoing randomized clinical trial for head and neck cancer patients who are treated with concomitant cisplatin and standard or adaptive high dose radiotherapy(26, 27). The high dose radiotherapy consists of a redistribution of dose to the primary tumor, in which the 50% of the gross tumor volume (GTV) with the highest uptake on F-18-fluorodeoxyglucose- positron emission tomography (FDG-PET) scan is defined and subsequently boosted in such a way that 2% is boosted to 84Gy, while the mean dose remains 70 Gy for the GTV. The adaptive part consist of a CT-scan in week two of treatment, with a new treatment plan per week three of treatment. This new plan has the same constraints for tumor and OAR dose as the original and does not strive for additional sparing of OAR, nor additional dose to the tumor. It is isotoxic, isotreatment ART, or ARTex_aequo. Unique about this trial is that it is indirect proof of the feasibility of multi-center and standardized ART. Unfortunately because the ART is done in the experimental arm, a comparison of toxicity or outcome discriminating only for adaptive radiotherapy will not be possible based on the results.
The future will require additional definitions of ART, as using images solely to keep treating what you set out to treat, or serial plan verification such as in ARTex_aequo will belong to the past. In fact, ART in head and neck cancer has already changed from ARTex_aequo to a regimen that seeks extra OAR sparing: ARTOAR. Alternatively, dose escalation to the primary tumor and/or adjusting the CTV based on images made during treatment can be performed. For this, we propose the following terms: ARTamplio to indicate ART with the purpose of dose–escalation to the CTV; ARTreduco to indicate ART in which the CTV is cropped to the new anatomy, and ARTtotale, in which both dose-escalation to the CTV and reduction of CTV to the new anatomy are goals (Table 3). ARTreduco is currently being clinically investigated using MR-guided adaptation in a prospective cohort, as will the safety and toxicity reduction of this regimen(28).
ART Techniques
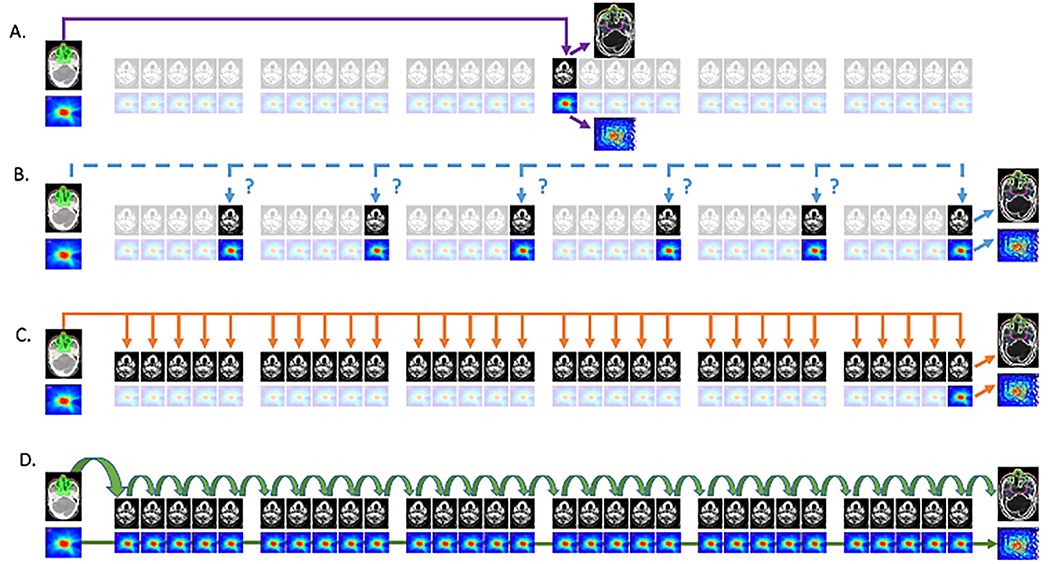
The same ambiguity remains, not only to the intent of an ART protocol, but to the mechanics or technique of its implementation. Figure 1 illustrates a selection of possible typologies of ART implementation for head and neck cancer, with increasing temporal resolution.
Figure 1. Possible typologies of ART implementation.
1A:fixed interval approach; 1B: ‘triggered’ ART; 1C: serial ART,1D cascade ART.
In Figure 1A, a “fixed-interval” approach is implemented, wherein the CTsimulation image and dose data are registered to a single (often mid-therapy) time-point. In many cases, the initial plan is recalculated or superimposed on the mid-therapy anatomy, and, if dose constraints are not met, a single adaptation is performed. This approach is computationally and workflow efficient, and may have particular utility in scenarios such as proton therapy, where contour and anatomic deviations may be mitigated by mid-therapy verification adaptation.
Figure 1B denotes an approach designated as “triggered” adaptation. In these scenarios, interval daily (or weekly) imaging is acquired, and iteratively reviewed throughout therapy for plan deviation based on qualitative or quantitative triggers, as typified by Yan et al, who developed this approach using EPID-based set-up assessment. If, rather than EPID, volumetric imaging for IGRT is used, ART can then be implemented on an “as needed” basis. Threshold “triggers” for replanning can encompass cachexia/weight loss, surface contour or mask fit changes, OAR/CTV volume alteration, increased daily or systematic set-up deviations. Furthermore, this approach can be combined with the “fixed interval” approach shown in XA, when both planned mid-therapy and ad hoc “triggered” adaptation are performed (e.g. Schwartz et al, wherein a single fixed interval adaptation was planned, with up to 2 additional “as needed” triggered adaptations utilized.(10, 29)
However, both fixed-interval and triggered ART approaches fail to incorporate any intervening image/dose data, and often eschew dose accumulation. Alternatively, both ‘serial’ and ‘cascade’ ART approaches incorporate dose accumulation in the ART procedure. For serial ART, this is done post-hoc, in cascade ART, the dose up to and including the most current image/fraction/day is accumulated, and this data available at the time of ART, which we will now elaborate further.
Figure 1C, which has been alternately referred to as “serial”, “one-to-many”, or “sequential” adaptation involves high-frequency (≥weekly) volumetric imaging and registration to the initial plan. However, though each ImageFraction to Imageplanning assessment is repeated, the cumulative deformation vector fields are not concatenated, meaning that aggregate dose accumulation is not occurring through-out the course of therapy. Consequently, interval OAR/GTV deformations during therapy are unincorporated, and dose is “forward projected” with increasingly large temporal and geometric differential(s) from the planning scan. This approach is currently the default implementation for the 0.35T ViewRay system for MR-guided RT systems (as detailed by Raghavan et al.(30), in a study where registration of the MRIFraction to the initial MRIplanning was performed prior to manual segmentation), and the initial 510k-compliant 1.5T Elekta MR-LinAc configuration (2ATL), which allows rigid registration of MRIplanning to daily fractions, and either a “adapt-to-point” (virtual isocentric alignment) or “adapt-to-shape” (volume-based replanning).
Ideally, in a computational resource unbounded space, the scenario in Figure 1D is preferred, dubbed “iterative” or “cascade” ART. In this approach, daily deformation in geometry and set-up error are incorporated subsequent to all fractions, meaning interval change in volume is minimized and preserving a DVF “chain” by which OAR/CTV voxel reduction/morphometric alteration can be tracked with increased precision. Dose accumulation in concert with therapy is a natural result, allowing “delivered cumulative dose” to be readily assessed. While such an approach is theoretically possible on several vendor systems, active implementation of such a data rich approach has yet to be done, and thus represents a demonstrable “post-modern ART” application in need of vendor/manufacturer support.
Perspective in ART
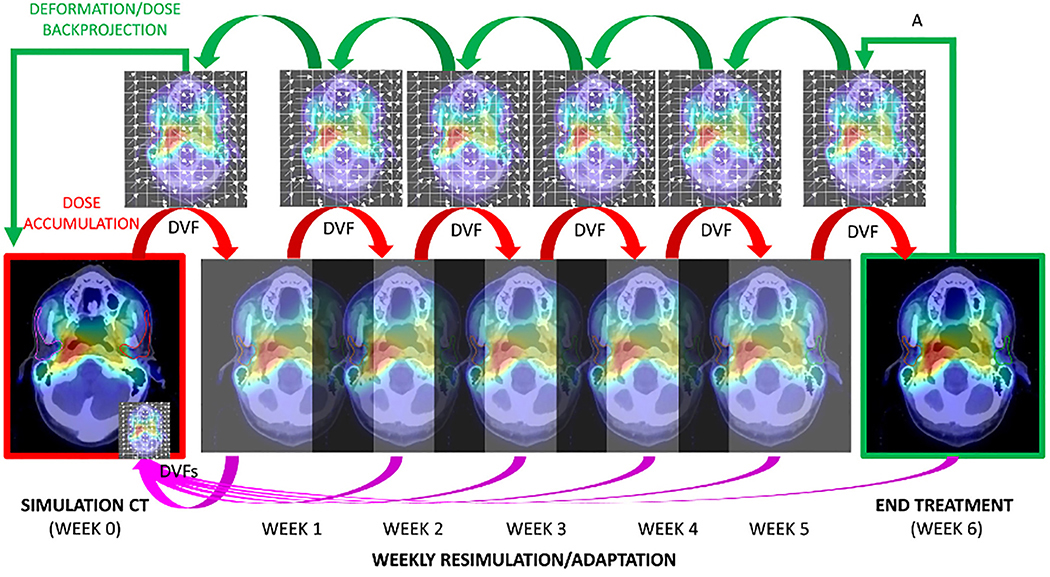
In addition to specification of the terms of ART and techniques of ART, a fundamental need exists for definition of the relative reference anatomy for reporting ART. That is to say, if anatomy is dynamically changing, is a plan judged by its conformance to the original “simulation plan”, or is are constraints judged on the accumulated dose after any given number of fractions? The importance of reference frame specification is illustrated in
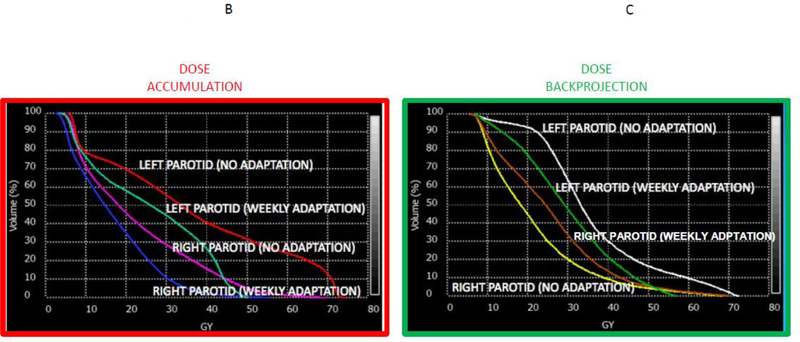
2A, which shows a series of weekly cascade registrations performed on an in silico case (red arrows) via interval deformable image registration/dose accumulation (denoted by visual representation of deformation vector fields (DVFs)). In this scenario, the parotid glands, which lost almost 25% of their total volume, have the cumulative ROI at simulation mapped iteratively via “DVF” chain to parotid ROI voxels at end therapy. In contrast, the reverse procedure, termed “dose back-projection” (green arrows), expands the end-therapy parotid ROI iteratively “back through time” to map the post-therapy volume to the simulation reference anatomy/dose grid. These distinct approaches can lead to disparate dose display, as in Figures 2B (DVF/dose accumulation) and 2C (deformation back-projection) which shows not only the effect of DVF/dose accumulation, but the capacity for parotid dose reduction if, at each registration step, weekly dose adaptation had been performed. Notably, the difference in frame of reference upon estimated parotid dose is in the same order of magnitude or greater than the alteration consequent to weekly adaptation. For this reason we recommend review of not only accumulated dose, but also deformation back-projection for prospective ART applications.
Figure 2. The importance of the reference frame in ART.
Figure 2A: forward calculation with dose accumulation, and back-projection; 2B: Dose-volume histogram (DVF/dose accumulation); 2C: dose-volume histogram (deformation back-projection).
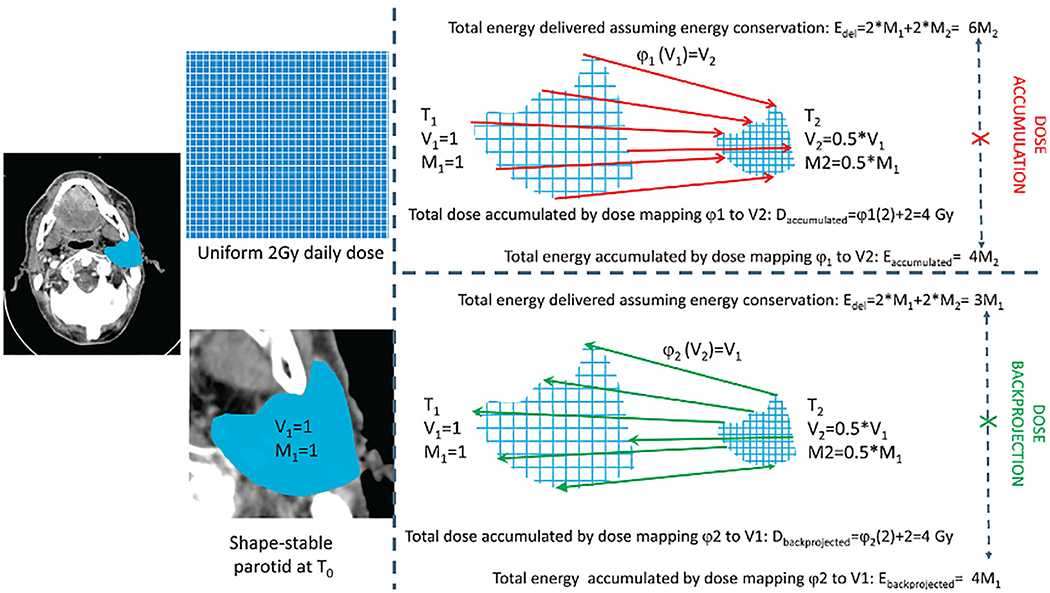
This “perspective problem” is especially pronounced when significant target or OAR volume shrinkage is observed. Indeed, substantial volume loss over the course of treatment can result in serious inaccuracies in dose accumulation, especially in head and neck cancer patients, because they may have observed significant volumetric changes with regard to tumor or tissue. These volumetric alterations, may inadvertently lead to intrinsic failures dose estimation of deformable registration algorithms, as eloquently explained by Zhong and Chetty(31), whose exemplary illustration is recapitulated for parotid volume reduction in Figure 3. In this example, a theoretical parotid gland from the initial time point (T1), receiving a uniform dose (D) of 2 Gy, experiences a 50% reduction in volume (Vi) shrinking to half its original size, such that the original volume (V1) is double the end volume (V2) at a second time point (T2), with a corresponding loss of mass (Mi; i.e. M1=2*M2. If a deformable registration algorithm, φ1, maps all voxels in V1 to V2, to measure the dose-to-date, the resultant dose accumulation (termed by Zhong & Chetty(31) as “deformable dose accumulation” (DDA), but here called simply dose accumulation) results in an underestimation of energy delivered (E). Similarly, dose back-projection (e.g. “dose mapping” (31)), the projection of dose from V2 to V1 via deformation φ2 results in underestimation of dose delivered when energy conservation is considered. A similar observation is seen in DIR algorithms that maintain “mass conservation” whereby additional structures (such as a mouth stent or flap reconstruction) are overfitted by intensity matching-or similarity-driven DIR approaches(32). Biomechanical models, which include prior knowledge of relational data in addition to intensity data can mitigate this effect(31, 33, 34). However, as the original authors note, caution must be used when large proportional volumetric changes are encountered in either tumor or tissue, and careful algorithm selection for particular applications and quality assurance thereof is thus de rigueur.
Figure 3. Volume loss and dose accumulation.
V: volume; M: mass; φ: deformable registration algorithm; EDEL: energy delivered;T0: start of treatment.
Cataloguing ART
In order to assess, nay appreciate ART, one must be able, in an analogical fashion, to determine not only the intent of the artist, but also the technique used, and allow for ART to be readily archived, catalogued and reproduced. In an effort to assist the interested reader, process-level considerations for potential standardization are detailed as queries (and easily converted to a checklist format) in Table 4. These disparate considerations point to unmet needs specific to adaptive radiotherapy. While several reports have defined recommendations for clinical trial implementation(35), dose prescription and reporting(36, 37), uncertainty margination(38–40), ROI and DVH nomenclature(41), commissioning of IGRT systems(42, 43), implementation and reporting of image registration techniques(44), modeling (45) and reporting guidelines(46), as yet no single standardized reporting structure exists to allow the massive amounts and permutations of image, margination, dose, clinical, and relational data generated by even a small adaptive head and neck clinical trial to be reportable in an efficient manner, let alone consistent with FAIR Guiding Principles for scientific data management (47) (vide infra, Table 5). The use of radiation oncology specific ontology systems at clinical scale remains exciting, but nascent (41, 48–50). Nonetheless, we believe that use of a series of self-directed queries and careful consideration of the multiple factors involved can assist even established programs with managing adaptive radiotherapy implementation, and allow programs building expertise a frame of reference for developmental protocols.
Table 4:
Practical process-level considerations and queries in design and reporting of adaptive clinical trials and observational adaptive regimens.
| Process | Clinical consideration | Query formulation | Sub-queries/examples | Suggested guidance document(s) |
|---|---|---|---|---|
| Adaptive regimen prescription/ intent documentation | A priori definition of clinical plan intent | -What is the physician- defined clinical intent of the adaptive regimen? | What conceptual approach underlies the
adaptive trial/regimen (viz Table
1)? -Define, if possible the expected magnitude of clinical benefit in terms of locoregional control or toxicity reduction. Examples: - “Reduce by 15% Grade 3 acute symptoms due to inadvertent elective risk PTV overdosage in the presence of >10% weight loss.” - “Improve local control probability by 15% via an isotoxic dose escalation of residual PET-derived high-risk regions on mid-therapy imaaina.” |
(35),(36, 37) (44, 81), |
| A priori definition of dosimetric aims of adaptive regimen. | What is the intended dosimetric intent of the adaptive regimen? | Examples: - “Shrinkage of CTV/PTV as tumor regression occurs through weekly offline adaptation, while ensuring >95% coverage of weekly PTVadapted”. - “Ensure parotid V15 overdosage of less than 5% deviation from pretherapy prescription via weight loss and deformation is prevented by mid-treatment verification.” - “Replan patient if systematic setup error exceeds a pre-specified tolerance of >3mm.” |
||
| Pre-therapy imaging/simulation | Annotation of worklow for initial planning image acquisition, as well as subsidiary images utilized for therapy planning. | What immobilization strategy/devices were implemented at simulation? | Was this method standardized for all patients in regimen/trial? | (81,82) |
| Was additional imaging (PET/MR/CT) used for pre-therapy treatm ent planning? | If so, have all images utilized been archived and registered with the simulation DICOM dataset? | (44) | ||
| Target delineation/OAR initial segmentation | Specification, in a reproducible manner, of segmentation process for initial planning. | Were TVs/OARs segmented manually? | If so, using what quality assurance procedures(83), guidelines(84–86) and nomenclature (41) in the TPS? | (84–88) (41) |
| Were any TVs/OARs segmented by automated/semi- automated processes? | -If so, using what
software/version/approach? -Are automated/semi-automated ROIs annotated to differentiate manual vs automated(18, 21,22, 89–93) vs. assisted(17, 24) segmentation? |
|||
| Initial dose prescription/evaluation | Reporting utilized parameters of interest for planning, as well as the reference model for potential dose modification. | What were pre-therapy dose-constraints implemented for TVs/OARS? | If using a reference constraint(s) (e.g. QUANTEC(94–100) or other extant models(3, 71, 101–109), note prior reference/model. | (45, 99, 110) |
| If constraints were based on a biological model, which one(s)? | ||||
| Serial on-treatment imaging | Explicit exposition of implemented processes for image acquisition and image-guided translational/set-up error modification. | Is reimaging performed online (e.g. CBCT, CT-on- rails, MRI-LinAc), or offline (inter-fraction CT resimulation)? | -What is the frequency of on- treatment
re-imaging? -Are all on-treatment images archived? |
(44) (42) (43) |
| Are additional offline image-data implemented (e.g. contrast CT, PET, diagnostic MRI), and if so, how utilized? | Is the method of offline-images (e.g.
PET-guided dose- painting (76,
78–80, 111–115))
clearly defined? Are all utilized offline-images archived with co-registration to closest interval online volumetric images? |
|||
| Are serial on-treatment translational corrections applied using IGRT in the presence/absence of simultaneous image- registration? | -If so, are translational shifts performed
relative to ROI(s), isocenter, or fiducial(s)? -Are all image-based shifts recorded and archived with matched IGRT image dataset? |
|||
| Replanning/ Plan adaptation | Overt description of the methodologlc approach to planned/delivered dose calculation, as well as associated ROI/segmentation and related dose-constraint monitoring. | Is the replanning strategy online (i.e. while patient is on treatment device) or offline (occurring between treatment fractions)? | -What is the frequency/interval of adaptive
replanning(116–118)? -What software/version/algorithm is utilized for replanning/adaptation? |
(44) |
| What if any, are the replanning criteria/action level specified (e.g. % underdosage of target, overdose of an OAR)? | -Are replanning criteria fixed interval,
reactive (e.g. once a dose constraint has been exceeded/unmet) or
proactive (triggered by a projected dose or dose trajectory
model)? -If so, specify action level. |
|||
| Are non-dosimetric surrogate criteria (e.g. systematic set-up error, morphometric alteration, ROI superimposition on daily IGRT) used as a trigger for replanning? | If so, specify action level. | |||
| Are ROIs for adaptation (re)segmented manually, semi-automated, or fully DIR-propagated? | -Are all propagated and manually generated
ROIs archived with daily images after IGRT/replanning review? -Is faculty/st aff approval required for relevant ROIs, and if so, are these annotated and timestamped? |
|||
| Is serial manual review of criteria/action level performed (i.e. does a human “check the DVH”) or is automated triggering performed? | -Are all generated/reviewed DVHs (or analogous
metrics(119))
archived? -If faculty/staff approval is performed, are relevant DVHs annotated and timestamped? |
(41) | ||
| Uncertainty margination | Estimation of the relative daily uncertainty accounted for by margin expansion, and disclosure of site- specific rationale/measurements used to calculate/justify utilized margins. | -Are isotropic margins implemented? If so,
provide an IGRT-system specific population estim ator. -For anisotropic approaches, describe the margin calculation approach. |
Are deformable phantoms(33, 120–125), digital phantoms(126–128), or other QA methods employed to generate trial/regimen-specific margins, or are standard institutional margins employed? | (35, 44, 129) |
| Are margins population- derived, or patient specific? | ||||
| How do margination strategies account (if at all) for registration uncertainty? | ||||
| Image Registration/ Dose accumulation assessment | Coherent and understandable explication of serial image/dose relational processes, allowing clear representation of how serially derived image and dose data are analyzed and assessed during treatment, as well as the manner by which the completed therapy course image and dose alterations are summarized. | By what method is image- registration performed: rigid, or deformable? | -If deformable, using what approach (e.g.
biomechanical atlas-based, B-spline, DEMONS) and via what
software/version?Describe performance metrics for software selection, if
available(32, 33). -To what reference data are images used for evaluation/replanning coregistered (e.g. planning simulation, previous daily on-line imaging, or offline imaging)? -Are all DVFs archived? -Are DVFs annotated so that it is readily determined whether they were actually utilized for treatment, or as a function of post hoc plan summation? |
(44, 130) |
| By what approach is dose- summation performed (e.g. iterative dose- accumulation, or superimposition of delivered dose)? | -What software/version/algorithm is used for
initial and replanning dose calculation(34)? -Describe utilized dose delivery quality assurance methods (e.g. pre-therapy phantom dosimetry(33, 120–125), EPID- dosimetry(131)) and frequency relative to imaging/plan adaptation. -Is accumulated dose iteratively recorded, archived and summarized? Are final accumulated dose and backprojected dose archived/summarized(31)? |
|||
| Data description/ dissemination | Collation of all relevant and
informative data elements of the adaptive trial/regimen into a coherent
and FAIR-compliant format for reporting of clinical, technical, and
dosimetric observations/outcomes and data sharing. |
Have relevant clinical outcome data been recorded using an established ontology/nomenclature(48–50)? | Example: If locoregional control is an
endpoint, are failure events mapped to delivered/accumulated dose and
acquired pre-therapy imaging(132–136)
using an accepted methodology/nomenclature(137, 138)? Are the data described, not just in free text format, but using a recognized informatics ontology(48–50)? |
(46) |
| Has relevant patient- and cohort-specific plan intent/TPS/IGRT/adaptive replanning/archival system data been collated into a single repository that meet FAIR criteria(47, 139)? | If so, are all data compatible with DICOM-RT linked (e.g. via DVF) to a common reference geometry and FAIR-compliant (i.e. machine searchable) image, ROI, and DVH nomenclature? When possible, are clinical data embedded within the DICOM-standard(140, 141)? | (41,44, 47, 49, 50, 139) | ||
| After trial/protocol completion and/or publication, can archived trial/adaptive protocol data be shared, either directly, or via distributed learning systems, to allow learning from extant data(64, 65, 142, 143)? |
Table 5.
The FAIR Guiding Principles (from Wilkinson et al.).
| Guiding Principle/Attribute | Definition | Adaptive trial example |
|---|---|---|
| Findable: | F1. (meta)data are assigned a globally unique
and persistent identifier F2. data are described with rich metadata (defined by R1 below) F3. metadata clearly and explicitly include the identifier of the data it describes F4. (meta)data are registered or indexed in a searchable resource |
After completion and publication of an adaptive trial, data are anonymized and deposited in a public repository (e.g. TCIA (64, 65)), and labeled with a permanent digital object identifier (DOI)(144). The DOI/repository and a data summary are then submitted as a data descriptor to a relevant journal (such as Medical Physics or Nature Scientific Data) and PubMed-indexed for easy searchability. |
| Accessible | A1. (meta)data are retrievable by their
identifier using a standardized communications protocol A1.1 the protocol is open, free, and universally implementable A1.2 the protocol allows for an authentication and authorization procedure, where necessary A2. metadata are accessible, even when the data are no longer available |
Other investigators, having located the data from PubMed or journal sites, can readily download the dataset from TCIA using the NBIA Data Retriever app (145) to query the permanent repository and download the anonymized image and dose data. |
| Interoperable | I1. (meta)data use a formal, accessible,
shared, and broadly applicable language for knowledge
representation. I2. (meta)data use vocabularies that follow FAIR principles I3. (meta)data include qualified references to other (meta)data |
Data from the adaptive trial, including all images, dose, and adaptive plans are stored using the DICOM-RT standard(146, 147). Additional clinical data and is either embedded within the DICOM header, referenced with relevant files/systems(140, 141), associated with semantic data (e.g. Resource Description Framework via the Radiation Oncology Ontology (48, 50, 51)framework) to allow the data to be used across multiple vendor and vendor neutral software(s) or for distributed learning. |
| Reusable | R1. meta(data) are richly described with a
plurality of accurate and relevant attributes R1.1. (meta)data are released with a clear and accessible data usage license R1.2. (meta)data are associated with detailed provenance R1.3. (meta)data meet domain-relevant community standard |
Using the aforementioned data, the adaptive protocol is reconstructed in silico, and used by several other sites to benchmark their internal adaptive processes and develop new segmentation and automated replanning approaches; the original data are cited in subsequent publications(148), and are widely a performance estimator for a new adaptive trial workflow development. |
As the field of radiotherapy has become increasing more complex in terms of the increasing number and complexity of disparate information sources which must be aggregated to extract meaningful data, ART represents, in some sense, the index case of an unmet need for “Big Data” information support. This is both for ensuring patient safety and for correlating image-dose-response data in a clinical utilizable manner(51), because the volume, temporal, and spatial correlation of multiple elements (patient data, accumulated dose, images, DVH, DVF, positional shifts, toxicity/outcome) must be carefully collated, organized, curated, recorded and reported(52–57). However, if the present situation persists, it will remain, as it is currently, almost impossible to effectively reconstruct an institutions’ specific adaptive protocol in the absence of identical vendor-supplied treatment planning, registration, archiving, electronic medical record, toxicity and patient-reported outcomes collection, and outcome monitoring, barring significant resource allocation(58).
Consequently, we must as a specialty, commit to making ART FAIR. While an art fair is the public display of many artists, ARTists in head and neck cancer should commit to public display of data, whenever possible, using the recently presented “FAIR Guiding Principles for scientific data management and stewardship”(47). The FAIR framework, if executed, would, in the authors’ estimation, do more to accelerate adaptive trials than any other technical or computational advance, as it would allow sites to evaluate their systems using established datasets, query alternative practices, and perform in-silico studies with shared normative “controls”. FAIR software and QA processes could allow “beta testing” on public adaptive head and neck datasets. To our knowledge, a limited number of FAIR-compliant head and neck radiotherapy datasets exist(59–63), primarily on the Cancer Imaging Archive (64, 65), and to our knowledge none are either of adaptive cases, or close to complete in terms of full reporting of dose/toxicity/response data. Thus, we call on our fellow ARTists to commit to FAIR-ness, data sharing and transparency in developing the tools and processes necessary to enable wide-scale, safe, easy and effective ART through becoming an invigorated, enthused and sharing ART collective.
Conclusion
In summary, this seminar has aimed to illustrate challenges and opportunities, in addition to a high-level survey of adaptive radiotherapy (ART) in head and neck cancer. Unfortunately, head and neck ART is, at present, not standardized nor widely utilized. We sought to provide possible guidelines for standardization in the various aspects of ART, i.e. specification of the intent with which ART is performed; the terms of ART used, or a disambiguation of the nomenclature; technical aspects considered at the implementation of ART; and, importantly, rigorous and standardized means of reporting. While some of these aspects are the responsibility of clinicians and physicists performing ART, continued creative and collaborative efforts with vendors will be necessary to make futuristic ART possible and beneficial to our shared patients.
Acknowledgments
Disclosures
This research is supported by the Andrew Sabin Family Foundation; Dr. Fuller is a Sabin Family Foundation Fellow. Dr. Fuller receive funding and project-relevant salary support from the National Institutes of Health (NIH), including: National Institute for Dental and Craniofacial Research Award (1R01DE025248–01/R56DE025248–01); National Cancer Institute (NCI) Early Phase Clinical Trials in Imaging and Image-Guided Interventions Program(1R01CA218148–01); NIH/NCI Head and Neck Specialized Programs of Research Excellence (SPORE) Developmental Research Program Award (P50 CA097007–10); National Science Foundation (NSF), Division of Mathematical Sciences; NIH Big Data to Knowledge (BD2K) Program of the National Cancer Institute Early Stage Development of Technologies in Biomedical Computing, Informatics, and Big Data Science Award (1R01CA214825–01); NIH/NCI Cancer Center Support Grant (CCSG) Pilot Research Program Award from the UT MD Anderson CCSG Radiation Oncology and Cancer Imaging Program (P30CA016672) and National Institute of Biomedical Imaging and Bioengineering (NIBIB) Research Education Program (R25EB025787). Dr. Fuller has received direct industry grant support and travel funding from Elekta AB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.LeWitt S Paragraphs on Conceptual Art. Artforum. 1967;10(Summer). [Google Scholar]
- 2.Ackerstaff AH, Rasch CR, Balm AJ, de Boer JP, Wiggenraad R, Rietveld DH, et al. Five-year quality of life results of the randomized clinical phase III (RADPLAT) trial, comparing concomitant intra-arterial versus intravenous chemoradiotherapy in locally advanced head and neck cancer. Head Neck. 2012;34(7):974–80. [DOI] [PubMed] [Google Scholar]
- 3.Langendijk JA, Doornaert P, Verdonck-de Leeuw IM, Leemans CR, Aaronson NK, Slotman BJ. Impact of late treatment-related toxicity on quality of life among patients with head and neck cancer treated with radiotherapy. J Clin Oncol. 2008;26(22):3770–6. [DOI] [PubMed] [Google Scholar]
- 4.Jensen AD, Nill S, Huber PE, Bendl R, Debus J, Munter MW. A clinical concept for interfractional adaptive radiation therapy in the treatment of head and neck cancer. Int J Radiat Oncol Biol Phys. 2012;82(2):590–6. [DOI] [PubMed] [Google Scholar]
- 5.Bhandari V, Patel P, Gurjar OP, Gupta KL. Impact of repeat computerized tomography replans in the radiation therapy of head and neck cancers. J Med Phys. 2014;39(3):164–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahmoud O, Reis IM, Samuels MM, Elsayyad N, Bossart E, Both J, et al. Prospective Pilot Study Comparing the Need for Adaptive Radiotherapy in Unresected Bulky Disease and in Postoperative Patients With Head and Neck Cancer. Technol Cancer Res Treat. 2017:1533034617717624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yan D, Lockman D, Martinez A, Wong J, Brabbins D, Vicini F, et al. Computed Tomography Guided Management of Interfractional Patient Variation. Seminars in Radiation Oncology. 2005;15(3):168–79. [DOI] [PubMed] [Google Scholar]
- 8.Jaffray DA, Siewerdsen JH. Cone-beam computed tomography with a flat-panel imager: initial performance characterization. Med Phys. 2000;27(6):1311–23. [DOI] [PubMed] [Google Scholar]
- 9.Barker JL Jr., Garden AS, Ang KK, O’Daniel JC, Wang H, Court LE, et al. Quantification of volumetric and geometric changes occurring during fractionated radiotherapy for head-and-neck cancer using an integrated CT/linear accelerator system. Int J Radiat Oncol Biol Phys. 2004;59(4):960–70. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz DL, Garden AS, Thomas J, Chen Y, Zhang Y, Lewin J, et al. Adaptive radiotherapy for head-and-neck cancer: initial clinical outcomes from a prospective trial. Int J Radiat Oncol Biol Phys. 2012;83(3):986–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castadot P, Lee JA, Geets X, Gregoire V. Adaptive radiotherapy of head and neck cancer. Semin Radiat Oncol. 2010;20(2):84–93. [DOI] [PubMed] [Google Scholar]
- 12.Zhang P, Simon A, Rigaud B, Castelli J, Ospina Arango JD, Nassef M, et al. Optimal adaptive IMRT strategy to spare the parotid glands in oropharyngeal cancer. Radiother Oncol. 2016;120(1):41–7. [DOI] [PubMed] [Google Scholar]
- 13.Brouwer CL, Steenbakkers RJ, Langendijk JA, Sijtsema NM. Identifying patients who may benefit from adaptive radiotherapy: Does the literature on anatomic and dosimetric changes in head and neck organs at risk during radiotherapy provide information to help? Radiother Oncol. 2015;115(3):285–94. [DOI] [PubMed] [Google Scholar]
- 14.Castelli J, Simon A, Lafond C, Perichon N, Rigaud B, Chajon E, et al. Adaptive radiotherapy for head and neck cancer. Acta Oncol. 2018;57(10):1284–92. [DOI] [PubMed] [Google Scholar]
- 15.Krishnatry R, Bhatia J, Murthy V, Agarwal JP. Survey on Adaptive Radiotherapy Practice. Clin Oncol (R Coll Radiol). 2018;30(12):819. [DOI] [PubMed] [Google Scholar]
- 16.Bondiau PY, Malandain G, Chanalet S, Marcy PY, Habrand JL, Fauchon F, et al. Atlas-based automatic segmentation of MR images: validation study on the brainstem in radiotherapy context. Int J Radiat Oncol Biol Phys. 2005;61(1):289–98. [DOI] [PubMed] [Google Scholar]
- 17.Walker GV, Awan M, Tao R, Koay EJ, Boehling NS, Grant JD, et al. Prospective randomized double-blind study of atlas-based organ-at-risk autosegmentation-assisted radiation planning in head and neck cancer. Radiother Oncol. 2014;112(3):321–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wardman K, Prestwich RJ, Gooding MJ, Speight RJ. The feasibility of atlas-based automatic segmentation of MRI for H&N radiotherapy planning. J Appl Clin Med Phys. 2016;17(4):146–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berthon B, Evans M, Marshall C, Palaniappan N, Cole N, Jayaprakasam V, et al. Head and neck target delineation using a novel PET automatic segmentation algorithm. Radiother Oncol. 2017;122(2):242–7. [DOI] [PubMed] [Google Scholar]
- 20.Tong N, Gou S, Yang S, Ruan D, Sheng K. Fully automatic multi-organ segmentation for head and neck cancer radiotherapy using shape representation model constrained fully convolutional neural networks. Med Phys. 2018;45(10):4558–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cardenas CE, Anderson BM, Aristophanous M, Yang J, Rhee DJ, McCarroll RE, et al. Auto-delineation of oropharyngeal clinical target volumes using 3D convolutional neural networks. Phys Med Biol. 2018;63(21):215026. [DOI] [PubMed] [Google Scholar]
- 22.Cardenas CE, McCarroll RE, Court LE, Elgohari BA, Elhalawani H, Fuller CD, et al. Deep Learning Algorithm for Auto-Delineation of High-Risk Oropharyngeal Clinical Target Volumes With Built-In Dice Similarity Coefficient Parameter Optimization Function. Int J Radiat Oncol Biol Phys. 2018;101(2):468–78. 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lim JY, Leech M. Use of auto-segmentation in the delineation of target volumes and organs at risk in head and neck. Acta Oncol. 2016;55(7):799–806. [DOI] [PubMed] [Google Scholar]
- 24.Voet PW, Dirkx ML, Teguh DN, Hoogeman MS, Levendag PC, Heijmen BJ. Does atlas-based autosegmentation of neck levels require subsequent manual contour editing to avoid risk of severe target underdosage? A dosimetric analysis. Radiother Oncol. 2011;98(3):373–7. [DOI] [PubMed] [Google Scholar]
- 25.Merriam-Webster. 2019. [Available from: https://www.merriam-webster.com/dictionary/term%20of%20art.
- 26.Heukelom J, Hamming O, Bartelink H, Hoebers F, Giralt J, Herlestam T, et al. Adaptive and innovative Radiation Treatment FOR improving Cancer treatment outcomE (ARTFORCE); a randomized controlled phase II trial for individualized treatment of head and neck cancer. BMC Cancer. 2013;13:84 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heukelom J, Lamers E, Slooten E, van Werkhoven E, Rasch C, Sonke J-J. Redistributed versus homogenous radiotherapy dose for head and neck cancer; a treatment planning study. Physics and Imaging in Radiation Oncology. 2017;3:17–20. [Google Scholar]
- 28.Bahig H, Yuan Y, Mohamed ASR, Brock KK, Ng SP, Wang J, et al. Magnetic Resonance-based Response Assessment and Dose Adaptation in Human Papilloma Virus Positive Tumors of the Oropharynx treated with Radiotherapy (MR-ADAPTOR): An R-IDEAL stage 2a-2b/Bayesian phase II trial. Clin Transl Radiat Oncol. 2018;13:19–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwartz DL, Garden AS, Shah SJ, Chronowski G, Sejpal S, Rosenthal DI, et al. Adaptive radiotherapy for head and neck cancer--dosimetric results from a prospective clinical trial. Radiother Oncol. 2013;106(1):80–4. [DOI] [PubMed] [Google Scholar]
- 30.Raghavan G, Kishan AU, Cao M, Chen AM. Anatomic and dosimetric changes in patients with head and neck cancer treated with an integrated MRI-tri-(60)Co teletherapy device. Br J Radiol. 2016;89(1067):20160624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhong H, Chetty IJ. Caution Must Be Exercised When Performing Deformable Dose Accumulation for Tumors Undergoing Mass Changes During Fractionated Radiation Therapy. Int J Radiat Oncol Biol Phys. 2017;97(1):182–3. [DOI] [PubMed] [Google Scholar]
- 32.Mohamed AS, Ruangskul MN, Awan MJ, Baron CA, Kalpathy-Cramer J, Castillo R, et al. Quality assurance assessment of diagnostic and radiation therapy-simulation CT image registration for head and neck radiation therapy: anatomic region of interest-based comparison of rigid and deformable algorithms. Radiology. 2015;274(3):752–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qin A, Ionascu D, Liang J, Han X, O’Connell N, Yan D. The evaluation of a hybrid biomechanical deformable registration method on a multistage physical phantom with reproducible deformation. Radiat Oncol. 2018;13(1):240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qin A, Liang J, Han X, O’Connell N, Yan D. Technical Note: The impact of deformable image registration methods on dose warping. Med Phys. 2018;45(3):1287–94. [DOI] [PubMed] [Google Scholar]
- 35.Moran JM, Molineu A, Kruse JJ, Oldham M, Jeraj R, Galvin JM, et al. Executive summary of AAPM Report Task Group 113: Guidance for the physics aspects of clinical trials. J Appl Clin Med Phys. 2018;19(5):335–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Report 83: Prescribing, Recording and Reporting Proton Beam Therapy. Journal of the International Commission on Radiation Units and Measurements. 2016;10(1):NP–NP. [Google Scholar]
- 37.Report 62: Prescribing, Recording and Reporting Photon Beam Therapy (Supplement to ICRU Report 50). Journal of the International Commission on Radiation Units and Measurements. 1999;os32(1):NP–NP. [Google Scholar]
- 38.van Herk M, Remeijer P, Lebesque JV. Inclusion of geometric uncertainties in treatment plan evaluation. Int J Radiat Oncol Biol Phys. 2002;52(5):1407–22. [DOI] [PubMed] [Google Scholar]
- 39.van Herk M Errors and margins in radiotherapy. Semin Radiat Oncol. 2004;14(1):52–64. [DOI] [PubMed] [Google Scholar]
- 40.Papiez L, Langer M. On probabilistically defined margins in radiation therapy. Phys Med Biol. 2006;51(16):3921–39. [DOI] [PubMed] [Google Scholar]
- 41.Mayo CS, Moran JM, Bosch W, Xiao Y, McNutt T, Popple R, et al. American Association of Physicists in Medicine Task Group 263: Standardizing Nomenclatures in Radiation Oncology. Int J Radiat Oncol Biol Phys. 2018;100(4):1057–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bissonnette JP, Balter PA, Dong L, Langen KM, Lovelock DM, Miften M, et al. Quality assurance for image-guided radiation therapy utilizing CT-based technologies: a report of the AAPM TG-179. Med Phys. 2012;39(4):1946–63. [DOI] [PubMed] [Google Scholar]
- 43.Potters L, Gaspar LE, Kavanagh B, Galvin JM, Hartford AC, Hevezi JM, et al. American Society for Therapeutic Radiology and Oncology (ASTRO) and American College of Radiology (ACR) practice guidelines for image-guided radiation therapy (IGRT). Int J Radiat Oncol Biol Phys. 2010;76(2):319–25. [DOI] [PubMed] [Google Scholar]
- 44.Brock KK, Mutic S, McNutt TR, Li H, Kessler ML. Use of image registration and fusion algorithms and techniques in radiotherapy: Report of the AAPM Radiation Therapy Committee Task Group No. 132. Med Phys 2017;44(7):e43–e76. [DOI] [PubMed] [Google Scholar]
- 45.Allen Li X, Alber M, Deasy JO, Jackson A, Ken Jee KW, Marks LB, et al. The use and QA of biologically related models for treatment planning: short report of the TG-166 of the therapy physics committee of the AAPM. Med Phys. 2012;39(3):1386–409. [DOI] [PubMed] [Google Scholar]
- 46.Bentzen SM. Towards evidence based radiation oncology: improving the design, analysis, and reporting of clinical outcome studies in radiotherapy. Radiother Oncol. 1998;46(1):5–18. [DOI] [PubMed] [Google Scholar]
- 47.Wilkinson MD, Dumontier M, Aalbersberg IJ, Appleton G, Axton M, Baak A, et al. The FAIR Guiding Principles for scientific data management and stewardship. Sci Data. 2016;3:160018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lustberg T, van Soest J, Fick P, Fijten R, Hendriks T, Puts S, et al. Radiation Oncology Terminology Linker: A Step Towards a Linked Data Knowledge Base. Stud Health Technol Inform. 2018;247:855–9. [PubMed] [Google Scholar]
- 49.Meldolesi E, van Soest J, Damiani A, Dekker A, Alitto AR, Campitelli M, et al. Standardized data collection to build prediction models in oncology: a prototype for rectal cancer. Future Oncol. 2016;12(1):119–36. [DOI] [PubMed] [Google Scholar]
- 50.Traverso A, van Soest J, Wee L, Dekker A. The radiation oncology ontology (ROO): Publishing linked data in radiation oncology using semantic web and ontology techniques. Med Phys. 2018;45(10):e854–e62. [DOI] [PubMed] [Google Scholar]
- 51.Bibault JE, Zapletal E, Rance B, Giraud P, Burgun A. Labeling for Big Data in radiation oncology: The Radiation Oncology Structures ontology. PLoS One. 2018;13(1):e0191263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vikram B Perspectives on potential research benefits from big data efforts in Radiation Oncology. Med Phys. 2018;45(10):e848–e9. [DOI] [PubMed] [Google Scholar]
- 53.Sanders JC, Showalter TN. How Big Data, Comparative Effectiveness Research, and Rapid-Learning Health-Care Systems Can Transform Patient Care in Radiation Oncology. Front Oncol. 2018;8:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McNutt TR, Moore KL, Quon H. Needs and Challenges for Big Data in Radiation Oncology. Int J Radiat Oncol Biol Phys. 2016;95(3):909–15. [DOI] [PubMed] [Google Scholar]
- 55.McNutt TR, Benedict SH, Low DA, Moore K, Shpitser I, Jiang W, et al. Using Big Data Analytics to Advance Precision Radiation Oncology. Int J Radiat Oncol Biol Phys. 2018;101(2):285–91. [DOI] [PubMed] [Google Scholar]
- 56.Mayo CS, Phillips M, McNutt TR, Palta J, Dekker A, Miller RC, et al. Treatment data and technical process challenges for practical big data efforts in radiation oncology. Med Phys. 2018;45(10):e793–e810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Matuszak MM, Fuller CD, Yock TI, Hess CB, McNutt T, Jolly S, et al. Performance/outcomes data and physician process challenges for practical big data efforts in radiation oncology. Med Phys. 2018;45(10):e811–e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kerkmeijer LG, Fuller CD, Verkooijen HM, Verheij M, Choudhury A, Harrington KJ, et al. The MRI-Linear Accelerator Consortium: Evidence-Based Clinical Introduction of an Innovation in Radiation Oncology Connecting Researchers, Methodology, Data Collection, Quality Assurance, and Technical Development. Front Oncol. 2016;6:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brock KK, Hollister SJ, Dawson LA, Balter JM. Technical note: creating a four-dimensional model of the liver using finite element analysis. Med Phys. 2002;29(7):1403–5. [DOI] [PubMed] [Google Scholar]
- 60.Brock KK, McShan DL, Ten Haken RK, Hollister SJ, Dawson LA, Balter JM. Inclusion of organ deformation in dose calculations. Med Phys. 2003;30(3):290–5. [DOI] [PubMed] [Google Scholar]
- 61.Park H, Bland PH, Brock KK, Meyer CR. Adaptive registration using local information measures. Med Image Anal. 2004;8(4):465–73. [DOI] [PubMed] [Google Scholar]
- 62.Dawson LA, Eccles C, Bissonnette JP, Brock KK. Accuracy of daily image guidance for hypofractionated liver radiotherapy with active breathing control. Int J Radiat Oncol Biol Phys. 2005;62(4):1247–52. [DOI] [PubMed] [Google Scholar]
- 63.Brock KK, Sharpe MB, Dawson LA, Kim SM, Jaffray DA. Accuracy of finite element model-based multi-organ deformable image registration. Med Phys. 2005;32(6):1647–59. [DOI] [PubMed] [Google Scholar]
- 64.Prior F, Smith K, Sharma A, Kirby J, Tarbox L, Clark K, et al. The public cancer radiology imaging collections of The Cancer Imaging Archive. Sci Data. 2017;4:170124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Prior FW, Clark K, Commean P, Freymann J, Jaffe C, Kirby J, et al. TCIA: An information resource to enable open science. Conf Proc IEEE Eng Med Biol Soc. 2013;2013:1282–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kataria T, Gupta D, Goyal S, Bisht SS, Basu T, Abhishek A, et al. Clinical outcomes of adaptive radiotherapy in head and neck cancers. Br J Radiol. 2016;89(1062):20160085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang H, Hu W, Wang W, Chen P, Ding W, Luo W. Replanning during intensity modulated radiation therapy improved quality of life in patients with nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2013;85(1):e47–54. [DOI] [PubMed] [Google Scholar]
- 68.Chen AM, Daly ME, Cui J, Mathai M, Benedict S, Purdy JA. Clinical outcomes among patients with head and neck cancer treated by intensity-modulated radiotherapy with and without adaptive replanning. Head Neck. 2014;36(11):1541–6. [DOI] [PubMed] [Google Scholar]
- 69.Zhao L, Wan Q, Zhou Y, Deng X, Xie C, Wu S. The role of replanning in fractionated intensity modulated radiotherapy for nasopharyngeal carcinoma. Radiother Oncol. 2011;98(1):23–7. [DOI] [PubMed] [Google Scholar]
- 70.Capelle L, Mackenzie M, Field C, Parliament M, Ghosh S, Scrimger R. Adaptive radiotherapy using helical tomotherapy for head and neck cancer in definitive and postoperative settings: initial results. Clin Oncol (R Coll Radiol). 2012;24(3):208–15. [DOI] [PubMed] [Google Scholar]
- 71.Castelli J, Simon A, Louvel G, Henry O, Chajon E, Nassef M, et al. Impact of head and neck cancer adaptive radiotherapy to spare the parotid glands and decrease the risk of xerostomia. Radiat Oncol. 2015;10:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dewan A, Sharma S, Dewan A, Srivastava H, Rawat S, Kakria A, et al. Impact of Adaptive Radiotherapy on Locally Advanced Head and Neck Cancer - A Dosimetric and Volumetric Study. Asian Pac J Cancer Prev. 2016;17(3):985–92. [DOI] [PubMed] [Google Scholar]
- 73.Duma MN, Kampfer S, Schuster T, Winkler C, Geinitz H. Adaptive radiotherapy for soft tissue changes during helical tomotherapy for head and neck cancer. Strahlenther Onkol. 2012;188(3):243–7. 74. [DOI] [PubMed] [Google Scholar]
- 74.Olteanu LA, Berwouts D, Madani I, De Gersem W, Vercauteren T, Duprez F, et al. Comparative dosimetry of three-phase adaptive and non-adaptive dose-painting IMRT for head-and-neck cancer. Radiother Oncol. 2014;111(3):348–53. [DOI] [PubMed] [Google Scholar]
- 75.Al Mamgani AG Z.A.R. Adaptive Dose-Escalated Multi-modality Image-guided RadiothErapy (ADMIRE) 2018 [Patients with primary head and neck squamous cell carcinoma (HNSCC) planned for treatment with radiotherapy with or without chemotherapy in curative setting will be treated with an adaptive radiotherapy scheme. An FDG-PET/CT scan for re-delineation and re-planning will be made at the end of the second and fourth of week of radiotherapy. The non-responding part of the tumor on FDG-PET will receive a mild dose-escalation. Depending on the metabolic response, the entire tumor will receive 70 Gy or the residual FDG-avid area will receive 4 or 8 Gy.]. Available from: https://clinicaltrials.gov/ct2/show/NCT03376386.
- 76.Berwouts D, Madani I, Duprez F, Olteanu AL, Vercauteren T, Boterberg T, et al. Long-term outcome of (18) F-fluorodeoxyglucose-positron emission tomography-guided dose painting for head and neck cancer: Matched case-control study. Head Neck. 2017;39(11):2264–75. [DOI] [PubMed] [Google Scholar]
- 77.Berwouts D, Olteanu LA, Duprez F, Vercauteren T, De Gersem W, De Neve W, et al. Three-phase adaptive dose-painting-by-numbers for head-and-neck cancer: initial results of the phase I clinical trial. Radiother Oncol. 2013;107(3):310–6. [DOI] [PubMed] [Google Scholar]
- 78.Berwouts D, Olteanu LA, Speleers B, Duprez F, Madani I, Vercauteren T, et al. Intensity modulated arc therapy implementation in a three phase adaptive (18)F-FDG-PET voxel intensity-based planning strategy for head-and-neck cancer. Radiat Oncol. 2016;11:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Duprez F, De Neve W, De Gersem W, Coghe M, Madani I. Adaptive dose painting by numbers for head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2011;80(4):1045–55. [DOI] [PubMed] [Google Scholar]
- 80.Schatteman J, Van Gestel D, Berwouts D, De Gersem W, De Kerf G, De Neve W, et al. A feasibility study on adaptive (18)F-FDG-PET-guided radiotherapy for recurrent and second primary head and neck cancer in the previously irradiated territory. Strahlenther Onkol. 2018;194(8):727–36. 81. [DOI] [PubMed] [Google Scholar]
- 81.Fraass B, Doppke K, Hunt M, Kutcher G, Starkschall G, Stern R, et al. American Association of Physicists in Medicine Radiation Therapy Committee Task Group 53: quality assurance for clinical radiotherapy treatment planning. Med Phys. 1998;25(10):1773–829. [DOI] [PubMed] [Google Scholar]
- 82.Mutic S, Palta JR, Butker EK, Das IJ, Huq MS, Loo LN, et al. Quality assurance for computed-tomography simulators and the computed-tomography-simulation process: report of the AAPM Radiation Therapy Committee Task Group No. 66. Med Phys. 2003;30(10):2762–92. [DOI] [PubMed] [Google Scholar]
- 83.Cardenas CE, Mohamed ASR, Tao R, Wong AJR, Awan MJ, Kuruvila S, et al. Prospective Qualitative and Quantitative Analysis of Real-Time Peer Review Quality Assurance Rounds Incorporating Direct Physical Examination for Head and Neck Cancer Radiation Therapy. Int J Radiat Oncol Biol Phys. 2017;98(3):532–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brouwer CL, Steenbakkers RJ, Bourhis J, Budach W, Grau C, Gregoire V, et al. CT-based delineation of organs at risk in the head and neck region: DAHANCA, EORTC, GORTEC, HKNPCSG, NCIC CTG, NCRI, NRG Oncology and TROG consensus guidelines. Radiother Oncol. 2015;117(1):83–90. [DOI] [PubMed] [Google Scholar]
- 85.Gregoire V, Ang K, Budach W, Grau C, Hamoir M, Langendijk JA, et al. Delineation of the neck node levels for head and neck tumors: a 2013 update. DAHANCA, EORTC, HKNPCSG, NCIC CTG, NCRI, RTOG, TROG consensus guidelines. Radiother Oncol. 2014;110(1):172–81. [DOI] [PubMed] [Google Scholar]
- 86.Gregoire V, Evans M, Le QT, Bourhis J, Budach V, Chen A, et al. Delineation of the primary tumour Clinical Target Volumes (CTV-P) in laryngeal, hypopharyngeal, oropharyngeal and oral cavity squamous cell carcinoma: AIRO, CACA, DAHANCA, EORTC, GEORCC, GORTEC, HKNPCSG, HNCIG, IAGKHT, LPRHHT, NCIC CTG, NCRI, NRG Oncology, PHNS, SBRT, SOMERA, SRO, SSHNO, TROG consensus guidelines. Radiother Oncol. 2018;126(1):3–24. [DOI] [PubMed] [Google Scholar]
- 87.Lee AW, Ng WT, Pan JJ, Poh SS, Ahn YC, AlHussain H, et al. International guideline for the delineation of the clinical target volumes (CTV) for nasopharyngeal carcinoma. Radiother Oncol. 2018;126(1):25–36. [DOI] [PubMed] [Google Scholar]
- 88.Leclerc M, Lartigau E, Lacornerie T, Daisne JF, Kramar A, Gregoire V. Primary tumor delineation based on (18)FDG PET for locally advanced head and neck cancer treated by chemo-radiotherapy. Radiother Oncol. 2015;116(1):87–93. [DOI] [PubMed] [Google Scholar]
- 89.Zhu W, Huang Y, Zeng L, Chen X, Liu Y, Qian Z, et al. AnatomyNet: Deep learning for fast and fully automated whole-volume segmentation of head and neck anatomy. Med Phys. 2018. [DOI] [PubMed] [Google Scholar]
- 90.Zhu M, Bzdusek K, Brink C, Eriksen JG, Hansen O, Jensen HA, et al. Multi-institutional quantitative evaluation and clinical validation of Smart Probabilistic Image Contouring Engine (SPICE) autosegmentation of target structures and normal tissues on computer tomography images in the head and neck, thorax, liver, and male pelvis areas. Int J Radiat Oncol Biol Phys. 2013;87(4):809–16. [DOI] [PubMed] [Google Scholar]
- 91.Yang J, Beadle BM, Garden AS, Schwartz DL, Aristophanous M. A multimodality segmentation framework for automatic target delineation in head and neck radiotherapy. Med Phys. 2015;42(9):5310–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hansch A, Schwier M, Gass T, Morgas T, Haas B, Dicken V, et al. Evaluation of deep learning methods for parotid gland segmentation from CT images. J Med Imaging (Bellingham). 2019;6(1):011005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fritscher KD, Peroni M, Zaffino P, Spadea MF, Schubert R, Sharp G. Automatic segmentation of head and neck CT images for radiotherapy treatment planning using multiple atlases, statistical appearance models, and geodesic active contours. Med Phys. 2014;41(5):051910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Moiseenko V, Wu J, Hovan A, Saleh Z, Apte A, Deasy JO, et al. Treatment planning constraints to avoid xerostomia in head-and-neck radiotherapy: an independent test of QUANTEC criteria using a prospectively collected dataset. Int J Radiat Oncol Biol Phys. 2012;82(3):1108–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lee TF, Fang FM. Quantitative analysis of normal tissue effects in the clinic (QUANTEC) guideline validation using quality of life questionnaire datasets for parotid gland constraints to avoid causing xerostomia during head-and-neck radiotherapy. Radiother Oncol. 2013;106(3):352–8. [DOI] [PubMed] [Google Scholar]
- 96.Lee TF, Chao PJ, Wang HY, Hsu HC, Chang P, Chen WC. Normal tissue complication probability model parameter estimation for xerostomia in head and neck cancer patients based on scintigraphy and quality of life assessments. BMC Cancer. 2012;12:567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gabrys HS, Buettner F, Sterzing F, Hauswald H, Bangert M. Parotid gland mean dose as a xerostomia predictor in low-dose domains. Acta Oncol. 2017;56(9):1197–203. [DOI] [PubMed] [Google Scholar]
- 98.Brodin NP, Tome WA. Revisiting the dose constraints for head and neck OARs in the current era of IMRT. Oral Oncol. 2018;86:8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Brodin NP, Kabarriti R, Garg MK, Guha C, Tome WA. Systematic Review of Normal Tissue Complication Models Relevant to Standard Fractionation Radiation Therapy of the Head and Neck Region Published After the QUANTEC Reports. Int J Radiat Oncol Biol Phys. 2018;100(2):391–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Anderson NJ, Wada M, Schneider-Kolsky M, Rolfo M, Joon DL, Khoo V. Dose-volume response in acute dysphagia toxicity: Validating QUANTEC recommendations into clinical practice for head and neck radiotherapy. Acta Oncol. 2014;53(10):1305–11. [DOI] [PubMed] [Google Scholar]
- 101.Xu CJ, van der Schaaf A, Van’t Veld AA, Langendijk JA, Schilstra C. Statistical validation of normal tissue complication probability models. Int J Radiat Oncol Biol Phys. 2012;84(1):e123–9. [DOI] [PubMed] [Google Scholar]
- 102.Wopken K, Bijl HP, Langendijk JA. Prognostic factors for tube feeding dependence after curative (chemo-) radiation in head and neck cancer: A systematic review of literature. Radiother Oncol. 2018;126(1):56–67. [DOI] [PubMed] [Google Scholar]
- 103.van Dijk LV, Noordzij W, Brouwer CL, Boellaard R, Burgerhof JGM, Langendijk JA, et al. (18)F-FDG PET image biomarkers improve prediction of late radiation-induced xerostomia. Radiother Oncol. 2018;126(1):89–95. [DOI] [PubMed] [Google Scholar]
- 104.van Dijk LV, Brouwer CL, van der Schaaf A, Burgerhof JGM, Beukinga RJ, Langendijk JA, et al. CT image biomarkers to improve patient-specific prediction of radiation-induced xerostomia and sticky saliva. Radiother Oncol. 2017;122(2):185–91. [DOI] [PubMed] [Google Scholar]
- 105.Kierkels RGJ, Wopken K, Visser R, Korevaar EW, van der Schaaf A, Bijl HP, et al. Multivariable normal tissue complication probability model-based treatment plan optimization for grade 2–4 dysphagia and tube feeding dependence in head and neck radiotherapy. Radiother Oncol. 2016;121(3):374–80. [DOI] [PubMed] [Google Scholar]
- 106.Kanayama N, Kierkels RGJ, van der Schaaf A, Steenbakkers R, Yoshioka Y, Nishiyama K, et al. External validation of a multifactorial normal tissue complication probability model for tube feeding dependence at 6months after definitive radiotherapy for head and neck cancer. Radiother Oncol. 2018;129(2):403–8. [DOI] [PubMed] [Google Scholar]
- 107.Christianen ME, Schilstra C, Beetz I, Muijs CT, Chouvalova O, Burlage FR, et al. Predictive modelling for swallowing dysfunction after primary (chemo)radiation: results of a prospective observational study. Radiother Oncol. 2012;105(1):107–14. [DOI] [PubMed] [Google Scholar]
- 108.Beetz I, Schilstra C, van der Schaaf A, van den Heuvel ER, Doornaert P, van Luijk P, et al. NTCP models for patient-rated xerostomia and sticky saliva after treatment with intensity modulated radiotherapy for head and neck cancer: the role of dosimetric and clinical factors. Radiother Oncol. 2012;105(1):101–6. [DOI] [PubMed] [Google Scholar]
- 109.Rwigema JM, Langendijk JA, Paul van der Laan H, Lukens JN, Swisher-McClure SD, Lin A. A model-based approach to predict short-term toxicity benefits with proton therapy for oropharyngeal cancer. Int J Radiat Oncol Biol Phys. 2019. [DOI] [PubMed] [Google Scholar]
- 110.Marks LB, Yorke ED, Jackson A, Ten Haken RK, Constine LS, Eisbruch A, et al. Use of normal tissue complication probability models in the clinic. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S10–9. 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Skorska M, Piotrowski T, Ryczkowski A. Comparison of dose distribution for head and neck cancer patients with and without dose painting escalation during radiotherapy realized with tomotherapy unit. Br J Radiol. 2017;90(1075):20170019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Skjotskift T, Evensen ME, Furre T, Moan JM, Amdal CD, Bogsrud TV, et al. Dose painting for reirradiation of head and neck cancer. Acta Oncol. 2018;57(12):1693–9. [DOI] [PubMed] [Google Scholar]
- 113. Rasmussen JH, Hakansson K, Vogelius IR, Aznar MC, Fischer BM, Friborg J, et al. Phase I trial of 18F-Fludeoxyglucose based radiation dose painting with concomitant cisplatin in head and neck cancer. Radiother Oncol. 2016;120(1):76–80. [DOI] [PubMed] [Google Scholar]
- 114. Differding S, Sterpin E, Hermand N, Vanstraelen B, Nuyts S, de Patoul N, et al. Radiation dose escalation based on FDG-PET driven dose painting by numbers in oropharyngeal squamous cell carcinoma: a dosimetric comparison between TomoTherapy-HA and RapidArc. Radiat Oncol. 2017;12(1):59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Barragan AM, Differding S, Janssens G, Lee JA, Sterpin E. Feasibility and robustness of dose painting by numbers in proton therapy with contour-driven plan optimization. Med Phys. 2015;42(4):2006–17. [DOI] [PubMed] [Google Scholar]
- 116.Richter A, Weick S, Krieger T, Exner F, Kellner S, Polat B, et al. Evaluation of a software module for adaptive treatment planning and re-irradiation. Radiat Oncol. 2017;12(1):205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Aly F, Miller AA, Jameson MG, Metcalfe PE. A prospective study of weekly intensity modulated radiation therapy plan adaptation for head and neck cancer: improved target coverage and organ at risk sparing. Australas Phys Eng Sci Med. 2018. [DOI] [PubMed] [Google Scholar]
- 118.Brown E, Owen R, Harden F, Mengersen K, Oestreich K, Houghton W, et al. Head and neck adaptive radiotherapy: Predicting the time to replan. Asia Pac J Clin Oncol. 2016;12(4):460–7. 119. [DOI] [PubMed] [Google Scholar]
- 119.Fiorino C, Maggiulli E, Broggi S, Liberini S, Cattaneo GM, Dell’oca I, et al. Introducing the Jacobian-volume-histogram of deforming organs: application to parotid shrinkage evaluation. Phys Med Biol. 2011;56(11):3301–12. [DOI] [PubMed] [Google Scholar]
- 120.Kashani R, Hub M, Balter JM, Kessler ML, Dong L, Zhang L, et al. Objective assessment of deformable image registration in radiotherapy: a multi-institution study. Med Phys. 2008;35(12):5944–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Liao YL, Chen HB, Zhou LH, Zhen X. Construction of an anthropopathic abdominal phantom for accuracy validation of deformable image registration. Technol Health Care. 2016;24 Suppl 2:S717–23. 122. [DOI] [PubMed] [Google Scholar]
- 122.Pukala J, Johnson PB, Shah AP, Langen KM, Bova FJ, Staton RJ, et al. Benchmarking of five commercial deformable image registration algorithms for head and neck patients. J Appl Clin Med Phys. 2016;17(3):25–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Singhrao K, Kirby N, Pouliot J. A three-dimensional head-and-neck phantom for validation of multimodality deformable image registration for adaptive radiotherapy. Med Phys. 2014;41(12):121709. [DOI] [PubMed] [Google Scholar]
- 124.Stanley N, Glide-Hurst C, Kim J, Adams J, Li S, Wen N, et al. Using patient-specific phantoms to evaluate deformable image registration algorithms for adaptive radiation therapy. J Appl Clin Med Phys. 2013;14(6):4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Varadhan R, Karangelis G, Krishnan K, Hui S. A framework for deformable image registration validation in radiotherapy clinical applications. J Appl Clin Med Phys. 2013;14(1):4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yang D, Zhang M, Chang X, Fu Y, Liu S, Li HH, et al. A method to detect landmark pairs accurately between intra-patient volumetric medical images. Med Phys. 2017;44(11):5859–72. [DOI] [PubMed] [Google Scholar]
- 127.Kim H, Park SB, Monroe JI, Traughber BJ, Zheng Y, Lo SS, et al. Quantitative Analysis Tools and Digital Phantoms for Deformable Image Registration Quality Assurance. Technol Cancer Res Treat. 2015;14(4):428–39. [DOI] [PubMed] [Google Scholar]
- 128.Ger RB, Yang J, Ding Y, Jacobsen MC, Fuller CD, Howell RM, et al. Accuracy of deformable image registration on magnetic resonance images in digital and physical phantoms. Med Phys. 2017;44(10):5153–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Huq MS, Fraass BA, Dunscombe PB, Gibbons JP Jr., Ibbott GS, Mundt AJ, et al. The report of Task Group 100 of the AAPM: Application of risk analysis methods to radiation therapy quality management. Med Phys. 2016;43(7):4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jaffray DA, Lindsay PE, Brock KK, Deasy JO, Tome WA. Accurate accumulation of dose for improved understanding of radiation effects in normal tissue. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S135–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lee L, Mao W, Xing L. The use of EPID-measured leaf sequence files for IMRT dose reconstruction in adaptive radiation therapy. Med Phys. 2008;35(11):5019–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Due AK, Vogelius IR, Aznar MC, Bentzen SM, Berthelsen AK, Korreman SS, et al. Recurrences after intensity modulated radiotherapy for head and neck squamous cell carcinoma more likely to originate from regions with high baseline [18F]-FDG uptake. Radiother Oncol. 2014;111(3):360–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Shakam A, Scrimger R, Liu D, Mohamed M, Parliament M, Field GC, et al. Dose-volume analysis of locoregional recurrences in head and neck IMRT, as determined by deformable registration: a prospective multi-institutional trial. Radiother Oncol. 2011;99(2):101–7. [DOI] [PubMed] [Google Scholar]
- 134.Mohamed ASR, Wong AJ, Fuller CD, Kamal M, Gunn GB, Phan J, et al. Patterns of locoregional failure following post-operative intensity-modulated radiotherapy to oral cavity cancer: quantitative spatial and dosimetric analysis using a deformable image registration workflow. Radiat Oncol. 2017;12(1):129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mohamed ASR, Cardenas CE, Garden AS, Awan MJ, Rock CD, Westergaard SA, et al. Patterns-of-failure guided biological target volume definition for head and neck cancer patients: FDG-PET and dosimetric analysis of dose escalation candidate subregions. Radiother Oncol. 2017;124(2):248–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gunn GB, Blanchard P, Garden AS, Zhu XR, Fuller CD, Mohamed AS, et al. Clinical Outcomes and Patterns of Disease Recurrence After Intensity Modulated Proton Therapy for Oropharyngeal Squamous Carcinoma. Int J Radiat Oncol Biol Phys. 2016;95(1):360–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Due AK, Vogelius IR, Aznar MC, Bentzen SM, Berthelsen AK, Korreman SS, et al. Methods for estimating the site of origin of locoregional recurrence in head and neck squamous cell carcinoma. Strahlenther Onkol. 2012;188(8):671–6. [DOI] [PubMed] [Google Scholar]
- 138.Mohamed AS, Rosenthal DI, Awan MJ, Garden AS, Kocak-Uzel E, Belal AM, et al. Methodology for analysis and reporting patterns of failure in the Era of IMRT: head and neck cancer applications. Radiat Oncol. 2016;11(1):95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wilkinson MD, Sansone SA, Schultes E, Doorn P, Bonino da Silva Santos LO, Dumontier M. A design framework and exemplar metrics for FAIRness. Sci Data. 2018;5:180118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.van Herk M Integration of a clinical trial database with a PACS. J Phys Conf Ser. 2014;489. [Google Scholar]
- 141.Haak D, Page CE, Reinartz S, Kruger T, Deserno TM. DICOM for Clinical Research: PACS-Integrated Electronic Data Capture in Multi-Center Trials. J Digit Imaging. 2015;28(5):558–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kalpathy-Cramer J, Freymann JB, Kirby JS, Kinahan PE, Prior FW. Quantitative Imaging Network: Data Sharing and Competitive AlgorithmValidation Leveraging The Cancer Imaging Archive. Transl Oncol. 2014;7(1):147–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Fedorov A, Clunie D, Ulrich E, Bauer C, Wahle A, Brown B, et al. DICOM for quantitative imaging biomarker development: a standards based approach to sharing clinical data and structured PET/CT analysis results in head and neck cancer research. PeerJ. 2016;4:e2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Meadows A, Haak L. How persistent identifiers can save scientists time. FEMS Microbiol Lett. 2018;365(15). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Pan Q NBIA Data Retriever 4+: A Data Retriever for NBIA/TCIA [ [Google Scholar]
- 146.Law MY, Liu B, Chan LW. Informatics in radiology: DICOM-RT-based electronic patient record information system for radiation therapy. Radiographics. 2009;29(4):961–72. [DOI] [PubMed] [Google Scholar]
- 147.Law MY, Liu B. Informatics in radiology: DICOM-RT and its utilization in radiation therapy. Radiographics. 2009;29(3):655–67. [DOI] [PubMed] [Google Scholar]
- 148.Cousijn H, Kenall A, Ganley E, Harrison M, Kernohan D, Lemberger T, et al. A data citation roadmap for scientific publishers. Sci Data. 2018;5:180259. [DOI] [PMC free article] [PubMed] [Google Scholar]