Abstract
Background
The prevalence and risk of concurrent unhealthy drinking, cigarette use, and depression on mortality among persons living with HIV (PLWH) is unclear. This study applied a syndemic framework to assess whether these co-occurring conditions increase mortality and whether such risk is differential by HIV status.
Methods
We evaluated 6721 participants (49.8% PLWH) without baseline cancer from the Veterans Aging Cohort Study, a prospective, observational cohort of PLWH and matched uninfected veterans enrolled in 2002 and followed through 2015. Multivariable Cox proportional hazards regressions estimated risk of a syndemic score (number of conditions: that is, unhealthy drinking, cigarette use, and depressive symptoms) on all-cause mortality by HIV status, adjusting for demographic, health status, and HIV-related factors.
Results
Fewer than 10% of participants had no conditions; 25.6% had 1, 51.0% had 2, and 15.0% had all 3. There were 1747 deaths (61.9% PLWH) during the median follow-up (11.4 years). Overall, age-adjusted mortality rates/1000 person-years increased with a greater number of conditions: (0: 12.0; 1: 21.2; 2: 30.4; 3: 36.3). For 3 conditions, the adjusted hazard ratio of mortality was 36% higher among PLWH compared with uninfected participants with 3 conditions (95% confidence interval, 1.07–1.72; P = .013), after adjusting for health status and HIV disease progression. Among PLWH and uninfected participants, mortality risk persisted after adjustment for time-updated health status.
Conclusions
Syndemic unhealthy drinking, cigarette use, and depression are common and are associated with higher mortality risk among PLWH, underscoring the need to screen for and treat these conditions.
Keywords: alcohol, depression, HIV, mortality, smoking
Globally, cigarette use and unhealthy alcohol consumption result in 7 million [1] and 3 million [2] deaths, respectively, each year. Three hundred million people in the world live with depression [3]. Cigarette use (20%–24%), unhealthy alcohol use (17%–25%), and depression (7%) are prevalent in the general population and are attributed to a combined $600 billion in health care costs and lost productivity in the United States [4]. Among persons living with HIV (PLWH), the prevalence rates of smoking (20% former, 44% current) [5], unhealthy alcohol use (25%) [6], including alcohol use disorder (10%) [7], and depression (18% to 81%) [8] are high.
Individually, cigarette use, unhealthy alcohol consumption, and depression are associated with an increased risk of death [9]. However, the extent to which these conditions cluster—known as a syndemic [10]—in this population is unclear. A syndemic refers to 2 or more adverse conditions—in this case, psychosocial and behavioral factors—that cluster and/or interact to exacerbate poor health outcomes in disadvantaged populations [11]. A longitudinal study among PLWH of the Veteran’s Aging Cohort Study (VACS) found that unhealthy alcohol use, smoking, and depressive symptoms were temporally concordant and that suspension of any 1 factor was associated with cessation of the other 2, suggesting that perhaps the co-occurrence of these 3 conditions constitutes a unique syndrome [12]. Although it is expected that co-occurring conditions will be associated with an increased risk of mortality, the magnitude of risk associated with clustering and the degree to which such syndemic risk is greater for PLWH as compared with uninfected people are not known. Given that each of these conditions, like HIV infection, are pro-inflammatory and that unhealthy alcohol use and depression are associated with antiretroviral therapy nonadherence, the clustering of these conditions, particularly if the prevalence of clustering is high, may result in a substantial excess risk of mortality among PLWH compared with uninfected people.
If unhealthy alcohol use, smoking, and depression do cluster, if they are associated with increased mortality, and if PLWH have particularly high risk for mortality when burdened with this syndemic, as hypothesized, then it is prudent that clinicians screen and provide treatment for these conditions in practice. Evidence suggests that all 3 of these conditions are behaviorally and biologically linked [13], making them amenable to combined treatment with behavior therapy and medication, with increasing evidence for use of a single medication/intervention to treat simultaneous conditions (eg, varenicline for alcohol and tobacco) [14] to minimize polypharmacy [15].
The objectives of this analysis were to determine (1) the prevalence of having more than 1 condition (ie, syndemic of unhealthy alcohol use, cigarette use, and/or depression); (2) the risk of death associated with the number of syndemic conditions among people living with and without HIV infection; and (3) whether the mortality risk associated with number of syndemic conditions was differential by HIV status.
METHODS
Study Design
The Veterans Aging Cohort Study Survey Cohort [16] is an observational, longitudinal cohort of veterans living with HIV infection and age/race/site-matched uninfected comparators enrolled in 2002 or thereafter. All participants were free of cancer at baseline and were followed from their baseline date until date of death, the last follow-up date, or were censored on September 30, 2015 (n = 7504). Exclusion criteria were people that seroconverted during follow-up, had unknown HIV status, had missing components of the Patient Health Questionnaire (PHQ-9), and those with last follow-up date on/before the baseline date (n = 446). We also excluded never-drinkers as there were too few to analyze (n = 256). We identified deaths from the VA Vital Status file, which uses data from the Social Security Death Master File, VA Beneficiary Identification and Records Locator Subsystem, and the Veterans Health Administration Medical Statistical Systems inpatient data sets.
Unhealthy alcohol use, cigarette use, and depressive symptoms were assessed at VACS survey baseline. We used the Alcohol Use Disorders Identification Test (AUDIT-C) [17] to assess unhealthy drinking (>14 drinks per week for men and >7 drinks per week for women) and heavy episodic drinking (5+ drinks for men and 4+ drinks for women on 1 occasion). Cigarette use was measured through self-report of past and current smoking. We assessed depressive symptoms using the PHQ-9 [18]; those with a PHQ-9 score ≥10 were considered positive for depressive symptoms. We used a count variable to assess the concurrence of unhealthy drinking, cigarette use, and depressive symptoms, ranging from 0 to 3 (all conditions).
Based on prior literature in the field, we adjusted for several baseline demographic and chronic disease variables as covariates. Baseline demographic data included age, race/ethnicity, and education. Diabetes was defined as having any of the following indicators: abnormal glucose measurement, use of medications to treat diabetes, or at least 1 inpatient or 2 outpatient International Classification of Diseases, Ninth Revision (ICD-9), codes for diabetes. Hypertension was categorized as having no hypertension (blood pressure [BP] < 140/90 mmHg with no antihypertensive medication use), controlled (BP < 140/90 mmHg with antihypertensive medication use), and uncontrolled (BP ≥ 140/90 mmHg), using the average BP measurement of 3 routine outpatient clinical assessments closest to the baseline date. Low-density lipoprotein, high-density lipoprotein, and triglycerides were measured during routine clinical practice and were considered continuous variables. Obesity was defined as a body mass index ≥30 kg/m2. Prevalent chronic obstructive pulmonary disease and cardiovascular disease were defined as having 1 inpatient or 2 outpatient ICD-9 codes at baseline. Additionally, the VACS Index [19] was used to adjust for general health status and includes 6 clinical variables (CD4 cell count, HIV RNA load, hepatitis C serostatus, hemoglobin, estimated glomerular filtration [eGFR], and fibrosis-4 [FIB-4]). Prevalent post-traumatic stress disorder was defined using the ICD-9 code for diagnosis at baseline. Illicit drug use was self-reported and included the use of cocaine/crack, other stimulants (amphetamines), and opioids (heroin, morphine, codeine, opium).
Statistical Analysis
First, we assessed baseline characteristics by number of syndemic conditions. Second, we calculated age-adjusted all-cause mortality rates per 1000 person-years and constructed age-adjusted survival curves by number of conditions for the total sample, stratified by HIV status. Third, we tested the interaction of HIV status and number of concurrent conditions. Because this interaction was significant (P = .02), we constructed an 8-level HIV/number of conditions variable and reported hazards ratios (HRs) and 95% confidence intervals (CIs) with uninfected people with 0 conditions as the reference group using a Cox proportional hazards model of the total sample, adjusting for the aforementioned covariates. We then constructed Cox proportional hazards models, stratified by HIV status.
Multiple imputations using chained equations with 5 separate imputed data sets were generated based on the predictive mean matching method using the Hmisc library of the R programming language. Cox survival models were fitted in each imputed data set and were combined to obtain pooled hazard ratios and standard errors. All analyses were performed using R software (version 3.3.3; www.r-project.org).
Ethical Considerations
Approval of the VACS was obtained from the institutional review board of Yale School of Medicine. These analyses were approved by the institutional review board of Vanderbilt University Medical Center.
RESULTS
The final evaluable data set consisted of 6721 people (3347 PLWH and 3374 uninfected) (Table 1). The majority were male (95.0%) and nonwhite (64.8% black), with a median age of 50.0 years. Fewer than 10% of the total sample had no conditions, with a majority having at least 2 conditions (65.2% in PLWH, 66.9% in uninfected) (Table 1). Prevalence and mortality rate estimates specific to each condition and combinations of conditions, stratified by HIV status, are presented in Supplementary Tables 1 and 2.
Table 1.
Baseline Characteristics of Study Population by Number of Syndemic Behavioral Conditions
| No. of Syndemic Conditions | |||||
|---|---|---|---|---|---|
| Characteristics | Total | 0 | 1 | 2 | 3 |
| n = 6721 | 560 (8.3%) | 1722 (25.6%) | 3429 (51.0%) | 1010 (15.0%) | |
| Age, median (IQR), y | 50.0 (44.4–55.3) | 45.65 (38.6–54.1) | 49.8 (43.0–56.4) | 50.6 (45.6–55.4) | 49.8 (45.8–54.3) |
| Sex, No. (%) | |||||
| Women | 338 (5.0) | 54 (9.6) | 125 (7.3) | 115 (3.4) | 44 (4.4) |
| Men | 6383 (95.0) | 506 (90.4) | 1597 (92.7) | 3314 (96.6) | 966 (95.6) |
| Race/ethnicity, No. (%) | |||||
| White, non-Hispanic | 1448 (21.5) | 137 (24.5) | 443 (25.7) | 648 (18.9) | 220 (21.8) |
| Black, non-Hispanic | 4358 (64.8) | 342 (61.1) | 1025 (59.5) | 2373 (69.2) | 618 (61.2) |
| Hispanic | 659 (9.8) | 65 (11.6) | 168 (9.8) | 296 (8.6) | 130 (12.9) |
| Other | 256 (3.8) | 16 (2.9) | 86 (5.0) | 112 (3.3) | 42 (4.2) |
| Education, No. (%) | |||||
| < High school | 492 (7.3) | 14 (2.5) | 88 (5.2) | 276 (8.1) | 114 (11.4) |
| High school or equivalent | 2205 (32.8) | 93 (16.8) | 480 (28.1) | 1233 (36.4) | 399 (39.9) |
| > High school | 3951 (58.8) | 447 (80.7) | 1139 (66.7) | 1879 (55.5) | 486 (48.6) |
| Diabetes (glucose measurement, antidiabetic agent use, and/ or at least 1 inpatient/2 outpatient ICD-9 codes for diabetes), No. (%) | 1201 (17.9) | 92 (16.4) | 284 (16.5) | 624 (18.2) | 201 (19.9) |
| Hypertension, No. (%) | |||||
| No (BP < 140/90 mm Hg, no antihypertensive medication) | 2338 (34.8) | 247 (44.1) | 610 (35.4) | 1155 (33.7) | 326 (32.3) |
| Controlled (BP < 140/90 mm Hg, on antihypertensive medication) | 2617 (38.9) | 187 (33.4) | 658 (38.2) | 1341 (39.1) | 431 (42.7) |
| Uncontrolled (BP ≥ 140/90 mm Hg) | 1762 (26.2) | 125 (22.3) | 453 (26.3) | 931 (27.2) | 253 (25.0) |
| LDL cholesterol ≥ 160 mg/dL, blood, No. (%) | 532 (7.9) | 49 (8.8) | 155 (9.0) | 261 (7.6) | 67 (6.6) |
| HDL cholesterol < 40 mg/dL, blood, No. (%) | 3074 (45.7) | 286 (51.1) | 862 (50.1) | 1482 (43.2) | 444 (44.0) |
| Triglycerides ≥ 200 mg/dL, blood, No. (%) | 1505 (22.4) | 108 (19.3) | 409 (23.8) | 752 (21.9) | 236 (23.4) |
| Obesity (body mass index ≥ 30 kg/m2), No. (%) | 1894 (28.2) | 186 (33.2) | 526 (30.5) | 913 (26.6) | 269 (26.6) |
| COPD, No. (%) | 762 (11.3) | 27 (4.8) | 136 (7.9) | 421 (12.3) | 178 (17.6) |
| Prevalent CVD, No. (%) | 1060 (15.8) | 40 (7.1) | 245 (14.2) | 585 (17.1) | 190 (18.8) |
| VACS Index, median (IQR) | 21.0 (10.0–33.0) | 12.0 (6.0–27.0) | 18.0 (10.0–33.0) | 22.0 (11.0–34.0) | 22.0 (12.0–35.0) |
| Hepatitis C infection, No. (%) | 2368 (35.2) | 54 (9.6) | 362 (21.0) | 1413 (41.2) | 539 (53.4) |
| Anemia (hemoglobin < 13 g/dL men; <12 g/dL women), No. (%) | 1382 (20.6) | 90 (16.1) | 326 (18.9) | 727 (21.2) | 239 (23.7) |
| Renal disease (estimated GFR, mL/min/1.73 m2), No. (%) | |||||
| <30 | 95 (1.4) | 5 (0.9) | 24 (1.4) | 52 (1.5) | 14 (1.4) |
| 30–59 | 357 (5.3) | 28 (5.0) | 109 (6.3) | 181 (5.3) | 39 (3.9) |
| ≥60 | 6174 (91.9) | 514 (91.8) | 1564 (90.8) | 3151 (91.9) | 945 (93.6) |
| PTSD (ICD-9 code), No. (%) | |||||
| No | 5695 (84.7) | 538 (96.1) | 1590 (92.3) | 2890 (84.3) | 677 (67.0) |
| Yes | 1026 (15.3) | 22 (3.9) | 132 (7.7) | 539 (15.7) | 333 (33.0) |
| Illicit drug use, No. (%) | 2682 (39.9) | 20 (3.6) | 321 (18.6) | 1675 (48.8) | 666 (65.9) |
| Cigarette use, No. (%) | |||||
| Current | 3426 (51.0) | 0 (0.0) | 486 (28.2) | 2155 (62.8) | 785 (77.7) |
| Former | 1690 (25.1) | 0 (0.0) | 377 (21.9) | 1088 (31.7) | 225 (22.3) |
| Never | 1605 (23.9) | 560 (100.0) | 859 (49.9) | 186 (5.4) | 0 (0.0) |
| Alcohol consumption, No. (%) | |||||
| Not current drinker | 2265 (33.7) | 0 (0.0) | 390 (22.6) | 1457 (42.5) | 418 (41.4) |
| Not hazardous | 1694 (25.2) | 560 (100.0) | 950 (55.2) | 184 (5.4) | 0 (0.0) |
| Unhealthy and/or alcohol use disorder | 2762 (41.1) | 0 (0.0) | 382 (22.2) | 1788 (52.1) | 592 (58.6) |
| Depressive symptoms (PHQ-9 ≥ 10), No. (%) | |||||
| No | 5254 (78.2) | 560 (100.0) | 1635 (94.9) | 3059 (89.2) | 0 (0.0) |
| Yes | 1467 (21.8) | 0 (0.0) | 87 (5.1) | 370 (10.8) | 1010 (100.0) |
| HIV status, No. (%) | |||||
| Uninfected (HIV-) | 3374 (50.2) | 298 (53.2) | 876 (50.9) | 1718 (50.1) | 482 (47.7) |
| Infected (HIV+) | 3347 (49.8) | 262 (46.8) | 846 (49.1) | 1711 (49.9) | 528 (52.3) |
| HIV-1 RNA viral load ≥ 400 copies/mL (% of HIV+) | 1752 (52.3) | 127 (48.5) | 446 (52.7) | 885 (51.7) | 294 (55.7) |
| CD4+ T-cell count < 500 cells/μL3 (% of HIV+) | 2267 (67.7) | 173 (66.0) | 572 (67.6) | 1151 (67.3) | 371 (70.3) |
| On antiretroviral therapy, No. (% of HIV+) | 2678 (80.0) | 211 (80.5) | 704 (83.2) | 1354 (79.1) | 409 (77.5) |
Diabetes was identified using a previously validated metric that incorporates glucose measurements, antidiabetic agent use, and/or at least 1 inpatient or 2 outpatient ICD-9 codes for diabetes; hepatitis C virus sero-positivity was defined as a positive hepatitis C virus antibody test result or at least 1 inpatient or 2 outpatient ICD-9 codes for this diagnosis. VACS Index includes 6 clinical variables (CD4 cell count, HIV RNA load, hepatitis C serostatus, hemoglobin, estimated glomerular filtration, and fibrosis-4). Total missing frequency = 81.
Abbreviations: AUDIT-C, Alcohol Use Disorders Identification Test; BMI, body mass index; BP, blood pressure; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; GFR, glomerular filtration rate; HDL, high-density lipoprotein; IQR, interquartile range; LDL, low-density lipoprotein; PHQ-9, Patient Health Questionnaire; PTSD, post-traumatic stress disorder.
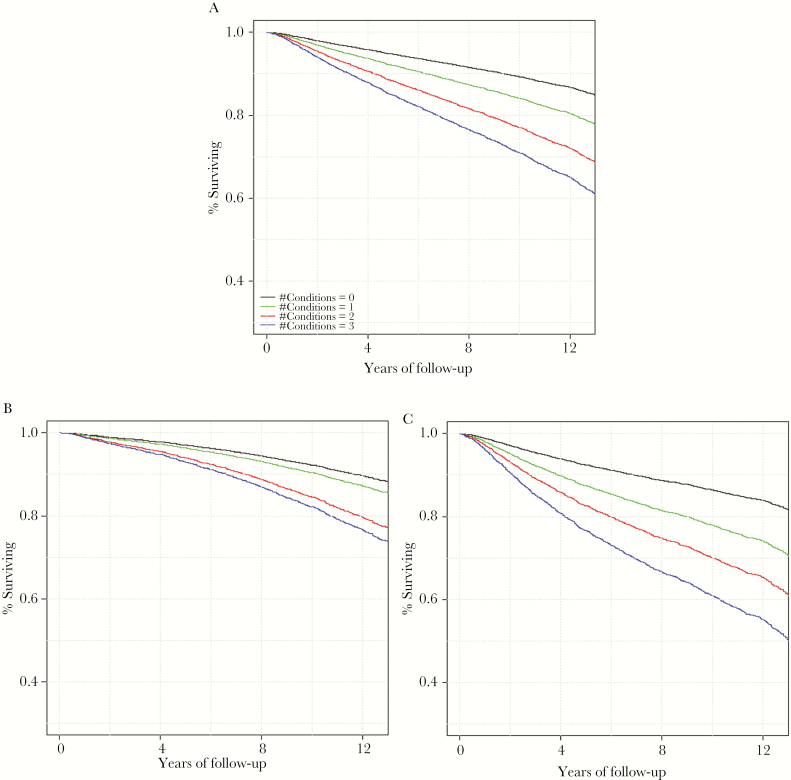
During the median follow-up period of 11.4 years, there were 1747 deaths (32.3% among PLWH, 19.7% among uninfected people). Of the total deaths, 12.5% occurred among those with no conditions, 21.4% among those with 1, 28.6% among those with 2, and 32.5% among those with all 3 conditions (Table 2). Mortality rates increased with number of conditions for people living with and without HIV (log-rank test P < .001) (Figure 1). For those with all 3 conditions, about 30% of uninfected people died during the follow-up period, whereas nearly 50% of PLWH died during that same time period.
Table 2.
Unadjusted Mortality and Survival by Number of Syndemic Behavioral Conditions
| No. of Concurrent Conditions | |||||
|---|---|---|---|---|---|
| Total | 0 | 1 | 2 | 3 | |
| n = 6721 | 560 (8.3%) | 1722 (25.6%) | 3429 (51.0%) | 1010 (15.0%) | |
| All-cause mortality, No. (%) | 1747 (27.0) | 70 (12.5) | 368 (21.4) | 981 (28.6) | 328 (32.5) |
| Survival, median (IQR), y | |||||
| HIV-uninfected | 12.0 (8.9–12.8) | 11.9 (8.7–12.6) | 11.5 (7.2–12.5) | 11.1 (7.3–12.4) | |
| HIV-infected | 12.0 (8.3–12.7) | 11.7 (7.1–12.5) | 11.0 (5.9–12.4) | 9.6 (4.5–12.2) | |
“Conditions” refers to unhealthy alcohol use, cigarette smoking, and depressive symptoms.
Abbreviation: IQR, interquartile range.
Figure 1. .
Age-adjusted survival curves by baseline number of syndemic conditions among the total sample and by HIV status. A, Age-adjusted (50.0 years) all-cause mortality among the total sample. B, Age-adjusted all-cause mortality among HIV-uninfected people. C, Age-adjusted all-cause mortality among HIV-infected people. “Conditions” refers to unhealthy alcohol use, cigarette smoking, and depressive symptoms.
There was a significant interaction between HIV status and number of conditions on all-cause mortality (P = .02) (Table 3). In adjusted models, compared with uninfected people with 0 conditions, PLWH with 3 conditions had over twice the mortality risk, whereas uninfected people with 3 conditions had 59% greater risk. In direct comparison, this excess risk in PLWH corresponds to 36% greater mortality risk compared with uninfected people (HR, 1.36; 95% CI, 1.07–1.72; P = .013) among those with 3 conditions. In HIV status–stratified models, the association between syndemic conditions and mortality among PLWH remained after further adjustment for time-updated health status and HIV viral load and CD4+ T-cell counts (included in the VACS Index) (Table 4).
Table 3.
Hazard Ratios (95% CI) for Interactive Effect of HIV Status and Syndemic Conditions on Mortality
| HIV-Uninfected | HIV-Infected | Pairwise Comparison | |||||
|---|---|---|---|---|---|---|---|
| No. of Concurrent Conditions | HR (95% CI) | P Value | No. of Concurrent Conditions | HR (95% CI) | P Value | HR (95% CI) | P Value |
| Adjusted for VACS Index | |||||||
| HIV-/0 conditions | 1.0 (ref) | — | HIV+/0 conditions | 0.89 (0.56–1.44) | .641 | ||
| HIV-/1 condition | 1.55 (1.05–2.30) | .028 | HIV+/1 condition | 1.50 (1.02–2.20) | .038 | 0.97 (0.78–1.19) | .752 |
| HIV-/2 conditions | 2.14 (1.48–3.11) | <.001 | HIV+/2 conditions | 1.93 (1.33–2.79) | .001 | 0.90 (0.78–1.03) | .126 |
| HIV-/3 conditions | 2.10 (1.40–3.15) | <.001 | HIV+/3 conditions | 2.53 (1.72–3.73) | <.001 | 1.21 (0.95–1.53) | .117 |
| Adjusted for VACS Index and other predictorsa | |||||||
| HIV-/0 conditions | 1.0 (ref) | — | HIV+/0 conditions | 0.95 (0.59–1.53) | .827 | ||
| HIV-/1 condition | 1.39 (0.94–2.06) | .102 | HIV+/1 condition | 1.49 (1.01–2.20) | .043 | 1.08 (0.87–1.33) | .502 |
| HIV-/2 conditions | 1.75 (1.20–2.55) | .004 | HIV+/2 conditions | 1.78 (1.22–2.60) | .003 | 1.02 (0.89–1.18) | .784 |
| HIV-/3 conditions | 1.59 (1.05–2.40) | .029 | HIV+/3 conditions | 2.15 (1.45–3.20) | <.001 | 1.36 (1.07–1.72) | .013 |
“Conditions” refers to unhealthy alcohol use, cigarette smoking, and depressive symptoms. HIV-infected and uninfected people with 2 conditions had 1.78 (95% CI, 1.22–2.60; P < .01) and 1.75 (95% CI, 1.20–2.55; P < .01) times greater mortality risk, respectively, compared with uninfected people with 0 conditions. HIV-infected and -uninfected people with 3 conditions had 2.15 (95% CI, 1.45–3.20; P < .001) and 1.59 (95% CI, 1.05–2.40; P = .029) times greater mortality risk, respectively, compared with uninfected people with 0 conditions. HIV-infected people with 3 conditions had 1.36 times greater mortality risk (95% CI, 1.07–1.72; P = .013) compared with HIV-uninfected people with 3 conditions.
Abbreviations: CI, confidence interval; HIV-, HIV-uninfected; HIV+, HIV-infected; HR, hazard ratio; VACS Index, Veterans Aging Cohort Study index.
a“Other predictors”: race/ethnicity, education, cardiovascular disease risk factors (hypertension, diabetes, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides, and obesity), illicit drug use, post-traumatic stress disorder, prevalent chronic obstructive pulmonary disease, and cardiovascular disease.
Table 4. .
Adjusted Hazard Ratios (95% CI) for Association Between Syndemic Conditions and Mortality by HIV Status
| No. of Concurrent Conditions | Deaths, No. (%) | Age-Adjusted Mortality Rate/1000 Person-Years | Model A | Model B | Model C | |||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |||
| Total sample | ||||||||
| 0 (n = 560) | 70 (12.5) | 12 (9.4–15.1) | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) | |||
| 1 (n = 1722) | 368 (21.4) | 21.2 (19.1–23.5) | 1.63 (1.26–2.10) | <.001 | 1.49 (1.15–1.93) | .002 | 1.40 (1.08–1.81) | .01 |
| 2 (n = 3429) | 981 (28.6) | 30.4 (28.5–32.3) | 2.15 (1.69–2.74) | <.001 | 1.82 (1.42–2.33) | <.001 | 1.70 (1.33–2.18) | <.001 |
| 3 (n = 1010) | 328 (32.5) | 36.3 (32.5–40.4) | 2.54 (1.96–3.29) | <.001 | 1.98 (1.51–2.60) | <.001 | 1.97 (1.50–2.58) | <.001 |
| HIV-uninfected | ||||||||
| 0 (n = 298) | 30 (10.1) | 9.6 (6.6–13.5) | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) | |||
| 1 (n = 576) | 146 (25.3) | 16.1 (13.6–18.8) | 1.50 (1.01–2.22) | .044 | 1.38 (0.93–2.06) | .107 | 1.42 (0.96–2.12) | .08 |
| 2 (n = 1718) | 385 (22.4) | 22.9 (20.7–25.2) | 2.09 (1.44–3.03) | <.001 | 1.73 (1.18–2.54) | .005 | 1.71 (1.16–2.50) | .006 |
| 3 (n = 482) | 105 (21.8) | 22.6 (18.5–27.2) | 2.03 (1.36–3.05) | .001 | 1.51 (0.98–2.31) | .061 | 1.55 (1.01–2.38) | .047 |
| HIV-infected | ||||||||
| 0 (n = 262) | 40 (15.3) | 14.8 (10.7–19.9) | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) | |||
| 1 (n = 846) | 222 (26.2) | 26.9 (23.5–30.5) | 1.69 (1.20–2.36) | .002 | 1.56 (1.12–2.20) | .01 | 1.42 (1.01–1.99) | .045 |
| 2 (n = 1711) | 596 (34.8) | 38.5 (35.5–41.7) | 2.18 (1.58–3.00) | <.001 | 1.87 (1.34–2.60) | <.001 | 1.70 (1.22–2.37) | .002 |
| 3 (n = 528) | 223 (42.2) | 50.9 (44.5–57.9) | 2.84 (2.03–3.98) | <.001 | 2.28 (1.60–3.25) | <.001 | 2.23 (1.56–3.18) | <.001 |
“Conditions” refers to unhealthy alcohol use, cigarette smoking, and depressive symptoms. Model A was adjusted for baseline Veterans Aging Cohort Index; Model B was adjusted for baseline Veterans Aging Cohort Index, race/ethnicity, education, cardiovascular disease risk factors (hypertension, diabetes, low-density lipoprotein [LDL] cholesterol, high-density lipoprotein [HDL] cholesterol, triglycerides, and obesity), illicit drug use, post-traumatic stress disorder (PTSD), prevalent chronic obstructive pulmonary disease, and prevalent cardiovascular diseases; Model C was adjusted for time-updated Veterans Aging Cohort Index, race/ethnicity, education, cardiovascular disease risk factors (hypertension, diabetes, LDL cholesterol, HDL cholesterol, triglycerides, and obesity), PTSD, illicit drug use, prevalent chronic obstructive pulmonary disease, and prevalent cardiovascular disease.
Abbreviations: CI, confidence interval; HR, hazards ratio.
DISCUSSION
In the VACS, the syndemic of unhealthy alcohol use, cigarette use, and depressive symptoms was common among people living with and without HIV: overall, two-thirds of participants had at least 2 of these conditions, and 15% had all 3. For both groups, the syndemic was associated with an increased risk of mortality. Of note, concurrent unhealthy alcohol use and smoking was the most prevalent combination among those with 2 syndemic conditions (89%) and was present in >50% of the deaths (Supplementary Table 2). The syndemic was independently associated with higher mortality risk for PLWH compared with their uninfected counterparts after controlling for comorbidity status and HIV progression.
Although prior studies have reported that unhealthy alcohol use [20], cigarette use [21], and depression [22] are individually associated with higher mortality, this study is the first to directly measure and compare the impact of syndemic unhealthy alcohol use, cigarette use, and depressive symptoms on mortality and between PLWH and uninfected people. Some research demonstrates these conditions to be temporally concordant (ie, maintenance of cessation of 1 condition was associated with maintenance or cessation of the other 2 conditions, respectively) [12]. Thus, our results have important implications for the integrated screening and management of unhealthy alcohol use, cigarette use, and depression, particularly among PLWH.
Based on the prevalence of each individual factor (Table 1), the expected prevalence of a participant having all 3 conditions was 6.8%, yet the observed syndemic prevalence of all 3 conditions (ie, unhealthy alcohol use, cigarette use, and depressive symptoms) was 15.0%, presenting more than by chance alone. This finding, along with prior VACS research showing temporal concordance of these factors over time [12], supports existing evidence that these conditions share underlying behavioral and biological mechanisms. Well-studied behavioral pathways among these conditions suggest that substance use, particularly unhealthy alcohol use, increases the risk for depression, and it has been observed that depressive symptoms decrease upon treatment for alcohol use disorder [23, 24]. Conversely, behavioral research has also indicated that those with depression are more likely to engage in substance use as a means to “self-medicate” or reduce negative affect [25].
The biological underpinnings of concurrent unhealthy alcohol use, cigarette use, and depression are less understood but stem in part from an underlying dysfunction of the executive system in the context of competing influences of impulsive and executive decision-making [13], encompassing dopaminergic, serotonergic, and cholinergic systems [26, 27]. Shared biological mechanisms of these conditions support the possibility of parsimonious behavioral and pharmacologic treatment, through cognitive behavioral therapy and medications such as varenicline, a partial agonist of the α4β2 nicotinic acetylcholine receptor, which has demonstrated effectiveness for smoking cessation and potential efficacy for treatment of alcohol use in humans [14], and bupropion, an antidepressant developed for the treatment of major depressive disorder and the first non-nicotine medication proven effective for smoking cessation [28].
Presently, the US Preventive Services Task Force [29] recommends that clinicians assess all adults aged 18 years and older for alcohol misuse, tobacco use, and depression and provide support to reduce alcohol and tobacco use and ensure effective diagnosis, treatment, and follow-up for those with depression. Regardless of these recommendations, unhealthy alcohol use, cigarette use, and depression remain underscreened and undertreated [30–32], particularly among PLWH compared with uninfected populations [33, 34]. Further, guidelines do not address the simultaneous assessment or management of these conditions. Given the syndemic occurrence of these conditions and the impact on mortality, health care professionals should screen for all 3 conditions, especially when a positive screening for any 1 condition occurs in clinical practice. Research supports the combination of behavioral therapy and pharmacotherapy as the most effective treatment for smoking cessation, alcohol use disorder, and depression [35]. This supports the need for increased implementation of these treatments in clinical care settings using integrated models of care [36]. The fact that these conditions are syndemic, are associated with increased risk of mortality, and may share similar biology is particularly important for PLWH and their providers because this clinical population is at great risk for polypharmacy [15].
Limitations
The current study has several limitations. First, the number of conditions was considered at baseline; therefore, we cannot comment on cause and effect among conditions or how changes in conditions may result in changes in risk for all-cause mortality. Second, as the sample consisted of mostly men, our findings may not be generalizable to women. Although the prevalence of smoking and depression among PLWH is similar to estimates found in other cohorts of PLWH, unhealthy alcohol use was markedly higher in this veteran cohort, which may not be representative of nonveteran PLWH. Third, this study was not powered to model the differential magnitude of effect of each specific condition, as it was beyond the scope of the current study, although we do provide rates in Supplementary Table 2. Fourth, although the PHQ-9 is a well-validated tool to measure major depressive symptoms in clinical settings, it is possible that use of the PHQ-9 could have misclassified people as having depressive symptoms who may have different mental health issues, such as bipolar disorder. Fifth, we did not include other substance use (ie, illicit drug use and/or nonmedical use of prescription drugs) as additional syndemic conditions. We focus specifically on the syndemic of unhealthy alcohol use, smoking, and depressive symptoms for 2 main reasons: (1) these 3 conditions commonly cluster together [12] through strong behavioral and biological links that are amenable to combined treatment, as previously discussed, and (2) standardized clinical data to evaluate the baseline prevalence of each condition are available. For these reasons, we expect that people who use other substances will have distinctly different risk profiles. Sixth, we are unable to provide insight on specific causes of death related to the number of syndemic conditions, as adjudicated causes of death are not currently available in the VACS.
CONCLUSIONS
The syndemic of unhealthy alcohol use, cigarette use, and depressive symptoms is common among PLWH. The number of conditions was incrementally associated with increased mortality among those with HIV, with a 36% greater effect among PLWH with all 3 conditions than their uninfected counterparts. This association persisted after controlling for important cofounders including general health status and HIV progression. These findings support aggressive screening and treatment efforts for unhealthy alcohol use, cigarette use, and depression as a syndemic and, when possible, adopting effective parsimonious treatments. Future research should focus on the longitudinal effects of this syndemic on morbidity and mortality, as well as investigation of other associated conditions such as polysubstance use.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We would like to thank the participants of the Veterans Aging Cohort Study.
Financial support. This work was supported by the National Center for Advancing Translational Sciences (TL1TR002244 to N.C.); ViTAL: The Vanderbilt Center for Tobacco, Addiction and Lifestyle (to H.T., M.F., N.C.); and V-CREATE: Vanderbilt Clinical Cardiovascular Outcomes Research and Trials Evaluation (to M.F., H.A.T., N.C.). The Veterans Aging Cohort Study was funded by the National Institute on Alcohol Abuse and Alcoholism (U24-AA020794, U01-AA020790, U01-AA02201, and U10 AA013566).
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Report on the global tobacco epidemic, 2017: monitoring tobacco use and prevention policies. Geneva: World Health Organization; 2017. Licence: CC BY-NC-SA 3.0 IGO. [Google Scholar]
- 2. World Health Organization. Global status report on alcohol and health. Geneva: World Health Organization; 2018. Licence: CC BY-NC-SA 3.0 IGO. [Google Scholar]
- 3. World Health Organization. Depression and other common mental disorders: Global Health Estimates. Geneva: World Health Organization; 2017. Licence: CC BY-NC-SA 3.0 IGO. [Google Scholar]
- 4. Brown RL, Smith MA. Population-level quality measures for behavioral screening and intervention. Am J Med Qual 2016; 31:323–30. [DOI] [PubMed] [Google Scholar]
- 5. Akhtar-Khaleel WZ, Cook RL, Shoptaw S, et al. . Trends and predictors of cigarette smoking among HIV seropositive and seronegative men: the multicenter aids cohort study. AIDS Behav 2016; 20:622–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Monroe AK, Lau B, Mugavero MJ, et al. . Heavy alcohol use is associated with worse retention in HIV care. J Acquir Immune Defic Syndr 2016; 73:419–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sullivan LE, Goulet JL, Justice AC, Fiellin DA. Alcohol consumption and depressive symptoms over time: a longitudinal study of patients with and without HIV infection. Drug Alcohol Depend 2011; 117:158–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Arseniou S, Arvaniti A, Samakouri M. HIV infection and depression. Psychiatry Clin Neurosci 2014; 68:96–109. [DOI] [PubMed] [Google Scholar]
- 9. Graham NA, Frost-Pineda K, Gold MS. Tobacco and psychiatric dual disorders. J Addict Dis 2007; 26(Suppl 1):5–12. [DOI] [PubMed] [Google Scholar]
- 10. Singer M, Bulled N, Ostrach B, Mendenhall E. Syndemics and the biosocial conception of health. Lancet 2017; 389:941–50. [DOI] [PubMed] [Google Scholar]
- 11. The Lancet. Syndemics: health in context. Lancet 2017; 389:881. [DOI] [PubMed] [Google Scholar]
- 12. Braithwaite RS, Fang Y, Tate J, et al. . Do alcohol misuse, smoking, and depression vary concordantly or sequentially? A longitudinal study of HIV-infected and matched uninfected veterans in care. AIDS Behav 2016; 20:566–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bickel WK, Moody L, Quisenberry AJ, et al. . A competing neurobehavioral decision systems model of SES-related health and behavioral disparities. Prev Med 2014; 68:37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Litten RZ, Ryan ML, Fertig JB, et al. ; NCIG (National Institute on Alcohol Abuse and Alcoholism Clinical Investigations Group) Study Group. A double-blind, placebo-controlled trial assessing the efficacy of varenicline tartrate for alcohol dependence. J Addict Med 2013; 7:277–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Justice AC, Gordon KS, Skanderson M, et al. ; VACS Project Team. Nonantiretroviral polypharmacy and adverse health outcomes among HIV-infected and uninfected individuals. AIDS 2018; 32:739–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Justice AC, Dombrowski E, Conigliaro J, et al. . Veterans Aging Cohort Study (VACS): overview and description. Med Care 2006; 44:S13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Saunders JB, Aasland OG, Babor TF, et al. . Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons With Harmful Alcohol Consumption–II. Addiction 1993; 88:791–804. [DOI] [PubMed] [Google Scholar]
- 18. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001; 16:606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Justice AC, Modur SP, Tate JP, et al. ; NA-ACCORD and VACS Project Teams. Predictive accuracy of the veterans aging cohort study index for mortality with HIV infection: a North American cross cohort analysis. J Acquir Immune Defic Syndr 2013; 62:149–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Justice AC, McGinnis KA, Tate JP, et al. . Risk of mortality and physiologic injury evident with lower alcohol exposure among HIV infected compared with uninfected men. Drug Alcohol Depend 2016; 161:95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Helleberg M, May MT, Ingle SM, et al. . Smoking and life expectancy among HIV-infected individuals on antiretroviral therapy in Europe and North America. AIDS 2015; 29:221–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Leserman J, Pence BW, Whetten K, et al. . Relation of lifetime trauma and depressive symptoms to mortality in HIV. Am J Psychiatry 2007; 164:1707–13. [DOI] [PubMed] [Google Scholar]
- 23. Kelly JF, Stout RL, Magill M, et al. . Mechanisms of behavior change in alcoholics anonymous: does alcoholics anonymous lead to better alcohol use outcomes by reducing depression symptoms? Addiction 2010; 105:626–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sullivan LE, Fiellin DA, O’Connor PG. The prevalence and impact of alcohol problems in major depression: a systematic review. Am J Med 2005; 118:330–41. [DOI] [PubMed] [Google Scholar]
- 25. Williams J, Jones SB, Pemberton MR, et al. . Measurement invariance of alcohol use motivations in junior military personnel at risk for depression or anxiety. Addict Behav 2010; 35:444–51. [DOI] [PubMed] [Google Scholar]
- 26. Koob GF. Addiction is a reward deficit and stress surfeit disorder. Front Psychiatry 2013; 4:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Koob GF. Theoretical frameworks and mechanistic aspects of alcohol addiction: alcohol addiction as a reward deficit disorder. Curr Top Behav Neurosci 2013; 13:3–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jorenby DE, Leischow SJ, Nides MA, et al. . A controlled trial of sustained-release bupropion, a nicotine patch, or both for smoking cessation. N Engl J Med 1999; 340:685–91. [DOI] [PubMed] [Google Scholar]
- 29. US Preventive Services Task Force. https://www.uspreventiveservicestaskforce.org/Page/Name/home. Accessed 22 August 2018. [Google Scholar]
- 30. Conigliaro J, Gordon AJ, McGinnis KA, et al. ; Veterans Aging Cohort 3-Site Study. How harmful is hazardous alcohol use and abuse in HIV infection: do health care providers know who is at risk? J Acquir Immune Defic Syndr 2003; 33:521–5. [DOI] [PubMed] [Google Scholar]
- 31. Strauss SM, Tiburcio NJ, Munoz-Plaza C, et al. . HIV care providers’ implementation of routine alcohol reduction support for their patients. AIDS Patient Care STDS 2009; 23:211–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Centers for Disease Control and Prevention. Vital signs: communication between health professionals and their patients about alcohol use — 44 states and the District of Columbia. 2011. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6301a4.htm?s_cid=mm6301a4_w. Accessed 17 August 2018. [Google Scholar]
- 33. Williams EC, Lapham GT, Shortreed SM, et al. . Among patients with unhealthy alcohol use, those with HIV are less likely than those without to receive evidence-based alcohol-related care: a national VA study. Drug Alcohol Depend 2017; 174:113–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Crothers K, Goulet JL, Rodriguez-Barradas MC, et al. . Decreased awareness of current smoking among health care providers of HIV-positive compared to HIV-negative veterans. J Gen Intern Med 2007; 22:749–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ait-Daoud N, Lynch WJ, Penberthy JK, et al. . Treating smoking dependence in depressed alcoholics. Alcohol Res Health 2006; 29:213–20. [PMC free article] [PubMed] [Google Scholar]
- 36. Edelman EJ, Maisto SA, Hansen NB, et al. . Integrated stepped alcohol treatment for patients with HIV and alcohol use disorder: a randomised controlled trial. Lancet HIV. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.