Abstract
Purpose: Gene therapies via Noggin small interfering (si)RNA (siNoggin) and bone morphogenetic protein (BMP)-2 plasmid DNA (pBMP-2) may be promising strategies for bone repair/regeneration, but their ideal delivery vectors, efficacy difference, and underlying mechanisms have not been explored, so these issues were probed here.
Methods: This study used lipopolysaccharide-amine nanopolymersomes (LNPs), an efficient cytosolic delivery vector developed by the research team, to mediate siNoggin and pBMP-2 to transfect MC3T3-E1 cells, respectively. The cytotoxicity, cell uptake, and gene knockdown efficiency of siNoggin-loaded LNPs (LNPs/siNoggin) were studied, then the osteogenic-differentiation efficacy of MC3T3-E1 cells treated by LNPs/pBMP-2 and LNPs/siNoggin, respectively, were compared by measuring the expression of osteogenesis-related genes and proteins, alkaline phosphatase (ALP) activity, and mineralization of the extracellular matrix at all osteogenic stages. Finally, the possible signaling pathways of the two treatments were explored.
Results: LNPs delivered siNoggin into cells efficiently to silence 50% of Noggin expression without obvious cytotoxicity. LNPs/siNoggin and LNPs/pBMP-2 enhanced the osteogenic differentiation of MC3T3 E1 cells, but LNPs/siNoggin was better than LNPs/pBMP-2. BMP/Mothers against decapentaplegic homolog (Smad) and glycogen synthase kinase (GSK)-3β/β-catenin signaling pathways appeared to be involved in osteogenic differentiation induced by LNPs/siNoggin, but GSK-3β/β-catenin was not stimulated upon LNPs/pBMP-2 treatment.
Conclusion: LNPs are safe and efficient delivery vectors for DNA and RNA, which may find wide applications in gene therapy. siNoggin treatment may be a more efficient strategy to enhance osteogenic differentiation than pBMP-2 treatment. LNPs loaded with siNoggin and/or pBMP-2 may provide new opportunities for the repair and regeneration of bone.
Keywords: gene delivery, nanopolymersomes, Noggin, small interfering RNA, bone morphogenetic proteins, osteogenesis
Introduction
Bone defects caused by trauma, tumor, inflammation, or other diseases are problems worldwide. They bring about not only suffering and inconvenience to patients, but also constitute a heavy financial burden to patients and society due to the huge expenditures from direct costs (medical treatments) and indirect costs (loss of productivity).1,2
For example, after blood, bone is the second most common type of transplanted tissue. In the US, about 0.5–1.5 million cases of bone grafting per year are done, leading to a market for bone grafting of $1.6–$2.5 billion.1 In Canada, the mean direct costs for established long-bone non-unions have been calculated to be CN$11,800, and the indirect costs for a tibia fracture to be 67–79% of total costs.2
Bone tissue has intrinsic regenerative capacity, but satisfactory restoration in structure and function as well as the recovery time remain challenges due to the limited natural regeneration ability of bone, especially in patients with large defects, comorbidity, or biomechanical instability.1,3 Therefore, development of intervention strategies to promote the repair and regeneration of bone in terms of quality and speed is important.
Intervention strategies such as bone grafting as well as use of osteoinductive/osteoconductive matrices, stem cells, signaling molecules, and genes, alone or in combination, have been developed, and much progress has been made.1,3–8 Among these strategies, gene-based therapies via delivery of osteogenesis-related nucleic acids (eg, DNA, small interfering (si)RNA into target cells have been demonstrated to be promising due to their high specificity and low toxicity.1,5,6,8,9
Bone morphogenetic protein (BMP) signaling has a pivotal role during osteogenesis. Increased expression of BMPs can enhance bone regeneration, which can be realized by “turning on” activator genes via DNA transfection, or by “turning off” inhibitor genes via siRNA transfection. Several genes can regulate BMP signaling, but BMP-2 has been used extensively due to its potent osteoinductive ability, and it is one of two proteins (the other one is BMP-7) approved for clinical use in bone defects in the US. Accordingly, Noggin (an antagonist of BMP), which can bind and inactivate BMP-2, -4, -5, -7, -13, and -14, has attracted much attention.10–12 Upregulating expression of BMP-2 or downregulating expression of Noggin alone or in combination can promote osteogenic differentiation of several cell types and bone-tissue formation in vitro and in vivo.11–19 However, the difference in efficacy and the underlying molecular mechanisms between treatment of BMP-2 and Noggin siRNA mediated by the same vector has not been reported.
Gene vectors are important factors for successful gene therapy. “Naked” genes must be transported to their action sites in cells by viral or nonviral vectors due to their nature, such as negative charge, susceptibility to degradation, and large size. Usually, nonviral vectors have superior safety and lower cost, but limited transfection efficiency compared with those of viral vectors. To improve the transfection efficiency of nonviral vectors, numerous strategies have been developed to modify vectors to overcome the barriers in gene delivery, as reviewed thoroughly by Zhou et al.20 However, the “ideal” vector has yet to be identified, and exploring safe and efficacious systems is a major concern for gene therapies for osteogenesis.
“Polymersomes” are vesicles self-assembled from amphiphilic copolymers. They consist of an aqueous core and enclosed hydrophobic membranes surrounded by hydrophilic coronas. As nonviral vectors, polymersomes have attracted considerable attention due to their controllable structure, nature (size, degradability, stability, and tailor-made surface chemistry for target delivery), and ability to load hydrophilic, hydrophobic, or amphiphilic compounds alone or in combination.21,22
Zhong and colleagues synthesized chimeric polymersomes composed of polyethylene glycol (PEG), P(TMC-DTC) and polyethylenimine (PEI) blocks, and then decorated them with different peptides targeting brain and tumor cells, respectively.23,24When using these functionalized polymersomes as vectors for anti-polo-like kinase 1 siRNA, they showed excellent packaging and protection of siRNA in their lumen while releasing “payloads” in a cytoplasmic reductive environment quickly. Such siRNA-loaded polymersomes could significantly boost targeted siRNA therapy against human lung cancer and glioblastoma in nude mice by prolonging the circulation time of siRNA, enhancing siRNA accumulation in cancer cells, silencing target genes, and suppressing the corresponding protein expression. Ge et al25 used a PEG-PCL-DEX polymersome–protamine vector to mediate siRNA to transfect SMMC-7721 cells, and expression of the target gene could be reduced to 61.73%±6.25%.
Our research team has developed a nonviral vector of lipopolysaccharideamine nanopolymersomes (LNPs) for gene delivery.26–29 LNPs are prepared from a synthesized water soluble and degradable three-block-graft copolymer containing oxidized sodium alginate (OA; which forms the backbone), and cholesteryl-graft-polyethylenimine (Cho-PEI; 1.8 kDa of MnPEI; which forms the side chains). We have demonstrated that LNPs have low cytotoxicity, degradability, excellent abilities to enter cells, and to escape from lysosomes, as well as high stability against dilution, pH, heparin, salts, and serum.29 LNPs have transfection efficiency >95% when delivering plasmids encoding enhanced green flurescent protein (pEGFP) into mesenchymal stem cells (MSCs)26 and induce significant angiogenesis in zebrafish when delivering plasmids encoding vascular endothelial growth factor (pVEGF).28 When using LNPs to deliver pBMP-2 into MSCs, expression of BMP-2 protein in MSCs can be enhanced.27
Based on the data mentioned above, to explore whether LNPs are good candidate vectors for siRNA delivery, we evaluated the knockdown efficiency of Noggin siRNA (siNoggin) mediated by LNPs. Meanwhile, we compared the osteogenic differentiation between LNPs/pBMP-2 and LNPs/siNoggin, and then investigated the underlying molecular mechanisms. In this way, we hope that greater understanding of bone repair via siRNA or pDNA (or both) can be obtained and, thus, more choices provided for clinical treatment.
Materials and methods
Materials
LNPs were synthesized following our established method.26 siNoggin (catalog numbers Line 1-10620318 and Line 2-10620319), Alexa Fluor®555 siRNA, Stealth™ RNAi Negative Control Duplexes (ctrRNA), lipofectamine3000 (lipo) (catalog number L3000015), Opti-MEM™ I Reduced Serum Media, trypsin, TRIzol® Reagent, α-minimum essential medium (MEM), fetal bovine serum (FBS) and Penicillin/Streptomycin were purchased from Thermo Scientific (Waltham, MA, USA). pBMP-2 (vector ID: VB160930-1048bkg), osteogenic medium, and Alizarin Red were obtained from Cyagen Biosciences. (Guangzhou, China). Cell Counting Kit-8 (CCK-8) was supplied by Dojindo (Tokyo, Japan). A bicinchoninic acid (BCA) assay kit was ordered from CWBIO (Beijing, China). An alkaline phosphatase (ALP) kit was supplied by Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Cetylpyridinum chloride was purchased from Sigma–Aldrich (Saint Louis, MO, USA). A Prime–Script™ Real Time (RT) reverse transcription kit was obtained from Takara Biotechnology (Shiga, Japan). LightCycler®480 SYBR® Green I Master was supplied by Roche Molecular Systems (Basel, Switzerland).
Antibodies against mouse Noggin were purchased from Novus Biologicals (Centennial, CO, USA). Antibodies against mouse BMP-2, osteopontin (OPN), and Mothers against decapentaplegic homolog (Smad)/1/5/9 were supplied by Abcam (Cambridge, UK). Antibodies against mouse β-catenin, Runt-related transcription factor (Runx)2, phosphorylated (p)-Smad/1/5/9, glycogen synthase kinase (GSK)-3β, p-GSK-3β(Ser9), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were obtained from Cell Signaling Technology (Danvers, MA, USA). The secondary antibody was anti-rabbit immunoglobulin G, horseradish peroxidase-linked antibody (catalog number 7074S), which was purchased from Cell Signaling Technology. The MC3T3-E1 cell line was obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China) (catalog number CRL-2593). Detailed information on other reagents can be found in the Supplementary Materials.
Complexation of siRNA or pDNA with vectors
First, stock solutions of LNPs (0.67 mg/mL) and siRNA (20 µM) in nuclease-free sterile water were prepared and stored at 4°C until further use. Before use, the two stock solutions were diluted separately with culture medium to a certain concentration. Then, equal volumes of the two diluted solutions were mixed thoroughly and incubated at room temperature for 10 minutes to allow formation of LNPs/siNoggin complexes. Complexes of LNPs/ctrRNA, which were used as controls to clarify the Noggin-targeted specificity of siNoggin, were prepared by the same method. Likewise, complexes of lipo/siNoggin and lipo/ctrRNA were prepared, whereby the lipo concentration followed manufacturer suggestions. In addition, 50 nM of siRNA (final concentration in the culture medium) was used for cell transfection according to manufacturer instructions and our preliminary results (data not shown). Complexes of LNPs/pBMP-2 (molar ratio of the amino groups in LNPs to the phosphate groups in pBMP-2 was 60) with optimal transfection were prepared according to a method reported by our research team.26,27 All complex solutions were used immediately after preparation.
Cell culture and osteoblast differentiation
MC3T3-E1 cells were cultured in basal growth medium (α-MEM, 10% FBS, 1% Penicillin/Streptomycin) at 37°C with 5% carbon dioxide. To induce osteogenic differentiation, cells were first cultured in basal growth medium until 60–70% confluence, and then transferred to osteogenic medium containing 0.1 µM of dexamethasone, 50 µg/mL of ascorbic acid, and 10 mM of β-glycerol phosphate.
Cytotoxicity
Toxicity of LNPs to MC3T3-E1 cells was evaluated using a CCK-8 kit, and lipo was used as a control. Briefly, cells were seeded in 96-well plates (104 cells/well). After overnight incubation, medium was replaced by 100 µL of fresh basal growth medium with different concentrations of LNPs and lipo. According to our preliminary data, optimal transfections could be achieved for LNPs/siNoggin with 3.35 µg/mL of LNPs and 50 nM of siRNA and for lipo/siNoggin with 2.5 µL/mL of lipo and 50 nM of siRNA), so the concentrations of LNPs were set as 0, 1.675, 3.35, 5.025, 6.70, 10.05, and 13.40 µg/mL, and the concentrations of lipo were set as 1.25, 2.50, 3.75, 5.00, 6.25, and 7.50 µL/mL, respectively. After 4 hours of incubation, the medium was replaced with fresh growth medium. Cells were cultured continuously for 48 hours, then 10 µL CCK-8 was added to each well for an additional 2 hours of incubation, and their absorbance was measured at 450 nm using a microplate reader (Infinite200; Tecan, Männedorf, Switzerland).
Cell uptake of siRNA
In this experiment, Alexa Fluor 555-labeled siRNA was used to prepare complexes of LNPs/siRNA and lipo/siRNA. The efficiency of cell uptake efficiency was defined as the percentage of cells with red fluorescence. MC3T3-E1 cells were seeded in six-well plates (106 cells/well) and cultured until 60% confluence. Complexes of vector/siRNA with different concentrations were added to culture medium, and cells were incubated for 4 hours. Then, medium was replaced with new growth medium, and cells were cultured continuously for 24 hours. Thereafter, some cells were observed under an automatic inverted fluorescence microscope (DMI8; Leica, Wetzlar, Germany), and the other cells were cultured continuously and harvested at 48 hours for measurement of cell-uptake efficiency with a flow cytometer (FC500MPL; Beckman Coulter, Fullerton, CA, USA), as described previously.26 Combining the results from cytotoxicity studies and this experiment, the final concentration of vectors in the culture medium for optimal transfection (maximal efficiency of cells with minimal cytotoxicity) were determined to be 3.35 μg/mL LNPs for LNPs/siRNA and 2.50 µL/mL lipo for lipo/siRNA, respectively, and they were used for subsequent experiments unless specified otherwise.
Cell proliferation assay
The effects of transfection upon cell proliferation were determined using a CCK-8 kit as described for the cytotoxicity test with some changes. That is, in the cell-proliferation test, cells were treated with LNPs/siNoggin instead of LNPs, and MC3T3-E1 cells were cultured continuously for 7 days after transfection, the absorbance of which was measured on days 1, 3, 5, and 7, respectively.
ALP activity in transfected cells
MC3T3-E1 cells were seeded in 6-well plates (1×106 cells/well) and cultured until 60% confluence. Then cells were treated with LNPs/siNoggin for 4 hours, followed by incubation in osteogenic medium for 7 days or 14 days. During this period, osteogenic medium was exchanged every 3 days. At predetermined time points, cells were lysed for assays of total-protein concentration and ALP activity. The protein concentration was detected by a BCA assay kit following manufacturer protocols. ALP activity was measured by an ALP kit according to manufacturer instructions.
Mineralization of the extracellular matrix (ECM)
Mineralization in MC3T3-E1 cells was determined by Alizarin Red staining.30 MC3T3-E1 cells were cultured and treated as described for the ALP-activity test. After 28 days of culture in osteogenic medium, cells were washed with phosphate-buffered saline (PBS), fixed in 4% paraformaldehyde for 30 minutes, rinsed with PBS, and stained in 2% Alizarin Red solution for 15 minutes at room temperature, followed by thorough washing with PBS. Thereafter, some stained cells were observed under an inverted microscope (Axio Observer Z1; Zeiss, Oberkochen, Germany) to evaluate the formation of calcified nodules. To quantify mineralization, the other stained cells were incubated in 10% cetylpyridinum chloride for 30 minutes to dissolve calcified nodules, and their absorbance at 562 nm was measured.
Real-time polymerase chain reaction (RT-PCR) quantification of Noggin mRNA and Runx2 mRNA
MC3T3-E1 cells were cultured and treated as described for the ALP-activity test. We undertook RT-PCR according to a reported method.6,8,30 Briefly, after culture in osteogenic medium for 2, 7, and 14 days, total RNA in cells was extracted using TRIzol Reagent. Then, 0.5 µg of total RNA was reverse-transcribed to cDNA using a Prime-Script RT kit according to manufacturer instructions. RT-PCR was carried out a RT-PCR instrument (LightCycler 480 SYBR Green I Master). Expression of target genes was calculated by the 2−Ct△△ method using expression of the housekeeping gene (GAPDH) as a control. The target primer sequences are listed in the Supplementary Materials.
Western blotting
Western blotting was done using a standard method. Briefly, MC3T3-E1 cells were cultured and treated as described above in the ALP-activity test. After culture in osteogenic medium for a predetermined time, cells were lysed for extraction of total protein. The concentration of total protein was determined with a BCA assay kit according to supplier protocols. Then, the proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis using 8% gels and transferred to polyvinylidene difluoride (PVDF) membranes. After blockade, PVDF membranes were incubated overnight using antibodies against mouse Noggin, BMP-2, OPN, β-catenin, Runx2, Smad/1/5/9, p-Smad/1/5/9, GSK-3β, p-GSK-3β(Ser9), and GAPDH. All antibodies were diluted to 1:1,000. Finally, the PVDF membranes were incubated with secondary antibody (1:2,000 dilution) for 1 hour at room temperature. Western-blotting signals were detected using ECL Plus (Millipore, Billerica, MA, USA).
Statistical analyses
Data are the mean±standard deviation (n=3). Statistical tests were undertaken using one-way analysis of variance by Prism 6.0 (GraphPad, La Jolla, CA, USA) or SPSS v-20.0 (IBM, Armonk, NY, USA). P<0.05 was considered significant.
Results
Toxicity of lnps to MC3T3-E1 cells
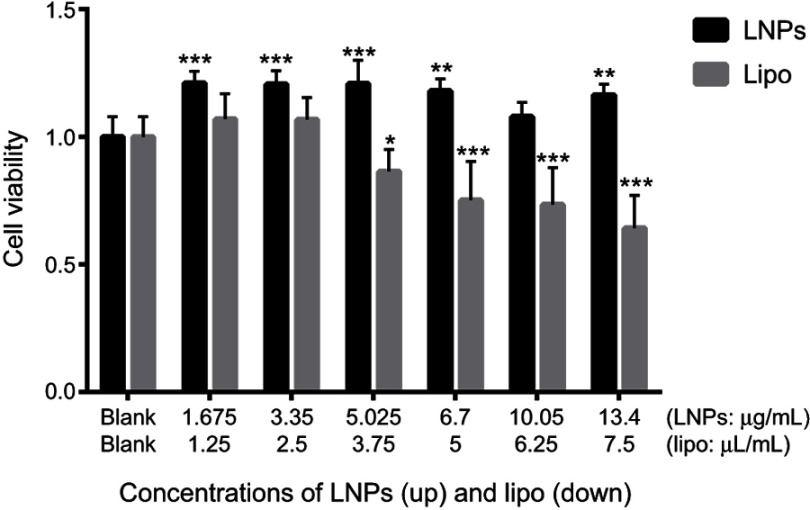
Safety is the most important parameter for a gene-delivery vector, and cytotoxicity is the basic mode of evaluation. The cytotoxicity of LNPs was measured by cell viability using the CCK-8 assay. Lipo3000, a commercially available transfection reagent, was used as a control, and MC3T3-E1 cells were used as “model” cells. In the concentration range tested, 48 hours after transfection, in the LNPs group, cell viability was ~20% higher than that in untreated cells (Figure 1), which could be ascribed to the rapid proliferation of untreated MC3T3-E1 cells. At 48 hours after transfection (at ~60 hours after seeding), untreated cells were crowded, and their metabolic activity decreased due to contact inhibition; whereas, after adaptation to transfection, the transfected cells had high metabolic activity. In the lipo group, cell viability was comparable with that of untreated cells at ≤2.5 µL/mL, but further increases in concentration (≥3.75 µL/mL) decreased cell viability. Cells treated with 3.35 µg/mL of LNPs (the concentration for optimal transfection) showed high viability, which was slightly higher than that for cells treated by 2.5 µL/mL of lipo, but the difference was not significant. The low cytotoxicity of LNPs was consistent with previous data from our research team, in which the viability of transfected MSCs was ~88% at 25 μg/mL of LNPs for 48 hours of incubation.26 However, in the present study, we used a lower concentration (the upper concentration of LNPs was 13.4 μg/mL) and, thus, cells showed higher viability.
Figure 1.
Cell viability 48 hours after transfection.
Notes: *P<0.05, **P<0.01 and ***P<0.001 vs the blank group. The error bars represent the mean±SD (n=3).
Abbreviations: LNPs, lipopolysaccharide-amine nanopolymersomes; lipo, lipofectamine3000.
Cell uptake
siRNAs must enter cells to exert their functions. Therefore, we measured the cellular uptake of LNPs/siNoggin labeled by Alexa Fluor 555 by fluorescence and flow cytometry. When the concentration of LNPs was ≤5.025 μg/mL, cells treated by LNPs/siNoggin emitted strong red fluorescence 24 hours after transfection (Figure 2A), suggesting successful uptake of LNPs/siNoggin. In addition, cells (bright-field images) exhibited a fusiform appearance with ~95% confluence, which was similar to that of blank (untreated) cells, indicating that the cells were healthy. These data further confirmed the low cytotoxicity of LNPs because cell morphology is a visual indicator of cytotoxicity. Upon increasing the concentration of LNPs to 6.7 μg/mL, strong red fluorescence remained, but cells shrank and decreased in number, and a few extracellular fragments could be seen, suggesting that use of this concentration may be slightly harmful to cells. When the concentration of lipo was ≤2.5 µL/mL, cells treated by lipo/siNoggin were healthy, with ~50% confluence (Figure 2B), which suggested the low toxicity of reagents. Also, the distribution of red fluorescence became denser with an increase in concentration, suggesting an increase in the cell-uptake efficiency of lipo/siNoggin; when the concentration of lipo increased to 3.75 µL/mL, the health of cells deteriorated obviously.
Figure 2.
(A and B) Images of MC3T3-E1 cells treated by different concentrations of LNPs/siRNA or lipo/siRNA 24 hours after transfection treatment. (C) Efficiency of cell uptake quantified by flow cytometry 48 hours after transfection.
Abbreviations: LNPs, lipopolysaccharide-amine nanopolymersomes; lipo, lipofectamine3000.
Figure 2C shows the cell uptake efficiency of LNPs/siNoggin and lipo/siNoggin 48 hours after transfection. The maximal cell-uptake efficiency was 97.8% for LNPs/siNoggin at 3.35 μg/mL of LNPs, and 74.6% for lipo/siNoggin at 2.5 µL/mL of lipo. From the results of cytotoxicity and cell uptake, we concluded that 3.35 μg/mL of LNPs for LNPs/siNoggin and 2.5 µL/mL of lipo for LNPs/siNoggin were the concentrations for optimal transfection (the concentration at which the gene-delivery systems showed maximal efficiency of cell uptake with minimal cytotoxicity).
Knockdown of Noggin by LNPs/siNoggin
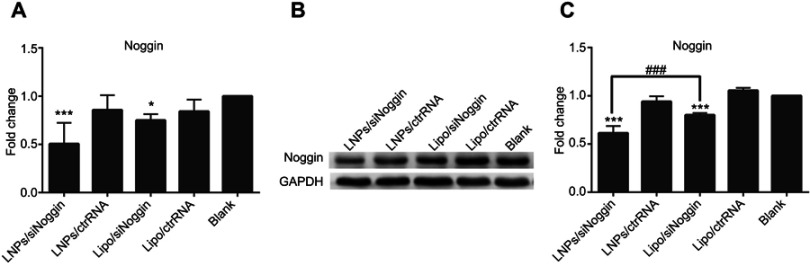
To evaluate gene-knockdown efficiency via LNPs/siNoggin, Noggin expression in transfected MC3T3-E1 cells was tested by RT-PCR (Figure 3A). To clarify the specificity of siRNA on target genes, we undertook transfection using Noggin-targeted siRNA (siNoggin) and non-targeted siRNA (ctrRNA). siNoggin treatment led to much lower expression of Noggin and Noggin protein in cells than ctrRNA treatment using the same vector. For example, compared with LNPs/ctrRNA treatment, LNPs/siNoggin treatment decreased expression of Noggin and Noggin protein by 40% and 35%, respectively. Compared with the blank, 2 days after transfection, the LNPs/siNoggin group had a knockdown efficiency for Noggin of 50%, but that of lipo/siNoggin was 25%. Then, to further verify gene suppression, expression of Noggin protein in transfected MC3T3-E1 cells was detected by Western blotting 3 days after transfection (Figures 3B and C). Expression of Noggin protein decreased by 40% in the LNPs/siNoggin group and 20% in the lipo/siNoggin group. These results of Noggin-protein expression were consistent with Noggin knockdown, and showed that LNPs: could deliver siNoggin into cells to suppress noggin expression; were a more efficient gene delivery vector than lipo; and could induce two-fold higher gene-knockdown efficiency than that induced by lipo.
Figure 3.
Expression of Noggin and Noggin protein in MC3T3-E1 cells transfected with different siRNA delivery systems. (A) Expression of Noggin on day-2 (PCR data). (B) Expression of Noggin protein on day-3 (data from Western blotting). (C) Semiquantitation of Noggin protein from Western blotting images analyzed by ImageJ software.
Notes: *P<0.05 and ***P<0.001 vs the blank group. ###P<0.001. The error bars represent the mean±SD (n=3).
Abbreviations: LNPs, lipopolysaccharide-amine nanopolymersomes; lipo, lipofectamine3000.
Effects of transfection via different delivery systems upon cell proliferation
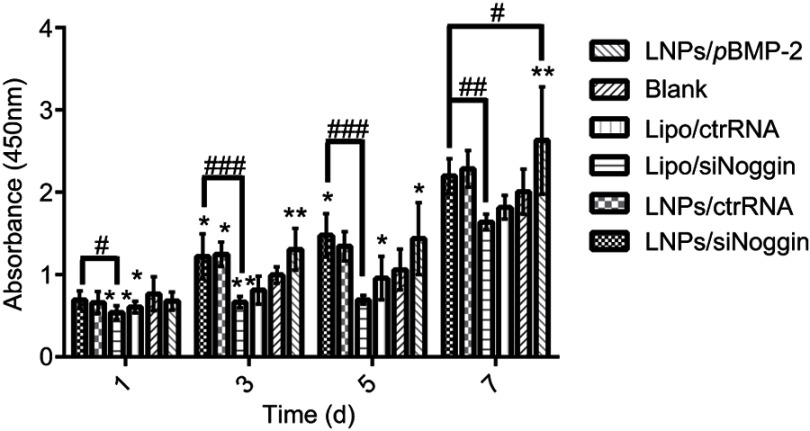
The effects of transfection via LNPs/siNoggin and LNPs/pBMP-2 upon cell proliferation were examined using the CCK-8 assay. During days 1–7 of culture after transfection, MC3T3-E1 cells proliferated with time in all groups (Figure 4). This finding is consistent with the reported developmental sequence of MC3T3-E1 cells, which replicate actively during the initial developmental phase (days 1–9 of culture), as evidenced by a progressive increase in cell number.31 As expected, cells treated by LNPs/(ctrRNA or siNoggin or pBMP-2) exhibited higher proliferation than cells untreated or treated by lipo/(ctrRNA or siNoggin), indicating that LNPs/(siNoggin or pBMP-2) could enhance proliferation of MC3T3-E1 cells. On day-7, the proliferation in LNPs/pBMP-2 is higher than that in LNPs/siNoggin, whereas no obvious difference was observed between them before day 7. Based on the results from experiments on the viability, morphology, and proliferation of cells, we concluded that LNPs and LNPs/(siNoggin or pBMP-2) did not show obvious toxicity, and could enhance proliferation within 7 days.
Figure 4.
Effects of transfection via different systems on proliferation of MC3T3-E1 cells on days 1, 3, 5, and 7. The error bars represent the mean±SD (n=3).
Notes: *P<0.05 and **P<0.01 vs the blank group. #P<0.05, ##P<0.01 and ###P<0.001. The error bars represent the mean±SD (n=3).
Abbreviations: LNPs, lipopolysaccharide-amine nanopolymersomes; lipo, lipofectamine3000.
ALP activity of MC3T3-E1 cells treated by LNPs/siNoggin
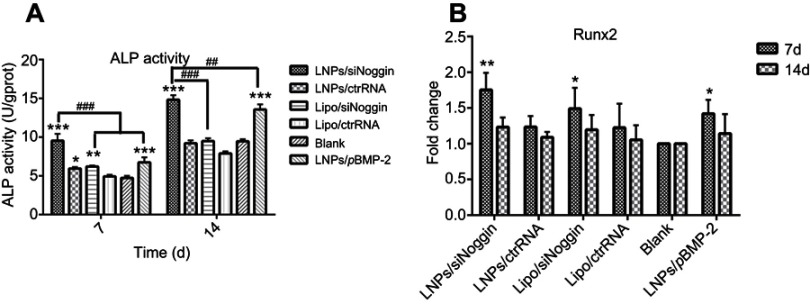
ALP activity was measured to assess the effects of LNPs/siNoggin treatment on osteogenesis of MC3T3-E1 cells (Figure 5A). ALP activity increased with culture time in all groups, and vector/siNoggin treatment led to higher ALP activity than that observed with vector/ctrRNA treatment. Compared with untreated cells, treatment with LNPs/siNoggin, LNPs/pBMP-2, or lipo/siNoggin enhanced ALP activity in cells by approximately 1.98-, 1.45-, and 1.28-fold on day 7, and by 1.56-, 1.42-, and 0.98-fold on day 14, respectively, suggesting that the ability of complexes for promoting ALP activity was in the order LNPs/siNoggin > LNPs/pBMP-2> lipo/siNoggin.
Figure 5.
ALP activity (A) and relative expression of Runx2 mRNA determined by RT-PCR (B) in transfected MC3T3-E1 cells after 7 days and 14 days of culture in osteogenic medium.
Notes: *P<0.05, **P<0.01 and ***P<0.001 vs the blank group. ##P<0.01 and ###P<0.001. The error bars represent the mean±SD (n=3).
Abbreviations: LNPs, lipopolysaccharide-amine nanopolymersomes; lipo, lipofectamine3000; ALP, alkaline phosphatase.
Effects of LNPs/siNoggin on expression of Runx2 mRNA
Runx2 is a major transcription gene that regulates osteogenic differentiation. Runx2 expression in MC3T3-E1 cells was examined by RT-PCR on days 7 and 14. Expression of Runx2 mRNA increased on days 7 and 14 in all groups compared with that in the blank (untreated) cells (Figure 5B). Compared with untreated cells, on day-7, Runx2 expression increased to 1.75-, 1.50-, and 1.50-fold in LNPs/siNoggin, LNPs/pBMP-2, and lipo/siNoggin groups, respectively; on day-14, the corresponding value was 1.22-, 1.14-, and 1.18-fold, respectively, but a significant difference was not found between groups at this time. Based on these results, we concluded that LNPs/siNoggin could contribute more to improving expression of Runx2 mRNA in MC3T3-E1 cells than LNPs/pBMP-2 and lipo/siNoggin.
ECM mineralization
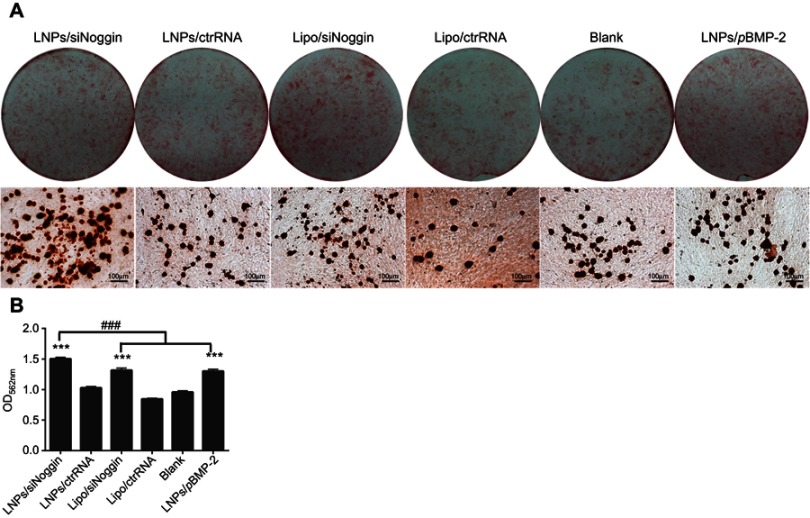
Late-stage osteogenesis was characterized further by examining ECM mineralization through Alizarin Red staining on day-28. Compared with the blank, all transfection groups had significantly enhanced formation of calcified nodules (stained red) in MC3T3-E1 cells (Figure 6A). The mineralization level in the LNPs/siNoggin group was ~1.2-fold higher than that in the LNPs/pBMP-2 group, and was comparable between the groups of LNPs/pBMP-2 and lipo/siNoggin (Figure 6B), indicating that promotion of mineralization regulated via LNPs/siNoggin was more powerful than that via LNPs/pBMP-2 at the late stage of osteogenesis.
Figure 6.
Mineralization of transfected MC3T3-E1 cells on day 28. (A) Alizarin Red staining. Photos (upper) and microscopic images (lower) show formation of calcified nodules (stained red). (B) Quantification of calcified nodules dissolved by 10% cetylpyridinium chloride solution.
Notes: ***P<0.001 vs the blank group. The error bars represent the mean±SD (n=3).
Abbreviations: LNPs, lipopolysaccharide-amine nanopolymersomes; lipo, lipofectamine3000.
Effects of gene transfection on expression of osteogenic proteins
After assessing the effect of gene transfection on osteogenic differentiation via measuring expression of related genes, we further assessed this effect by determining the expression of related proteins in transfected MC3T3-E1 cells at different osteogenesis stages using Western blotting.
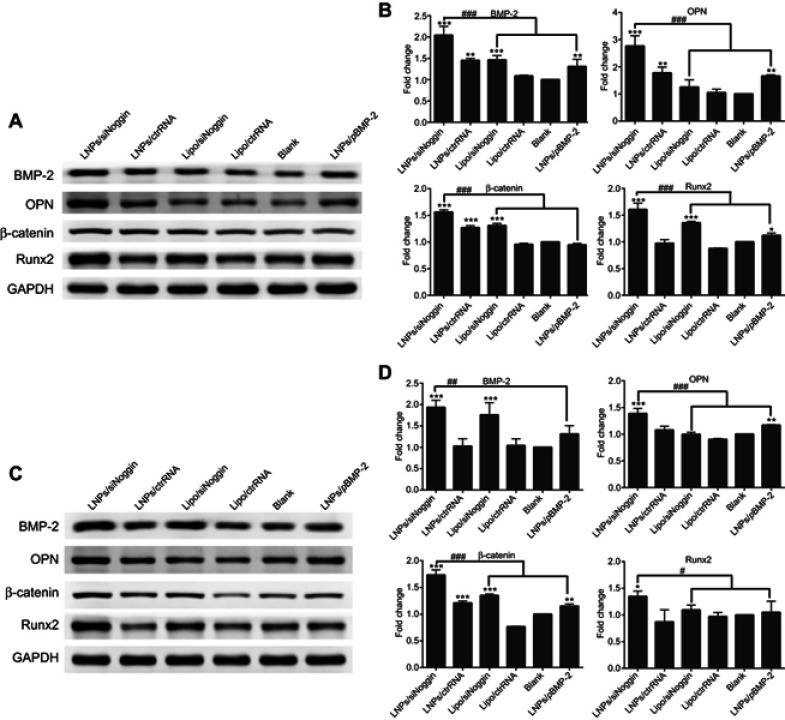
At the early stage of osteogenesis (1–7 days of culture), compared with untreated cells, transfection increased protein expression of BMP-2, OPN, Runx2, and β-catenin in cells. However, expression of OPN protein in the lipo/siNoggin group and β-catenin protein in the LNPs/pBMP-2 group on day-3 was not significantly different from that of blank (untreated) cells. The same was true for expression of OPN and Runx2 proteins in the lipo/siNoggin group and BMP-2 and Runx2 proteins in the LNPs/pBMP-2 group on day-7 (Figure 7). LNPs/siNoggin led to higher expression of protein than that elicited by LNPs/pBMP-2 or lipo/siNoggin treatment. For example, compared with the LNPs/pBMP-2 group, on day-3, protein expression of BMP-2, OPN, Runx2 and β-catenin in the LNPs/siNoggin group increased by 1.57-, 1.66-, 1.64-, and 1.43-fold (Figures 7A and B) andon day 7, it increased by 1.48-, 1.19-, 1.50-, and 1.28-fold, respectively (Figures 7C and D).
Figure 7.
Effects of siNoggin and pBMP-2 treatment on expression of osteogenic-related proteins on day 3 (A, B) and 7 (C, D). (A, C) Representative Western blots of each protein. (B, D) Semiquantitative expression of BMP-2, OPN, β-catenin, and Runx2 proteins by ImageJ software.
Notes: *P<0.05, **P<0.01 and ***P<0.001 vs the blank group. #P<0.05, ##P<0.01 and ###P<0.001. The error bars represent the mean±SD (n=3).
Abbreviations: LNPs, lipopolysaccharide-amine nanopolymersomes; lipo, lipofectamine3000.
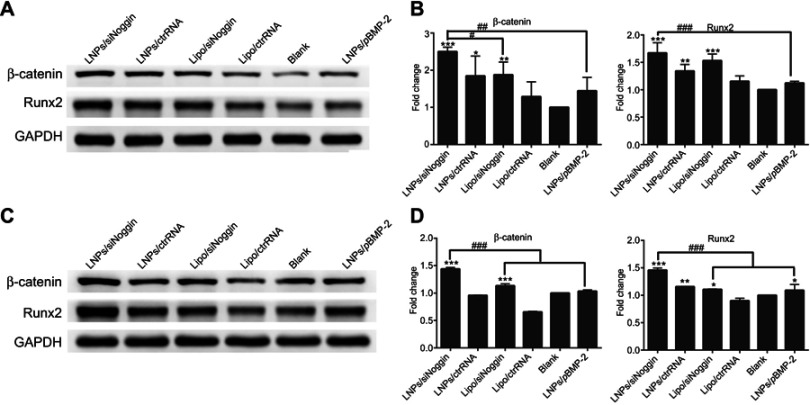
At the intermediate stage of osteogenesis (8–14 days of culture), on day-14, protein expression of β-catenin and Runx2 was enhanced significantly in cells treated by LNPs/siNoggin or lipo/siNoggin compared with that in untreated cells, but there was no significant difference in protein expression between cells untreated and cells treated by LNPs/pBMP-2 (Figures 8A and B). In addition, LNPs/siNoggin treatment led to higher expression of β-catenin protein and Runx2 protein than that by lipo/siNoggin treatment.
Figure 8.
Effects of siNoggin and pBMP-2 treatment on expression of osteogenic-related proteins on days 14 (A, B) and 21 (C, D). (A, C) Representative Western blots of each protein. (B, D) Semiquantitative expression of β-catenin protein and Runx2 protein by ImageJ software. The error bars represent the mean±SD (n=3).
Notes: *P<0.05, **P<0.01 and ***P<0.001 vs the blank group. #P<0.05, ##P<0.01 and ###P<0.001. The error bars represent the mean±SD (n=3).
Abbreviations: LNPs, lipopolysaccharide-amine nanopolymersomes; lipo, lipofectamine3000.
At the late stage of osteogenesis (15–21 days of culture), on day 21 (Figures 8C and D), LNPs/siNoggin treatment enhanced expression of β-catenin protein and Runx2 protein significantly to 1.40- and 1.34-fold compared with that in untreated cells, but expression of these two proteins in LNPs/pBMP-2 and lipo/siNoggin groups was almost identical to that in untreated cells.
Taken together, during all stages of osteogenesis, in the case of osteogenesis-related protein expression, LNPs/siNoggin exhibited the strongest ability to promote osteogenic differentiation of MC3T3-E1 cells among LNPs/siNoggin, LNPs/pBMP-2, and lipo/siNoggin. At early and intermediate stages, lipo/siNoggin showed a stronger ability to promote osteogenic differentiation than that by LNPs/pBMP-2. At the late stage of osteogenesis, lipo/siNoggin or LNPs/pBMP-2 contributed little to osteogenic differentiation, and their osteogenesis-related protein expression was almost identical to that in untreated cells. Therefore, we may conclude that LNPs are more efficient gene-delivery vectors compared with lipo, and that the negative regulation of suppressors via LNPs/siNoggin may be better in osteogenic differentiation than positive regulation of promoters via LNPs/pBMP-2.
Effects of LNPs/siNoggin on BMP/Smad and GSK-3β/β-catenin signaling pathways
Noggin is an antagonist of BMP-2/4/5/6/7/13/14. BMP-2/4/6/7/14 has been demonstrated to enhance osteogenic differentiation and bone formation. Binding of Noggin to BMP-2/4/7/14 inhibits signaling pathways induced by BMPs through blockade of binding sites for the type-I and type-II receptors of BMPs.8,10,30,32 It is clear that the BMP/Smad signaling pathway is involved in osteogenic differentiation and bone formation upon siNoggin treatment, but whether other signaling pathways are involved is not clear.
Among signals stimulating bone formation, BMP/Smad and GSK-3β/β-catenin signaling pathways are two of the most critical. In the BMP/Smad signaling pathway, BMPs bind to and activate receptors to phosphorylate Smad/1/5/9 proteins, which further drives osteogenic differentiation. In the GSK-3β/β-catenin signaling pathway, inhibition of GSK-3β activity is a key step that protects β-catenin from degradation and leads to its accumulation in cytoplasm, and then β-catenin translocates to the nucleus to drive osteogenic differentiation.33,34 GSK-3 is a widely expressed and highly conserved serine/threonine protein kinase. It is encoded by two genes that generate two related proteins (GSK-3α and GSK-3β) and acts downstream of several essential osteogenesis-related signaling pathways such as phosphatidylinositol 3‘ kinase (PI3K), Wnt/β-catenin, Hedgehog, and Notch pathways. The mechanism of GSK-3 regulation is not fully understood, but it has been demonstrated that GSK-3 activity can be suppressed by: phosphorylation of GSK-3β (p-GSK-3β) induced by agonists (eg, eurotrophins, growth factors); formation of protein complexes of GSK-3β induced by Wnt ligands; intracellular localization.33
Therefore, we tested if LNPs/siNoggin treatment activated BMP/Smad and GSK-3β/β-catenin signaling pathways by measuring their respective protein expression of Smad/1/5/9 and p-Smad/1/5/9, GSK-3β, and p-GSK-3β (Ser9) and β-catenin by Western blotting.
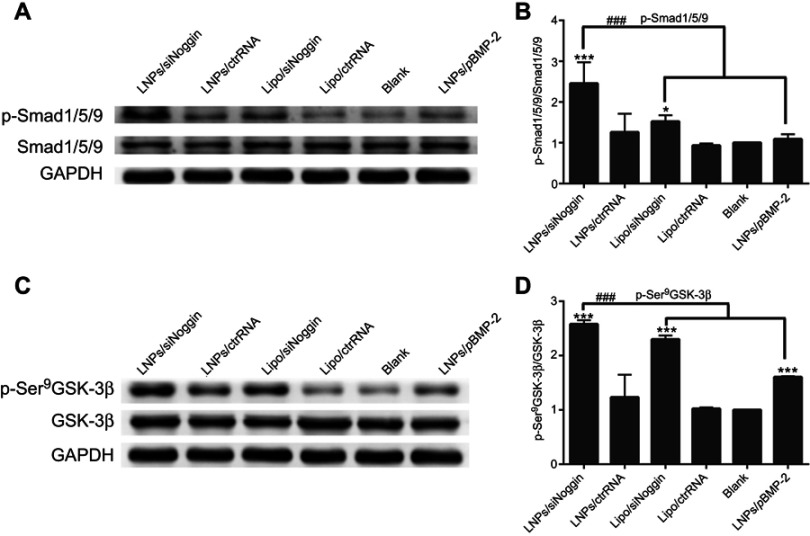
Compared with untreated cells, treatment of LNPs/siNoggin, lipo/siNoggin, or LNPs/pBMP-2 up-regulated protein expression of p-Smad/1/5/9 by 2.38-, 1.52-, and 1.05-fold, and of p-GSK-3β (Ser9) by 2.53-, 2.26-, and 1.61-fold, respectively (Figure 9). Up-regulation of p-GSK-3β further increased expression of β-catenin protein by 1.61-, 1.32-, and 0.95-fold in LNPs/siNoggin, lipo/siNoggin, and LNPs/pBMP-2 groups, respectively (Figure 7). Finally, the osteogenic differentiation marked by ALP activity, mineralization, and the genes and proteins of osteogenic markers was enhanced (Figures 5–7). These results suggested that: Noggin suppression from siNoggin treatment promoted osteogenesis differentiation possibly by the BMP/Smad and GSK-3β/β-catenin signaling pathways; LNPs may be more efficient gene-delivery vectors than lipo; siNoggin treatment may be more efficient for stimulating osteogenic differentiation than pBMP-2 treatment via delivery of nonviral vectors. We obtained some evidence that siNoggin treatment affects the GSK-3β/β-catenin signaling pathway. However, the detailed underlying molecular mechanisms (how the regulation of GSK-3β activity is triggered, the ratio of phosphorylated and unphosphorylated β-catenin, and the concentration of unphosphorylated β-catenin in nuclei) were not revealed, but merit detailed study.
Figure 9.
LNPs/siNoggin promotes osteogenesis differentiation by activating BMP/Smad and GSK-3β/β-catenin signaling pathways in MC3T3-E1 cells on day-3. (A) Effects on the expression of phosphor-Smad/1/5/9 proteins. (C) Effects on the expression of phosphor-GSK-3β (Ser9) protein. (B, D) Semiquantitative expression of phosphor-Smad/1/5/9 or phosphor-GSK-3β (Ser9) proteins by ImageJ software.
Notes: *P<0.05, **P<0.01 and ***P<0.001 vs the blank group. The error bars represent the mean±SD (n=3).
Abbreviations: LNPs, lipopolysaccharide-amine nanopolymersomes; lipo, lipofectamine3000.
Discussion
Gene therapy based on RNA or DNA can offer a new, efficacious option for treatment of bone defects. Theoretically, osteogenesis can be enhanced through downregulation of negative (eg, by gene silencing) or upregulation of the positive (eg, by gene expression) regulators of osteogenic signaling. In osteogenic gene therapy, BMP-2 (DNA) and siNoggin (siRNA) are used extensively alone or in combination, and have been demonstrated to be potent in promoting osteogenesis in vitro or in vivo.1,5,6,8,16,20,35 The safety and efficacy of gene therapy are dependent largely on gene-delivery vectors, and an ideal gene-delivery vector has not been identified.
Therefore, the first goal of our work was to explore whether the LNPs, which were developed by our research team and have been shown to mediate the transient transfection of pDNA,26–29 were also good candidates for siRNA delivery. MC3T3-E1 cells were chosen as target cells for the study of transfection and osteogenic differentiation. MC3T3-E1 cells are clonal osteoblast-like cells from murine calvaria with distinct proliferative and differentiated stages in culture. They are a recognized in vitro model of osteoblast development due to their convenience and a developmental sequence similar to osteoblasts in bone tissue.31 Hence, MC3T3-E1 cells are used widely in the study of osteogenic differentiation.6,31,36,37
LNPs could deliver siNoggin into MC3T3-E1 cells in the present study. Optimal transfection could be achieved at 3.35 μg/mL of LNPs and 50 nM of siRNA with uptake efficiency of ~98% and minimal cytotoxicity. The latter was evidenced by the higher viability, similar morphology, and enhanced proliferation of cells compared with those of the untreated cells (Figures 1 and 2). At this condition, delivery of LNPs/siNoggin into MC3T3-E1 cells led to a silencing efficiency of Noggin of 50% (Figure 3A). This value is not the higstest among reported nonviral vectors, but is attractive because it is relatively higher than that for most reported nonviral vector systems and close to the viral vector system for siNoggin delivery. For example, for a viral vector delivery system, about 30–70% of gene knockdown can be achieved using lentiviral particles containing shRNA targeting Noggin to transduce adipose-derived mesenchymal stem cells (ASCs).30,38 For nonviral vector/siNoggin systems, the efficiency of gene knockdown is about 45–50% in ASCs using a vector of cationic sterosomes of stearylamine/cholesterol vesicles,17 ~25% in ASCs when using a vector of commercially available lipo 2000,17 ~25% in MC3T3-E1 cells when using a vector of lipo in the present study (Figure 3A), and <20% in human MSCs when using a vector of PEI.15 Recently, 68% of Noggin knockdown has been reported using a supramolecular vector comprising anionic fusogenic peptides and biocleavable cationic polyrotaxanes (GALA/DMAE-SS-PRX) to transfect MC3T3-E1 cells.6 Such high knockdown efficiency is attributed to the improved escape of siRNA polyplexes from endosomes-/lysosomes whose membranes are destabilized by the protonation of fusogenic peptides at acidic pH. Also, in vitro osteogenic differentiation at intermediate and late development stages and in vivo efficacy of bone formation mediated by this vector have been postulated.
LNPs/siRNA had higher transfection efficiency compared with lipo/siRNA, which may be ascribed to their different delivery mechanisms. Lipo is thought to facilitate transfection in the early stages by mediating nucleic-acid condensation and nucleic acid-cell interactions (https://www.thermofisher.com/cn/zh/home/references/gibco-cell-culture-basics/transfection-basics/gene-delivery-technologies/cationic-lipid-mediated-delivery/how-cationic-lipid-mediated-transfection-works.html). With regard to LNPs, the natural merits of three blocks (PEI 1.8k, cholesteryl, OA) and the newly acquired properties of lipopolysaccharide-amine copolymer and formation of polymersomes overcome the barriers in different steps during cytosolic delivery, which has been explained in detail in our previous publications.26,28 For example, LNPs/siRNA can efficiently enter cells, which is attributed to synergistic facilitation of endocytosis via ionic attraction from cationic PEI blocks and a receptor-mediated cholesterol-uptake pathway from the cholesteryl block. Then, LNPs/siRNA can escape efficiently from endosomes/lysosomes, which is ascribed to an increased membrane-disrupting ability of LNPs at an acidic pH of endosomes/lysosomes. That is, at an acidic pH, PEI and OA are protonated sufficiently, which induces a “proton sponge effect” (from PEI protonation) and enhances interactions between LNPs and endosomes/lysosomes membranes due to the increases in positive charge density (PEI protonation) and hydrophobicity (OA protonation).
Comparing vectors is difficult due to differences in the: cell types and their culture conditions, concentrations of nucleic acid and vectors; transfection timing; characterization methods; and way data are presented. For example, protein expression can be an absolute value from enzyme-linked immunosorbent assays or fold-change compared with the control from Western blots. Therefore, setting standards for evaluation of nucleic acid-based delivery therapy is important. Controllable expression of Noggin in a reasonable range is necessary for a good vector, and the final efficacy is the only “gold standard”. As such, for appropriate and controlled regeneration of bone, optimal expression of Noggin should be explored in efficacy experiments.
Based on the concepts mentioned above, after probing the knockdown efficiency of LNPs/siNoggin, we investigated the effects of LNPs/siNoggin on the expression of Noggin protein and osteogenic differentiation of MC3T3-E1 cells at different development stages. Suppression of Noggin led to reduced expression of Noggin protein. In the LNPs/siNoggin group, the expression of Noggin protein decreased by 40% compared with that in the blank, which was significantly lower than that in the lipo/siNoggin group (decreased by 20%) (Figure 3). Scholars have shown that a decrease in levels of endogenous Noggin up-regulates BMP activity and thereby affects BMP-driven actions such as osteogenic differentiation,8,15,17,18,30,39 and our results are consistent with those studies. In detail, LNPs/siNoggin stimulated ALP activity, increased expression of Runx2 mRNA, and BMP-2 protein at an early stage, and enhanced expression of osteogenic proteins and mineralization of MC3T3-E1 cells significantly at the medium and late stages of cell differentiation (Figures 5–9). Taken together, these results suggest that LNPs are good nonviral vectors for siNoggin delivery, and that LNPs/siNoggin can promote osteogenic differentiation of MC3T3-E1 cells, thereby indicating their promising future in bone repair and regeneration.
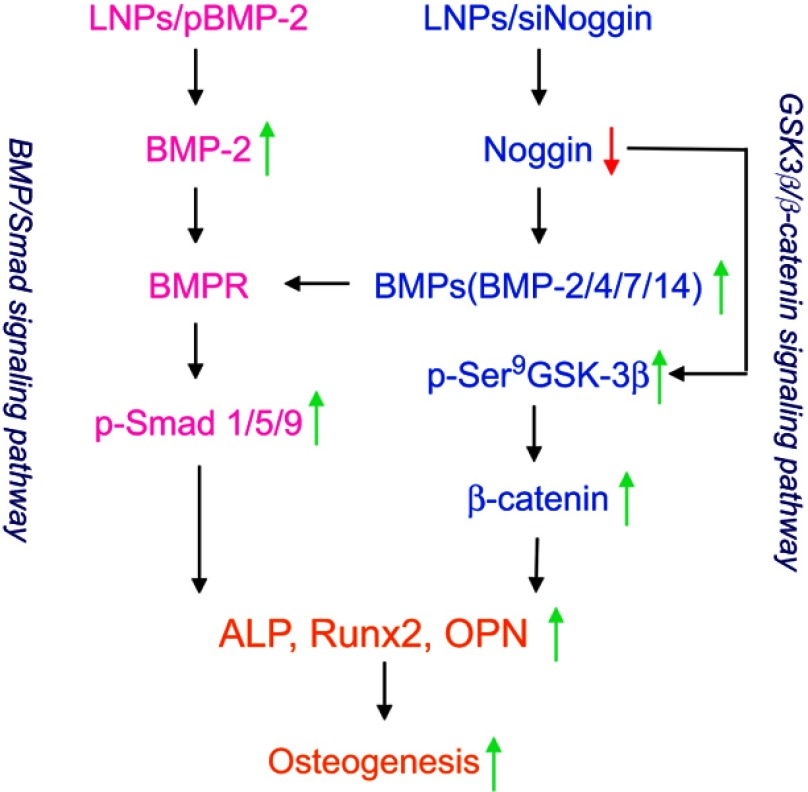
After confirming that LNPs/siNoggin can promote osteogenic differentiation, we further tested the possible signaling pathways involved in siNoggin and pBMP-2 treatment (our second goal) (Scheme 1). For siNoggin treatment, it has been shown that the BMP/Smad signaling pathway is involved in osteogenic differentiation.10,11,30,32,38 However, other signaling pathways might be involved, and this hypothesis was confirmed by our results.
Scheme 1.
Possible signaling pathways and roles of LNPs/siNoggin and LNPs/pBMP-2 for osteogenesis.
First, after LNPs/siNoggin treatment, expression of BMP-2 protein and phosphorylated Smad/1/5/9 protein increased significantly (Figures 7–9) which, in turn, promoted the expression of Runx2 and protein (Figures 5, 7, and 8), key osteogenic transcription factors, and, finally, improved the osteogenic differentiation of MC3T3-E1 cells (as evidenced by an increase in ECM mineralization) (Figure 6). These results confirmed that the BMP/Smad signaling pathway played an important part in the osteogenic differentiation of LNPs/siNoggin-treated MC3T3-E1 cells. Second, LNPs/siNoggin treatment up-regulated protein expression of β-catenin, which can drive osteogenic differentiation, and β-catenin is also the key component in the canonical Wnt signaling pathway. In general, β-caten
in expression can be regulated by GSK-3β in the cytoplasm. Hence, GSK-3β can drive the phosphorylation and degradation of β-catenin, and, conversely, suppression of GSK-3β activity leads to the β-catenin stabilization, and induces its accumulation in cytoplasm and translocation to the nucleus to drive osteogenic differentiation.33 GSK-3β activity can be inhibited through phosphorylation induced by agonists (eg, neurotrophins, growth factors) or complex formation induced by Wnt ligands (canonical Wnt/β-catenin signaling pathway). LNPs/siNoggin treatment enhanced phosphorylation of GSK-3β at Ser9 (Figure 9), and promoted intracellular accumulation of β-catenin (Figures 7, 8). However, β-catenin accumulation might have been caused by canonical Wnt pathways or other pathways, which merits further investigation. Taken together, LNPs/siNoggin promoted osteogenic differentiation, possibly by activating BMP/Smad and GSK-3β/β-catenin signaling pathways, but the detailed molecular mechanisms must be studied further. With respect to pBMP-2 treatment, the BMP/Smad signaling pathway has been demonstrated to be involved in osteogenic differentiation.12 Our results are consistent with this observation, whereby LNPs/pBMP-2 treatment increased expression of pSmad1/5/9 and Runx2 slightly, but could not increase protein expression of β-catenin (0.946-fold, compared with untreated cells).
Our third goal was to compare the in vitro efficacy of pBMP-2 and siNoggin delivered by LNPs. BMP-2 is considered to be a powerful osteoinductive factor, and is approved for clinical application for bone-defect treatment. Noggin is a potent extracellular antagonist of BMPs encoded by NOG. Noggin is a secreted homodimeric glycoprotein with a molecular mass of 64 kDa. Through specific binding with BMPs to shield the binding sites for BMP receptors on cell surfaces, Noggin inhibits the action mediated by BMP-2, -4, -5, -7, -13, and -14, such as osteoblast differentiation.10 BMP-2 and siNoggin have been used extensively alone or in combination in large studies, but few scholars have compared their efficacy.
Clinical treatments must be safe and efficacious. Hence, comparison of the efficacy of BMP-2 and siNoggin delivered by nonviral vectors is needed to provide evidence for treating physicians and patients to make choices in bone-defect treatments. As expected, LNPs/siNoggin and LNPs/pBMP-2 enhanced osteogenic differentiation by increasing ALP activity, expression of osteogenesis-related gene/proteins, and ECM mineralization, but LNPs/siNoggin was more powerful than LNPs/pBMP-2 and, at a late stage of cell differentiation, LNPs/pBMP-2 could not enhance expression of Runx2 protein or β-catenin. These data suggest that regulation via LNPs/siNoggin may be a more effective way to enhance osteogenic differentiation than that via LNPs/pBMP-2.
Despite the fact that few studies have assessed the difference in the osteogenic efficacy between pBMP-2 and siNoggin delivered by the same nonviral vector, we found some clues to support this conjecture. For example, Ramasubramanian et al19 reported that delivery of pBMP-2 alone upregulated expression of osteogenic markers slightly in hADSCs, whereas co-delivery of siNoggin and pBMP-2 (cells first treated by siNoggin/lipid and then pBMP-2/PBAE C32-122 polymer) accelerated their osteogenic differentiation significantly with a marked increase in bone-marker expression and ECM mineralization. The first reason for this difference in efficacy may be because Noggin binds and inactivates BMP-2/4/5/7/13/14.10–12 BMP-2/4/7/14 have been reported to induce osteogenic differentiation of MSCs, ADSCs, and MC3T3-E1 cells in vitro and in vivo.11,12,16 As a result, Noggin silencing leads to activation of other downstream pathways besides BMP-2, and their synergistic actions may be more beneficial to osteogenesis than the activation of BMP-2 signaling alone induced by LNPs/pBMP-2. Protein expression of p-Smad/1/5/9, p-GSK-3β, Runx2 and β-catenin provide evidence of this hypothesis (Figure 9), which suggests that LNPs/pBMP-2 treatment causes activation of the BMP/Smad signaling pathway, but LNPs/siNoggin treatment may (at least) activate BMP/Smad and GSK-3β/β-catenin signaling pathways. The second reason may be the different mechanisms of action of siRNA and pDNA. pDNA delivery undergoes a longer path with more barriers than that of siRNA because the action site is in the cell nucleus for DNA and in the cytoplasm for siRNA. Also, production of the target protein via pDNA delivery can occur only in dividing cells, but suppression of the target protein via siRNA delivery can occur in dividing and non-dividing cells. In addition, unwanted genetic changes may occur due to integration of exogenous DNA sequences into host DNA.8 Also, Noggin knockdown can increase angiogenesis, which will further facilitate bone formation.40
Collectively, for biomaterial-mediated delivery of nucleic acids, siNoggin therapy may be a more efficacious, safer, and easier alternative technology than pBMP-2 therapy to regulate expression of the target protein for tissue regeneration. Further (especially efficacy verification in vivo) studies will be undertaken in the future.
Conclusions
We demonstrated that LNPs can deliver siNoggin into MC3T3-E1 cells efficiently to knock down Noggin in MC3T3-E1 cells with minimal cytotoxicity. This action significantly enhanced the expression of osteogenic markers and in vitro osteogenic differentiation, possibly through the BMP/Smad and GSK-3β/β-catenin signaling pathways. Compared with LNPs/pBMP-2, LNPs/siNoggin had better efficacy for osteogenic differentiation of MC3T3-E1 cells. Our results suggest that LNPs are efficient vectors for delivery of nucleic acids such as pDNA and siRNA. siNoggin delivery via nonviral vectors may be a more effective and alternative way to promote osteogenic differentiation than pBMP-2 delivery. LNPs/siNoggin alone or in combination with other stimulators may have great potential in the repair and regeneration of bone.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81571015 and No. 81371665), the Guangdong Science and Technology Project (2016A050502012), the Natural Science Foundation of Guangdong Province (2015A030313087), and the Guangzhou Science and Technology Plan Project (201707010011 and 201803010102).
Disclosure
The authors report no conflicts of interest in this work.
Supplementary materials
Table S1.
Primer sequences
| Gene | 5ʹ-3ʹ Sequence |
|---|---|
| Noggin | Forward-TGCTGTACGCGTGGAATGA |
| Reverse-TGAGGTGCACAGACTTGGATG | |
| Runx2 | Forward-AAGTGTTCTGTGGTCTCTGAGTTGA |
| Reverse-GCTGTATGGTGAGGCTGGTAGG | |
| GAPDH | Forward-AAGAAGGTGGTGAAGCAGG |
| Reverse-GAAGGTGGAAGAGTGGGAGT |
Cat No. of materials
MC3T3-E1 cell line (Cat No. CRL-2593)
Noggin siRNA (siNoggin) (Line 1 - Cat No. 10620318; Line 2 - Cat No. 10620319)
Alexa Fluor®555 siRNA (Cat No. 14750100)
Stealth TM RNAi Negative Control Duplexes (ctrRNA) (Cat No. 12935400)
lipofectamine3000 (lipo) (Cat No. L3000015)
Opti-MEM™ I Reduced Serum Media (Cat No. 31985062)
trypsin (Cat No. 15050065)
Trizol (Cat No. 15596-026)
α-MEM (Cat No. C12571500bt)
Fetal Bovine Serum (FBS) (Cat No. 10099141)
Penicillin/Streptomycin (Cat No. 15140122)
pBMP-2 (Vector ID: VB160930-1048bkg)
osteogenic medium (Cat No. MUBMX-90021)
Alizarin Red (Cat No. Alizarin Red S)
Cell Counting Kit-8 (CCK-8) (Cat No. ck04)
BCA assay kit (Cat No. CW0014S)
alkaline phosphatase kit (Cat No. A059-2)
Cetylpyridinum chloride (Cat No. C9002-100G)
Prime Script TM RT transcription kit (Cat No. RR036A)
LightCycler®480 SYBR Green I Master (Cat No. 4887352001-1)
Antibodies against mouse noggin (Cat No. NB110-40413)
Antibodies against mouse BMP-2 (Cat No. ab214821), OPN (Cat No. ab8448), Smad/1/5/9 (Cat No. ab66737)
Antibodies against mouse β-catenin (Cat No. 8480), Runx2 (Cat No. 12556), p-Smad/1/5/9 (Cat No. 13820), GSK-3β (Cat No. 12456S), p-GSK-3β(Ser9) (Cat No. 5558S) and GAPDH (Cat No. 2118)
The secondary antibody is Anti-rabbit IgG, HRP-linked Antibody (Cat No. 7074S)
References
- 1.Kowalczewski CJ, Saul JM. Biomaterials for the delivery of growth factors and other therapeutic agents in tissue engineering approaches to bone regeneration. Front Pharmacol. 2018;9:513. doi: 10.3389/fphar.2018.00513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hak DJ, Fitzpatrick D, Bishop JA, et al. Delayed union and nonunions: epidemiology, clinical issues, and financial aspects. Injury. 2014;45(Suppl 2):S3–S7. doi: 10.1016/j.injury.2014.04.002 [DOI] [PubMed] [Google Scholar]
- 3.Haumer A, Bourgine PE, Occhetta P, et al. Delivery of cellular factors to regulate bone healing. Adv Drug Deliv Rev. 2018;129:285–294. doi: 10.1016/j.addr.2018.01.010 [DOI] [PubMed] [Google Scholar]
- 4.Bai X, Gao M, Syed S, et al. Bioactive hydrogels for bone regeneration. Bioact Mater. 2018;3(4):401–417. doi: 10.1016/j.bioactmat.2018.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hasan A, Byambaa B, Morshed M, et al. Advances in osteobiologic materials for bone substitutes. J Tissue Eng Regen Med. 2018;12(6):1448–1468. doi: 10.1002/term.2677 [DOI] [PubMed] [Google Scholar]
- 6.Inada T, Tamura A, Terauchi M, Yamaguchi S, Yui N. A silencing-mediated enhancement of osteogenic differentiation by supramolecular ternary siRNA polyplexes comprising biocleavable cationic polyrotaxanes and anionic fusogenic peptides. Biomater Sci. 2018;6(2):440–450. doi: 10.1039/c8bm00675j [DOI] [PubMed] [Google Scholar]
- 7.Liu H, Li D, Zhang Y, Li M. Inflammation, mesenchymal stem cells and bone regeneration. Histochem Cell Biol. 2018;149(4):393–404. doi: 10.1007/s00418-018-1643-3 [DOI] [PubMed] [Google Scholar]
- 8.Nguyen MK, Jeon O, Dang PN, et al. RNA interfering molecule delivery from in situ forming biodegradable hydrogels for enhancement of bone formation in rat calvarial bone defects. Acta Biomater. 2018;75:105–114. doi: 10.1016/j.actbio.2018.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tatiparti K, Sau S, Kashaw SK, Iyer AK. siRNA delivery strategies: a comprehensive review of recent developments. Nanomaterials (Basel). 2017;7(4). doi: 10.3390/nano7120458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krause C, Guzman A, Knaus P. Noggin. Int J Biochem Cell Biol. 2011;43(4):478–481. doi: 10.1016/j.biocel.2010.11.015 [DOI] [PubMed] [Google Scholar]
- 11.Pensak MJ, Lieberman JR. Gene therapy for bone regeneration. Curr Pharm Des. 2013;19(19):3466–3473. doi: 10.2174/1381612811319190012 [DOI] [PubMed] [Google Scholar]
- 12.Zhang X, Guo J, Zhou Y, Wu G. The roles of bone morphogenetic proteins and their signaling in the osteogenesis of adipose-derived stem cells. Tissue Eng Part B Rev. 2014;20(1):84–92. doi: 10.1089/ten.teb.2013.0204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wagner DO, Sieber C, Bhushan R, et al. BMPs: from bone to body morphogenetic proteins. Sci Signal. 2010;3(107):mr1. [DOI] [PubMed] [Google Scholar]
- 14.Molina CS, Stinner DJ, Obremskey WT. Treatment of traumatic segmental long-bone defects: a critical analysis review. JBJS Rev. 2014;2(4). doi: 10.2106/JBJS.RVW.M.00062 [DOI] [PubMed] [Google Scholar]
- 15.Nguyen MK, Jeon O, Krebs MD, Schapira D, Alsberg E. Sustained localized presentation of RNA interfering molecules from in situ forming hydrogels to guide stem cell osteogenic differentiation. Biomaterials. 2014;35(24):6278–6286. doi: 10.1016/j.biomaterials.2014.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carreira AC, Zambuzzi WF, Rossi MC, et al. Bone morphogenetic proteins: promising molecules for bone healing, bioengineering, and regenerative medicine. Vitam Horm. 2015;99:293–322. [DOI] [PubMed] [Google Scholar]
- 17.Cui ZK, Fan J, Kim S, et al. Delivery of siRNA via cationic Sterosomes to enhance osteogenic differentiation of mesenchymal stem cells. J Control Release. 2015;217:42–52. doi: 10.1016/j.jconrel.2015.08.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orciani M, Fini M, Di Primio R, Mattioli-Belmonte M. Biofabrication and bone tissue regeneration: cell source, approaches, and challenges. Front Bioeng Biotechnol. 2017;5:17. doi: 10.3389/fbioe.2017.00017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramasubramanian A, Shiigi S, Lee GK, Yang F. Non-viral delivery of inductive and suppressive genes to adipose-derived stem cells for osteogenic differentiation. Pharm Res. 2011;28(6):1328–1337. doi: 10.1007/s11095-011-0406-9 [DOI] [PubMed] [Google Scholar]
- 20.Zhou Z, Liu X, Zhu D, et al. Nonviral cancer gene therapy: delivery cascade and vector nanoproperty integration. Adv Drug Deliv Rev. 2017;115:115–154. doi: 10.1016/j.addr.2017.07.021 [DOI] [PubMed] [Google Scholar]
- 21.Leong J, Teo JY, Aakalu VK, Yang YY, Kong H. Engineering polymersomes for diagnostics and therapy. Adv Healthc Mater. 2018;7(8):e1701276. doi: 10.1002/adhm.201701276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chidanguro T, Ghimire E, Liu CH, Simon YC. Polymersomes: breaking the glass ceiling? Small. 2018;14(46):e1802734. doi: 10.1002/smll.v14.46 [DOI] [PubMed] [Google Scholar]
- 23.Shi Y, Jiang Y, Cao J, et al. Boosting RNAi therapy for orthotopic glioblastoma with nontoxic brain-targeting chimaeric polymersomes. J Control Release. 2018;292:163–171. doi: 10.1016/j.jconrel.2018.10.034 [DOI] [PubMed] [Google Scholar]
- 24.Zou Y, Zheng M, Yang W, et al. Virus-mimicking chimaeric polymersomes boost targeted cancer siRNA therapy in vivo. Adv Mater. 2017;29(42). doi: 10.1002/adma.201700681 [DOI] [PubMed] [Google Scholar]
- 25.Ge X, Zhang Q, Cai Y, et al. PEG-PCL-DEX polymersome-protamine vector as an efficient gene delivery system via PEG-guided self-assembly. Nanomedicine (Lond). 2014;9(8):1193–1207. doi: 10.2217/nnm.13.83 [DOI] [PubMed] [Google Scholar]
- 26.Huang Z, Teng W, Liu L, et al. Efficient cytosolic delivery mediated by polymersomes facilely prepared from a degradable, amphiphilic, and amphoteric copolymer. Nanotechnology. 2013;24(26):265104. doi: 10.1088/0957-4484/24/26/265104 [DOI] [PubMed] [Google Scholar]
- 27.Guan Y, Wang Q, Cheng Y, Teng W, Huang H. Study on gene transfection in bone marrow mesenchymal stem cells mediated by plasmid of bone morphogenetic protein 2 loaded lipopolysaccharide-amine nanopolymersomes. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2014;28(10):1292–1297. [PubMed] [Google Scholar]
- 28.Teng W, Huang Z, Chen Y, et al. pVEGF-loaded lipopolysaccharide-amine nanopolymersomes for therapeutic angiogenesis. Nanotechnology. 2014;25(6):065702. doi: 10.1088/0957-4484/25/6/065702 [DOI] [PubMed] [Google Scholar]
- 29.Wang Q, Chen Y, Wang L, et al. Stability and toxicity of empty or gene-loaded lipopolysaccharide-amine nanopolymersomes. Int J Nanomedicine. 2015;10:597–608. doi: 10.2147/IJN.S74156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fan J, Im CS, Guo M, et al. Enhanced osteogenesis of adipose-derived stem cells by regulating bone morphogenetic protein signaling antagonists and agonists. Stem Cells Transl Med. 2016;5(4):539–551. doi: 10.5966/sctm.2015-0249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quarles LD, Yohay DA, Lever LW, Caton R, Wenstrup RJ. Distinct proliferative and differentiated stages of murine MC3T3-E1 cells in culture: an in vitro model of osteoblast development. J Bone Mineral Res. 1992;7(6):683–692. doi: 10.1002/jbmr.5650070613 [DOI] [PubMed] [Google Scholar]
- 32.Groppe J, Greenwald J, Wiater E, et al. Structural basis of BMP signalling inhibition by the cystine knot protein Noggin. Nature. 2002;420(6916):636–642. doi: 10.1038/nature01131 [DOI] [PubMed] [Google Scholar]
- 33.Kaidanovich-Beilin O, Woodgett JR. GSK-3: functional insights from cell biology and animal models. Front Mol Neurosci. 2011;4:40. doi: 10.3389/fnmol.2011.00040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin FX, Zheng GZ, Chang B, et al. Connexin 43 modulates osteogenic differentiation of bone marrow stromal cells through GSK-3beta/beta-catenin signaling pathways. Cell Physiol Biochem. 2018;47(1):161–175. doi: 10.1159/000491620 [DOI] [PubMed] [Google Scholar]
- 35.Shapiro G, Lieber R, Gazit D, Pelled G. Recent advances and future of gene therapy for bone regeneration. Curr Osteoporos Rep. 2018;16:504–511. doi: 10.1007/s11914-018-0459-3 [DOI] [PubMed] [Google Scholar]
- 36.Casati L, Pagani F, Fibiani M, Lo Scalzo R, Sibilia V. Potential of delphinidin-3-rutinoside extracted from Solanum melongena L. as promoter of osteoblastic MC3T3-E1 function and antagonist of oxidative damage. Eur J Nutr. 2018. doi: 10.1007/s00394-018-1618-0 [DOI] [PubMed] [Google Scholar]
- 37.Qing T, Mahmood M, Zheng Y, et al. A genomic characterization of the influence of silver nanoparticles on bone differentiation in MC3T3-E1 cells. J Appl Toxicol. 2018;38(2):172–179. doi: 10.1002/jat.3528 [DOI] [PubMed] [Google Scholar]
- 38.Fan J, Park H, Tan S, Lee M. Enhanced osteogenesis of adipose derived stem cells with Noggin suppression and delivery of BMP-2. PLoS One. 2013;8(8):e72474. doi: 10.1371/journal.pone.0072474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fan J, Park H, Lee MK, et al. Adipose-derived stem cells and BMP-2 delivery in chitosan-based 3D constructs to enhance bone regeneration in a rat mandibular defect model. Tissue Eng Part A. 2014;20(15–16):2169–2179. doi: 10.1089/ten.tea.2013.0523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Levi B, Nelson ER, Hyun JS, et al. Enhancement of human adipose-derived stromal cell angiogenesis through knockdown of a BMP-2 inhibitor. Plast Reconstr Surg. 2012;129(1):53–66. doi: 10.1097/PRS.0b013e3182361ff5 [DOI] [PMC free article] [PubMed] [Google Scholar]