Abstract
Background
Acute decompensated heart failure (ADHF) can be confused with other conditions that cause dyspnea. Patients with ADHF are often simultaneously treated for community-acquired pneumonia (CAP), even when evidence for infection is lacking. We hypothesized that the fluid and sodium content of potentially unnecessary intravenous antibiotic (IVAB) therapy could worsen outcomes of ADHF patients.
Methods
We reviewed 144 ADHF patients at low risk of pneumonia based on diagnostic findings and clinical documentation. The primary end point was length of stay. Secondary outcomes were mortality, readmission rates, amount of diuretic received, and fluid volume and quantity of sodium administered as part of IVAB therapy.
Results
Of the 144 admissions reviewed, 88 did not and 56 did receive IVAB. IVAB-treated patients received an average of 1.7 L of additional fluid (230 mL/d) and 9311 mg of additional sodium (1381 mg/d) as a result of IVAB therapy. Length of stay was longer in the IVAB arm (6.6 days) compared with the no-IVAB arm (3.0 days; P < .001). Patients required more furosemide in the IVAB arm (930 mg) compared with the no-IVAB arm (320 mg; P < .001). Patients who received IVAB were also 2.51 times more likely to be readmitted compared with patients who did not receive IVAB (P = .04).
Conclusions
ADHF patients who received IVAB without evidence of infection had longer lengths of stay, required more diuretics, and were more likely to be readmitted compared with ADHF patients not exposed to IVAB. ADHF patients are a promising target of antibiotic stewardship interventions.
Keywords: antibiotic stewardship, heart failure, pneumonia, procalcitonin, sodium
Patients admitted with a diagnosis of heart failure often receive intravenous antibiotic therapy, even when there is a low likelihood of concurrent infection. This article describes potential adverse effects of the excess fluid and sodium administered as part of intravenous antibiotic therapy in this patient population.
Both ADHF and CAP may present as acute dyspnea, and making a definitive diagnosis of one vs the other can be a challenge. In many cases, the 2 conditions are simultaneously present in the same patient, and other studies have demonstrated that a large proportion of ADHF patients are concurrently treated for suspected pneumonia [1]. To our knowledge, the potential adverse effects of concurrent treatment, especially with respect to the additional sodium and fluid administration from IVAB in ADHF patients, have not been assessed. In this study, we compared the length of stay between patients admitted with ADHF who were given IVABs with patients with the same diagnosis who were not exposed to IVAB therapy. We also evaluated the volume of fluid and quantity of sodium provided as a result of antibiotic infusions to patients admitted with ADHF to assess the potential impact on outcomes.
METHODS
In a single-center, level I trauma center in the United States, patients admitted and discharged with ADHF were retrospectively analyzed. The institutional review board granted approval for the study.
Eligible patients were ≥18 years of age, had a previous diagnosis of heart failure, had been admitted with a diagnosis of ADHF or heart failure exacerbation based on coding data, and had a chest radiograph on the day of admission. Patients were excluded if they were pregnant, had documentation of an infection before admission, had proven infection during admission, or were on antibiotics before admission. Patients were also excluded if they did not have a chest radiograph on admission or had a brain-type natriuretic peptide (BNP) value <100 ng/mL. Patients who had radiographic documentation by a radiologist suggesting pneumonia, consolidation, or questionable pneumonia were also excluded. Example phrases from radiology reports of excluded patients include “adjacent atelectasis/consolidation” and “perihilar airspace opacities may represent multifocal pneumonia.” Example phrases from radiology reports of included patients include “interstitial edema compatible with volume overload” and “pulmonary vascular congestion,” as well as any report that stated “no acute cardiopulmonary findings.” Any patient on hemodialysis or any form of continuous renal replacement therapy was also excluded. Any patient documented to have developed infection during admission (eg, urinary tract infection [UTI], cellulitis, pneumonia by chest radiograph, sepsis, etc.) was excluded from analysis.
Patients who met inclusion criteria were divided into 2 arms: those who were initiated on IVABs at admission and those who did not receive IVAB therapy.
Outcome Measures
The primary outcome of the study was the length of stay between the 2 study arms. Other outcomes that were evaluated included the amount of furosemide administered between the 2 groups and the amount of sodium and volume provided by the IVABs. Admission values for APACHE II scores, procalcitonin (when available), and BNP values were also compared between the study arms. Incidence of Clostridioides difficile infection during hospital admission was evaluated between the groups. Mortality outcomes were based on in-hospital mortality from any cause. Thirty-day readmission rates for any cause and readmissions specifically for heart failure exacerbations were also analyzed between the 2 study groups.
Statistical Analysis
Frequency and cross-tabulation statistics were conducted on all categorical variables. Chi-square tests were used to test for association between independent groups and categorical outcomes. The statistical assumption of normality was assessed using skewness and kurtosis statistics. If either statistic was above an absolute value of 2.0, then the assumption was assumed to be violated. Nonparametric Mann-Whitney U tests were used to compare independent groups on non-normal continuous outcomes. Medians and interquartile ranges were reported and interpreted for the nonparametric comparisons. Statistical significance was assumed at an alpha value of 0.05, and all analyses were conducted using SPSS, version 22.
RESULTS
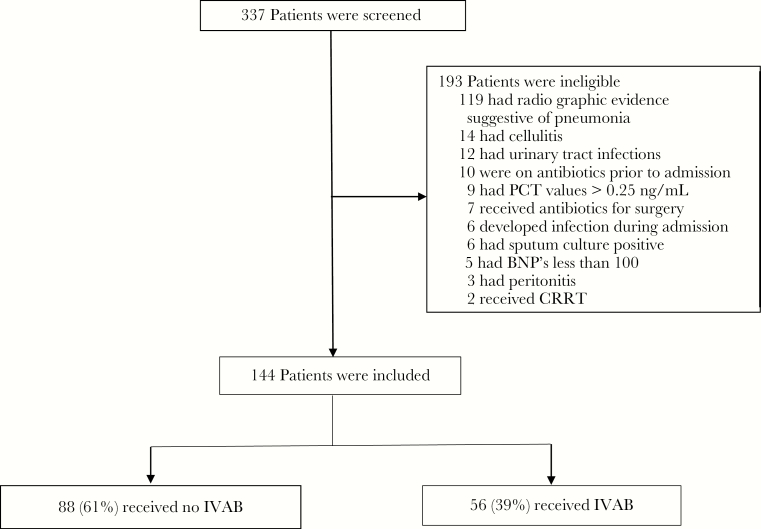
Consecutive patients admitted with a diagnosis of ADHF from July 28, 2016, through August 15, 2017, were retrospectively analyzed. Out of 337 patients screened, 144 met criteria to be included for evaluation. Of the 193 patients excluded, the majority (119) were not enrolled based on radiographic evidence suggestive of concurrent pneumonia. Other patients not enrolled included 12 with documented UTIs, 14 with a diagnosis of concurrent cellulitis, 10 patients who were on antibiotics before admission, 9 with procalcitonin (PCT) values >0.25 ng/mL, 7 patients receiving antibiotics as surgical prophylaxis, 5 with BNP values <100 pg/mL, 6 patients with sputum culture positivity, 2 patients on renal replacement therapy, and 9 patients with other diagnosed infections during their heart failure admission (Figure 1).
Figure 1.
Process of patient selection. Abbreviations: BNP, brain-type natriuretic peptide; CRRT, ; IVAB, intravenous antibiotic; PCT, procalcitonin.
Of the remaining 144 patients, 56 patients (39%) were started on IVAB therapy at the time of their ADHF admission. Patients who received IVAB at admission had higher APACHE II scores than those who did not (mean APACHE II score, 10.1 in the IVAB arm and 8.1 in the no-IVAB arm; P = .001). Otherwise, baseline demographic and laboratory data were similar between the IVAB and no-IVAB groups. Procalcitonin values were similar in the antibiotic (0.10 ng/mL) arm compared with the nonantibiotic (0.09 ng/mL) arm (P = .51) (Table 1).
Table 1.
Baseline Characteristics and Outcomes
| Variable | No Antibiotics (n = 88) | Antibiotics (n = 56) | P Value |
|---|---|---|---|
| Sexc | |||
| Male | 52 (59%) | 31 (55%) | .65 |
| Racec | |||
| Caucasian | 73 (83%) | 48 (86%) | |
| African American | 11 (12.5%) | 5 (9%) | |
| Other | 4 (4.5%) | 3 (5%) | |
| Age,d y | 71 (22) | 73 (18) | .3 |
| BMId | 28.6 (8.1) | 28.6 (7.6) | .99 |
| APACHE IId | 8.1 (3.4) | 10.1 (4.0) | .002 |
| Lab data | |||
| BNP, pg/mLb,e | 1099 (1147) | 927 (1098) | .5 |
| Procalcitonin, ng/mLa,d | 0.09 (0.06) | 0.10 (0.05) | .51 |
| Outcomes | |||
| LOSd | 3.0 (2.90) | 6.60 (5) | <.001 |
| Readmissionsc | 9 (10.1%) | 14 (22.2%) | .043 |
| Furosemide, mge | 320 (440) | 930 (1420) | <.001 |
| Clostridium difficile | 0 (0.00%) | 0 (0.00%) | - |
| Mortality | 0 (0.00%) | 1 (1.80%) | .81 |
Abbreviations: BMI, body mass index; BNP, brain-type natriuretic peptide; IVAB, intravenous antibiotic; LOS, length of stay.
aProcalcitonin values were available for 43 patients (77%) in the IVAB arm and 27 patients (31%) in the no-IVAB arm.
bBNP values were available for 55 patients (98%) in the IVAB arm and 85 patients (97%) in the no-IVAB arm.
cFrequency (%).
dM (SD).
eMedian (interquartile range).
Vancomycin and levofloxacin were the most common IVABs administered to the patients reviewed in this study. Twenty-six received at least 1 dose of vancomycin, and 22 received at least 1 dose of levofloxacin. Supplementary Table 1 lists the number of patients receiving different IVABs during the study period, as well as the sodium content and fluid volume per dose and the median doses of each antibiotic administered. Twenty-six patients in the IVAB arm received 3 or more different antibiotics, and 1 patient received a total of 6 different antibiotics. One patient received 60 intravenous doses of aztreonam and 19 of doxycycline, accounting for 4.9 L of fluid and 44.1 g of sodium for that patient alone.
Length of stay was significantly longer in the antibiotic arm (6.6 days) compared with the nonantibiotic arm (3.0 days; P < .001). Patients required higher total doses of furosemide in the antibiotic arm (mean total dose, 930 mg) compared with the nonantibiotic arm (mean total dose, 320 mg; P < .001). The antibiotic arm of the trial also received approximately 1.7 L of additional fluid (230 mL/d) and 9311 mg of additional sodium (1381 mg/d) per patient. Patients who received antibiotics were also 2.2 times more likely to be readmitted compared with patients who did not receive antibiotics (P = .043) (Table 1).
DISCUSSION
Heart failure is a common cause of dyspnea, with estimates of approximately 23 million people currently affected worldwide and projections for a continued rise in prevalence in the United States over the coming decades. Some estimate the number of new cases to reach 772 000 in the year 2040 [2, 3]. With the growing number of heart failure patients expected and the burden this diagnosis places on the patient and the health care system, it is imperative that we utilize our best clinical judgment in our plan of care toward those admitted with an exacerbation. In our study, we found that patients who received IVABs during an acute heart failure exacerbation had significantly longer lengths of stay compared with patients with the same diagnosis who did not receive antibiotics. The patients who received IVABs also received significantly more doses of furosemide during their hospitalizations. It is standard to volume- and sodium-restrict patients admitted with a heart failure exacerbation in the absence of evidence of volume depletion. As such, it is important to note the amount of sodium and fluid provided to these patients by antibiotics alone. On average, each patient in the antibiotic arm received approximately 1381 mg of sodium daily from antibiotics alone. The patients in the antibiotic arm also received approximately 1.7 L of volume per patient entirely attributed to the antibiotic infusions. Patients with PCT values >0.25 μg/L were excluded from this analysis, as antibiotic therapy is encouraged for patients with PCT values above that threshold [4, 5].
No patient in our study population developed C. difficile infection during the reviewed hospital stay, but incident C. difficile infection is an uncommon event at our institution and our study was not powered to detect this potentially harmful effect of antibiotic therapy. There are several limitations to our study. Given the retrospective cohort design, there is the potential for unobtained confounding variables that could have contributed to the difference in the length of stay for the antibiotic arm. Also, given the retrospective study design, we were unable to control for antibiotic exposure in a prospective fashion and therefore had to rely on other physicians and practitioners to accurately document their reasoning behind antibiotic initiation. Considering the relatively small sample size and single-center design, external validity may be limited.
The patient population we focused on in this study could be deemed at low risk of concurrent infection during their ADHF admission based on laboratory and imaging findings and clinical documentation. The majority of patients treated with IVAB had procalcitonin testing performed, but despite low-risk results, IVAB therapy was still prescribed. The modestly higher mean APACHE II scores noted in the IVAB group may indicate that severity of illness at presentation was a driver of the decision to initiate IVAB therapy. Overall, 39% of the patients we reviewed with ADHF received IVAB therapy during their hospital stay. This reflected a higher rate of antibiotic use in heart failure admissions than described in the study by Dharmarajan et al., where ~29% of patients with ADHF received antibiotic therapy [1]. That study did not review patient-level data to determine if such use was justified based on lab or imaging findings, but it does indicate that antibiotic use is a common practice during ADHF admissions. Their study also found that patients with ADHF who received simultaneous antibiotic therapy had worse outcomes than patients treated solely for heart failure. Whether the worsened outcome was a result of the true presence of multiple simultaneous acute conditions or potentially related to adverse effects of multiple concurrent therapies was not a focus of that study. A prospective intervention focusing on the possibility of direct patient harm from excess fluid volume and sodium from potentially inappropriate antibiotic therapy might prove to be an effective way of impacting prescribing behavior. Additional interventions to encourage early transition from intravenous to oral antibiotic agents could help minimize excess fluid and sodium in cases where concurrent antibiotic therapy is clinically warranted.
In summary, we found that patients admitted with ADHF were significantly more likely to have a longer length of stay if given IVABs on admission, even without radiographic or laboratory evidence to suggest that infection was present. As physicians generally order volume and sodium restriction in patients with ADHF, one should keep in mind that significant amounts of each that are given with common IVABs and recognize the potential harm, additional treatment required, and prolonged length of stay that can be associated with such treatment. Giving patients at low risk of infection antibiotic therapy “just to be safe” may not actually be the safest option.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Gratitude is expressed to the University of Tennessee Pharmacy Department, especially Nichole Szczerbowski, PharmD, BCPS, and Brian McCullough, PharmD, BCPS, for their contribution of volume and sodium data provided by individual antibiotic formulations and to Jeevan Keepa for assistance with data collation.
Financial support. The study was conducted without funding.
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Dharmarajan K, Strait KM, Tinetti ME, et al. Treatment for multiple acute cardiopulmonary conditions in older adults hospitalized with pneumonia, chronic obstructive pulmonary disease, or heart failure. J Am Geriatr Soc 2016; 64:1574–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McMurray JJ, Petrie MC, Murdoch DR, Davie AP. Clinical epidemiology of heart failure: public and private health burden. Eur Heart J 1998; 19(Suppl P):P9–16. [PubMed] [Google Scholar]
- 3. Owan TE, Redfield MM. Epidemiology of diastolic heart failure. Prog Cardiovasc Dis 2005; 47:320–32. [DOI] [PubMed] [Google Scholar]
- 4. Christ-Crain M, Stolz D, Bingisser R, et al. Procalcitonin guidance of antibiotic therapy in community-acquired pneumonia: a randomized trial. Am J Respir Crit Care Med 2006; 174:84–93. [DOI] [PubMed] [Google Scholar]
- 5. Schuetz P, Christ-Crain M, Thomann R, et al. ; ProHOSP Study Group Effect of procalcitonin-based guidelines vs standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. JAMA 2009; 302:1059–66. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.