Summary
Komagataeibacter xylinus ATCC 23770 was statically cultivated in eight culture media based on different carbon sources, viz. seven biomass‐derived sugars and one sugar mixture. The productivity and quality of the bacterial nanocellulose (BNC) produced in the different media were compared. Highest volumetric productivity, yield on consumed sugar, viscometric degree of polymerization (DP v, 4350–4400) and thermal stability were achieved using media based on glucose or maltose. Growth in media based on xylose, mannose or galactose resulted in lower volumetric productivity and DP v, but in larger fibril diameter and higher crystallinity (76–78%). Growth in medium based on a synthetic sugar mixture resembling the composition of a lignocellulosic hydrolysate promoted BNC productivity and yield, but decreased fibril diameter, DP v, crystallinity and thermal stability. This work shows that volumetric productivity, yield and properties of BNC are highly affected by the carbon source, and indicates how industrially relevant sugar mixtures would affect these characteristics.
Introduction
Bacterial nanocellulose (BNC), also known as microbial cellulose or bacterial cellulose, is a high value‐added biomaterial synthesized mainly by acetic acid bacteria. It possesses unique structure and properties, such as nanofibrillar structure, high degree of polymerization and high mechanical strength, which endow BNC with great potential in the areas of textile manufacturing, fibre‐based paper and packaging products, food industry, biomedical materials, and advanced functional bionanocomposites (Gama et al., 2012; Lin et al., 2013; Lee et al., 2014; Mohite and Patil, 2014). However, many potential applications for BNC are restricted by its relatively high price. The high price is in part due to the high cost of the culture medium including the carbon source.
Previous investigations have addressed production of BNC from agro‐industrial by‐products and cellulosic residues such as konjac glucomannan (Hong and Qiu, 2008), wheat straw (Hong et al., 2011; Chen et al., 2013), waste fibre sludge (Cavka et al., 2013; Chen et al., 2017), spruce wood residues (Guo et al., 2016), waste cotton textile (Hong et al., 2012; Guo et al., 2013) and sweet sorghum bagasse (Chen et al., 2018a,2018b). Media based on different feedstocks contain many different sugars. The exact effects of these different sugars on the production and quality of BNC are not well understood, and need to be further investigated. It is important to identify low‐value carbon sources that are converted with high productivity to BNC of high quality.
There have been some previous efforts aiming at investigating BNC productivity using media based on different sugars (Keshk and Sameshima, 2005; Dahman, et al., 2010; Mikkelsen et al., 2009). However, these studies were not comprehensive, and the quality of the BNC produced was not thoroughly investigated. Only four biomass‐derived sugars were studied, and very limited quality characterization was performed. For instance, there was no characterization of the degree of polymerization (DP). Quality characterization would include features such as DP, crystallinity, fibril diameter and thermal stability. Therefore, it is important to perform a comprehensive investigation of the effects of media based on different sugars on the productivity and quality of BNC using the same bacterial strain and the same cultivation conditions.
In this study, media based on eight different carbon sources were investigated. These carbon sources included seven common sugars derived from plant biomass, and one sugar mixture designed to resemble the composition of a lignocellulosic hydrolysate. The sugars included glucose, xylose, mannose, arabinose and galactose, all of which are common monosaccharide sugars derived from lignocellulosic biomass. The sugar mixture designed to resemble a lignocellulosic hydrolysate included these five monosaccharides. Sucrose, which is a common disaccharide obtained from sugar cane and sugar beet, was also included. The eighth carbon source was maltose, which is a common disaccharide obtained from starch. BNC quantity and quality as well as pH and residual sugar concentrations were monitored. The analyses covered surface morphology, fibril diameter, fourier‐transform infrared (FTIR) spectroscopy, DP, crystallinity and thermal stability.
Results and discussion
Comparison of BNC production from eight carbon sources
The bacterial strain studied was Komagataeibacter xylinus ATCC 23770. Static cultivations were carried out for 14 days. In the first series of experiments, the effects of the eight carbon sources on pH evolution (Fig. 1), sugar consumption (Fig. 1), and BNC productivity and yield (Fig. 2) were compared.
Figure 1.
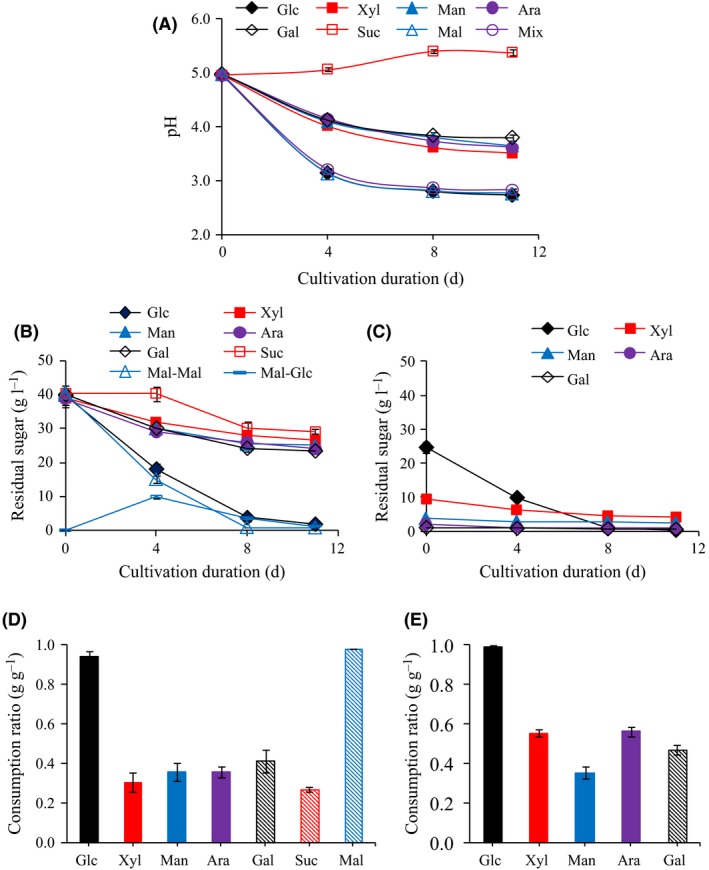
Time‐course of pH (A), sugar concentrations (B and C) and sugar consumption ratios (D and E) for cultures with eight different carbon sources. C and E show residual sugar concentrations and sugar consumption ratios in medium with a sugar mixture. Maltose would be partially hydrolysed to glucose, and therefore, the concentrations of maltose and glucose in the media are shown as Mal‐Mal and Mal‐Glc (B).
Figure 2.
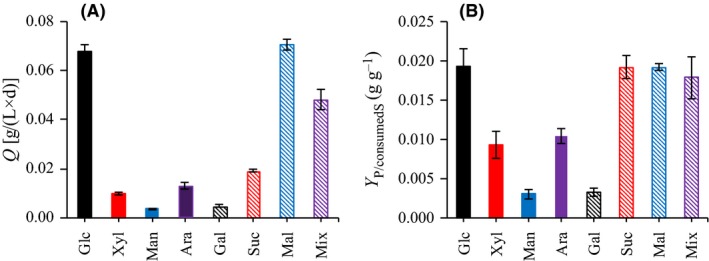
Volumetric bacterial nanocellulose (BNC) productivity (A) and yield of BNC on consumed sugar (B) from cultures with eight carbon sources. ‘P’ and ‘S’ refer to product and sugar respectively.
In media based on glucose, maltose and the sugar mixture, the pH decreased quickly to around 3 in 4 days, and then, in the next 7 days, it decreased slowly (Fig. 1A). In media based on xylose, mannose, arabinose or galactose, the decrease in pH was smoother (Fig. 1A). Among the seven media based on single sugars, glucose and maltose were consumed most rapidly and were exhausted after 11 days (Fig. 1B and D). The presence of glucose in the medium with maltose as carbon source was due to the hydrolysis of maltose (Fig. 1B). The other sugars were consumed much slower than glucose and maltose, and the consumption ratios were less than half of those of glucose and maltose (Fig. 1B and D). For sucrose, there was even a 4‐day lag phase (Fig. 1B). Thus, the two disaccharides in the study, sucrose and maltose, were metabolized with very different rate. In the medium with the sugar mixture (Fig. 1C and E), the consumption ratios of almost all the sugars were higher than in the media with single sugars, except for mannose, which exhibited similar consumption ratios (Fig. 1D and E). The consumption ratios of xylose and arabinose were enhanced by more than 0.20 g g−1, while the consumption ratios of glucose and galactose were enhanced by 0.05–0.06 g g−1. An evaluation using t‐test showed that these enhancements were statistically significant (P < 0.05).
The volumetric BNC productivity and the BNC yield on consumed sugars are shown in Fig. 2. As seen in Fig. 2A, the volumetric BNC productivity for media with glucose and maltose [around 0.07 g/(L×d)] was significantly higher than for the other media (P < 0.05). Cultures with mixed sugar medium exhibited the third highest volumetric BNC productivity (Fig. 2A). The volumetric BNC productivity of the mixed sugar cultures [0.048 g/(L×d)] was 1.1 times higher than the sum of the productivities of single sugar media with 24 g l−1 glucose, 9 g l−1 xylose, 4 g l−1 mannose, 2 g l−1 arabinose and 1 g l−1 galactose [theoretically 0.044 g/(L×d)] (significant difference with P < 0.05). The theoretical value, 0.044 g/(L×d), was calculated based on the assumption that the contribution to the productivity of each of the single sugars, i.e. glucose, xylose, mannose, arabinose and galactose, was ideally linear to that of the initial concentration of the sugar. Thus, the volumetric BNC productivities of cultures with single sugars at a concentration of 40 g l−1 (Fig. 2A) were used for the calculation of the theoretical value. The enhanced BNC productivity of the mixed sugar cultures compared to single sugar cultures should be related to the accelerated sugar consumption observed for the mixed sugar cultures (Fig. 1E). The volumetric BNC productivity of sucrose‐based cultures was much lower than that of maltose‐based cultures (significant difference with P < 0.05). This was expected considering the obvious difference in assimilation between sucrose and maltose (Fig. 1B).
The BNC yield on consumed sugar for cultures with sucrose medium was similar to those of cultures with glucose and maltose media (around 0.019 g g−1) (Fig. 2B), although the BNC productivity for cultures with sucrose medium was far lower than for cultures with glucose and maltose media (Fig. 2A). Furthermore, the BNC yield on consumed sugar for cultures with the sugar mixture was almost as high as the corresponding values for cultures with glucose and maltose media. The BNC yield on consumed sugar for cultures with the sugar mixture (0.018 g g−1) was 1.2 times higher (significant difference with P < 0.05) than the sum of the theoretical yields for cultures with media based on 24 g l−1 glucose, 9 g l−1 xylose, 4 g l−1 mannose, 2 g l−1 arabinose and 1 g l−1 galactose (which was 0.015 g g−1, calculated as indicated above). For cultures based on xylose or arabinose, the BNC yields on consumed sugar were lower than for cultures based on glucose, maltose, sucrose, or the sugar mixture, and for cultures based on mannose or galactose they were even lower (significant differences with P < 0.01) (Fig. 2B).
The BNC yields on consumed sugar are comparable to those found in other studies. Kurosumi et al. (2009) studied BNC production by K. xylinus NBRC 13693 in synthetic media based on glucose, fructose and sucrose. For removal of microbial contaminants, pellicles were washed successively with water, 2% (w/v) NaOH, 2% (v/v) acetic acid and water. Depending on the type of sugar, the BNC yield on consumed sugar was in the range 0.003–0.021 g g−1 (Kurosumi et al., 2009). In the study of Singhsa et al. (2018), BNC was produced from the K. xylinus strains TISTR 086, TISTR 428, TISTR 975, and TISTR 1011, and with media based on glucose, fructose, lactose, maltitol, sucralose or xylitol. The crude BNC was washed with 2% (w/v) NaOH at 80°C for 1 h and then with deionized water. The BNC yield on consumed sugar was in the range 0.002–0.031 g g−1 depending on the bacterial strain and the carbon source (Singhsa et al., 2018).
The enhanced BNC productivity and yield on consumed sugar observed for cultures with the sugar mixture agree with the previous study of Dahman et al. (2010). They studied the effect of using a sugar mixture (based on the sugar composition of hydrolysates of wheat straw, corn fibre and distillers grain) for BNC production and showed enhanced BNC yield on consumed sugar compared to that of single sugar cultures (Dahman et al., 2010). Differences between the two studies include that the current study is the first to show increased sugar consumption ratios for cultures based on individual sugars in the sugar mixture and that Dahman et al. (2010) used shaking cultivation rather than static cultivation. Moreover, in contrast to previous work in the field (Dahman et al., 2010), the current study covers the effects of individual sugars and a sugar mixture on the properties of the BNC.
Morphology of BNC
The macroscopical appearance of the BNC from cultures based on the eight carbon sources was similar, as gelatinous pellicles were formed on the surface of the culture medium. This observation agrees with previous reports (Watanabe et al., 1998; Bi et al., 2014).
The scanning electron microscopy (SEM) micrographs and the fibril diameters of the BNC are shown in Fig. 3. All BNC samples exhibited a three‐dimensional reticulated structure consisting of ultrafine cellulose fibrils. The fibril diameter of BNC from media based on glucose, maltose and the sugar mixture (19–22 nm) was significantly (P < 0.05) lower than that of BNC from media based on the other carbon sources (27–31 nm). As the use of media with glucose, maltose or the sugar mixture had higher volumetric BNC productivity (Fig. 3), it is a possibility that high volumetric productivity and low fibril diameter are related. The average fibril diameter of 19–31 nm of BNC in the current work (Fig. 3A3–H3) is comparable to that of 30–40 nm from strain ATCC 23770 as reported previously (Cavka et al., 2013).
Figure 3.
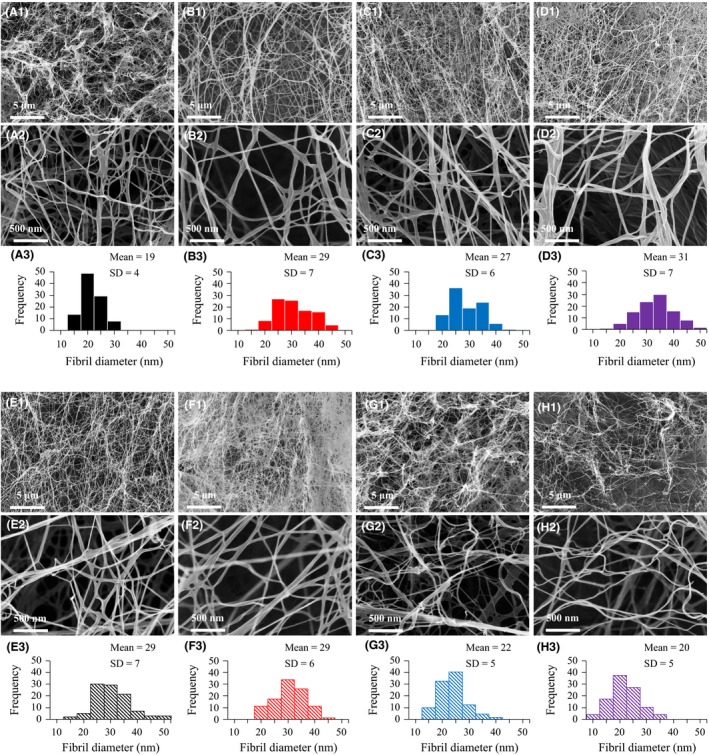
Scanning electron microscopy micrographs and fibril diameter of bacterial nanocellulose (BNC) produced from eight carbon sources. The eight carbon sources for the BNC were glucose (A1–A3), xylose (B1–B3), mannose (C1–C3), arabinose (D1–D3), galactose (E1–E3), sucrose (F1–F3), maltose (G1–G3) and sugar mixture (H1–H3). Amplifications: A1–H1, ×5000; A2–H2, ×50 000. The diameters of fibrils in bundles were calculated separately.
FTIR spectra of BNC
Fig. 4 displays the FTIR spectra of BNC produced in culture media based on the eight carbon sources. The peaks at around 1158 cm−1 are characteristic for the glycosidic links of cellulose (Dokken et al., 2005). The peaks at 895 and 1427 cm−1 can be assigned to deformation of anomeric CH and CH2 bending in cellulose respectively (Kataoka and Kondo, 1999). The peaks at 710 and 750 cm−1 are characteristic for cellulose Iβ and cellulose Iα, respectively (Kataoka and Kondo, 1999). The peaks at around 3350 cm−1 can be assigned to the vibration of OH in cellulose (Lu and Jiang, 2014). The peak at 2900 cm−1 can be assigned to the C‐H stretching vibration in cellulose (Lu and Jiang, 2014). These results are consistent with the characterization of the specimens as cellulose, and, as judged by FTIR spectroscopy, there was no significant difference among the BNC preparations from the culture media with the eight carbon sources.
Figure 4.
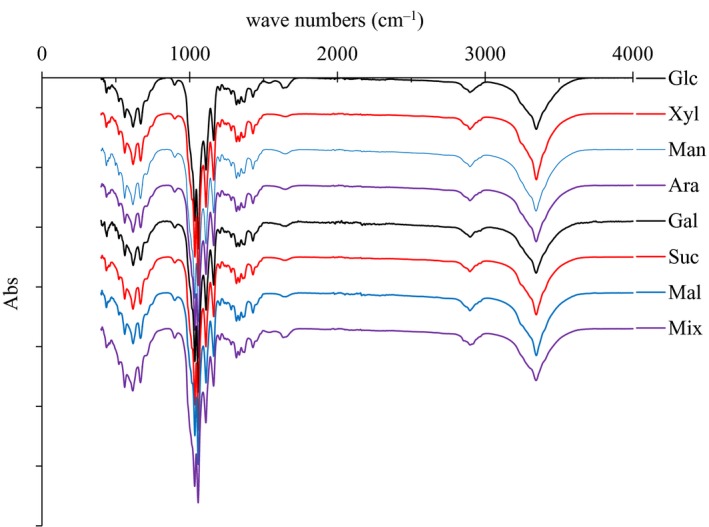
Fourier‐transform infrared spectra of bacterial nanocellulose produced from eight carbon sources.
Viscometric degree of polymerization of BNC
The average viscometric degree of polymerization (DPv) of the BNC produced from the eight carbon sources is shown in Fig. 5. The DPv values of BNC from cultures with glucose and maltose were similar (4350–4400) and significantly (P < 0.05) higher than the DPv values for BNC from the other cultures. The DPv of BNC from cultures with mannose (2340) was far lower than the DPv of BNC from cultures with the other carbon sources (significant difference with P < 0.05). The high DPv of BNC from cultures with glucose and maltose and the low DPv of BNC from cultures with mannose coincided with differences in BNC productivity and BNC yield for cultures based on these sugars. The DPv of BNC from cultures with the sugar mixture was on an intermediate level (Fig. 5).
Figure 5.
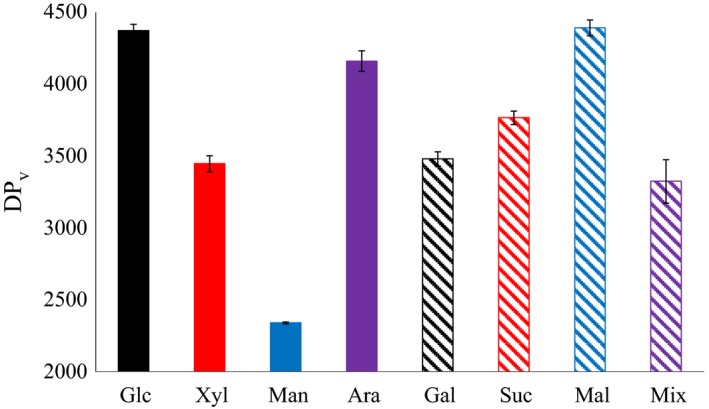
Average viscometric degree of polymerization of bacterial nanocellulose produced from eight carbon sources.
Compared with other studies, the DPv of the BNC in the current investigation was relatively high, except for the BNC from cultures with mannose‐based medium (Fig. 5). Using K. xylinus ATCC 23769 and measuring DP with a similar method as in the current investigation, Shibazaki et al. (1997) reported a DPv of 2000 for BNC and 2280 for cotton linter.
The difference in DPv of BNC from cultures with media based on different sugars is consistent with previous reports. Shi et al. (2013) found that the DP of BNC from cultures with xylose‐based medium was much lower than that of BNC from cultures with glucose‐based medium. However, the current investigation is the first that shows the effects of as many as eight carbon sources on the DPv of BNC, and also the first that shows that BNC from cultures with medium based on a sugar mixture can have lower DPv than BNC from cultures with medium based on individual sugars.
X‐ray diffraction spectra of BNC
Fig. 6 shows XRD (X‐ray diffraction) spectra of BNC produced from cultures with media based on the eight carbon sources. Data on the crystal structure are shown in Table 1. The crystallinity indices of BNC from cultures with galactose, xylose and mannose (76–78%, shown in bold) were the highest among the BNC preparations, whereas the crystallinity index of BNC from cultures with the sugar mixture (54%) was the lowest. The higher crystallinity indices of BNC from galactose, xylose and mannose coincide with low productivity, low yield and low DPv. Regarding the difference in Bragg angle between the peaks of plane (101) and plane (10i), the BNC from cultures with medium based on the sugar mixture had the highest value, while the BNC from cultures with mannose‐based medium had the lowest value. This suggests that the BNC from cultures with the sugar mixture had the highest content of cellulose Iα and that the BNC from cultures with mannose‐based medium had the lowest content of cellulose Iα (Watanabe et al., 1998). The crystallite size of the different crystallite planes of BNC from cultures with different carbon sources varied (Table 1). BNC from cultures with sucrose‐based medium had the largest crystallite size in all planes (Table 1, in bold).
Figure 6.
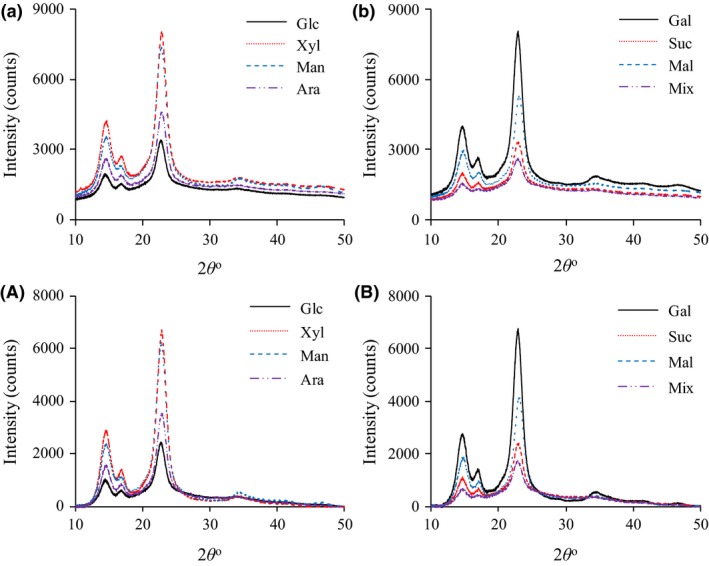
X‐ray diffraction spectra of bacterial nanocellulose produced from eight carbon sources (a and b: spectra before baseline subtraction; A and B: spectra after baseline subtraction).
Table 1.
Crystal properties of BNC produced from cultures with eight different carbon sources
| Crystallinity index (%) | Difference in Bragg angle (degrees)a | Crystallite size (nm) | ||||
|---|---|---|---|---|---|---|
| L (101) | L (10i) | L (002) | L (040) | |||
| Glucose | 61 | 2.34 | 4.27 | 4.32 | 4.49 | 4.99 |
| Xylose | 77 | 2.29 | 3.95 | 4.17 | 4.53 | 4.84 |
| Mannose | 76 | 2.17 | 4.24 | 4.28 | 4.45 | 4.98 |
| Arabinose | 67 | 2.28 | 5.07 | 5.13 | 5.32 | 5.96 |
| Galactose | 78 | 2.32 | 4.73 | 3.40 | 5.58 | 2.39 |
| Sucrose | 61 | 2.39 | 5.17 | 5.24 | 5.43 | 6.04 |
| Maltose | 71 | 2.33 | 4.14 | 4.37 | 4.76 | 5.08 |
| Sugar mix | 54 | 2.43 | 4.21 | 4.26 | 4.42 | 4.91 |
a. Difference in Bragg angle between peaks of plane (101) and plane (10i) in Fig. 6A and B.
Compared with other studies (Watanabe et al., 1998; Lu and Jiang, 2014), the BNC in the current study exhibited similar crystallinity index but smaller crystallite size. For example, Watanabe et al. (1998) found that the crystallinity index of BNC produced from static cultures with fructose‐based medium was 71% and that the crystallite size of plane (101) was 7.4 nm. Krystynowicz et al. (2002) found that the crystallinity index of BNC produced from static cultivation of K. xylinus E25 in glucose‐based medium was 50%.
There are few comparisons of the crystallinity of BNC produced in media based on different sugars. Molina‐Ramírez et al. (2017) found that the crystallinity index of BNC from cultures with sucrose‐based medium was slightly higher than that of BNC from cultures with glucose‐based medium. The current study, which covers BNC production from eight carbon sources and determination of both crystallinity and crystallite size, suggests that sugars that give low BNC productivity and low BNC yield could give high BNC crystallinity. Furthermore, the results suggest that a sugar mixture, as in a lignocellulosic hydrolysate, would give low BNC crystallinity but high BNC productivity and yield.
Thermogravimetric analysis of BNC
The results of thermogravimetric analysis (TGA) are shown in Fig. 7a and b, whereas results from analysis with differential thermogravimetry (DTG) are shown in Fig. 7A and B. The corresponding onset degradation temperature and temperature at maximum degradation rate for the BNC samples are shown in Fig. 7c and d. The BNC from cultures with glucose‐based medium had the highest onset degradation temperature, while the BNC from cultures with galactose‐based medium had the lowest. Regarding the temperature at the maximum degradation rate, the BNC from cultures with glucose‐based medium still had the highest value (361°C), whereas BNC from cultures with mannose‐based medium had the lowest value (349°C). Plots of the DPv and the crystallinity index of the BNC against the temperature at the maximum degradation rate (Fig. 8A and B) indicated that the temperature at the maximum degradation rate of the BNC exhibited a weak positive correlation with the DPv (R 2 = 0.65), whereas there was no correlation for the crystallinity index (R 2 = 0.17). However, it should be noticed that the correlation between the temperature at the maximum degradation rate and the DPv could be more complicated than a simple linear relationship.
Figure 7.
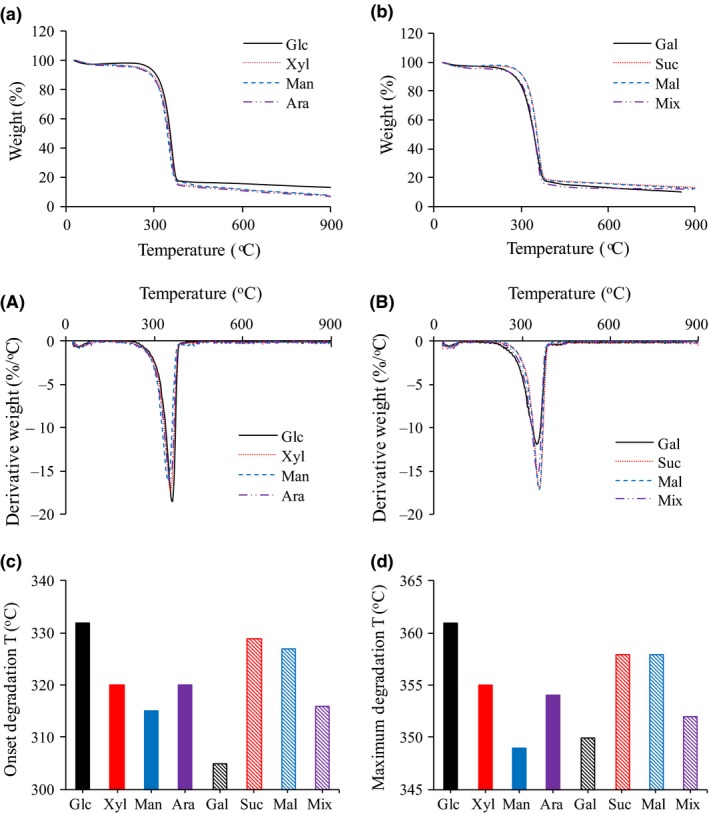
The figure shows results from thermogravimetric analysis (a and b), differential thermogravimetry (A and B), the corresponding onset degradation temperature (c) and the temperature at the maximum degradation rate (d) for bacterial nanocellulose produced from eight carbon sources.
Figure 8.
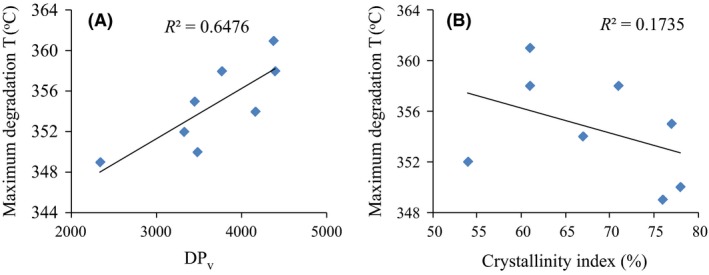
Correlation between degree of polymerizationv (A) and crystallinity index (B) of bacterial nanocellulose (BNC) and the temperature at the maximum degradation rate of BNC.
The thermal stability of BNC has been analysed previously. Generally, the results of previous studies have been similar to those of the current investigation, except that the thermal stability of BNC from cultures with a sugar mixture has not been thoroughly investigated before. Chandrasekaran et al. (2017) found that BNC from K. xylinus ATCC 11142 had a maximum degradation rate at around 300°C. The thermal stability of BNC produced by K. xylinus PTCC 1734 using media with mannitol or sucrose was analysed, and it was reported that the decomposition of the BNC samples resulted in a significant weight loss (70–80%) at around 360–390°C (Mohammadkazemi et al., 2015). The difference in thermal stability of the BNC samples in the current study could be attributed to structural differences in BNC produced in cultures with different sugars.
In conclusion, this investigation elucidated the significant impact of the carbon source in the culture medium on the productivity, structure and properties of BNC. Whereas some carbon sources gave high BNC productivity, yield and DPv, others gave high BNC fibril diameter and high crystallinity. The sugar mixture resembling a lignocellulosic hydrolysate gave relatively high BNC productivity and BNC yield on consumed sugar, but relatively low DPv, crystallinity and thermal stability. Further investigations are needed to clarify the metabolic pathways of the different sugars and how they contribute to different BNC productivity and quality. The large difference in consumption rate between the two industrially important disaccharides sucrose and maltose needs to be better understood. The current study suggests that the yield and DP of BNC produced from cultures with mannose need to be improved, if BNC is to be produced using feedstocks with a high content of mannan, such as softwood. Furthermore, the low BNC yield from culture medium based on xylose suggests that research on xylose utilization by K. xylinus warrants further attention, especially as most lignocellulosic feedstocks contain considerable amounts of xylan.
Experimental procedures
Microorganism and chemicals
Komagataeibacter xylinus (formerly Gluconacetobacter xylinus) ATCC 23770 was obtained from the American Type Culture Collection (Manassas, VA, USA) and was maintained as glycerol stocks at −80°C. K. xylinus ATCC 23770 was used as it is a widely studied BNC‐producing bacterial strain (Thompson and Hamilton, 2001; Hong and Qiu, 2008; Cavka et al., 2013). The sugars used for preparing media, i.e. glucose (glc), xylose (xyl), mannose (man), arabinose (ara), galactose (gal), sucrose (suc) and maltose (mal), were of spectral grade. The peptone was from Merck KGaA (Darmstadt, Germany). The yeast extract was from VWR Chemicals (Radnor, PA, USA). Deionized water was used in all the experiments.
Culture media
The seed culture medium used for preparing inocula contained 25 g l−1 glucose, 5 g l−1 peptone and 3 g l−1 yeast extract. The initial pH was adjusted to 5.0 by using an 8 M aqueous solution of NaOH.
In the comparison of the eight carbon sources, the culture medium contained 40 g l−1 sugar, 10 g l−1 peptone and 6 g l−1 yeast extract. Glucose, xylose, mannose, arabinose and galactose were included as they are common in lignocellulosic hydrolysates. Sucrose is found in sugar industry by‐products, such as molasses and bagasse. Maltose is obtained from hydrolysis of starch. The 40 g l−1 carbon source in the sugar mixture consisted of 24 g l−1 glucose, 9 g l−1 xylose, 4 g l−1 mannose, 2 g l−1 arabinose and 1 g l−1 galactose. This mixture was designed to resemble a hydrolysate of lignocellulose, which is an abundant source of carbohydrate in nature (Humbird et al., 2011; Guo et al., 2013; Domanski et al., 2016; Normark et al., 2016). The final pH was adjusted to 5.0 using the 8 M aqueous solution of NaOH. The volume of medium in each 250 ml flask was 100 ml.
BNC production using different carbon sources
Seed cultures were prepared, and inoculation was conducted as previously reported (Chen et al., 2018a,b). Static cultivations of K. xylinus in 250 ml flasks were performed at 30°C. Samples consisting of 1 ml culture broth were taken every 4 days for determination of pH and residual sugar. After 11 days, the BNC was harvested by centrifugation at 12 000 g. Triplicate cultivations were performed, and mean values are reported. The metabolic mechanisms behind the differences in BNC production were not within the scope of the current study. The current study was designed to show the differences with regard to the quantity and quality of the BNC among culture media based on different sugars.
Due to current limitations in analytical techniques, the biomass evolution was not followed during the cultivations. In static cultivation, K. xylinus cells are mostly embedded in the BNC membrane. Currently, there is no convenient and reliable method to count cells during static cultivation (Zou et al., 2017). More work is needed in the future to develop analytical techniques for measuring biomass evolution in static cultures.
The harvested crude BNC was washed five times at 80°C, each time for four h, using a 0.1 M aqueous solution of NaOH. After that, it was washed five times with deionized water at 80°C, each time for four h. The washing with the NaOH solution was performed a larger number of times and for longer period of time than in most other studies, in which washing was done only once and for no more than one h (Watanabe et al., 1998; Bae et al., 2004). Extensive washing would give a purer product, but might negatively affect the yield. The purified BNC was then freeze‐dried and weighed to calculate BNC productivity and yield. After washing and weighing, the quality of freeze‐dried BNC was analysed.
Measurement of sugar
Analysis of the sugars was performed by using high‐performance liquid chromatography (HPLC). A Bio‐Rad Aminex HPX‐87P Column (7.8 × 300 mm) was used in an Agilent 1260 Infinity series system (Agilent, Santa Clara, CA, USA) equipped with a 1260 series diode array and multiple wavelength detector (DAD). Elution was performed with isocratic flow of high‐quality nano‐pure water. The flow rate was 0.6 ml min−1, and the column temperature was set to 80°C. Agilent software was used for data analysis. The sugar consumption ratio was calculated as follows:
Characterization of BNC from eight carbon sources
Analyses of BNC using SEM, FTIR spectroscopy and DPv were conducted according to methods described previously (Chen et al., 2017, 2018a,b). XRD analysis was performed with an AXS d8 Advance X‐ray diffractometer (Bruker, Germany) using Cu Kα‐radiation (40 kV, 40 mA) with a line‐focus tube and with a two‐dimensional detector. The scan range was 2θ 10–50° with a step size of 0.005°. Calculations of the crystallinity index (expressing the relative degree of crystallinity) without any baseline subtraction of the XRD spectra were based on the empirical method reported by Segal et al. (1959). The apparent crystallite sizes (L) of the crystallographic planes of (101), (10i), (002) and (040) were calculated from the corresponding Bragg angle in baseline‐subtracted spectra as previously reported (Chen et al., 2018a,b).
Thermogravimetric analysis was carried out by using a TG‐209‐F1 Libra gravimetric analyser (Netzsch, Selb, Germany). BNC samples (3–5 mg) were weighed and loaded into an alumina pan, and were heated from 25 to 900°C with a heating rate of 10 K min−1 under a flow of nitrogen gas (250 ml min−1).
Conflict of interest
None declared.
Acknowledgements
Financial support was provided by the National Key Research and Development Program of China (2018YFC1105501), the Fundamental Research Funds for the Central Universities (2232019A3‐08 and 2232018A3‐06), the National Natural Science Foundation of China (No. 51373031), the Swedish strategic research environment Bio4Energy (http://www.bio4energy.se/), the Kempe Foundations (http://www.kempe.com/), the Innovation Foundation of Donghua University for Doctoral Candidates (CUSF‐DH‐D‐2015044) and the China Scholarship Council (201506630057).
Microbial Biotechnology (2019) 12(4), 677–687
Funding Information
Financial support was provided by the National Key Research and Development Program of China (2018YFC1105501), the Fundamental Research Funds for the Central Universities (2232019A3‐08 and 2232018A3‐06), the National Natural Science Foundation of China (No. 51373031), the Swedish strategic research environment Bio4Energy (http://www.bio4energy.se/), the Kempe Foundations (http://www.kempe.com/), the Innovation Foundation of Donghua University for Doctoral Candidates (CUSF‐DH‐D‐2015044) and the China Scholarship Council (201506630057).
Contributor Information
Feng F. Hong, Email: fhong@dhu.edu.cn.
Leif J. Jönsson, Email: leif.jonsson@umu.se.
References
- Bae, S. , Sugano, Y. , and Shoda, M. (2004) Improvement of bacterial cellulose production by addition of agar in a jar fermentor. J Biosci Bioeng 97: 33–38. [DOI] [PubMed] [Google Scholar]
- Bi, J.C. , Liu, S.X. , Li, C.F. , Li, J. , Liu, L.X. , Deng, J. , and Yang, Y.C. (2014) Morphology and structure characterization of bacterial celluloses produced by different strains in agitated culture. J Appl Microbiol 117: 1305–1311. [DOI] [PubMed] [Google Scholar]
- Cavka, A. , Guo, X. , Tang, S.‐J. , Winestrand, S. , Jönsson, L.J. , and Hong, F. (2013) Production of bacterial cellulose and enzyme from waste fiber sludge. Biotechnol Biofuels 6: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekaran, P.T. , Bari, N.K. , and Sinha, S. (2017) Enhanced bacterial cellulose production from Gluconobacter xylinus using super optimal broth. Cellulose 24: 4367–4381. [Google Scholar]
- Chen, L. , Hong, F. , Yang, X.‐X. , and Han, S.‐F. (2013) Biotransformation of wheat straw to bacterial cellulose and its mechanism. Bioresour Technol 135: 464–468. [DOI] [PubMed] [Google Scholar]
- Chen, G. , Wu, G. , Alriksson, B. , Wang, W. , Hong, F.F. , and Jönsson, L.J. (2017) Bioconversion of waste fiber sludge to bacterial nanocellulose and use for reinforcement of CTMP paper sheets. Polymers 9: 458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, G. , Chen, L. , Wang, W. , and Hong, F.F. (2018a) Evaluation of six ionic liquids and application in pretreatment of sweet sorghum bagasse for bacterial nanocellulose production. J Chem Technol Biotechnol 93: 3452–3461. [Google Scholar]
- Chen, G. , Wu, G. , Alriksson, B. , Chen, L. , Wang, W. , Hong, F.F. , and Jönsson, L.J. (2018b) Scale‐up of production of bacterial nanocellulose using submerged cultivation. J Chem Technol Biotechnol 93: 3418–3427. [Google Scholar]
- Dahman, Y. , Jayasuriya, K.E. , and Kalis, M. (2010) Potential of biocellulose nanofibers production from agricultural renewable resources: preliminary study. Appl Biochem Biotechnol 162: 1647–1659. [DOI] [PubMed] [Google Scholar]
- Dokken, K.M. , Davis, L.C. , and Marinkovic, N.S. (2005) Use of infrared microspectroscopy in plant growth and development. Appl Spectrosc Rev 40: 301–326. [Google Scholar]
- Domanski, J. , Borowski, S. , Marchut‐Mikolajczyk, O. , and Kubacki, P. (2016) Pretreatment of rye straw with aqueous ammonia for conversion to fermentable sugars as a potential substrates in biotechnological processes. Biomass Bioenerg 91: 91–97. [Google Scholar]
- Gama, M. , Gatenholm, P. , and Klemm, D. (2012) Bacterial Nanocellulose: A Sophisticated Multifunctional Material. Boca Raton, FL: CRC Press. [Google Scholar]
- Guo, X. , Cavka, A. , Jönsson, L.J. , and Hong, F. (2013) Comparison of methods for detoxification of spruce hydrolysate for bacterial cellulose production. Microb Cell Fact 12: 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, X. , Chen, L. , Tang, J. , Jönsson, L.J. , and Hong, F.F. (2016) Production of bacterial nanocellulose and enzyme from [AMIM]Cl‐pretreated waste cotton fabrics: effects of dyes on enzymatic saccharification and nanocellulose production. J Chem Technol Biotechnol 91: 1413–1421. [Google Scholar]
- Hong, F. , and Qiu, K. (2008) An alternative carbon source from konjac powder for enhancing production of bacterial cellulose in static cultures by a model strain Acetobacter aceti subsp. xylinus ATCC 23770. Carbohydr Polym 72: 545–549. [Google Scholar]
- Hong, F. , Zhu, Y.X. , Yang, G. , and Yang, X.X. (2011) Wheat straw acid hydrolysate as a potential cost‐effective feedstock for production of bacterial cellulose. J Chem Technol Biotechnol 86: 675–680. [Google Scholar]
- Hong, F. , Guo, X. , Zhang, S. , Han, S.‐F. , Yang, G. , and Jönsson, L.J. (2012) Bacterial cellulose production from cotton‐based waste textiles: enzymatic saccharification enhanced by ionic liquid pretreatment. Bioresour Technol 104: 503–508. [DOI] [PubMed] [Google Scholar]
- Humbird, D. , Davis, R. , Tao, L. , Kinchin, C. , Hsu, D. , Aden, A. , et al (2011) Process Design and Economics for Biochemical Conversion of Lignocellulosic Biomass to Ethanol: Dilute‐acid Pretreatment and Enzymatic Hydrolysis of Corn Stover. Golden, CO: National Renewable Energy Lab. No. NREL/TP‐5100‐47764. [Google Scholar]
- Kataoka, Y. , and Kondo, T. (1999) Quantitative analysis for the cellulose Iα crystalline phase in developing wood cell walls. Int J Biol Macromol 24: 37–41. [DOI] [PubMed] [Google Scholar]
- Keshk, S.M. , and Sameshima, K. (2005) Evaluation of different carbon sources for bacterial cellulose production. Afr J Biotechnol 4: 478–482. [Google Scholar]
- Krystynowicz, A. , Czaja, W. , Wiktorowska‐Jezierska, A. , Gonçalves‐Miśkiewicz, M. , Turkiewicz, M. , and Bielecki, S. (2002) Factors affecting the yield and properties of bacterial cellulose. J Ind Microbiol Biotechnol 29: 189–195. [DOI] [PubMed] [Google Scholar]
- Kurosumi, A. , Sasaki, C. , Yamashita, Y. , and Nakamura, Y. (2009) Utilization of various fruit juices as carbon source for production of bacterial cellulose by Acetobacter xylinum NBRC 13693. Carbohydr Polym 76: 333–335. [Google Scholar]
- Lee, K.‐Y. , Buldum, G. , Mantalaris, A. , and Bismarck, A. (2014) More than meets the eye in bacterial cellulose: Biosynthesis, bioprocessing, and applications in advanced fiber composites. Macromol Biosci 14: 10–32. [DOI] [PubMed] [Google Scholar]
- Lin, S.‐P. , Loira Calvar, I. , Catchmark, J.M. , Liu, J.‐R. , Demirci, A. , and Cheng, K.‐C. (2013) Biosynthesis, production and applications of bacterial cellulose. Cellulose 20: 2191–2219. [Google Scholar]
- Lu, H. , and Jiang, X. (2014) Structure and properties of bacterial cellulose produced using a trickling bed reactor. Appl Biochem Biotechnol 172: 3844–3861. [DOI] [PubMed] [Google Scholar]
- Mikkelsen, D. , Flanagan, B.M. , Dykes, G.A. , and Gidley, M.J. (2009) Influence of different carbon sources on bacterial cellulose production by Gluconacetobacter xylinus strain ATCC 53524. J Appl Microbiol 107: 576–583. [DOI] [PubMed] [Google Scholar]
- Mohammadkazemi, F. , Azin, M. , and Ashori, A. (2015) Production of bacterial cellulose using different carbon sources and culture media. Carbohydr Polym 117: 518–523. [DOI] [PubMed] [Google Scholar]
- Mohite, B.V. , and Patil, S.V. (2014) A novel biomaterial: Bacterial cellulose and its new era applications. Biotechnol Appl Biochem 61: 101–110. [DOI] [PubMed] [Google Scholar]
- Molina‐Ramírez, C. , Castro, M. , Osorio, M. , Torres‐Taborda, M. , Gómez, B. , Zuluaga, R. , et al (2017) Effect of different carbon sources on bacterial nanocellulose production and structure using the low pH resistant strain Komagataeibacter medellinensis . Materials 10: 639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normark, M. , Pommer, L. , Gräsvik, J. , Hedenström, M. , Gorzsás, A. , Winestrand, S. , and Jönsson, L.J. (2016) Biochemical conversion of torrefied Norway spruce after pretreatment with acid or ionic liquid. Bioenergy Res 9: 355–368. [Google Scholar]
- Segal, L. , Creely, J. , Martin, A. , and Conrad, C. (1959) An empirical method for estimating the degree of crystallinity of native cellulose using the X‐ray diffractometer. Text Res J 29: 786–794. [Google Scholar]
- Shi, Q.‐S. , Feng, J. , Li, W.‐R. , Zhou, G. , Chen, A.‐M. , Ouyang, Y.‐S. , and Chen, Y.‐B. (2013) Effect of different conditions on the average degree of polymerization of bacterial cellulose produced by Gluconacetobacter intermedius BC‐41. Cell Chem Technol 47: 503–508. [Google Scholar]
- Shibazaki, H. , Kuga, S. , and Okano, T. (1997) Mercerization and acid hydrolysis of bacterial cellulose. Cellulose 4: 75–87. [Google Scholar]
- Singhsa, P. , Narain, R. , and Manuspiya, H. (2018) Physical structure variations of bacterial cellulose produced by different Komagataeibacter xylinus strains and carbon sources in static and agitated conditions. Cellulose 25: 1571–1581. [Google Scholar]
- Thompson, D.N. , and Hamilton, M.A. (2001) Production of bacterial cellulose from alternate feedstocks. Appl Biochem Biotechnol 91: 503. [DOI] [PubMed] [Google Scholar]
- Watanabe, K. , Tabuchi, M. , Morinaga, Y. , and Yoshinaga, F. (1998) Structural features and properties of bacterial cellulose produced in agitated culture. Cellulose 5: 187–200. [Google Scholar]
- Zou, X. , Wu, G. , Stagge, S. , Chen, L. , Jönsson, L.J. , and Hong, F.F. (2017) Comparison of tolerance of four bacterial nanocellulose‐producing strains to lignocellulose‐derived inhibitors. Microb Cell Fact 16: 229. [DOI] [PMC free article] [PubMed] [Google Scholar]


