Abstract
Objective
Conduct an active case finding study in Tbilisi, Georgia, for pulmonary tuberculosis (TB) among people living with HIV (PLWH).
Methods
Newly diagnosed HIV patients were assessed for symptoms and asked to submit sputum samples for smear microscopy, culture, and molecular diagnostic testing (Xpert MTB/RIF).
Results
Among 276 PLWH, 131 agreed to participate and 103 submitted sputum samples. Most participants were male (70%) and mean age of 43 years. There were high rates of a positive hepatitis C virus (HCV) antibody test (46%) and the median CD4 count was 122 cells/mm3. A total of 15 (11.5%) persons were diagnosed with pulmonary TB, including 1 each with multidrug-resistant and isoniazid-resistant disease. Twelve had a positive culture for Mycobacterium tuberculosis and Xpert TB/RIF assay, and 4 had positive smear microscopy. Patients with pulmonary TB were more likely to use injection drugs (67% vs 36%, P = .02) and have a positive HCV antibody (73% vs 42%, P = .02). The presence and absence of any TB symptom had a sensitivity and negative predictive value for TB of 93% and 98%, respectively.
Conclusion
Our findings highlight the high prevalence of TB among newly diagnosed HIV-infected patients in an area with high rates of drug-resistant TB and the utility of an active case finding strategy for TB diagnosis.
Keywords: Case finding, Georgia, Tbilisi, TB symptoms
Utilizing an active case finding strategy including molecular diagnostic testing we found a high rate of pulmonary tuberculosis among newly diagnosed HIV patients in an area of the world (Eastern Europe) more known for high rates of drug-resistant tuberculosis.
There have been major advancements in the fight against tuberculosis (TB), including the implementation of rapid molecular diagnostic tests, new drugs [1], shorter regimens for drug-resistant disease, and, most recently in September 2018, the first ever United Nations high-level meeting on TB [2]. However, despite modest progress, TB has emerged as the leading cause of death due to an infectious disease. TB/HIV coinfection is one of the major challenges of global TB control, and TB remains the leading cause of mortality among people living with HIV (PLWH). There remains large gaps in both the detection and treatment of HIV-associated TB; out of the almost 1 million new cases of TB among PLWH in 2017, only half were diagnosed and reported [2]. Many PLWH with TB disease may not present to care until late in their disease course, as TB disease in this population typically manifests with nonspecific symptoms or can be asymptomatic [3]. Even after presenting at a health facility, confirmatory diagnosis of TB in PLWH is difficult due to nonspecific symptoms, atypical chest radiography, and a higher prevalence of sputum-smear negative disease [3–5].
Globally, TB accounts for one-third of all HIV-related deaths, with a diverging pattern seen in Europe. TB-related HIV-mortality in Eastern Europe (EE) is 27%, which is in contrast to the rest of Europe (<10%) [3, 6, 7]. Specifically, in the country of Georgia, TB remains the leading cause of death in PLWH [8]. A combination of factors, including increasing HIV prevalence and drug-resistant TB, high rates of late HIV diagnosis, and overlapping risk factors for TB and HIV, makes Georgia particularly vulnerable to a TB/HIV epidemic [6, 9, 10]. In order to alleviate both morbidity and mortality associated with TB in PLWH, active case finding (ACF) for TB has been recommended as a strategy for regions with high TB and HIV incidence [7, 11–13]. Although the World Health Organization (WHO) recommends a symptom-based screening strategy for TB in PLWH as an essential component of HIV care, it has been shown to have low accuracy in detecting active TB, especially in high-burden TB areas [2, 14, 15]. In contrast, due to the prevalence of nonspecific, or lack of, TB symptoms in PLWH, ACF utilizing the Xpert MTB/RIF for all patients could potentially identify a significant number of missed cases [4, 12, 14]. However, limited data currently exists on the potential success of ACF using the Xpert MTB/RIF in PLWH in EE, where TB burden remains high.
In this cross-sectional study conducted in the country of Georgia, we sought to determine whether the rapid molecular diagnostic test, Xpert MTB/RIF, would improve active pulmonary TB case finding in PLWH. Our primary aims included determining the prevalence of pulmonary TB among newly diagnosed HIV-infected patients utilizing Xpert MTB/RIF and culture testing and evaluating the yield of a WHO-recommended symptom-based screening approach versus testing all patients for active TB. The overall goal of this research is to identify ways to improve the diagnosis of TB and, thus, close the “detection” gap among PLWH in Georgia and other similar settings.
METHODS
Study Population and Setting
This cross-sectional study was conducted at the Infectious Diseases, AIDS, and Clinical Immunology Research Center (IDACIRC) and the National Center for Tuberculosis and Lung Disease (NCTLD) in Tbilisi, Georgia. IDACIRC is the national reference center for HIV diagnosis and treatment and provides care to approximately 65% of all HIV patients in the country. All newly diagnosed adult (≥18 years) HIV patients who presented for clinical care to the IDACIRC from February 2014 through May 2015 were eligible for enrollment. Receipt of anti-TB medications in the prior 60 days was an exclusion criterion. Ethics approval was obtained from the institutional review boards of IDACIRC, NCTLD, and Emory University, and written informed consent was required for participation.
Sample Collection and Laboratory Analysis
All study participants were asked to submit 2 sputum samples, including a spot and morning sample for analysis. A portion of obtained sputum samples underwent Xpert TB/RIF testing at the onsite IDACIRC laboratory, while the remaining portion (minimum of 2 ml) was transported to the NCTLD National Reference Laboratory (NRL) for AFB sputum smear microscopy and culture. At the NRL, all sputum samples were decontaminated and centrifuged before Ziehl-Nielsen staining for microscopy and inoculation onto solid egg-based Löwenstein–Jensen media for culture as previously described [16]. Mycobacterium tuberculosis was confirmed by the use of the MTBDRplus assay (Hain Lifescience GmbH, Nehren, Germany). First-line drug susceptibility testing was carried out for all positive cultures as previously described [16].
Data Collection and Analysis
Study participants underwent a brief interview by routine clinical staff, who were also study collaborators to collect information on demographics, medical history, and the presence of pulmonary TB symptoms. Additionally, medical chart abstraction was carried out to collect similar information for HIV-infected patients hospitalized at the NCTLD hospital during the study period. All data was entered into an online REDCap database and analyses were performed with SAS, version 9.4 (Cary, NC). Study data were collected and managed using REDCap electronic data capture tools hosted at Emory University.
Our main outcome was the prevalence of pulmonary TB among newly diagnosed PLWH. A case of pulmonary TB was defined as either a positive sputum Xpert TB/RIF or positive culture for M. tuberculosis. We compared characteristics among HIV-infected patients with and without pulmonary TB and also evaluated the performance of the WHO symptom screen in detecting pulmonary TB. Additionally, we compared characteristics between HIV patients diagnosed with TB through our study (ACF) and HIV-infected patients who presented to the NCTLD with active TB during the study period (passive case finding). This comparison was undertaken to determine if TB was occurring at different stages of HIV infection in those who were diagnosed through active versus passive case finding. For all descriptive statistics, differences in categorical variables were tested using chi square test and for continuous variables a 2-sample t test. A P value < .05 was considered significant.
RESULTS
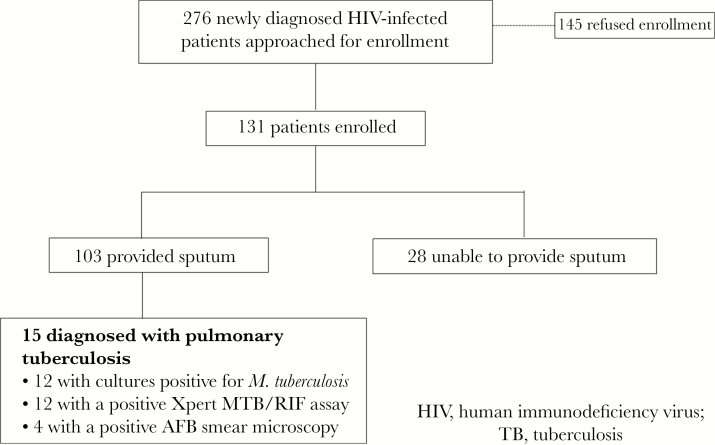
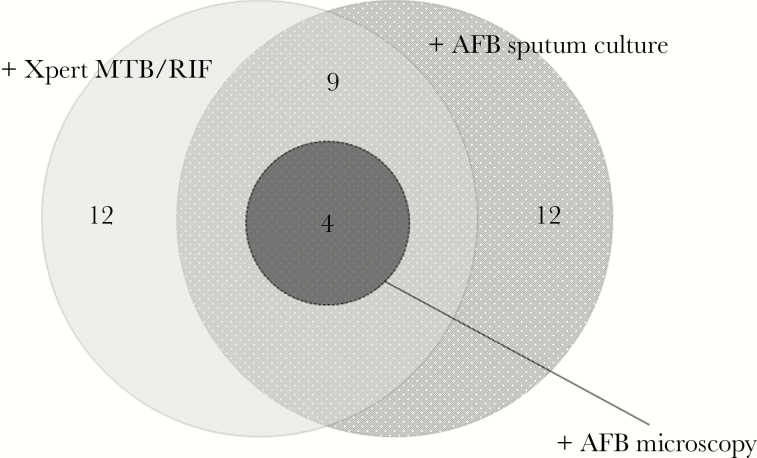
A total of 276 newly diagnosed HIV-infected patients approached for study enrollment at the IDACIRC and 131 agreed to participate (Figure 1). The median time from HIV diagnosis to study enrollment was 3 days (interquartile range 6–12). Among study participants, 103 of 131 (79%) were able to provide a sputum sample, and 15 (11.5% of those enrolled and 14.6% of those who provided a sputum sample) were found to have pulmonary TB. Among the 15 patients found to have pulmonary TB, 12 had a positive culture, 12 a positive Xpert TB/RIF assay, and 4 had a positive sputum smear microscopy (Figure 2). All 4 patients with a positive sputum smear microscopy result had both a positive sputum culture and Xpert TB/RIF result. One patient each had multi-drug resistant (MDR) and isoniazid monoresistant disease, while the remaining 13 had drug-susceptible disease. The number of patients needed to test via ACF (utilizing Xpert MT/RIF and culture testing) to detect 1 case of pulmonary tuberculosis was 9 (131/15), and, if including only patients who submitted sputum samples, 7 (103/15).
Figure 1.
Flowchart of Patient Enrollment and Study Results
Figure 2.
Venn Diagram, Sputum Xpert MTB/RIF, Culture and Microscopy Results Among HIV-Infected Patients Screened for Active Tuberculosis
The majority of the 131 enrolled HIV-infected patients were male (70%), and the mean age of 42 years. There were high rates of alcohol (42%) and tobacco use (58%). Close to half of the patients (46%) had a positive HCV antibody test and the median CD4 count of those enrolled was 122 cells/mm3. At presentation and enrollment, 26% of the cohort had oral candida infection, 29% had presumed bacterial pneumonia, and 9% had a positive serum cryptococcal antigen test. Many of those enrolled (63%) had at least 1 of the following symptoms: cough, shortness of breath, night sweats, weight loss, or fever. Further characteristics are shown in Table 1. Patients with pulmonary TB were more likely to have a history of injection drug use (67% vs 36%, P = .02) and a positive HCV antibody test (73% vs 42%, P = .02) compared to patients without TB as well as higher rates of oral candida infection (60% vs 22%, P < .01). Patients with pulmonary TB were significantly more likely to have cough, night sweats, weight loss, and fever than those without pulmonary TB (see Table 1). There was 1 death (7%) during TB treatment in our ACF group.
Table 1.
Characteristics of the HIV-Infected Patient Cohort Overall and by TB Status
| Demographics | N = 131 (%) | Pulmonary TB Detected (n = 15) | No Pulmonary TB (n = 116) | P |
|---|---|---|---|---|
| Males | 92 (70) | 11 (73) | 81 (70) | .78 |
| Mean age in years (SD) | 42 (10) | 42 (10) | 44 (12) | .56 |
| Mean BMI [kg/m2] (SD) | 22 (2.6) | 21.4 (3.3) | 22.6 (2.5) | .22 |
| Unemployed | 86 (66) | 13 (87) | 73 (63) | .07 |
| Prison history | 21 (16) | 3 (20) | 18 (16) | .66 |
| Current tobacco use | 76 (58) | 10 (67) | 66 (57) | .47 |
| Alcohol use | 55 (42) | 6 (40) | 49 (42) | .87 |
| Injection drug use | 52 (40) | 10 (67) | 42 (36) | .02 |
| Hepatitis C virus antibody positive | 60 (46) | 11 (73) | 49 (42) | .02 |
| Prior TB treatment for active TB | 4 (3) | 0 | 4 (4) | .47 |
| HIV | ||||
| Mean CD4 (SD) | 174 (184) | 133 (146) | 179 (188) | .28 |
| Median CD4 (IQR) | 122 (24–282) | 79 (33–198) | 123 (21–295) | .61 |
| Presenting opportunistic infections | ||||
| Oral thrush | 34 (26) | 9 (60) | 25 (22) | <.01 |
| Cryptococcemia | 12 (9) | 2 (13) | 10 (9) | .55 |
| PCP pneumonia | 11 (8) | 0 | 11 (10) | .36 |
| Bacterial pneumonia | 38 (29) | 7 (47) | 31 (27) | .11 |
| Symptoms at study enrollment | ||||
| Cough | 37 (28) | 9 (60) | 28 (24) | <.01 |
| Shortness of breath | 15 (12) | 3 (20) | 12 (10) | .27 |
| Night sweats | 34 (26) | 8 (53) | 26 (22) | .01 |
| Weight loss | 57 (44) | 11 (73) | 46 (40) | .01 |
| Fever | 75 (57) | 12 (80) | 63 (54) | .01 |
| Temperature ≥ 38.0○C | 64 (49) | 12 (80) | 52 (45) | .01 |
| ≥1 symptom | 83 (63) | 14 (93) | 69 (60) | .01 |
BMI indicates body mass index; HIV, human immunodeficiency virus; IQR, interquartile range; PCP, pneumocystis pneumonia; SD, standard deviation; TB, tuberculosis.
The presence of any 1 symptom (fever, cough, weight loss, or night sweats) had a sensitivity of 93% in detecting patients with pulmonary TB and a negative predictive value of 98%. If only the 83 patients with at least 1 TB related symptom underwent diagnostic testing, 14 of 15 cases would have been detected and the number needed to test to detect 1 case would be 6. Table 2 contains further performance characteristics of individual symptoms in detecting pulmonary TB.
Table 2.
Performance of Certain Signs and Symptoms in Detecting Patients With Pulmonary Tuberculosis
| Sign(s)/Symptoms(s) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|
| Fever | 80 | 46 | 16 | 95 |
| Cough | 60 | 76 | 24 | 94 |
| Weight loss | 73 | 60 | 19 | 95 |
| Night sweats | 53 | 78 | 24 | 93 |
| Any symptom | 93a | 41 | 17 | 98 |
a1 patient with tuberculosis had no symptoms; they were Xpert MTB/RIF and sputum culture positive for M. tuberculosis.
NPV indicates negative predictive value; PPV, positive predictive value.
During our study period, there were 28 HIV-infected patients who presented to the NCTLD with active TB. All patients were newly diagnosed with HIV after being diagnosed with TB. There were no significant differences among patients detected via our ACF study compared to HIV-infected patients presenting to the NCTLD with active TB in regards to age, gender, drug use, smear positivity, or CD4 count (Table 3).
Table 3.
Comparison of Selected Characteristics Between Confirmed Tuberculosis Among HIV-Infected Patients Diagnosed by Active and Passive Case Finding
| TB Diagnosed via Active Case Finding | TB via Diagnosed Through Passive Case Finding | ||
|---|---|---|---|
| Characteristic | (n = 15) | (n = 28) | P |
| Mean age | 43.6 | 42.6 | |
| Male gender | 11 (73) | 23 (82) | .59 |
| Hepatitis C antibody positive | 11 (73) | 16 (57) | .30 |
| Current tobacco user | 10 (67) | 18 (65) | .88 |
| Alcohol use | 6 (40) | 16 (57) | .28 |
| IDU | 10 (67) | 46 (46) | .21 |
| Prior TB treatment | 0 | 3 (14) | .25 |
| Mean CD4 | 133 | 147 | .73 |
| Median CD4 | 79 | 111 | |
| Sputum smear positive | 4 (27) | 9 (32) | .71 |
| Death | 1 (7) | 4 (14) | .45 |
IDU indicates injection drug user; TB, tuberculosis.
Discussion
Utilizing an ACF strategy, we found a very high prevalence (11.5%) of pulmonary TB among all patients with newly diagnosed HIV who agreed to participate in our study in the country of Georgia. Our findings confirm TB/HIV coinfection as a major problem in Georgia and the utility of ACF as a strategy to enhance TB control activities. This is important to highlight and recognize given that Georgia and surrounding European and former Soviet Union countries are known more for their high rates of MDR and extensively drug-resistant TB with less attention given to TB/HIV coinfection. Our findings also highlight a high rate of HCV infection among persons with TB/HIV coinfection in Georgia. This is likely driven by high rates of injection drug use (67% among TB/HIV patients in our study), similar to what has been found in other surrounding former Soviet republics [17]. Although our sample size was not powered to determine if a symptom-based screening strategy versus universal TB testing strategy was best among PLWH, we did find Xpert MTB/RIF testing was comparable to culture, thus confirming the effectiveness of an ACF strategy using molecular testing and supporting WHO recommendations on using Xpert MTB/RIF testing for the initial diagnostic work for TB among PLWH [18].
According to the latest WHO TB report, the prevalence of HIV among incident TB cases worldwide is 9.2%; however, there is no detailed information included on the rate of active TB among patients with newly diagnosed HIV cases. Focusing only on the HIV prevalence rate among incident TB cases can mislead one into thinking TB/HIV coinfection is not a major problem in certain countries, such as Georgia. Prior data along with current national TB program data have found that only 1%–3% of incident TB cases in Georgia are coinfected with HIV, results that do not indicate TB/HIV as a major problem [19]. However, our study, which focuses on a newly diagnosed HIV population that is much smaller than the number of incident TB cases per year in Georgia, clearly demonstrates the high burden of TB among HIV patients. Our TB prevalence rate (11.5%) was higher than the overall TB rate (5.8%) found in the meta-analyses by Getahun and colleagues, which included ACF studies that systematically included sputum samples from HIV-infected patients regardless of signs and symptoms. The 12 studies included >9000 patients, all of whom were from countries in Southeast Asia or Sub-Saharan Africa [20]. We could not find any published studies from EE or former Soviet Union countries utilizing a universal TB ACF among HIV-infected patients with which to compare our results. Along with prior data finding TB as the leading cause of death among HIV patients in Georgia [21], our findings confirm that TB/HIV coinfection is a major problem in the country, and one that needs more attention, especially considering the 2-fold increase in HIV incidence during the last decade (18.1 new HIV cases per 100, 000 in 2016) [22]. Given the late stage of HIV and high rates of injection drug use among our cohort, potential areas to target for TB prevention include earlier detection of HIV, harm-reduction strategies, and opiate substitution treatment for person using intravenous drugs [23].
The WHO recommends ACF with a preliminary symptom screen followed by confirmatory testing to exclude TB among people living with HIV [24]. This strategy has been limited in part by a low specificity of symptom screening leading to a large number needed to test and consequent laboratory burden [3, 25, 26]. Our results found a comparable sensitivity (93%) and specificity (41%) of the WHO recommended symptom screen as compared to studies outside EE, and our study is one of the first studies providing data in the region [3]. The low specificity of symptom screening for TB is far below the minimum proposed specificity requirement of 70% proposed by the WHO for a TB triage test [27]. The low number of patients with any positive symptom needed to test to detect 1 TB case via Xpert MTB/RIF and culture testing makes a symptom screening strategy feasible to implement in Georgia. As expected in a cohort with paucibacillary disease, the Xpert MTB/RIF outperformed sputum microscopy in our cohort [28, 29] and performed similarly to solid culture.
We found similar demographics and severity of HIV disease among patients whose active TB was detected by either an active or passive case finding strategy. These results demonstrate a need to detect and treat HIV earlier as almost all patients in both cohorts presented with a CD4 count < 200 cells/mm3. Given that initiation of antiretroviral therapy prevents TB, earlier detection and treatment of HIV is essential to decrease TB/HIV burden [30, 31]. The high rate of injection drug use present in both our active and passive TB cohorts is alarming. It presents challenges for care as injection drug use has been associated with poor linkage to HIV care in EE [32], and it suggests that harm-reduction policies may be a potentially effective means to reduce HIV and, subsequently, TB [23]. Addressing the injection drug use epidemic in Georgia and other EE countries is paramount to HIV, HCV, and TB control [32, 33]. Additionally, studies evaluating the yield and implementation challenges of screening persons infected with HCV or who use injection drugs for TB are needed. Finally, the prevalence of cryptococcemia in our cohort was high at 9%. Although TB is the leading cause of death among PLWH in Georgia, cryptococcal disease should not be neglected as it is a major cause of death among people living with HIV in Georgia and can occur simultaneously with TB [8].
Our study is subject to several limitations. About half of those with newly diagnosed HIV infection who were approached for the study declined to participate, and given that the characteristics of patients who refused participation are unknown, our results may not be generalizable to all HIV patients in Georgia. Assuming patients with symptoms were more likely to participate and that all persons not enrolled did not have TB, the estimated prevalence of pulmonary TB among all HIV-infected patients approached for enrollment would still be high at 5.4% (15 out of 276). In addition, 21% of those enrolled in the study were unable to produce a sputum sample. The inability of some participants to provide a sputum sample reflects real world practice and speaks to the need for the development of nonsputum-based diagnostic tests, such as the urine lipoarabinomannan for TB [34]. Collecting sputum samples allowed us to diagnose pulmonary TB only; thus, we likely underestimated the overall prevalence of TB given that extrapulmonary disease is common among PLWH [35]. Additionally, chest x-ray (CXR) information was not performed as part of this study and, thus, information was not available to evaluate and compare the value of radiography for TB case finding.
In summary, we found a very high prevalence of pulmonary TB (11.5%) among patients with newly diagnosed HIV in the country of Georgia by utilizing an ACF strategy. Our findings led to the implementation of a similar national strategy among HIV-infected patients in Georgia with all patients who have a positive symptom screen undergoing Xpert MTB/RIF testing and importantly highlight the need for more attention and research on the prevalence of TB and ACF strategies among HIV-infected patients in Georgia and surrounding countries.
Acknowledgments
N.T., N.C., and R.R.K. contributed to the conception and design of the study. N.T., N.C., I.K., and L.D. carried out data acquisition. N.T., N.C., D.G., H.M.B., C.D.R., M.C.S., K.H., and R.R.K. performed analysis, interpretation, or both, of data. All authors took part in drafting and revising of the article and approved the final version of the article.
Financial support. This work has been supported by the National Institutes of Health Fogarty International Center (D43TW007124), the National Institute of Allergy and Infectious Diseases (K23AI103044; R21AI122001), the Atlanta Clinical and Translational Science Institute (UL1TR000454), the Center for AIDs Research at Emory University (P30AI050409), and the Emory Global Health Institute.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Tornheim JA, Dooley KE. The global landscape of tuberculosis therapeutics. Annu Rev Med 2019; 70:105–20. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization. Global Tuberculosis Report 2018. Geneva, Switzerland: World Health Organization; 2018. [Google Scholar]
- 3. Hamada Y, Lujan J, Schenkel K, et al. Sensitivity and specificity of WHO’s recommended four-symptom screening rule for tuberculosis in people living with HIV: a systematic review and meta-analysis. Lancet HIV 2018; 5:e515–23. [DOI] [PubMed] [Google Scholar]
- 4. Floridia M, Ciccacci F, Andreotti M, et al. Tuberculosis case finding with combined rapid point-of-care assays (Xpert MTB/RIF and determine TB LAM) in HIV-positive individuals starting antiretroviral therapy in Mozambique. Clin Infect Dis 2017; 65:1878–83. [DOI] [PubMed] [Google Scholar]
- 5. Efsen AM, Schultze A, Post FA, et al. ; for the TB:HIV study group in EuroCoord. Major challenges in clinical management of TB/HIV coinfected patients in Eastern Europe compared with Western Europe and Latin America. PLOS ONE 2015; 10:e0145380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Podlekareva DN, Mocroft A, Post FA, et al. ; HIV/TB Study Writing Group Mortality from HIV and TB coinfections is higher in Eastern Europe than in Western Europe and Argentina. AIDS 2009; 23:2485–95. [DOI] [PubMed] [Google Scholar]
- 7. Alemayehu M, Gelaw B, Abate E, et al. Active tuberculosis case finding and detection of drug resistance among HIV-infected patients: a cross-sectional study in a TB endemic area, Gondar, Northwest Ethiopia. Int J Mycobacteriol 2014; 3:132–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chkhartishvili N, Sharvadze L, Chokoshvili O, et al. Mortality and causes of death among HIV-infected individuals in the country of Georgia: 1989–2012. AIDS Res Hum Retroviruses 2014; 30:560–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mansfeld M, Skrahina A, Shepherd L, et al. ; for the TB:HIV study group in EuroCoord Major differences in organization and availability of health care and medicines for HIV/TB coinfected patients across Europe. HIV Med 2015; 16:544–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chkhartishvili N, Chokoshvili O, Bolokadze N, et al. Late presentation of HIV infection in the country of Georgia: 2012–2015. PLOS ONE 2017; 12:e0186835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. World Health Organization. Recommendations For Investigating Contacts of Persons with Infectious Tuberculosis in Low- and Middle-Income Countries. Geneva, Switzerland: World Health Organization; 2012. [PubMed] [Google Scholar]
- 12. Dodd PJ, White RG, Corbett EL. Periodic active case finding for TB: when to look? PLOS ONE 2011; 6:e29130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Balcha TT, Skogmar S, Sturegård E, et al. Outcome of tuberculosis treatment in HIV-positive adults diagnosed through active versus passive case-finding. Glob Health Action 2015; 8:27048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shapiro AE, Variava E, Rakgokong MH, et al. Community-based targeted case finding for tuberculosis and HIV in household contacts of patients with tuberculosis in South Africa. Am J Respir Crit Care Med 2012; 185:1110–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ahmad Khan F, Verkuijl S, Parrish A, et al. Performance of symptom-based tuberculosis screening among people living with HIV: not as great as hoped. AIDS 2014; 28:1463–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tukvadze N, Kempker RR, Kalandadze I, et al. Use of a molecular diagnostic test in AFB smear positive tuberculosis suspects greatly reduces time to detection of multidrug resistant tuberculosis. PLOS ONE 2012; 7:e31563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Altice FL, Azbel L, Stone J, et al. The perfect storm: incarceration and the high-risk environment perpetuating transmission of HIV, hepatitis C virus, and tuberculosis in Eastern Europe and Central Asia. Lancet 2016; 388:1228–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. World Health Organization. A Guide to Monitoring and Evaluation for Collaborative TB/HIV Activities. Geneva, Switzerland: World Health Organization; 2015. [Google Scholar]
- 19. Richards DC, Mikiashvili T, Parris JJ, et al. High prevalence of hepatitis C virus but not HIV co-infection among patients with tuberculosis in Georgia. Int J Tuberc Lung Dis 2006; 10:396–401. [PubMed] [Google Scholar]
- 20. Getahun H, Kittikraisak W, Heilig CM, et al. Development of a standardized screening rule for tuberculosis in people living with HIV in resource-constrained settings: individual participant data meta-analysis of observational studies. PLOS Med 2011; 8:e1000391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chkhartishvili N, Sharvadze L, Chokoshvili O, et al. Mortality and causes of death among HIV-infected individuals in the country of Georgia: 1989–2012. AIDS Res Hum Retroviruses 2014; 30:560–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. European Centre for Disease Prevention and Control/World Health Organization Regional Office for Europe. HIV/AIDS Surveillance in Europe 2017 — 2016 Data. Stockholm, Sweden: European Center for Disease Prevention and Control; 2017. [Google Scholar]
- 23. MacArthur GJ, Minozzi S, Martin N, et al. Opiate substitution treatment and HIV transmission in people who inject drugs: systematic review and meta-analysis. BMJ 2012; 345:e5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. World Health Organization. Systematic screening for active tuberculosis: principles and recommendations. http://www.who.int/tb/tbscreening/en/. [PubMed] [Google Scholar]
- 25. Adelman MW, Tsegaye M, Kempker RR, et al. Intensified tuberculosis case finding among HIV-infected persons using a WHO symptom screen and Xpert(®) MTB/RIF. Int J Tuberc Lung Dis 2015; 19:1197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Martinez L, Andrews JR. Improving tuberculosis case finding in persons living with advanced HIV through new diagnostic algorithms. Am J Respir Crit Care Med 2019; 199:559–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. World Health Organization. High-Priority Target Product Profiles for New Tuberculosis Diagnostics:Report of a Consensus Meeting. Geneva, Switzerland: World Health Organization; 2014. [Google Scholar]
- 28. Lawn SD, Brooks SV, Kranzer K, et al. Screening for HIV-associated tuberculosis and rifampicin resistance before antiretroviral therapy using the Xpert MTB/RIF assay: a prospective study. PLOS Med 2011; 8:e1001067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Al-Darraji HA, Abd Razak H, Ng KP, et al. The diagnostic performance of a single GeneXpert MTB/RIF assay in an intensified tuberculosis case finding survey among HIV-infected prisoners in Malaysia. PLOS ONE 2013; 8:e73717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. INSIGHT START Study Group, Lundgren JD, Babiker AG, et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med 2015; 373:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. TEMPRANO ANRS 12136 Study Group, Danel C, Moh R, et al. A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med 2015; 373:808–22. [DOI] [PubMed] [Google Scholar]
- 32. Croxford S, Yin Z, Burns F, et al. ; on behalf of the OptTEST project. Linkage to HIV care following diagnosis in the WHO European Region: A systematic review and meta-analysis, 2006-2017. PLOS ONE 2018; 13:e0192403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kaplan R, Caldwell J, Middelkoop K, et al. Impact of ART on TB case fatality stratified by CD4 count for HIV-positive TB patients in Cape Town, South Africa (2009-2011). J Acquir Immune Defic Syndr 2014; 66:487–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dorman S. Advances in the diagnosis of tuberculosis: current status and future prospects. Int J Tuberc Lung Dis 2015; 19:504–16. [DOI] [PubMed] [Google Scholar]
- 35. Kwan CK, Ernst JD. HIV and tuberculosis: a deadly human syndemic. Clin Microbiol Rev 2011; 24:351–76. [DOI] [PMC free article] [PubMed] [Google Scholar]