Abstract
Background
Although existing literature supports durations of 5–7 days for skin and soft tissue infections (SSTIs), longer durations are commonly used. Obesity and heart failure (HF) have been associated with increased risk for treatment failure of SSTIs; however, whether prolonged antibiotic durations reduce the risk of treatment failure is unknown. We evaluated practice patterns for SSTIs in patients with obesity and/or HF and whether short antibiotic durations (≤8 days) were associated with treatment failure.
Methods
We performed a single-center, retrospective cohort study of inpatients between January 1, 2006, and December 30, 2016, with SSTIs based on International Classification of Diseases (ICD) coding, and obesity and/or HF. Charts were manually reviewed to collect demographic, clinical, treatment, and outcome data. Propensity score matching was used to estimate the risk of treatment failure between the 2 groups. Secondary outcomes included length of stay, 30-day readmission, and Clostridium difficile infection rates.
Results
A total of 207 patients were included. Forty-nine (23.7%) received a short antibiotic duration and 158 (76.3%) a long duration. The median duration of therapy (interquartile range [IQR]) was 7 (7–8) days in the short group and 14 (10–15) days in the long group. In the propensity score–matched cohort, 28 (28.6%) treatment failures occurred in the long group, as compared with 5 (10.2%) in the short group (P = .02), as well as a shorter length of stay (IQR) in the short- vs long-duration group (2 [2–3] vs 3 [2–5] days, respectively; P = .002). There was no difference in other secondary outcomes.
Conclusions
The majority of patients with obesity or HF received a longer antibiotic course for SSTIs; however, a longer antibiotic course was not associated with lower treatment failure rates. Higher failure rates in the long-duration group may be reflective of clinical decisions made in the face of diagnostic uncertainty and warrant further evaluation.
Keywords: antibiotic, cellulitis, heart failure, obesity, skin and soft tissue infections
Skin and soft tissue infections (SSTIs) are among the most common infections in hospitalized patients, with an estimated annual hospitalization rate of 156.2 per 100 000 persons in the United States, with cellulitis or abscesses accounting for 10% of all infectious disease–related hospitalizations [1]. SSTI treatment failure or recurrence rates range from 10% to 20%, with identified risk factors for treatment failure including obesity, lymphedema, heart failure (HF), and history of cancer [2–5]. Treatment failures often lead to subsequent courses of broad-spectrum antibiotics, readmission, and the perceived need for longer durations of therapy to prevent recurrence [6, 7].
Infectious Diseases Society of America (IDSA) guidelines recommend a 5-day antibiotic course for cellulitis and erysipelas but mention that treatment should be extended if symptoms have not improved [8]. Limited data exist regarding the optimal length of treatment for SSTI. The most commonly recommended and studied lengths of treatment range from 5 to 10 days [8–12]. Two randomized controlled trials compared 5- to 6-day antibiotic courses with 10 days for uncomplicated cellulitis in varying patient populations and found no difference in outcomes [12, 13]. Despite these recommendations, providers commonly prescribe longer antibiotic courses for SSTIs [14–17], with nearly a third of patients treated longer than 14 days [14]. Whether longer antibiotic durations for SSTI prevent treatment failure in patients with obesity or HF is unknown.
With rising rates of antibiotic-resistant organisms, there is an urgent need to understand the shortest effective duration of antibiotic therapy for all infections in order to avoid unnecessary antibiotic exposure. Prolonged durations of antibiotic therapy are associated with increasing rates of adverse drug events and antibiotic resistance [18]. Providers and antibiotic stewards need further tools to inform antibiotic prescribing recommendations and encourage prescribing of an optimal treatment duration that balances clinical outcomes with adverse drug events. Our primary objective was to determine whether a short duration of antibiotic therapy (≤8 days) as compared with a long duration (>8 days) was associated with an increased rate of SSTI treatment failure in patients with obesity and/or HF. We also aimed to evaluate practice patterns for SSTIs in patients with obesity and/or HF.
METHODS
Study Setting and Participants
We performed a retrospective cohort study of all adult inpatients at the Salt Lake City Veterans Affairs (VA) Medical Center with SSTI and obesity and/or HF between January 2006 and December 2016 to assess the impact of treatment duration on treatment response. The Salt Lake City VA Medical Center is a 122-bed hospital with 5000 discharges per year that serves veterans throughout the Intermountain West. Inpatients over the age of 18 were identified retrospectively based on International Classification of Diseases, 9th and 10th revision (ICD-9/10), codes for SSTI present on admission. Patients were further included if they had a history of obesity and/or HF and received at least 1 empiric SSTI targeted antibiotic within 48 hours of admission and for a minimum of 5 days. Patients were excluded if (1) the SSTI was complicated by deep underlying tissue infection (eg, osteomyelitis, septic arthritis, fasciitis), infected wounds, and/or bacteremia; (2) they had previous treatment for an SSTI in the 30 days before admission; (3) they had infection localized to the head and neck area, rectum, perineum, or genitals; (4) they were immunosuppressed; (5) they were transferred to or from another facility where hospitalization occurred; (6) they received antibiotics for a non-SSTI indication; or (7) they died within 5 days of admission.
Data Collection and Definitions
After initial derivation of the cohort based on SSTI-related coding present on admission, further inclusion and exclusion criteria were applied based on ICD-9/10 coding and manual review of the electronic medical record. Further demographic, comorbid medical, severity of illness, microbiological, treatment, and outcomes data were retrieved by manual review and entered into a secure REDCap database. History of HF was defined as HF-related ICD-9/10 codes within 2 years before the date of admission. Obesity was defined as a body mass index (BMI) ≥30 on admission. The Charlson comorbidity index was used to assess chronic comorbidities. Immunosuppression was defined as a concurrent coded diagnosis of neutropenia, absolute neutrophil count (ANC) ≤500 cells/mm3 during index hospitalization, hematopoietic stem cell transplant or solid organ transplantation at any time before the hospital visit, receipt of antirejection, biologic, or disease-modifying antirheumatic drugs (DMARDs) in the previous 3 months, systemic chemotherapy in the previous 6 weeks or use of systemic corticosteroids ≥20 mg prednisone equivalents for ≥2 of the 4 previous weeks. SSTI targeted antibiotics were adapted from IDSA guidelines and local susceptibilities and defined as any antibiotic with activity against Streptococci and/or Staphylococcus aureus, including methicillin-resistant S. aureus (MRSA) [8]. Cutaneous cultures were defined as cultures for the current encounter collected from abscess, tissue, or wound swab. History of SSTI was defined as a prior episode per the provider’s admission note or problem list, excluding patients with SSTI in the last 30 days. SSTIs were classified as purulent or nonpurulent based on an evaluation of the provider’s assessment, differential diagnosis, and physical exam of the affected site using a predefined list of terms consistent with purulent infection. Lower extremity edema was defined as evidence for the current episode documented in the provider’s admission note. Need for intensive care unit (ICU)–level care, leukocytosis, presence of fever, vasopressors, and mechanical ventilation within 48 hours of admission were collected to assess for severity of illness.
The primary exposure was duration of antibiotic therapy, defined as short duration (≤8 days) and long duration (>8 days). A cutoff of ≤8 days for short duration was chosen based on existing literature, observed durations of therapy, and rounding errors that commonly occur at the time of discharge. Antibiotic duration included antibiotics during inpatient admission and those continued after hospital discharge prescribed for SSTI. Anti-MRSA active antibiotic agents included trimethoprim-sulfamethoxazole, trimethoprim, ceftaroline, vancomycin, linezolid, daptomycin, clindamycin, doxycycline, tetracycline, minocycline, telavancin, dalbavancin, or oritavancin administered for ≥48 hours. The primary outcome was treatment failure for SSTI, defined as any of the following in the 30 days after discharge: (1) extending therapy beyond the originally planned treatment course as determined at the time of hospital discharge, (2) changing or adding antimicrobials after hospital discharge, (3) reinitiating antimicrobials after completion of the originally planned treatment course, or (4) incision and drainage after the end of the original planned antibiotic course. Secondary outcomes included length of hospital stay, 30-day readmission, 30-day SSTI-related readmission, and Clostridium difficile infection (CDI) within 60 days of admission.
Data Analysis
The 2 treatment groups were compared based on duration of antibiotic treatment. Propensity score matching was used to reduce the impact of confounding by indication as patients prescribed long antibiotic durations may differ on baseline characteristics in comparison with those prescribed short durations. Propensity scores were calculated using a multivariable regression model in which the dependent variable was antibiotic duration. Covariates included in the model generating propensity scores included age, history of SSTI, obesity, lower extremity edema, and Charlson comorbidity index. Assessments were made for multicollinearity and goodness of fit. Using a 0.2 caliper width, 1:2 nearest neighbor matching without replacement was performed [19]. Additionally, as a sensitivity analysis, a propensity score–weighted logistic regression was performed for the full cohort, evaluating heart failure, obesity, and treatment duration as predictors of treatment failure. The χ2 and Fisher exact tests were used to compare baseline categorical variables as appropriate between matched pairs. The Wilcoxon rank-sum test was used to compare baseline continuous variables. For all statistical tests, 2-sided P values of <.05 were considered statistically significant. All statistical analyses were performed using R, version 3.5.0 (R Foundation for Statistical Computing, Vienna, Austria). The University of Utah Institutional Review Board and the Salt Lake City VA Office of Research and Development approved this study.
RESULTS
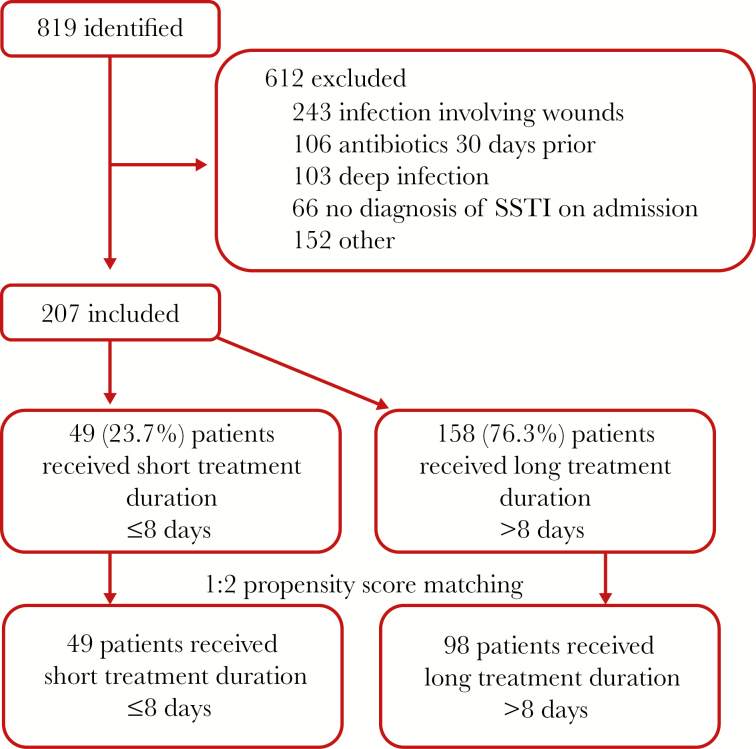
We identified 819 unique patients, of whom 207 met inclusion criteria (Figure 1). The most common reasons for exclusion were SSTIs involving wounds (39.7%), antibiotics in the previous 30 days (17.3%), and deep tissue infection (16.8%). The majority of patients were male (203/207, 98%) (Table 1). Eighty-six percent (178/207) of patients were obese, and 30% (62/207) had HF. Nearly half (97/207, 46.9%) of patients had lower extremity edema, and 32.9% (68/207) of patients were diabetic. Forty-nine patients (23.7%) received a short antibiotic duration and 158 (76.3%) a long duration. The median duration of therapy (interquartile range [IQR]) was 7 (7–8) days in the short-duration group and 14 (10–15) days in the long-duration group (P < .001). Patients receiving a long duration were more likely to have a history of SSTI (22.8% vs 10.2%; P = .08) and receive MRSA active antibiotics for >48 hours (68.4% vs 36.7%; P < .001). There was a nonsignificantly higher rate of obesity (88.0% vs 79.6%; P = .21), lower extremity edema (49.4% vs 38.8%; P = .26), and a white blood cell count >12 000 cells/mm3 on admission (46.2% vs 34.7%; P = .21) in patients receiving a long duration as compared with a short duration. Twenty-four percent of patients overall (50/207) had a purulent infection with a similar distribution in the short- and long-duration groups (26.5% vs 23.4%, respectively; P = .80). There was no difference in proportion of patients colonized with MRSA between the short- and long-duration groups (12.2% vs 16.5%; P = .65) (Table 2). Additionally, there was no difference in acquisition of cutaneous cultures (14.3% vs 22.2%; P = .57) or growth of MRSA (4.1% vs 6.3%; P = .56) comparing the short- vs long-duration groups.
Figure 1. .
Study flowchart. Abbreviation: SSTI, skin and soft tissue infection.
Table 1. .
Demographic and Clinical Characteristics of Hospitalized Adults With Skin and Soft Tissue Infections Receiving Short (≤8 Days) or Long (>8 Days) Durations of Antibiotic Therapy
| Overall Cohort | Propensity Score–Matched Cohort | |||||
|---|---|---|---|---|---|---|
| Characteristic | Short Duration (n = 49) | Long Duration (n = 158) | P Value | Short Duration (n = 49) | Long Duration (n = 98) | P Value |
| Age, median (IQR), y | 68 (57–75) | 64 (59–73) | .38 | 68 (57–75) | 67 (76–59.3) | .90 |
| Gender (male) | 49 (100.0) | 154 (97.5) | .60 | 49 (100) | 96 (98.0) | .80 |
| BMI, median (IQR), kg/m2 | 34 (30–40) | 34.4 (31–40) | .90 | 34 (30–40) | 33.5 (30–39.5) | .53 |
| Comorbidities | ||||||
| Heart failure only | 10 (20.4) | 19 (12.0) | .14 | 10 (20.4) | 16 (16.3) | .54 |
| Obesity only | 33 (67.6) | 112 (70.9) | .64 | 33 (67.3) | 67 (68.4) | .90 |
| Heart failure & obesity | 6 (12.2) | 27 (17.1) | .42 | 6 (12.2) | 15 (15.3) | .62 |
| Diabetes with complications | 9 (18.4) | 25 (15.8) | .84 | 9 (18.4) | 15 (15.3) | .81 |
| Lower extremity edema | 19 (38.8) | 78 (49.4) | .26 | 19 (38.8) | 41 (42.9) | .77 |
| Lymphedema | 0 (0) | 2 (1.3) | 1.00 | 0 (0) | 2 (2.0) | .80 |
| History of SSTI | 5 (10.2) | 36 (22.8) | .08 | 5 (10.2) | 9 (9.2) | 1.00 |
| Injection drug use | 4 (8.2) | 7 (4.4) | .51 | 4 (8.2) | 4 (4.1) | .52 |
| Charlson comorbidity index, median (IQR) | 2 (1–3) | 2 (1–3) | .75 | 2 (1–3) | 2 (1–3) | .59 |
| Type of infection | ||||||
| Nonpurulent cellulitis | 36 (73.5) | 121 (76.6) | .80 | 36 (73.5) | 74 (75.5) | .95 |
| Purulent cellulitis | 13 (26.5) | 37 (23.4) | .80 | 13 (26.5) | 24 (24.5) | .95 |
| Cutaneous abscess | 7 (14.3) | 21 (13.3) | 1.0 | 7 (14.3) | 13 (13.3) | 1.00 |
| Location of infection | ||||||
| Torso | 1 (8.2) | 11 (7.0) | 1.0 | 4 (8.2) | 4 (4.1) | .52 |
| Upper extremity | 12 (24.5) | 37 (23.4) | 1.0 | 12 (24.5) | 24 (24.5) | 1.00 |
| Lower extremity | 31 (63.3) | 108 (68.4) | .63 | 31 (63.3) | 70 (71.4) | .41 |
| Head and neck | 2 (4.1) | 1 (0.6) | .28 | 2 (4.1) | 0 (0) | .21 |
| Buttock | 1 (2.0) | 2 (1.3) | 1.0 | 1 (2.0) | 1 (1.0) | 1.00 |
| Bilateral lower extremity | 2 (4.1) | 5 (3.2) | 1.0 | 2 (4.1) | 4 (4.1) | 1.00 |
| Clinical characteristics | ||||||
| ICU on admission | 1 (2.0) | 4 (2.5) | 1.00 | 1 (2.0) | 2 (2.0) | 1.00 |
| Fever on admission | 13 (26.5) | 47 (29.7) | .80 | 13 (26.5) | 29 (29.6) | .85 |
| WBC ≥12 000 cells/mm3 on admission | 17 (34.7) | 73 (46.2) | .21 | 17 (34.7) | 45 (45.9) | .26 |
| ICU on day 5 | 0 (0) | 1 (0.6) | 1.0 | 0 (0) | 0 (0) | 1.00 |
| Fever on day 5 | 0 (0) | 1 (0.6) | 1.0 | 0 (0) | 0 (0) | 1.00 |
| WBC ≥12 000 cells/mm3 on day 5 | 3 (6.1) | 15 (9.5) | .66 | 3 (6.1) | 10 (10.2) | .61 |
| Antibiotic therapy | ||||||
| Anti-MRSA active agent for ≥48 h | 18 (36.7) | 108 (68.4) | <.001 | 18 (36.7) | 65 (66.3) | .001 |
| Days of intravenous antibiotics, median (IQR) | 2 (1–3) | 4 (3–6) | <.001 | 2 (1–3) | 4 (3–6.8) | <.001 |
| Total antibiotic days, median (IQR) | 7 (7–8) | 14 (10–15) | <.001 | 7 (7–8) | 13 (10–15) | <.001 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: BMI, body mass index; ICU, intensive care unit; IQR, interquartile range; MRSA, methicillin-resistant Staphylococcus aureus; SSTI, skin and soft tissue infection; WBC, white blood cell.
Table 2. .
Microbiologic Characteristics of Hospitalized Adults With Skin and Soft Tissue Infections Receiving Short (≤8 Days) and Long (>8 Days) Durations of Antibiotic Therapy
| Overall Cohort | Propensity Score–Matched Cohort | |||||
|---|---|---|---|---|---|---|
| Characteristic | Short Duration (n = 49) | Long Duration (n = 158) | P Value | Short Duration (n = 49) | Long Duration (n = 98) | P Value |
| MRSA colonized | 6 (12.2) | 26 (16.5) | .65 | 6 (12.2) | 13 (13.3) | .71 |
| Any cutaneous culture obtained | 7 (14.3) | 35 (22.2) | .23 | 7 (14.3) | 22 (22.5) | .24 |
| Wound or tissue culture | 3 (6.1) | 18 (11.4) | .68 | 3 (6.1) | 13 (13.3) | .45 |
| Abscess culture | 4 (8.2) | 17 (10.8) | 4 (8.2) | 9 (9.2) | ||
| Any microorganism identified | 7 (14.3) | 29 (18.4) | .51 | 7 (14.3) | 18 (18.4) | .54 |
| Polymicrobial | 0 (0) | 11 (7.0) | .06 | 0 (0) | 9 (9.2) | .03 |
| Staphylococcus aureus | 3 (6.1) | 17 (10.8) | .34 | 3 (6.1) | 10 (10.2) | .41 |
| MSSA | 1 (2.0) | 7 (4.4) | .45 | 1 (2.0) | 5 (5.1) | .38 |
| MRSA | 2 (4.1) | 10 (6.3) | .56 | 2 (4.1) | 5 (5.1) | .78 |
| Streptococcal species | 3 (6.1) | 8 (5.1) | .77 | 3 (6.1) | 5 (5.1) | .80 |
| Other gram-positive | 1 (2.0) | 9 (5.7) | .30 | 1 (2.0) | 7 (7.1) | .20 |
| Aerobic gram-negative | 0 (0) | 4 (2.5) | .26 | 0 (0) | 3 (3.1) | .22 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-susceptible Staphylococcus aureus.
Outcomes
Overall treatment failure occurred in 21.7% (45/207) of patients (Table 3). After propensity score matching, there were 49 patients in the short-duration group matched to 98 patients in the long-duration group. In the propensity score–matched cohort, there were 28 (28.6%) treatment failures in the long-duration group, as compared with 5 (10.2%) in the short-duration group (P = .02). In a sensitivity analysis among the full cohort using a propensity score–weighted regression, short treatment duration remained significantly associated with reduced treatment failure (adjusted odds ratio, 0.36; 95% confidence interval, 0.13 to 0.99), whereas heart failure and obesity were not associated with treatment failure. When evaluating secondary outcomes in the propensity score–matched cohort, patients in the short-duration group had a reduced median length of stay (IQR) as compared with the long-duration group (2 [2–3] vs 3 [3–5] days, respectively; P ≤ .001). There was a nonsignificantly higher readmission rate in the long-duration group as compared with the short-duration group (16.3% vs 8.2%, respectively; P = .27). There was no difference in CDI rates (4.1% [2/49] vs 0%, short vs long, respectively; P = .21).
Table 3. .
Primary Secondary Outcomes in Hospitalized Adults With Skin and Soft Tissue Infections Receiving Short (≤8 Days) and Long (>8 Days) Durations of Antibiotic Therapy
| Overall Cohort | Propensity Score–Matched Cohort | |||||
|---|---|---|---|---|---|---|
| Characteristic | Short Duration (n = 49) | Long Duration (n = 158) | P Value | Short Duration (n = 49) | Long Duration (n = 98) | P Value |
| Treatment failure | 5 (10.2) | 40 (25.3) | .04 | 5 (10.2) | 28 (28.6) | .02 |
| Length of stay, median (IQR), d | 2 (2–3) | 3 (2–5) | .001 | 2 (2–3) | 3 (3–5) | <.001 |
| 30-d readmission | 4 (8.2) | 27 (17.1) | .19 | 4 (8.2) | 16 (16.3) | .27 |
| 30-d SSTI-related readmission | 1 (2.0) | 8 (5.1) | .61 | 1 (2.0) | 5 (5.1) | .66 |
| Clostridium difficile infection | 2 (4.1) | 1 (0.6) | .28 | 2 (4.1) | 0 (0) | .21 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: BMI, body mass index; IQR, interquartile range; SSTI, skin and soft tissue infection.
DISCUSSION
Although obesity and HF have been identified as risk factors for SSTI treatment failure, the optimal duration of therapy that balances clinical outcomes with risk of antibiotic-associated adverse events is unknown [5]. IDSA guidelines make a strong recommendation for 5 days of antibiotic therapy for uncomplicated cellulitis; however, guidelines also state that treatment should be extended if the infection has not improved within this time period [8]. Unfortunately, no definition of clinical improvement is provided, and retrospective evaluations show that treatment durations of 14 days are commonly prescribed [17]. The US Food and Drug Administration (FDA) now recommends a primary efficacy end point of clinical response at 48–72 hours for clinical trials for SSTIs [20]. Although the clinical importance of this definition has been questioned, recent trials evaluating new antibiotics at varying durations for SSTIs have shown no difference in secondary outcomes including clinical treatment success 1–2 weeks after the end of treatment even in patients without early response [10, 21, 22]. Our goal was to evaluate treatment patterns for SSTI in patients with obesity and/or HF and whether antibiotic duration is associated with treatment failure in this population. Our findings indicate that patients with obesity and/or HF commonly receive but do not benefit from longer antibiotic treatment durations for SSTIs. We evaluated propensity-matched pairs and found a higher treatment failure rate and longer length of stay in the group treated with a longer antibiotic duration, with no difference in CDI or readmission rates. Our observations may benefit stewardship efforts aimed at minimizing unnecessary durations of therapy, but perhaps more importantly, they engender questions regarding definitions of SSTI treatment failure for future studies.
Although those prescribed longer durations were more likely to experience treatment failure, we do not believe this was as a direct result of their antibiotic treatment course. We hypothesize that the higher rate of treatment failure was related to the following 3 factors: (1) patients in the long group having a higher rate of conditions associated with failure and recurrence, (2) misinterpretation of expected response to antibiotic treatment, and (3) misdiagnosis.
Patients in the long-course group had a higher rate of conditions known to predispose to SSTIs and treatment failure, such as a history of SSTI, lower extremity edema, and obesity. It is possible that these patients simply experienced higher rates of recurrence given known risk factors for SSTIs.
Alternatively, uncertainty about the expected response may have contributed to higher failure rates in patients who received a long duration. Prospective studies evaluating treatment responses in cellulitis suggest that at day 3 of treatment, 17% of patients still have a fever, 23% have persistent leukocytosis, and 4% still have lesion spread [23, 24]. Additionally, in 1 study, >50% of patients had residual inflammation at the end of treatment [23], and the time required for resolution of redness and swelling was reported to exceed 10 days [25]. Interestingly, higher BMI and cardiovascular disease were predictors of nonresponse at day 3 [23]. No difference in treatment success rates has been seen in the subset of patients with nonresponse in the first 48–72 hours in randomized controlled trials comparing short (6 days) vs long (10 days) antibiotic durations for SSTIs [21]. We did not evaluate time to follow-up, but it is possible that patients in the long-duration group were evaluated for resolution sooner than patients in the short-duration group or, more likely, had delayed resolution beyond the expected course given higher rates of obesity and lower extremity edema, resulting in perceived treatment failure and retreatment.
Moreover, there is often diagnostic ambiguity in patients with presumed cellulitis and clinical mimickers such as lower extremity edema, leading to a misdiagnosis rate of 30% to 90% [26–28]. It is possible that treatment failure based on our definition was reflective of these diagnostic inaccuracies in a population with cellulitis clinical mimickers (eg, lower extremity edema) and other dermatoses misdiagnosed as SSTI, potentially explaining the higher failure rate with longer antibiotic duration.
Our study has several limitations. First, given the retrospective observational study design, it is possible that there are confounding factors associated with treatment decisions and clinical outcomes we did not include. We tried to account for this by use of propensity score matching to isolate the effect of treatment duration. Second, this is a single-center study involving a predominantly male VA cohort and may not be generalizable to other settings. Arguing against this are data from other VA and non-VA settings reflecting similar treatment patterns for SSTI and clinical failure rates [5, 14]. Given our reliance on ICD-9/10 coding for the diagnosis of HF, we were unable to collect information retrospectively on HF severity. It is possible that there were more patients in the long-duration group with more severe heart failure (eg, New York Heart Association functional classification class III/IV), placing them at higher risk of relapse. Additionally, given the retrospective nature of our study, we were unable to collect physical exam data on cellulitis severity aside from fever and leukocytosis. To our knowledge, there is no validated severity measure for SSTIs; however, it is possible that there were more patients in the long-duration group with more severe disease, leading to higher relapse rates. Even if these imbalances in severity of disease exist, our data suggest that longer durations do not prevent treatment failures. Additionally, we excluded a significant number of patients with complicated infections to arrive at the final cohort, which may limit the impact of our findings. However, estimates of annual hospitalizations for SSTI derive from patient populations similar to our cohort, suggesting potential for far-reaching implications should our findings lead to shorter durations of antibiotic therapy. Our definition of clinical failure involved observed clinical decisions to extend or change antibiotic therapy and did not involve any standardized confirmation of infection, as this was not possible retrospectively. Although it is likely that our definition of clinical failure included patients who did not have ongoing or recurrent infection but rather lower extremity edema or other noninfectious dermatoses, the definition used is consistent with other retrospective studies evaluating management of SSTIs [14] and reflects real-world diagnosis and treatment patterns for possible SSTIs. Lastly, we did not collect information on antibiotic-associated adverse events other than CDI, and a thorough evaluation of additional impacts of prolonged antibiotic courses could serve to strengthen our argument in support of shorter courses.
Despite these limitations, our study suggests that there is no benefit to longer antibiotic durations for SSTIs in patients with obesity or HF. Furthermore, longer antibiotic durations were associated with increased treatment failure, which may highlight patients who could benefit from more in-depth diagnostic evaluation. Our findings not only have implications for individual patient care and health care utilization, but also importantly may inform much needed stewardship interventions aimed at minimizing unnecessary antibiotic durations of therapy and their untoward effects. Moreover, prospective studies evaluating duration of therapy for patients at high risk of SSTI relapse or recurrence are needed that utilize standard objective measures of treatment failure rather than rely on individual provider treatment decisions.
Acknowledgment
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Christensen KL, Holman RC, Steiner CA, et al. Infectious disease hospitalizations in the United States. Clin Infect Dis 2009; 49:1025–35. [DOI] [PubMed] [Google Scholar]
- 2. Inghammar M, Rasmussen M, Linder A. Recurrent erysipelas—risk factors and clinical presentation. BMC Infect Dis 2014; 14:270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McNamara DR, Tleyjeh IM, Berbari EF, et al. A predictive model of recurrent lower extremity cellulitis in a population-based cohort. Arch Intern Med 2007; 167:709–15. [DOI] [PubMed] [Google Scholar]
- 4. Halilovic J, Heintz BH, Brown J. Risk factors for clinical failure in patients hospitalized with cellulitis and cutaneous abscess. J Infect 2012; 65:128–34. [DOI] [PubMed] [Google Scholar]
- 5. Conway EL, Sellick JA, Kurtzhalts K, Mergenhagen KA. Obesity and heart failure as predictors of failure in outpatient skin and soft tissue infections. Antimicrob. Agents Chemother 2017; 61:e02389–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jenkins TC, Knepper BC, McCollister BD, et al. Failure of outpatient antibiotics among patients hospitalized for acute bacterial skin infections: what is the clinical relevance? Am J Emerg Med 2016; 34:957–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Almarzoky Abuhussain SS, Burak MA, Adams DK, et al. Variability in emergency medicine provider decisions on hospital admission and antibiotic treatment in a survey study for acute bacterial skin and skin structure infections: opportunities for antimicrobial stewardship education. Open Forum Infect Dis 2018; 5(X):XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stevens DL, Bisno AL, Chambers HF, et al. ; Infectious Diseases Society of America Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis 2014; 59:e10–52. [DOI] [PubMed] [Google Scholar]
- 9. Wintenberger C, Guery B, Bonnet E, et al. ; Recommendation Group of the SPILF Proposal for shorter antibiotic therapies. Med Mal Infect 2017; 47:92–141. [DOI] [PubMed] [Google Scholar]
- 10. Prokocimer P, De Anda C, Fang E, et al. Tedizolid phosphate vs linezolid for treatment of acute bacterial skin and skin structure infections: the ESTABLISH-1 randomized trial. JAMA 2013; 309:559–69. [DOI] [PubMed] [Google Scholar]
- 11. Kilburn SA, Featherstone P, Higgins B, Brindle R. Interventions for cellulitis and erysipelas. Cochrane Database Syst Rev 2010; 29:CD004299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hepburn MJ, Dooley DP, Skidmore PJ, et al. Comparison of short-course (5 days) and standard (10 days) treatment for uncomplicated cellulitis. Arch Intern Med 2004; 164:1669–74. [DOI] [PubMed] [Google Scholar]
- 13. Sandison T, De Anda C, Fang E, et al. Clinical response of tedizolid versus linezolid in acute bacterial skin and skin structure infections by severity measure using a pooled analysis from two phase 3 double-blind trials. Antimicrob Agents Chemother 2017; 61:e02687–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jenkins TC, Knepper BC, Moore SJ, et al. Antibiotic prescribing practices in a multicenter cohort of patients hospitalized for acute bacterial skin and skin structure infection. Infect Control Hosp Epidemiol 2014; 35:1241–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gibbons JA, Smith HL, Kumar SC, et al. Antimicrobial stewardship in the treatment of skin and soft tissue infections. Am J Infect Control 2017; 45:1203–7. [DOI] [PubMed] [Google Scholar]
- 16. Walsh TL, Chan L, Konopka CI, et al. Appropriateness of antibiotic management of uncomplicated skin and soft tissue infections in hospitalized adult patients. BMC Infect Dis 2016. Nov 29;16(1):721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jenkins TC, Sabel AL, Sarcone EE, et al. Skin and soft-tissue infections requiring hospitalization at an academic medical center: opportunities for antimicrobial stewardship. Clin Infect Dis 2010; 51:895–903. [DOI] [PubMed] [Google Scholar]
- 18. Tamma PD, Avdic E, Li DX, et al. Association of adverse events with antibiotic use in hospitalized patients. JAMA Intern Med 2017; 177:1308–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rosenbaum PR, Rubin DB. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat 2012; 39:33–8. [Google Scholar]
- 20.Food and Drug Administration. Guidance for industry. 2013. www.fda.gov/downloads/Drugs/Guidances/ucm071185.pdf. Accessed January 10, 2019.
- 21. Nathwani D, Corey R, Das AF, et al. Early clinical response as a predictor of late treatment success in patients with acute bacterial skin and skin structure infections: retrospective analysis of 2 randomized controlled trials. Clin Infect Dis 2017; 64:214–7. [DOI] [PubMed] [Google Scholar]
- 22. Corey GR, Jiang H, Moeck G. Dalbavancin or oritavancin for skin infections. N Engl J Med 2014; 371:1162–3. [DOI] [PubMed] [Google Scholar]
- 23. Bruun T, Oppegaard O, Hufthammer KO, et al. Early response in cellulitis: a prospective study of dynamics and predictors. Clin Infect Dis 2016; 63:1034–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dall L, Peterson S, Simmons T, Dall A. Rapid resolution of cellulitis in patients managed with combination antibiotic and anti-inflammatory therapy. Cutis 2005; 75:177–80. [PubMed] [Google Scholar]
- 25. Bergkvist PI, Sjöbeck K. Antibiotic and prednisolone therapy of erysipelas: a randomized, double blind, placebo-controlled study. Scand J Infect Dis 1997; 29:377–82. [DOI] [PubMed] [Google Scholar]
- 26. Li DG, Xia FD, Khosravi H, et al. Outcomes of early dermatology consultation for inpatients diagnosed with cellulitis. JAMA Dermatol 2018; 154:537–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Caterino JM, Leininger R, Kline DM, et al. Accuracy of current diagnostic criteria for acute bacterial infection in older adults in the emergency department. J Am Geriatr Soc 2017; 65:1802–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ko LN, Garza-Mayers AC, St John J, et al. Effect of dermatology consultation on outcomes for patients with presumed cellulitis: a randomized clinical trial. JAMA Dermatol 2018; 154:529–36. [DOI] [PMC free article] [PubMed] [Google Scholar]