Abstract
Background
Severe fever with thrombocytopenia syndrome (SFTS) is a typical tick-borne, natural focal disease. The natural foci of SFTS were considered to exist in hilly and mountainous areas before 2015. A cluster of 3 patients exposed to a patient with a fulminant disease consistent with SFTS occurred from July to August 2015 in Dongtai County, which is characterized by alluvial plains; this prompted investigation.
Methods
The epidemiological, clinical, and laboratory features of 4 patients in the cluster were analyzed. Serum samples from the indigenous healthy population and native domesticated animals were collected to conduct laboratory tests, along with small wild animals and ticks.
Results
In 3 secondary case patients, high fever, thrombocytopenia and leukopenia developed within 8–13 days after contact with blood or bloody secretions from the index patient; SFTS was then diagnosed by means of reverse-transcription polymerase chain reaction. Genomic sequencing and analysis of S and L segments of 2 viral strains isolated from 2 secondary case patients showed that they shared 99.8%–99.9% homology in nucleotide sequence. The seroprevalences among indigenous healthy population, native livestock, native poultry, and small wild animals was 0.74%, 17.54%, 6.67%, and 1.12%, respectively. Three questing ticks, 61 feeding ticks, and 178 small wild animals were collected in August 2015. Survey on tick density and seasonal fluctuation in 2016 showed that ticks were active from March to October. All ticks were identified as Haemaphysalis longicornis. Severe fever with thrombocytopenia bunyavirus (SFTSV)–specific RNA was detected in the ticks collected in 2016, and the minimum SFTSV infection rate in these ticks was 0.54% (1 of 185).Wild mammals and ticks collected in August 2015 tested negative for SFTSV-specific RNA.
Conclusions
Aside from hilly or mountainous area, a coastal plain was identified as the natural foci of SFTSV in Dongtai County, China. The involvement of migration in the evolution of SFTSV might lead to a transregional transmission event of SFTSV.
Keywords: Bunyavirus, Endemic region, Human-to-human transmission, Severe fever with thrombocytopenia syndrome (SFTS)
A cluster of patients with severe fever with thrombocytopenia syndrome was identified in Dongtai County from July to August 2015, suggesting that the natural foci of severe fever with thrombocytopenia bunyavirus (SFTSV) are not limited to hilly or mountainous areas. A transregional SFTSV transmission event occurred in the cluster, further evidence of migration in the evolution of SFTSV.
Severe fever with thrombocytopenia syndrome (SFTS) is a tick-borne zoonosis caused by severe fever with thrombocytopenia bunyavirus (SFTSV), which is classified in the Phlebovirus genus, Phenuiviridae family, Bunyavirales order. This disease, characterized by high fever, thrombocytopenia, and leukopenia, was first identified in China in 2009, and the earliest cases can be traced back to 1996 [1, 2]. It has an mean case-fatality rate of 12%, but this rate can be as high as 30% [3]. SFTS cases were reported in 23 provinces of China, and annual case numbers increased year by year, with the highest number recorded in 2016 (1306 cases), according to surveillance data from the Chinese Center for Disease Control and Prevention (CDC) [4, 5]. SFTS has also been reported in South Korea and Japan [6, 7]. There is no licensed vaccine or therapeutic against SFTSV currently, and avoiding tick exposure is an important way to prevent SFTSV infection [8, 9]. The disease was listed as 1 of the 9 infectious diseases on the World Health Organization priority list in 2017 because of a substantial risk to public health [10].
SFTS has been known as a typical tick-borne, natural focal disease. In China, the peak outbreak months are May–July, coinciding with the high tick density during these months, and farmers in endemic areas of hilly and mountainous environments are at greatest risk of infection [11, 12]. SFTSV, tick vectors [13–15], and animal hosts (eg, goats, cattle, rats, and hedgehogs) [16, 17] form a biocoenosis in the natural foci, within which the infection circulates independently of humans as long as humans do not come in contact with the foci [18].
Exposure to and bites from infected ticks are thought to be the primary transmission route of SFTSV [8, 9], and secondary cases can be caused by human-to-human transmission, probably through inhalation of virus-containing aerosol [19] and direct contact with blood or bloody secretions bearing SFTSV [20, 21]. The natural foci are thus the basis for the survival, sustaining, and transmission of SFTSV, constituting a potential epidemiological danger. It is important that their existence and localization be recognized beforehand, so they can be avoided or brought under control. In the current study, we revealed a cluster of SFTSV infection in Dongtai County of China, and we used epidemiological investigation and laboratory testing to verify that SFTSV natural foci existed in plain areas.
MATERIALS AND METHODS
Study Site
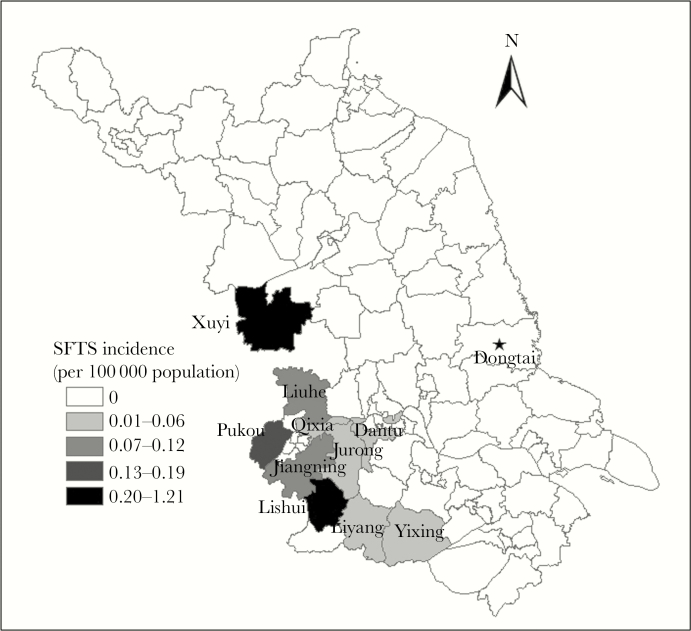
Dongtai County is located in the central part of Jiangsu Province, China (latitude, 32°33′~32°57′N; longitude, 120°07′~120°53'E) (Figure 1) and connected to the Yellow Sea in the east with 85-km coastlines. The county covers 3240 km [2] of alluvial plains, with an mean altitude of 1.4–5.6 m. There are no mountains or hills in the county. It has a subtropical monsoon climate (mean annual temperature, 14.6°C), with adequate rainfall (mean annual precipitation, approximately 1050 mm) and sunlight (mean annual sunshine duration, approximately 2200 hours).
Figure 1.
Location of Dongtai County in Jiangsu Province and spatial distribution of mean annual incidence of severe fever with thrombocytopenia syndrome (SFTS) in Jiangsu Province, 2010–2015.
Endemic arthropod-borne infectious diseases are mainly scrub typhus and hemorrhagic fever with renal syndrome. No SFTS cases were reported before 2015.
Patients and Clinical Samples
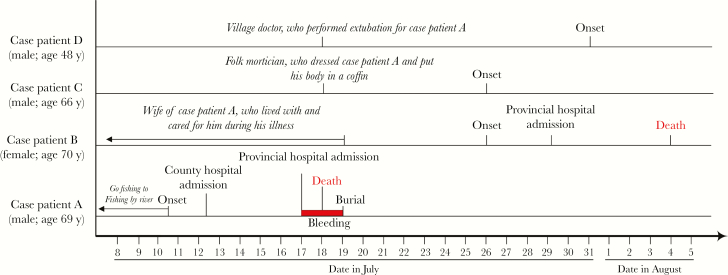
In 2015, a cluster of 4 suspected SFTS cases occurred in Dongtai County, Jiangsu Province. The index patient (case patient A) was a 69-year-old retired cadre worker who experienced a sudden onset of fever, fatigue, and diarrhea on 10 July and died at home in the afternoon on 18 July, after treatment was stopped because of his critical condition. The 3 secondary patients included the index patient’s wife (case patient B), who lived with case patient A and cared for him during his illness; a folk mortician (case patient C), who dressed the index patient’s body in a traditional “longevity” garment after death and placed the body in a coffin; and a village doctor (case patient D), who helped remove urethral and tracheal intubation and an intravenous catheter from the index patient’s body after he died at home. Case patients B, C, and D had illness onset 8–13 days after the death of case patient A. Case patient B died the evening of 4 August, and the other 2 survived and were eventually discharged.
The cluster was unveiled 17 days after the index patient’s death. A clinician from a township health center in Dongtai County consulted 2 febrile patients (case patients C and D) with thrombocytopenia and leukopenia of unknown cause on 3 and 4 August, respectively. He reported these 2 cases to the Dongtai County CDC at once.
Serum specimens were collected from case patients B, C, and D to detect SFTSV on 4 August. SFTSV testing of these samples was considered by the Jiangsu Provincial CDC owing to the patients’ clinical manifestations.
Case Investigation
Case investigation was conducted to inquire about information including illness onset and course, clinical manifestations, case patients’ surroundings, history of tick bites and animal contacts, and the routes of possible exposure to risk factors. All case patients’ family members, neighbors, and medical staffs and the 2 surviving case patients were interviewed, and their clinical records were checked.
Small Wild Animals and Ticks Collection
Epidemiological survey showed that case patient A often went fishing by a river (latitude, 32°44′51″N; longitude, 120°53′58″E; altitude, ‒2 m) in southeast Dongtai County (the distance from the river to the seaside is about 3 km); this was his only opportunity for exposure to ticks before illness onset. Moreover, the index patient did not raise any animals, and there was no vegetation in or around his residence. Therefore, all the sampling sites chosen were near the river.
Small mammals were collected with live traps in August 2015. We followed established safety guidelines for small mammals capture and processing [22]. All trapped animals were anesthetized using ketamine, blood was collected from the retro-orbital sinus, and animals were then killed by cervical dislocation. We performed necropsies of the small wild animals on site and collected hearts, kidneys, spleens, livers, lungs, and brains, which were subsequently stored in liquid nitrogen.
Questing ticks were collected by dragging a white flannel cloth flag (1 × 1-m2) over the weeds and bushes along the river bank, and feeding ticks were collected from small wild animals in August 2015. Furthermore, the density and seasonal fluctuation of questing ticks were analyzed from March to November 2016, according to protocols described elsewhere [22]. Ticks were pooled according to species, and developmental stages were identified morphologically.
Tick pools and animal organs were homogenized in 500 μL of TLR buffer supplied in the RNeasy kit (Qiagen) with a tissue lyser, Tissuelyser LT (Qiagen). The homogenates were used for RNA extraction and SFTSV RNA detection.
Collection of Serum Samples From General Population and Domesticated Animals
A total of 269 indigenous healthy persons from different age groups and 174 native domesticated animals were selected randomly in 2 townships near the river where case patient A had fished from September to November 2015. The sampled population was divided into 7 age groups (10–19, 20–29, 30–39, 40–49, 50–59, 60–69, and ≥70 years). Blood samples from participants and domesticated animals were collected directly in serum tubes. The serum were separated from blood samples by centrifugation, and serum samples then were kept at ‒18°C until the time of analysis.
Experimental Detection and Phylogenic Analysis of SFTSV
Viral RNA was extracted from 140-μL serum samples collected from suspected case patients in the acute phase, using QIAamp Viral RNA Mini kit (Qiagen), according to the manufacturer’s instructions. SFTSV RNA was detected with a real-time of reverse-transcription polymerase chain reaction (RT-PCR) assay, described elsewhere [23]. The reaction conditions for real-time RT-PCR were as follows: 50°C for 30 minutes, 95°C for 15 minutes, and 40 cycles of 95°C for 15 seconds and 60°C for 1 minute. Data were analyzed using the software supplied by the manufacturer.
Anti-SFTSV immunoglobulin M antibodies were detected in acute-phase serum specimens from patients with SFTS using an enzyme-linked immunosorbent assay (ELISA) kit (Xinlianxin), according to the manufacturer’s protocol. Total antibodies, including immunoglobulin G and M against SFTSV, were determined using another ELISA kit (Xinlianxin).
Virus isolation was performed using Vero cells with SFTSV RNA–positive serum specimens, as described elsewhere [22]. Isolated viruses were further identified by means of real-time RT-PCR. S and L segments of the genome of SFTSV strains isolated from SFTS cases were amplified by RT-PCR. The products were sent to Sangon Biotech for Sanger DNA sequencing. Complete S and L segments of SFTSV strains generated from this study and S and L segments of 75 SFTSV strains obtained from GenBank were aligned using the multiple alignment program ClustalX implemented in BioEdit software (version 7.1.3.0; Ibis Biosciences). Phylogenetic trees were generated using the maximum likelihood method with Molecular Evolutionary Genetics Analyses (MEGA) software, version 5.10. Statistical support of the clades was measured by a heuristic search with 1000 bootstrap replicates.
Ethical Approval
The study was approved by the Ethics Committee of the Jiangsu Provincial CDC, and informed consent was obtained from all participants after they were provided with detailed descriptions of the potential benefits of the study. All data were analyzed anonymously.
RESULTS
Clinical and Epidemiological Profile of the cluster
The index patient (case patient A) experienced fever and diarrhea on 10 July 2015. He was admitted to People’s Hospital of Dongtai County on 12 July, transferred initially to the hematology department of a provincial hospital owing to severe leukopenia and thrombocytopenia on 14 July, and then to the hospital’s intensive care unit owing to dysphoria, drowsiness, and coma on 17 July. His family members decided to stop his treatment owing to his critical condition. He was taken back home, with urethral and tracheal intubation and a central intravenous catheter that was removed after his death on 18 July. His condition was diagnosed only as a blood disorder during the course of disease. Case patients B and C experienced fever on 26 July, and case patient D on 31 July. All 3 secondary case patients had high fever, leukopenia, and thrombocytopenia. Case patient B died on 4 August, and 2 other case patients finally recovered after symptomatic and supportive treatment. The timeline of key events is shown in Figure 2. Demographic and clinical information for all 4 patients is shown in Table 1.
Figure 2.
Epidemic curve shows the timeline of key events in the cluster.
Table 1.
Demographic and Clinical Characteristics of Severe Fever With Thrombocytopenia Syndrome Cluster in Dongtai County
| Case Patient | ||||
|---|---|---|---|---|
| Characteristics | Index/A | B | C | D |
| General | ||||
| Sex | Male | Female | Male | Male |
| Age, y | 70 | 69 | 65 | 46 |
| Occupation | Retired cadre worker | Housewife | Folk mortician | Village doctor |
| Medical history | Hypertension, thyroidectomy | No relevant history | Hypertension | Hepatitis B, diabetes |
| Relationship with index case patient (case patient A) | Self | Spouse | Prepared body for burial (dressed and placed in coffin) | Provided medical service |
| Date of illness onset | 10 July 2015 | 26 July 2015 | 26 July 2015 | 31 July 2015 |
| Date of hospitalization | 12 July 2015 | 29 July 2015 | 4 August 2015 | 4 August 2015 |
| Clinical symptoms | ||||
| Fever, maximum body temperature, °C | 38.8 | 39.6 | 39.0 | 40.0 |
| Chills | Yes | Yes | Yes | No |
| Headache | Yes | Yes | Yes | No |
| Muscular stiffness | Yes | Yes | Yes | Yes |
| Low back pain | Yes | No | Yes | No |
| Fatigue | Yes | Yes | Yes | Yes |
| Anoxia | Yes | Yes | Yes | Yes |
| Nausea | Yes | Yes | No | No |
| Vomiting | Yes | Yes | Yes | No |
| Abdominal pain and/or distension | No | No | Yes | No |
| Diarrhea | Yes | Yes | No | No |
| Neurological symptoms | Yes | Yes | No | No |
| Skin petechiae | Yes | No | No | No |
| Superficial lymphadenopathy | No | No | No | No |
| Hematemesis | Yes | No | No | No |
| Hematuria | Yes | Yes | No | No |
| Hematochezia | Yes | Yes | No | No |
| Laboratory findings | ||||
| Cell count, ×109/L | ||||
| WBCs | 3.21 | 1.00 | 1.74 | 1.07 |
| Lymphocytes | 0.80 | 0.41 | 0.66 | 0.42 |
| Platelets | 44 | 96 | 89 | 88 |
| ALT, U/L | 138 | 41 | 20 | 202 |
| AST, U/L | 1154 | 86 | 35 | 219 |
| Prothrombin time, s | 19.1 | Not tested | 11.9 | 13.4 |
| aPTT, s | 106.8 | Not tested | 37.7 | 46.9 |
| Urine protein | Not tested | Not tested | 1+ | 2+ |
Abbreviations: ALT, alanine aminotransferase; aPTT, activated partial thromboplastin time; AST, aspartate aminotransferase; WBCs, white blood cells, 1+, urinary protein concentration of 0.2-1.0g/L; 2+, urinary protein concentration of 1.0-2.0g/L.
The index patient, a 69-year-old retired cadre worker, was healthy before his illness and lived with his wife in the center of a township in Dongtai County. He and his wife did not raise any animals. They had a good dwelling environment without weeds or bushes in and around their residence. His hobby was fishing, and he used fish by a river, wearing a short-sleeved shirt, shorts, and slippers. The fishing spot was a mudflat with low weeds and bushes on the river bank. His family members did not know whether he had been bitten by ticks while fishing, but they stated unequivocally that he had neither left Dongtai County nor been in contact with anyone with a similar illness in the month before his illness onset.
Case patient B, a 70-year-old housewife, was married to the index patient, took care of his daily affairs at home and provided bed care for him during his illness. She asked for help from case patient D and 2 folk morticians (including case patient C) after the index patient died at home.
Case patient D, a 48-year-old village doctor, helped remove urethral and tracheal intubation and B intravenous catheter from the index patient’s body without protective measures on 18 July. Case patient D stated that the index patient had petechiae and ecchymoses on the skin before extubation, with persistent bleeding at these deep puncture sites on the skin after extubation. Case patients B and D therefore applied pressure with clean and dry clothes to stop the bleeding.
Case patient C, a 66-year-old folk mortician, dressed the index patient’s body in a longevity garment after extubation (on 18 July) and then helped another folk mortician put the body in a coffin . All of the secondary case patients had no history of tick bite or animal contact within 2 weeks before illness onset but were directly exposed to blood or bloody secretion from the index patient shortly after his death.
Laboratory Virology Test of the Cluster
The cluster was unveiled 17 days after case patient A died, so we could not collect his tissue or serum sample for laboratory confirmation. SFTSV-specific RNA was detected from the blood specimens of 3 secondary case patients. The blood viral loads in case patients B, C, and D were up to 108, 106, and 104 copies/mL, respectively. Two viral strains were successfully isolated from case patients B and C.
The sequence of the viral S segment from case patient B shared 99.8% nucleotide identity with case patient C and that of the viral L segment from case patient B shared 99.9% nucleotide identity with case patient C. Phylogenic analysis revealed that the S and L segments of SFTSV strains from case patients B and C are closest to the SFTSV strain from an SFTS case patient from Jiangning County in southwest Jiangsu Province in 2012 (Figure 3). Jiangning County is a previously identified endemic region for SFTSV. Thus, the results suggested that the SFTSV strains from case patients B and C might share the same origins, which provided genetic evidence for the possible transmission route of SFTSV in the cluster.
Figure 3.
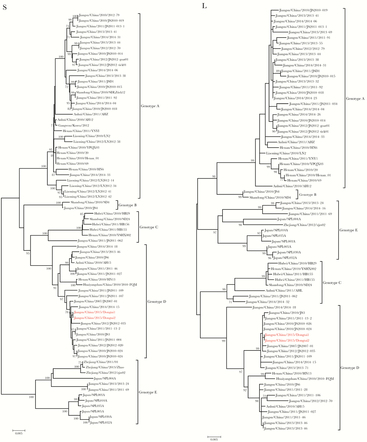
Phylogenetic analysis of severe fever with thrombocytopenia bunyavirus (SFTSV) strains from the current cluster, compared with SFTSV strains from other areas. The phylogenetic trees were constructed using the maximum likelihood method with MEGA5.1 software, based on S and L segments of SFTSV strains from different severe fever with thrombocytopenia syndrome–endemic areas. The reliability values indicated at the branch nodes were determined using 1000 bootstrap replications, and bootstrap values ≥70 were labeled at nodes. The sequences were named according to the province, country, year of collection or isolation, and strain. Red taxon names represent the SFTSV strains from case patients B (Dongtai1) and C (Dongtai2). The scale bar indicates the branch length and corresponds to 0.005 estimated amino acid substitutions per site.
Seroprevalence of Antibodies Against SFTSV in General Population and Native Animals
We sampled 174 native domesticated animals, including 114 livestock and dogs (60 goats, 52 pigs, 1 cattle, 1 dog) and 60 poultry (chickens) in 2 townships near the river where the index patient had fished from September to November 2015. In addition, a total of 178 small wild animals, including 171 rodents and 7 hedgehogs, were captured and sampled near the river in August 2015 (Table 2). The difference in seroprevalence among the 3 groups was statistically significant (P < .001). Further comparison between any 2 groups with adjusted statistical significance level (.05/3 = .017) showed that the seroprevalence in livestock was higher than that in small wild animals (P < .001), whereas no difference in seroprevalence was noted between livestock/dogs and poultry (P = .048) or between poultry and small wild animals (P = .018).
Table 2.
Seroprevalence of Antibodies Against Severe Fever With Thrombocytopenia Bunyavirus in Animals
| Animals | All Sampled Animals, No. | Animals With Antibodies to SFTSV, No. (%)a |
|---|---|---|
| Livestock and dogs | ||
| Goats | 60 | 6 (10.00) |
| Pigs | 52 | 13 (25.00) |
| Cattle | 1 | 1 (100.00) |
| Dogs | 1 | 0 (0) |
| Total | 114 | 20 (17.54) |
| Poultry (chicken) | 60 | 4 (6.67) |
| Wild animals | ||
| Rodents | 171 | 1 (0.58) |
| Hedgehogs | 7 | 1 (14.29) |
| Total | 178 | 2 (1.12) |
Abbreviation: SFTSV, severe fever with thrombocytopenia bunyavirus.
aSampled animals with antibodies to SFTSV, including immunoglobulin G and M.
A total of 269 indigenous participants (median age, 45 years; age range, 10–83 years) were enrolled in this study, and 2 participants in the 60–69-year age group were seropositive for SFTSV total antibodies. The overall seroprevalence of antibodies against SFTSV was 0.74% (Table 3).
Table 3.
Seroprevalence of Antibodies Against Severe Fever With Thrombocytopenia Bunyavirus in General Population
| Persons in General Population | ||
|---|---|---|
| Age Group, y | No. Sampled | No. (%) With Antibodies to SFTSV |
| 10–19 | 23 | 0 (0) |
| 20–29 | 32 | 0 (0) |
| 30–39 | 48 | 0 (0) |
| 40–49 | 52 | 0 (0) |
| 50–59 years | 56 | 0 (0) |
| 60–69 | 48 | 2 (4.17) |
| ≥70 | 10 | 0 (0) |
| Total | 269 | 2 (0.74) |
Abbreviation: SFTSV, severe fever with thrombocytopenia bunyavirus.
aPersons in general population with antibodies to SFTSV, including immunoglobulin G and M.
Species, Density and Seasonal Fluctuation of Ticks
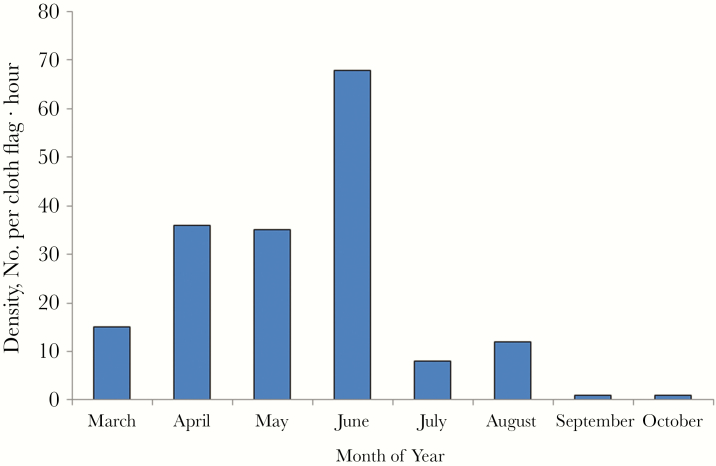
Three questing ticks were collected from weeds and bushes along the bank of the river where the index patient had fished, and 61 feeding ticks were collected from 5 goats (9 feeding ticks), 8 dogs (2 feeding ticks), and 6 hedgehogs (50 feeding ticks) in August 2015. Survey on ticks in 2016 showed that they were active from March to October and had a significant seasonal variation (Figure 4). The mean density was 22 per cloth flag · hour and the peak was from April to June with density 36, 35, and 68 per cloth hour, respectively. All ticks were identified as Haemaphysalis longicornis. Per cloth flag · hour was the unit for measuring the density of questing ticks. 1 per cloth flag · hour denoted that 1 cloth flag could sample 1 tick per hour.
Figure 4.
Density and seasonal fluctuation of ticks in 2016. Per cloth flag · hour was the unit for measuring the density of questing ticks. 1 per cloth flag · hour denoted that 1 cloth flag could sample 1 tick per hour.
Detection of SFTSV RNA in Wild Mammals and Ticks
The SFTSV RNA results at RT-PCR were negative in 171 rodents, 7 hedgehogs, and all questing and feeding ticks collected in August 2015. However, viral RNA was detected in the questing ticks collected from weeds and bushes in 2016. The minimum infection rate of SFTSV in these ticks was 0.54% (1 in 185). The minimum infection rate was defined as the number of positive pools divided by the total number of ticks tested.
DISCUSSION
Three cases of SFTSV infection in Dongtai County were confirmed by the Jiangsu Provincial CDC in 2015. All case patients presented with high fever, leukopenia and thrombocytopenia and fulfilled the laboratory-confirmed criteria for SFTSV infection established by the Chinese Ministry of Health [24]. The infection route in these patients was closely associated with exposure to blood or bloody secretion from an index patient who died of a fulminant febrile illness with hemorrhage. Although the index patient can only be categorized as a suspected case patient, his epidemiological history and clinical manifestations support the diagnosis of SFTSV infection. The index patient was exposed to weeds and bushes while fishing outdoors before the onset of illness and had a clinical presentation compatible with severe SFTSV infection. Further investigation showed that ticks were found in the weeds and bushes of the fishing site.
Moreover, the epidemiological investigation and viral phylogenetic analysis of subsequent individuals with SFTSV infection implicates this patient as the index case patient. The secondary case patients had illness onset within 8–13 days after contact with the index patient’s blood or bloody secretions, which is consistent with the incubation period and transmission pattern of SFTSV reported elsewhere in the literature [20, 21, 25]. The isolated viruses from case patients B and C were highly similar in genomic sequences. Unfortunately, no tissue or serum sample is available to confirm retrospectively the index patient’s diagnosis, and we did not know whether he was exposed to ticks, owing to lag identification of the cluster.
Numerous clusters of SFTSV infection caused by human-to-human transmission have been reported [20, 21, 25–29], so we can never overemphasize the importance of protecting healthcare workers and patients’ family members from exposure to blood or bloody secretions. The features of these clusters are summarized as following: a final outcome of death in the index patient, a superior clinical outcome for secondary infection, an incubation period of 6–15 days, and a transmission route involving contact with blood and/or bloody secretions. The cluster in Dongtai County had the above 3 characteristics, but in 1 of the secondary case patients (case patient B) experienced severe hemorrhage and eventually died. We speculate that this death was due to a high viral load. Viral load has been found to be independently predictive of severe disease outcome throughout the hospitalization, and a viral load of >107 copies/mL is predictive of a fatal outcome [30]. The blood viral load in case patient B was up to 108 copies/mL on the day of his death, whereas the viral loads of case patients C and D were <107 copies/mL.
Dongtai County is characterized by alluvial plains and has no mountains or hills in the prefecture. It is not in line with the geomorphic feature of SFTS natural foci. However, there are 3 reasons to confirm that humans have been affected by SFTSV in Dongtai County, which was considered not to be affected or without human cases officially reported before 2015. First, we found H. longicornis ticks from animal bodies and vegetation inside the county, and SFTSV was detected in ticks from vegetation, showing a minimum infection rate of 0.54%. H. longicornis, the most abundant human-biting tick species in most SFTS-endemic areas of China, is known as the major vector, because SFTSV is successfully isolated from H. longicornis ticks, and transtadial and transovarial transmission of SFTSV occurs in them [13, 22, 31, 32]. Ticks collected in August 2015 were negative for SFTSV RNA in our study, perhaps because the sample size was relatively small and August is not the peak outbreak month.
Second, results of a serological survey of native animals suggest that SFTSV circulates widely in livestock, poultry, and small wild animals in the county. They act as the host amplifier in which SFTSV multiplies rapidly to high levels only during a short period of time, providing an important source of infection for ticks. Last but not least, not only were 2 asymptomatic infected persons found through a serological survey of the indigenous healthy population, but the index patient in the cluster was also identified. These constitute the direct evidence that SFTSV had infected humans in the county. Based on all of these findings, we can infer that Dongtai County is a previously unidentified endemic region for SFTSV, with natural foci in some areas.
One highlight of our study is that the natural foci of SFTSV are not limited to hilly or mountainous areas. Epidemiological investigation showed that SFTSV had naturally flourished in Dongtai County, characterized by alluvial plains, before 2015. It is noteworthy that the county area is very large and includes many different geographic landscapes. The natural foci of SFTSV should be located only within geographic landscapes suited for a biological system formed by SFTSV, tick vectors, and animal hosts. Therefore, it is necessary to determine the structure and borders of the natural foci through further study. This will make it possible to assess the risks to the public, apply prevention policies correctly, and control the activity of natural foci.
The other highlight is a transregional transmission of SFTSV from Jiangning County to Dongtai County, which provides further evidence for involvement of migration in the evolution of SFTSV. Phylogenetic analysis indicated that the S and L segments of SFTSV strains from case patients B and C are closest to the SFTSV strain from an SFTS case patient from Jiangning County, which is endemic for SFTS in the southwestern part of Jiangsu Province, 260 km away from Dongtai County in the eastern part of the province. All case patients in the cluster were local indigenous persons and did not leave Dongtai County in the month before illness onset. Migration of SFTSV from Jiangning County to Dongtai County might be due to the trade of nursery-grown plants suited to the tick habitat and animals such as goats, cattle, and pigs, and, especially, migratory birds infected by SFTSV or carrying SFTSV-infected ticks, as described elsewhere [22, 33].
In conclusion, the cluster in Dongtai County resulted in identification of a previously unidentified endemic region for SFTSV. Our findings suggest that the natural foci of SFTSV are not limited to hilly or mountainous areas. In the future, we should carry out intensive studies of the ecological conditions under which SFTSV infection can be maintained among the animal hosts and tick vectors, which could provide a rational basis for the control of SFTS.
Acknowledgments
Disclaimer. The funders had no role in study design, data collection and analysis, the decision to publish, or the preparation of the manuscript.
Financial support. This work was supported by the Natural Science Foundation of China (grants 81601794 and 81703284), the Jiangsu Provincial Key Medical Discipline of Epidemiology (ZDXKA2016008), the Jiangsu Provincial Medical & Youth Talent (grants ZDRCA2016032 and QNRC2016545), and the Jiangsu Provincial Nature Science Foundation (grant BK20161584).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Yu XJ, Liang MF, Zhang SY, et al. Fever with thrombocytopenia associated with a novel bunyavirus in China. N Engl J Med 2011; 364:1523–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hu J, Shi C, Li Z, et al. A cluster of cases of severe fever with thrombocytopenia syndrome bunyavirus infection in China, 1996: a retrospective serological study. PLoS Negl Trop Dis 2018; 12:e0006603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liu K, Zhou H, Sun RX, et al. A national assessment of the epidemiology of severe fever with thrombocytopenia syndrome, China. Sci Rep 2015; 5:9679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhan J, Wang Q, Cheng J, et al. Current status of severe fever with thrombocytopenia syndrome in China. Virol Sin 2017; 32:51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sun J, Lu L, Wu H, et al. The changing epidemiological characteristics of severe fever with thrombocytopenia syndrome in China, 2011-2016. Sci Rep 2017; 7:9236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kim KH, Yi J, Kim G, et al. Severe fever with thrombocytopenia syndrome, South Korea, 2012. Emerg Infect Dis 2013; 19:1892–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Takahashi T, Maeda K, Suzuki T, et al. The first identification and retrospective study of severe fever with thrombocytopenia syndrome in Japan. J Infect Dis 2014; 209:816–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hu JL, Li ZF, Wang XC, et al. Risk factors for bunyavirus-associated severe fever with thrombocytopenia syndrome: a community-based case-control study. PLoS One 2016; 11:e0166611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ding F, Guan XH, Kang K, et al. Risk factors for bunyavirus-associated severe fever with thrombocytopenia syndrome, China. PLoS Negl Trop Dis 2014; 8:e3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. World Health Organization. Annual review of diseases prioritized under the Research and Development Blueprint. 2017. Available at: https://www.who.int/blueprint/what/research-development/2017-Prioritization-Long-Report.pdf?ua=1. Accessed 31 March 2019.
- 11. Liu Q, He B, Huang SY, et al. Severe fever with thrombocytopenia syndrome, an emerging tick-borne zoonosis. Lancet Infect Dis 2014; 14:763–72. [DOI] [PubMed] [Google Scholar]
- 12. Silvas JA, Aguilar PV. The emergence of severe fever with thrombocytopenia syndrome virus. Am J Trop Med Hyg 2017; 97:992–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jiao Y, Qi X, Liu D, et al. Experimental and natural infections of goats with severe fever with thrombocytopenia syndrome virus: evidence for ticks as viral vector. PLoS Negl Trop Dis 2015; 9:e0004092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Luo LM, Zhao L, Wen HL, et al. Haemaphysalis longicornis ticks as reservoir and vector of severe fever with thrombocytopenia syndrome virus in China. Emerg Infect Dis 2015; 21:1770–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhuang L, Sun Y, Cui XM, et al. Transmission of severe fever with thrombocytopenia syndrome virus by Haemaphysalis longicornis ticks, China. Emerging Infectious Diseases. 2018;24:868–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li Z, Hu J, Bao C, et al. Seroprevalence of antibodies against SFTS virus infection in farmers and animals, Jiangsu, China. J Clin Virol 2014; 60:185–9. [DOI] [PubMed] [Google Scholar]
- 17. Niu G, Li J, Liang M, et al. Severe fever with thrombocytopenia syndrome virus among domesticated animals, China. Emerg Infect Dis 2013; 19:756–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vynograd N. Natural foci diseases as a stable biological threat. Arch Immunol Ther Exp (Warsz) 2014; 62:445–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gong Z, Gu S, Zhang Y, et al. Probable aerosol transmission of severe fever with thrombocytopenia syndrome virus in southeastern China. Clin Microbiol Infect 2015; 21:1115–20. [DOI] [PubMed] [Google Scholar]
- 20. Gai Z, Liang M, Zhang Y, et al. Person-to-person transmission of severe fever with thrombocytopenia syndrome bunyavirus through blood contact. Clin Infect Dis 2012; 54:249–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bao CJ, Guo XL, Qi X, et al. A family cluster of infections by a newly recognized bunyavirus in eastern China, 2007: further evidence of person-to-person transmission. Clin Infect Dis 2011; 53:1208–14. [DOI] [PubMed] [Google Scholar]
- 22. Li Z, Bao C, Hu J, et al. Ecology of the tick-borne phlebovirus causing severe fever with thrombocytopenia syndrome in an endemic area of China. PLoS Negl Trop Dis 2016; 10:e0004574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li Z, Cui L, Zhou M, et al. Development and application of a one-step real-time RT-PCR using a minor-groove-binding probe for the detection of a novel bunyavirus in clinical specimens. J Med Virol 2013; 85:370–7. [DOI] [PubMed] [Google Scholar]
- 24. Ministry of Health. National guideline for prevention and control of severe fever with thrombocytopenia syndrome (2010 edition). Chin J Clin Infect Dis. 2011; 04:193–4. [Google Scholar]
- 25. Liu Y, Li Q, Hu W, et al. Person-to-person transmission of severe fever with thrombocytopenia syndrome virus. Vector Borne Zoonotic Dis 2012; 12:156–60. [DOI] [PubMed] [Google Scholar]
- 26. Yoo JR, Heo ST, Park D, et al. Family cluster analysis of severe fever with thrombocytopenia syndrome virus infection in Korea. Am J Trop Med Hyg 2016; 95:1351–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Huang D, Jiang Y, Liu X, et al. A cluster of symptomatic and asymptomatic infections of severe fever with thrombocytopenia syndrome caused by person-to-person transmission. Am J Trop Med Hyg 2017; 97:396–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang Y, Deng B, Zhang J, et al. Person-to-person asymptomatic infection of severe fever with thrombocytopenia syndrome virus through blood contact. Intern Med 2014; 53:903–6. [DOI] [PubMed] [Google Scholar]
- 29. Jiang XL, Zhang S, Jiang M, et al. A cluster of person-to-person transmission cases caused by SFTS virus in Penglai, China. Clin Microbiol Infect 2015; 21:274–9. [DOI] [PubMed] [Google Scholar]
- 30. Yang ZD, Hu JG, Lu QB, et al. The prospective evaluation of viral loads in patients with severe fever with thrombocytopenia syndrome. J Clin Virol 2016; 78:123–8. [DOI] [PubMed] [Google Scholar]
- 31. Zhang YZ, Zhou DJ, Qin XC, et al. The ecology, genetic diversity, and phylogeny of Huaiyangshan virus in China. J Virol 2012; 86:2864–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang S, Li J, Niu G, et al. SFTS virus in ticks in an endemic area of China. Am J Trop Med Hyg 2015; 92:684–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shi J, Hu S, Liu X, et al. Migration, recombination, and reassortment are involved in the evolution of severe fever with thrombocytopenia syndrome bunyavirus. Infect Genet Evol 2017; 47:109–17. [DOI] [PubMed] [Google Scholar]