Abstract
Background
Talaromycosis caused by Talaromyces marneffei infection is a fatal systemic mycosis in immunosuppressed individuals, such as patients with AIDS. Cytokines and immunocytes play a central role against fungus infection. However, how the host immune system responds to infection and treatment has not been reported to date.
Methods
Forty-one Talaromyces marneffei coinfected AIDS patients were followed up, their immunocytes and cytokine profiles were obtained at different antifungal treatment stages, and data on clinical features and laboratory examinations were collected. Correlation analysis was used to identify factors associated with host immunity against Talaromyces marneffei infection in AIDS patients.
Results
Common diseases and conditions of these 41 patients were lymphadenopathy, hepatomegaly, and splenomegaly. CD4+ T cells were extremely low in all of them. Moreover, significant increases of proinflammatory cytokines (IL-12, IL-17A, TNF-α, IFN-γ, IL-18, and IL-1β), anti-inflammatory cytokines (IL-10), and chemokines (IP-10) were observed in talaromycosis before treatment (P < .05), comparing to both AIDS patients and healthy controls. The cytokines IL-6, IL-8, TNF-α, IL-18, IL-17A, IL-7, IP-10, and IL-1β reached peak levels 3 days after initial antifungal therapy, and then gradually decreased. The symptoms of the patients gradually decreased. Furthermore, patients who died showed the highest levels of IL-6, TNF-α, IL-8, IL-1β, and IP-10, which were 1.4- to 164-fold higher than in surviving patients.
Conclusions
Our findings indicate that innate immune-cell-derived cytokines are critical for host defense against AIDS-associated Talaromyces marneffei infection; furthermore, excessive inflammatory cytokines are associated with poor outcomes.
Keywords: AIDS, antifungal therapy, cytokines, innate immunity, Talaromyces marneffei
Talaromyces marneffei (T. marneffei), formerly designated as Penicillium marneffei, is a dimorphic fungus and is endemic in Southeast Asian countries, such as Thailand, Vietnam, and China [1–3]. T. marneffei can cause fatal systemic mycosis (talaromycosis) in immunocompromised individuals [4]. In patients infected HIV and AIDS, talaromycosis, after tuberculosis and cryptococosis, ranks third among the most fatal common opportunistic infections in Southeast Asia [4, 5], particularly in individuals with a CD4+ T cells count < 50 cells/μL.
Inhalation of T. marneffei conidia without normally occurring clearance due to immunologic dysfunction may results in conidia dissemination throughout the body. Host immunity initiates inflammatory mechanisms to eliminate pathogens in response to T. marneffei invasion [6]. During the immunoinflammatory reaction, the activated immunocytes produce pro- and anti-inflammatory cytokines, which simultaneously interact and form a complex inflammatory network, the dynamic balance of which influences the progression and outcome of the disease [7]. The intracellular infection of macrophages is characteristic of T. marneffei pathology, and recent studies reported that the activation of macrophage-derived cytokines (ie, TNF-α and IFN-γ) is elevated and responsible for T. marneffei clearance [8, 9]. However, these experiments were performed in vitro by stimulating macrophages with T. marneffei or in animal models. In AIDS patients with severe adaptive immune deficiency, the host defense against T. marneffei infection remains to be elucidated. Additionally, the dynamic changes in immune parameters during both infection and treatment have not been reported. Furthermore, little is known about the role of inflammatory factors in vivo and the correlation between cytokines and both disease course and prognosis.
This work dynamically detected changes of inflammatory factors and immune cells at different treatment stages of AIDS co-infected with T. marneffei, AIDS patients without T. marneffei coinfection and healthy individuals were enrolled as controls. Clinical manifestations and routine laboratory examinations were analyzed and elucidated immunological changes during infection and treatment were investigated. These data provide a clinical reference for the further exploration of the relationship between immunological mechanisms and disease prognosis. This study showed that in innate immune cells, macrophage-derived cytokines and chemokines are particularly vital for the host defense against T. marneffei in inadequate CD4+ T cells and excessive inflammatory factors may relate to poor disease prognosis and outcomes.
METHODS
Ethical Approval
Participant samples were collected according to protocols approved by the respective institutional review boards. This study was approved by the Ethics Committee of the First Affiliated Hospital of Kunming Medical University (2018L-45). Study participants provided written informed consent in accordance with the Declaration of Helsinki.
Study Subjects
Sixty-eight AIDS patients who were coinfected with T. marneffei were recruited in the Yunnan Infectious Disease Hospital between May 2016 and July 2018. Patients with ongoing acute or chronic coinfections, such as viral hepatitis, tuberculosis, pneumonia, candidiasis, and cryptococcosis, were excluded and, consequently, 41 patients were recruited for this study. Among these, 14 patients were followed up before (as pretreatment) as well as 3 days, 7 days, 15 days, 30 days, 90 days, and 180 days after initial antifungal therapy. All subjects were treated and both physical examination and laboratory tests were conducted. Twelve healthy subjects were used as control to rule out possible medical conditions and a further 12 HIV-1 infected patients (HIV-1-only), who did not suffer from any other infectious diseases, were enrolled as control group.
Clinical Data Collection
The clinical data of patients with AIDS and T. marneffei coinfection, including their routine laboratory examinations, chest computed tomography (CT) scans, chest and abdominal ultrasonography, treatment, and outcomes were collected. The demographic characteristics of all subjects were collected.
Sample Collection and Treatment
Peripheral venous blood was drawn from HIV-1-only patients, healthy control individuals, and AIDS and T. marneffei coinfection patients at pretreatment, as well as 3 days, 7 days, 15 days, 30 days, 90 days, and 180 days after initiation of the antifungal therapy, respectively. Every subject received an EDTA tube for the collection of peripheral blood. The blood samples were centrifugated at 3500 rpm for 10 minutes to separate plasma and blood cells. Once obtained, all plasma samples were immediately stored at -80°C for cytokine and chemokine detection.
Sample Culture and T. marneffei Identification
Whole blood samples, other body fluid samples, or both, were collected at different stages of antifungal therapy and were cultured at 37°C and 25°C for 7–14 days. Pathogens were identified under a microscope after medan dyeing. Brain heart infusion agar (BHI agar) was used as T. marneffei culture medium.
Measurement of Serum Concentrations of Cytokines
The plasma concentrantions of IL-4, IL-6, IL-7, IL-10, IL-12, IL-17A, IL-18, IL-1β, TNF-α, IFN-γ, IL-8, IP-10, and SDF-1α were measured by using the Magnetic Luminex assay with the Human Premixed Multi-Analyte kit (R&D Systems, Minneapolis, MN) according to the manufacturer’s guidelines.
Statistical Analyses
Clinical data were presented as number (%), mean (), and standard deviation, or median and interquartile range, as appropriate. Comparisons of demographic data and clinical characteristics between 2 groups were performed using t test, Chi-square test, or Fisher’s exact test, as appropriate. One-way analysis of variance was used for statistical calculations among multigroups. A P value of < 0.05 was considered statistically significant. Data analyses were performed using SPSS 22.0 software (SPSS Inc, Chicago, IL). Figures were designed by using GraphPad Prism 7 (GraphPad Software, San Diego, CA) and OriginPro software (OriginLab Corporation, Northampton, MA).
RESULTS
Demographic and Clinical Characteristics of AIDS-Associated T. marneffei Fungemia Patients
Forty-one T. marneffei strains were isolated from the blood of patients, and 8 further strains were isolated from bodily fluid samples, including bone marrow, skin lesion, sputum, and cerebrospinal fluid. The average age of the surviving group and the deceased group was 35.00 ± 9.76 years and 42.00 ± 10.29 years, respectively. No statistical difference of clinical symptoms was found between the group of patients that died and the group with noticeable improvements. In addition, no significant differences were observed for age, gender, occupation, marital status, and route of transmission between the 2 groups (Table 1).
Table 1.
Demographic and Clinical Characteristics in T. marneffei Coinfected AIDS Patients
| Total cases (n = 41) | |||
|---|---|---|---|
| Surviving group (n = 37) | Deceased group (n = 4) | P value | |
| Age (years) | 35.00 ± 9.76 | 42.00 ± 10.29 | .271 |
| Gender | .517 | ||
| Male | 31 (88.6%) | 4 (11.4%) | |
| Female | 6 (100%) | 0 (0%) | |
| Nationality | .668 | ||
| Han | 26 (89.7%) | 3 (10.3%) | |
| Other | 11 (91.7%) | 1 (8.3%) | |
| Marriage | .533 | ||
| Married | 23 (88.5%) | 3 (11.5%) | |
| Other | 14 (93.3%) | 1 (6.7%) | |
| Occupation | .703 | ||
| Farmers | 12 (85.7%) | 2 (14.3%) | |
| Unemployed | 17 (94.4%) | 1 (5.6%) | |
| Other | 8 (88.9%) | 1 (11.1%) | |
| HIV transmission route | .716 | ||
| Heterosexual transmission | 22 (91.7%) | 2 (8.3%) | |
| Homosexual transmission | 5 (100%) | 0 (0%) | |
| Intravenous drug use | 4 (80%) | 1 (20%) | |
| Unknown | 6 (85.7%) | 1 (14.3%) | |
| Clinical symptoms | |||
| Fever | 33 (91.7%) | 3 (8.3%) | .418 |
| No fever | 4 (80.0%) | 1 (20.0%) | |
| Cutaneous lesions | .512 | ||
| Yes | 23 (92.0%) | 2 (8.0%) | |
| No | 14 (87.5%) | 2 (12.5%) | |
| Respiratory symptoms | .598 | ||
| Yes | 21 (91.3%) | 2 (8.7%) | |
| No | 16 (88.9%) | 2 (11.1%) | |
| Gastrointestinal symptoms | .598 | ||
| Yes | 16 (88.9%) | 2 (11.1%) | |
| No | 21 (91.3%) | 2 (8.7%) | |
| Weight loss | .467 | ||
| Yes | 13 (86.7%) | 2 (13.3%) | |
| No | 24 (92.3%) | 2 (7.7%) | |
| Other symptoms | .052 | ||
| Yes | 8 (72.7%) | 3 (27.3%) | |
| No | 29 (96.7%) | 1 (3.3%) | |
| Lymphadenopathy | .533 | ||
| Yes | 23 (88.5%) | 3 (11.5%) | |
| No | 14 (93.3%) | 1 (6.7%) | |
| Hepatomegaly | .245 | ||
| Yes | 8 (80.0%) | 2 (20.0%) | |
| No | 29 (93.5%) | 2 (6.5%) | |
| Splenomegaly | .678 | ||
| Yes | 18 (90%) | 2 (10.0%) | |
| No | 19 (90.5%) | 2 (9.5%) | |
| Abdominal or pleural cavity effusion | .623 | ||
| Yes | 12 (92.3%) | 1 (7.7%) | |
| No | 25 (89.3%) | 3 (10.7%) | |
| Lung CT examination | .278 | ||
| Alveolar infiltration | 22 (84.6%) | 4 (15.4%) | |
| Interstitial infiltration | 8 (100%) | 0 (0%) | |
| Normal | 7 (100%) | 0 (0%) | |
| Initial antifungal regimen | .398 | ||
| Amphotericin B + Fluconazole | 12 (100%) | 0 (0%) | |
| Amphotericin B | 12 (85.7%) | 2 (14.3%) | |
| Voriconazole + Amphotericin B | 13 (86.7%) | 2 (13.3%) | |
| ART before hospitalization | .332 | ||
| Yes | 10 (83.3%) | 2 (16.7%) | |
| No | 27 (93.1%) | 2 (6.9%) | |
| CD4 count (cells/μl) | 15 (10–28) | 7 (3–28) | .187 |
| CD8 count (cells/μl) | 193 (125–346) | 73 (49–105) | .012a |
| Viral load (copies/ml) | 1.0 × 106 (3.5 × 103–6.2 × 106) | 3.0 × 107 (2.0 × 107–3.4 × 107) | .007b |
Abbreviations: ART, antiretroviral therapy; CT, computed tomography.
a P < .05.
b P < .01.
The CD8+ cell count in the deceased group (73 cells/μl, (49–105)) was significantly lower than in the surviving group (193 cells/μl, (125–346)) (P = .012). Furthermore, viral load of the deceased group (3.0 × 107 copies/ml, (2.0 × 107–3.4 × 107)) was significantly higher than in the surviving group (1.0 × 106 copies/ml, (3.5 × 103–6.2 × 106)) (P = .007) (Table 1).
Dynamics of Routine Laboratory Examinations
The count and percentage of lymphocytes at pretreatment were lower than those of healthy control and HIV-infected patients, temporarily decreased during antifungal therapy, and then gradually increased and returned to normal levels within 90 days of antifungal treatment. The neutrophil percentage (NEUT%) and absolute neutrophil count at baseline were higher than in healthy control and HIV-infected patients and peaked at 3 and 7 days after initial treatment, respectively, before gradually reuturning to normal levels in response to antifungal therapy. The percentage of monocytes in T. marneffei coinfected AIDS patients was at a high level pretreatment; however, it deceased significantly during the 3 days after treatment, and then returned to normal levels after 15 days of treatment (Table 2). The CD4+ T (14 cells/μl, (9–28)) and CD8+ T (169 cells/μl, (103–331)) counts significantly decreased compared to those of HIV-infected patients (351 ± 46 and 899, (703–1522), respectively), and significantly increased in response to 30 days of treatment (130 ± 17 and 741 ± 55, respectively) (Table 2).
Table 2.
Dynamics of Laboratory Examinations During Antifungal Therapy in T. marneffei Coinfected AIDS Patients
| DPT | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Control (n = 12) | HIV (n = 12) | Pre-T (n = 14) | 3 (n = 14) | 7 (n = 14) | 15 (n = 14) | 30 (n = 14) | 90 (n = 14) | 180 (n = 14) | |
| WBC (×109/L) | 5.89 ± 0.83a | 5.1 ± 1.08b | 4.11 ± 2.04c | 3.83 ± 3.39 | 4.30 ± 2.28 d | 3.45 ± 2.13 | 4.01 ± 1.6f | 5.39 ± 2.24g | 5.92 ± 2.17h |
| Neutrophil (×109/L) | 3.38 ± 0.53 | 3.00 ± 0.78 | 3.18 ± 2.12 | 4.47 ± 3.90 | 4.66 ± 1.74 | 2.84 ± 2.61 | 2.28 ± 1.41 | 2.92 ± 1.69 | 3.61 ± 1.58 |
| Neutrophil (%) | 58.57 ± 6.04a | 58.7 ± 7.06bi | 72.54 ± 19.98 | 84.54 ± 12.54 | 83.33 ± 6.61 | 60.08 ± 19.37e | 57.28 ± 15.54fj | 53.2 ± 16.1g | 59.86 ± 7.3h |
| Lymphocyte (×109/L) | 2.06 ± 0.47a | 1.89 ± 0.55bi | 0.59 ± 0.31 | 0.34 ± 0.25 | 0.51 ± 0.23 | 0.77 ± 0.38e | 0.97 ± 0.53fj | 1.67 ± .090g | 1.54 ± 0.49h |
| Lymphocyte (%) | 34.98 ± 4.96a | 37.69 ± 6.95bi | 15.63 ± 9.7 | 11.34 ± 6.08 | 9.32 ± 0.82 | 22.07 ± 11.45e | 24.31 ± 9.28fj | 31.30 ± 13.46g | 26.73 ± 6.32h |
| Monocyte (×109/L) | 0.31 ± 0.04 | 0.21 ± 0.08 | 0.24 ± 0.15 | 0.16 ± 0.10 | 0.35 ± 0.21 | 0.48 ± 0.28 | 0.41 ± 0.14 | 0.39 ± 0.13 | 0.41 ± 0.15 |
| Monocyte (%) | 5.38 ± 1.03 | 3.97 ± 0.54 | 7.19 ± 5.50 | 3.66 ± 2.05 | 6.80 ± 3.97 | 13.13 ± 6.00 e | 10.97 ± 4.18 fj | 7.91 ± 3.23 | 7.58 ± 3.79 |
| Hemoglobin (g/L) | 156.28 ± 16.29a | 137.36 ± 23.17bi | 95.73 ± 22.25 | 93.00 ± 19.35 | 77.00 ± 25.01d | 96. 43 ± 19.17 | 101.45 ± 15.69 | 137.22 ± 31.21g | 152.62 ± 27.41h |
| Platelet (×109/L) | 258.28 ± 67.36a | 156.81 ± 58.71 | 149.42 ± 95.79 | 147.75 ± 55.48 | 104.40 ± 61.31 | 177.62 ± 25.30 | 205.55 ± 73.86fj | 261.77 ± 107.44g | 235.00 ± 76.71h |
| ALT (U/L) | - | 46.18 ± 27.13i | 38.42 ± 33.97 | 47.40 ± 20.03 | 42.20 ± 27.66 | 33.31 ± 26.72 | 23.85 ± 15.00j | 21.25 ± 10.55g | 21.25 ± 7.26h |
| AST (U/L) | - | 30.63 ± 7.63 | 84.86 ± 20.48 | 85.40 ± 27.90 | 119.14 ± 34.61 | 54.91 ± 12.01 | 43.88 ± 11.2 | 33.50 ± 8.33 | 26.25 ± 2.90 |
| CD4+ T (cells/μl) | - | 351 ± 46i | 14 (9–28) | - | - | - | 129.8 ± 17.3j | - | - |
| CD8+ T (cells/μl) | - | 899 (703–1522)i | 169 (103–331) | - | - | - | 740.9 ± 55.4j | - | - |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; DPT, days posttreatment; pre-T, pretreatment; WBC, white blood cell.
a Statistically significant (P < .05) between DPT-3 group and Health control group.
b Statistically significant (P < .05) between DPT-3 group and HIV-1-only group.
c Statistically significant (P < .05) between DPT-3 group and pre-T group.
d Statistically significant (P < .05) between DPT-3 group and DPT-7 group.
e Statistically significant (P < .05) between DPT-3 group and DPT-15 group.
f Statistically significant (P < .05) between DPT-3 group and DPT-30 group.
g Statistically significant (P < .05) between DPT-3 group and DPT-90 group.
h Statistically significant (P < .05) between DPT-3 group and DPT-180 group.
i Statistically significant (P < .05) between pre-T group and HIV-1-only group.
j Statistically significant (P < .05) between pre-T group and DPT-30 group.
Hemoglobin levels significantly decreased from pretreatment to 3 days and 7 days after treatment (95.73 ± 22.25, 93.00 ± 19.35, 77.00 ± 25.01 g/L, respectively) in both HIV-1-only patients (P < .001) and healthy controls (P < .001). With ongoing treatment, the level of hemoglobin returned to normal after 90 days of treatment. Platelet levels decreased and returned to normal level with ongoing treatment. The levels of alanine aminotransferase and aspartate aminotransferase increased markedly from pretreatment (38.42 ± 33.97 IU/L, 84.86 ± 20.48 IU/L, respectively) to 7 days after treatment (47.40 ± 20.03 IU/L, 85.40 ± 27.90 IU/L, respectively), and then gradually decreased to normal levels (Table 2).
Dynamics of Pro- and Anti-Inflammatory Cytokines and Chemokines
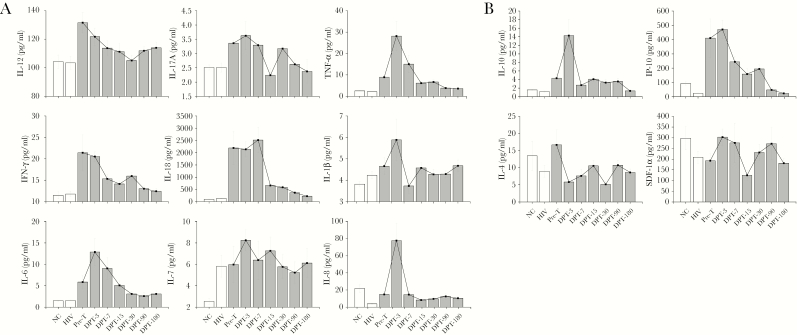
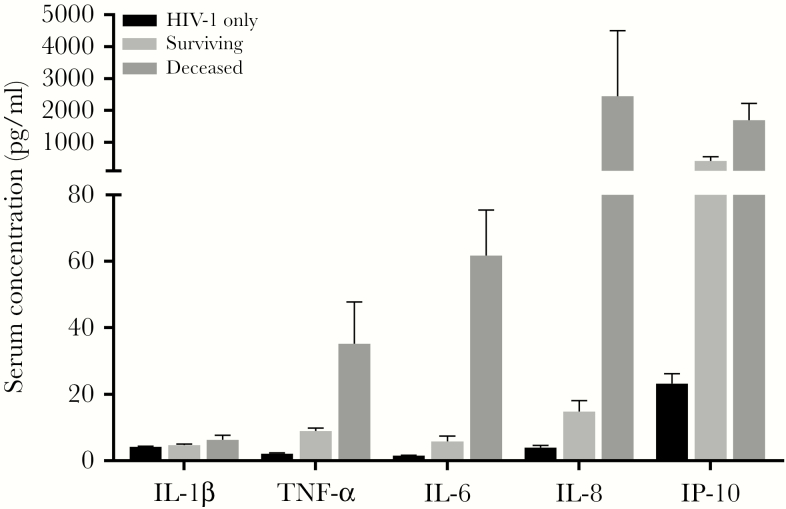
Most proinflammatory and anti-inflammatory cytokines and chemokines of the AIDS and T. marneffei coinfected patients at the pretreatment stage were higher than those of healthy controls and HIV-1-only patients. Among these, TNF-α, IL-6, IL-17A, IL-1β, and IL-7 continued to increase and reached peak levels 3 days after treatment (Figure 1). Thereafter, these levels gradually decreased 7 days after treatment. IL-18 significantly increased at baseline, 3 days, and 7 days, and then sharply decreased to normal levels 15 days after treatment. However, plasma levels of IL-12 and IFN-γ had peak levels at baseline and gradually decreased after treatment, returning to nomal levels day 7. The levels of the chemokines IL-8, SDF-1α, and IP-10 increased significantly and peaked 3 days after treatment, showing a decreasing trend on day 7 (Figure 1). The level of IL-10 quickly peaked at 3 days and returned to nearly baseline level at 7 days. IL-4 showed the highest level at pretreatment and began to gradually decrease in response to treatment (Figure 1). The levels of TNF-α, IL-6, IL-8, IL-1β, and IP-10 in deceased patients were 1.4–164 fold higher than in surviving patients before treatment (P < .05) (Figure 2).
Figure 1.
Cytokine profile during antifungal therapy in T. marneffei coinfected AIDS patients. (A) Levels of proinflammatory cytokines. TNF-α, IL-6, IL-8, IL-1β, IL-17A, IL-7, IL-18 at pretreatment were higher than those of healthy control and HIV-infected group. Besides IL-18, they continued to rise and reached the peak levels 3 days after treatment. The levels of IL-12 and IFN-γ reached peak levels at pretreatment compared to healthy controls and HIV-1-only group and then gradually decreased after treatment and returned to normal levels 7 days after treatment. (B) Levels of anti-inflammatory cytokines and chemokines. SDF-1α and IP-10 showed high levels at 3 days, and 7 days after treatment. Anti-inflammatory cytokines IL-10 quickly peaked 3 days after treatment and soon returned to almost baseline level 7 days after treatment. However, IL-4 reached peak levels at baseline and began to decrease gradually after treatment. IL indicates interleukin; IFN-γ, interferon γ; TNF-α, tumor necrosis factor-α; SDF-1α, stromal cell-derived factor-1α; IP-10, Interferon-induced protein 10.
Figure 2.
Plasma level of proinflammatory cytokines and chemokines in survivin and deceased patients. Pretreatment levels of TNF-α, IL-6, IL-8, IL-1β, and IP-10 in deceased patients were significantly higher than surviving patients (P < .05). HIV-1-only indicates the cytokine levels in patients with HIV-1 infection but without T. marneffei coinfection; surviving represents the cytokine levels at pretreatment stage in AIDS patients with T. marneffei coinfection; deceased indicates cytokine levels in the AIDS-patients with T. marneffei coinfection who died despite antifungal treatment.
Correlations Between Innate Immunocytes and Inflammatory Cytokine Levels
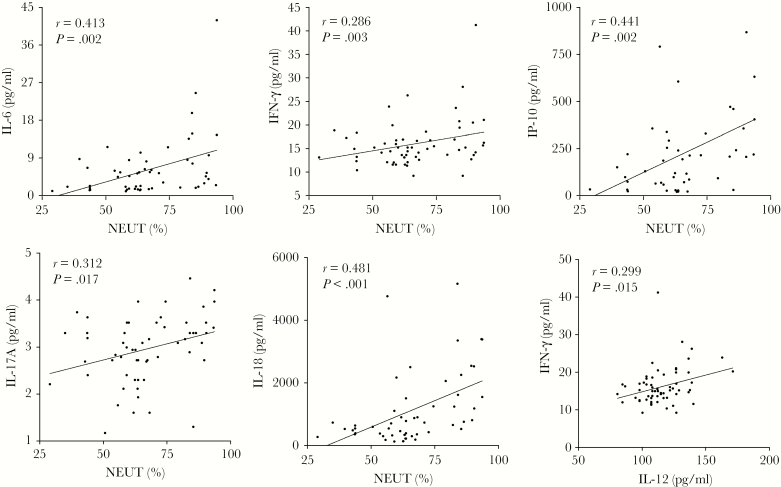
The correlation between levels of plasma inflammatory agents levels and innate immunocytes in patients indicated positive correlations between NEUT (%) and IFN-γ (r = 0.286; P = .03), IL-18 (r = 0.481; P < .001), IL-17A (r = 0.312; P = .017), IP-10 (r = 0.441; P = .002), and IL-6 (r = 0.413; P = .002). IFN-γ had a significant positive correlation with IL-12 (r = 0.299; P = .015) (Figure 3).
Figure 3.
Correlation between levels of inflammatory cytokines and innate immunocytes. Positive correlations were found between percentage of neutrophil (%) and IFN-γ, IL-18, IL-17A, IP-10, and IL-6 (P < .05). IFN-γ had a significant positive correlation with IL-12 (P = .002).
DISCUSSION
For the first time, this study described the elaborate dynamics of the immune status of AIDS-associated T. marneffei infection both pre- and post-antifungal treatment. In innate immune cells, mainly macrophages-derived inflammatory cytokines (TNF-α, IFN-γ, IL-6, IL-12, IL-18, IL-1β) and chemokines (IL-8, IP-10) were evaluated and found to that play an important role in resistance to T. marneffei in patients who suffer from extreme deficiency of the adaptive immune system as a result of HIV infection. More interestingly, on the third day after initial antifungal treatment, a substantial number of inflammatory factors showed a secretion peak compared to pretreatment, while these which should have decreased in response to effective treatment. An overactive immune response may be associated with poor disease progression and disease outcomes, because inflammatory factors increased sharply in deceased patients.
Inhalation of T. marneffei conidia causes transient fungal pneumonia in immunodeficient patients, resulting in lung lesions and associated respiratory symptoms, such as cough and expectoration. Conidia turn into pathogenic yeast infecting alveolar macrophages, which can follow the pulmonary circulation spread to the whole body causing fungaemia and exhibit sepsis like manifestations, such as fever, chills, and fatigue. Mononuclear or macrophages phagocytisis follows intracellular infection of T. marneffei after infection [10], as indicated by the fact that patients infected with T. marneffei showed hepatomegaly, splenomegaly, and lymphadenopathy in multiple organs that are rich in mononuclear-macrophages. In addition, digestive symptoms such as abdominal pain, diarrhea, poor appetite, weight loss, anemia, and other non-specific symptoms like hematuria, nausea, and a sore throat. These often lead to misdiagnosis. These reported signs and symptoms were similar to those reported previously [11, 12].
T cell immunodeficient individuals are at risk for developing disseminated fungal infections. In nude or T-cell-depleted mice, mycosis is fatal. This study showed that T. marneffei coinfected AIDS patients had severe adaptive immunodeficiency with low levels of lymphocytes, especially CD4+ T cells (Table 2). When the CD4 levels decrease below 50 cells/μl, the host enters a state of severe immunodeficiency. CD4 cell subtypes can be divided into Th1, Th2, Th17, and Treg cells. CD4+ T cells are the main or sole source of Th2 anti-inflammatory cytokines (IL-4, IL-5, and IL-10), and CD4 deficiency resulted in a loss of IL-4, IL-5, and IL-10. However, residual CD8+ T cells still secrete IL-2 and IFN-γ [13]. This was also found in the present study, where IL-4 increased with low amplitude due to low CD4+ T levels (Figure 1 and Table 1). However, other proinflammatory factors (IFN-γ, TNF-α, IL-17A, IL-12, IL-6, and IL-10) can be secreted by Th1, Th17, and other intrinsic immunity cells. Therefore, the immune inflammatory response was much stronger in the investigated cohort compared to both HIV-1-only patients and healthy individuals, although these were at an immunosuppressed state. This indicates that substantial inflammatory factors in these patients were not only secreted by CD4+ T cells. The peaks of a number of proinflammatory cytokines (IFN-γ and IL-17A) that mainly are released by T cell types were not as high as those of cytokines secreted by innate immune cells. Moreover, proinflammatory cytokines (IL-6, IL-12, IL-18, and TNF-α) and chemokines (IL-8 and IP-10) that are predominantly secreted by macrophages, increased strongly, especially in deceased patients. Thus, the phagocytes of the innate immune system, including monocytes, tissue macrophages [10], and neutrophils [14], play a prominent role in the response against T. marneffei infection when T cells are compromised.
As a type of multiple biological effect monocytes, macrophages are essential for mediating the first steps of an effective antifungal host defense [15]. In vitro studies have suggested that both human and mouse macrophages are central for the crucial of T. marneffei growth and the killing of intracellular yeast cells by secreting cytokines [16, 17]. Activated macrophages produce cytokines such as IL-6, TNF-α, IL-8, IL-18, IL-12, IL-10, and IFN-γ. In addition, the proinflammatory cytokine TNF-α could stimulate the antifungal effector functions of macrophages and neutrophils [18].
A strongly positive correlation between IL-12 and IFN-γ levels was observed (Figure 3), and both of these levels were significantly increased compared to HIV-1-only patients and healthy controls (Figure 1). It has been reported that the IFN-γ and IL-12 pathways are essential for the initiation of adaptive responses in mucocutaneous diseases [19]. IFN-γ deficiency resulted in a higher susceptibility to T. marneffei infection [20] and increased T. marneffei proliferation [14]. Patients with anti-IFN-γ autoantibodies are more susceptible to infections T. marneffei [21, 22]. Because the antibody could block IFN-γ activation, inhibited STAT1 phosphorylation and IL-12 production result in a reduction of host resistance to fungal infection [23]. Mice dendritic cells also could produce IL-12 for defense against T. marneffei by TLR2 and dectin-1 [24]. The present study shows that the levels of IL-12 and IFN-γ increased, which may be significant for host defense against T. marneffei infection in vivo.
Monocyte- or macrophages-derived IL-1β and IL-18 can recruit more proinflammatory cytokines and chemokines for fungicidic efficacy, causing a cascade of inflammatory responses in the process of pyroptosis [25]. One particularly interesting observation in this study was that IL-1β and IL-18 levels increased and reached peak levels 3 days posttreatment, which were accompanied by peaks in the production of other inflammatory cytokines (TNF-α, IL-6, and IL-17A) and chemokines (IP-10 and IL-8), and they all showed the same decreasing trend in response to treatment (Figure 1). Srinoulprasert et al also demonstrated that human monocytes could recognize T. marneffei conidia though pattern recognition receptors and initiate the production of proinflammatory cytokines TNF-α and IL-1β [6]. Monocytes and macrophages provide the largest contribution of chemokine IP-10 [26]. High IP-10 levels were reported in HIV infection and have been associated with lower CD4 counts [27]. IL-6 is also is promptly secreted by activated macrophages. This study provides novel information about IL-6 and IP-10 in T. marneffei infection in AIDS patients.
In addition to macrophages, neutrophils also exhibit strong fungicidal activity in T. marneffei infection [14]. Various neutropenic mouse models clearly have demonstrated a significant role for neutrophils in disseminating fungal infection [28]. A relevant case report provides information on a patient with dysplastic neutrophils infected with T.marneffei [29]. Ellett et al reported that tissue macrophages could enhance T. marneffei exposure to effective neutrophil fungicidal mechanisms [14]. On the one hand, the antigens of the pathogen are capable of attracting the neutrophils to reach the inflammatory site [28]. In the present study, both the count and percentage of neutrophils in AIDS patients coinfected with T. marneffei increased at pretreatment and 3 days posttreatment compared to healthy controls. Therefore, it was hypothesized that it is likely that activated neutrophils were chemotactically recruited to the inflammatory site, where they activated and enhanced the function of neutrophils and promoted the fungicidic activity of the host.
On the other hand, activated neutrophils also recruit and activate other phagocytes, cytokines, and chemotaxis to kill fungal cells [28]. These results may help to understand that neutrophils showed a significant positive correlation with proinflammatory cytokines and chemokines (IL-17A, IFN-γ, IL-6, IP-10, and IL-18). IL-17A is a proinflammatory cytokine produced by Th17 cells, neutrophils, and resident macrophages, and it is a potent mediator of neutrophil recruitment, chemotaxis, and activation [30]. IL-17A could enhance host defenses against invading fungi, including Cryptococcus neoformans, Aspergillus, and Histoplasma capsulatum by affecting leukocyte recruitment, IFN-γ production, and antimicrobial peptides [31, 32]. It has been shown that the neutrophil relies on IL-17RA-independent antifungi activity [33].
Therefore, higher levels of innate cells and cytokines, especially monocytes and macrophage-derived cytokines (IL-8, IL-18, TNF-α, IL-12, IL-6, IP-10, and IL-1β) may play a prominent role in the response to T. marneffei infection in AIDS patients with substantial loss of CD4+ T cells. Interestingly, the host immune inflammatory response should decline with treatment as it promotes disease improvement. However, these inflammatory factors peaked again on the third day of treatment, while the lymphocyte and monocyte counts were at their lowest level. Antifungal drugs may promote intracellular T. marneffei release, further causing fungaemia to promote an immune inflammatory response, and recruit further immunocytes (mainly monocytes) and inflammatory factors to the sites of infection. Systemic sepsis-like clinical manifestations in patients appear to be associated with a high inflammatory response. In surviving patients, most inflammatory factors gradually decreased after 7 days of therapy and nearly returned to normal levels after 15 days of therapy. Furthermore, the patient’s clinical symptoms and signs gradually reduced about 2 weeks after treatment.
Excessive inflammatory response can lead to tissue damage and poor prognosis after infection. Immune activation and exaggerated production of various cytokines (MCP-1, TNF-α, IL-1, IL-6, CXCL-8, IL-10, IL-18, and IFN-γ) result in proinflammatory cascades or a “cytokine-storm” [34]. This has been associated with activating cell signaling pathways and overproduction of free radicals, which in turn results in oxidative stress, and might contribute to disease severity and poor clinical outcomes [35]. Both IL-4 and IL-10 moderate and balance potentially excessive immune responses during infection [36]. They have been reported to impair clearance cryptococcosis in mouse models [37]. IL-10 has been shown to inhibit the secretion of proinflammatory cytokines (IFN-γ and IL-2) and CD4+ T cell proliferation [37]. In the present study, excessive immune response was found in patients who died in the hospital and who showed the highest levels of IL-6, TNF-α, IP-10, IL-1β, and IL-8. However, anti-inflammatory cytokines IL-4 and IL-10 did not increase significantly in deceased patients due to lack of CD4+ T cells. It has been reported that high levels of IL-1β and IL-6 were consistently associated with the severity of sepsis and the highest levels were associated with the worst outcomes [38, 39]. Patients who died also had a higher HIV viral load compared to patients who showed improvement; the exudation of highly inflammatory factors may be associated with high levels of HIV viral stimulation [3]. The poor outcome likely was associated with an overly strong immune response. In addition, effective antifungal treatment seems to recover overexuberant inflammatory factors.
Although the present study discovered several cryptic aspects of the immunological profile during T. marneffei infecton and antifugal theatment, a number of limitations remain. First, appropriate clinical samples for cytokines and chemokines observed were not obtained, and precise subcellular localization of cytokines and chemokines are not available. Second, due to low compliance of the patients and their actual treatment situation, relatively few patients could be followed up for half a year. Third, for the first time, it was observed that inflammatory factors did not decrease but increased after 3 days of antifungal treatment in AIDS patients with T. marneffei fungal coinfection; however, the underlying mechanisms were further explored.
In conclusion, our finding provides a deep understanding of the underlying immunological mechanisms that are responsible for host defense against fungal infection. Moreover, this information is useful to guide a more in-depth investigation on the mechanisms of intracellular parasitism pathogenesis. Additional studies are required to explore the most significant causes of changes during the peak of host inflammatory response at the third day of treatment and the specific mechanisms of innate immunity against T. marneffei infection.
Notes
We are grateful to all participants of this study.
Financial support. This work was partially supported by the National Science and Technology Major Project of China (2018ZX10301-101), the National Natural Science Foundation of China (81560325 and 81860553), the Joint Special Fund of Science and Technology Department of Yunnan–Kunming Medical University (2017FE467-(005)), the Science and Technology Innovation Team of Sexually Transmitted Diseases of Kunming Medical University (2018HC005), the Medical Leadership Foundation of the Health and Family Planning Commission of Yunnan Province (L-201613), and the Foundation of Health Technology Project of Yunnan Province (2016NS298).
Potential conflict of interests. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Bulterys PL, Le T, Quang VM, et al. Environmental predictors and incubation period of AIDS-associated Penicillium marneffei infection in Ho Chi Minh City, Vietnam. Clin Infect Dis 2013; 56:1273–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Supparatpinyo K, Khamwan C, Baosoung V, et al. Disseminated Penicillium marneffei infection in southeast Asia. Lancet 1994; 344:110–3. [DOI] [PubMed] [Google Scholar]
- 3. Jiang J, Meng S, Huang S, et al. Effects of Talaromyces marneffei infection on mortality of HIV/AIDS patients in southern China: a retrospective cohort study. Clin Microbiol Infect 2019; 25:233–41. [DOI] [PubMed] [Google Scholar]
- 4. Le T, Hong Chau TT, Kim Cuc NT, et al. AIDS‐associated Cryptococcus neoformans and Penicillium marneffei coinfection: a therapeutic dilemma in resource‐limited settings. Clin Infect Dis 2010; 51:e65–8. [DOI] [PubMed] [Google Scholar]
- 5. Li YY, Saeed U, Wei SS, et al. Both coinfections of Penicillium marneffei and Cryptococcus neoformans in AIDS patient: a report of rare case. AIDS 2017; 31:2171–2. [DOI] [PubMed] [Google Scholar]
- 6. Srinoulprasert Y, Pongtanalert P, Chawengkirttikul R, Chaiyaroj SC. Engagement of Penicillium marneffei conidia with multiple pattern recognition receptors on human monocytes. Microbiol Immunol 2009; 53:162–72. [DOI] [PubMed] [Google Scholar]
- 7. Tisoncik JR, Korth MJ, Simmons CP, et al. Into the eye of the cytokine storm. Microbiol Mol Biol Rev 2012; 76:16–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen R, Ji G, Wang L, et al. Activation of ERK1/2 and TNF-α production are regulated by calcium/calmodulin signaling pathway during Penicillium marneffei infection within human macrophages. Microb Pathog 2016; 93:95–9. [DOI] [PubMed] [Google Scholar]
- 9. Dai X, Mao C, Lan X, et al. Acute Penicillium marneffei infection stimulates host M1/M2a macrophages polarization in BALB/C mice. BMC Microbiol 2017; 17:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pongpom M, Vanittanakom P, Nimmanee P, et al. Adaptation to macrophage killing by Talaromyces marneffei. Future Sci OA 2017; 3:FSO215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Larsson M, Nguyen LH, Wertheim HF, et al. Clinical characteristics and outcome of Penicillium marneffei infection among HIV-infected patients in northern Vietnam. AIDS Res Ther 2012; 9:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kawila R, Chaiwarith R, Supparatpinyo K. Clinical and laboratory characteristics of Penicilliosis marneffei among patients with and without HIV infection in Northern Thailand: a retrospective study. BMC Infect Dis 2013; 13:464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huffnagle GB, Lipscomb MF, Lovchik JA, et al. The role of CD4+ and CD8+ T cells in the protective inflammatory response to a pulmonary cryptococcal infection. J Leukoc Biol 1994; 55:35–42. [DOI] [PubMed] [Google Scholar]
- 14. Ellett F, Pazhakh V, Pase L, et al. Macrophages protect Talaromyces marneffei conidia from myeloperoxidase-dependent neutrophil fungicidal activity during infection establishment in vivo. PLoS Pathog 2018; 14:e1007063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ma T, Chen R, Li X, et al. The in vitro fungicidal activity of human macrophages against Penicillium marneffei is suppressed by dexamethasone. Microb Pathog 2015; 86:26–31. [DOI] [PubMed] [Google Scholar]
- 16. Chen R, Li X, Lu S, et al. Role of extracellular signal-regulated kinases 1 and 2 and p38 mitogen-activated protein kinase pathways in regulating replication of Penicillium marneffei in human macrophages. Microbes Infect 2014; 16:401–8. [DOI] [PubMed] [Google Scholar]
- 17. Chen R, Ji G, Ma T, et al. Role of intracellular free calcium in killing Penicillium marneffei within human macrophages. Microb Pathog 2015; 83-84:29–34. [DOI] [PubMed] [Google Scholar]
- 18. Kawakami K, Qureshi MH, Koguchi Y, et al. Role of TNF-alpha in the induction of fungicidal activity of mouse peritoneal exudate cells against Cryptococcus neoformans by IL-12 and IL-18. Cell Immunol 1999; 193:9–16. [DOI] [PubMed] [Google Scholar]
- 19. Tran DQ. Susceptibility to mycobacterial infections due to interferon-gamma and interleukin-12 pathway defects. Allergy Asthma Proc 2005; 26:418–21. [PubMed] [Google Scholar]
- 20. Chan JF, Lau SK, Yuen KY, Woo PC. Talaromyces (Penicillium) marneffei infection in non-HIV-infected patients. Emerg Microbes Infect 2016; 5:e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pruetpongpun N, Khawcharoenporn T, Damronglerd P, et al. Disseminated Talaromyces marneffei and Mycobacterium abscessus in a patient with anti-interferon-gamma autoantibodies. Open Forum Infect Dis 2016; 3:ofw093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lee PP, Mao H, Yang W, et al. Penicillium marneffei infection and impaired IFN-γ immunity in humans with autosomal-dominant gain-of-phosphorylation STAT1 mutations. J Allergy Clin Immunol 2014; 133:894–6 e5. [DOI] [PubMed] [Google Scholar]
- 23. Cenci E, Mencacci A, Del Sero G, et al. IFN-gamma is required for IL-12 responsiveness in mice with Candida albicans infection. J Immunol 1998; 161:3543–50. [PubMed] [Google Scholar]
- 24. Nakamura K, Miyazato A, Koguchi Y, et al. Toll-like receptor 2 (TLR2) and dectin-1 contribute to the production of IL-12p40 by bone marrow-derived dendritic cells infected with Penicillium marneffei. Microbes Infect 2008; 10:1223–7. [DOI] [PubMed] [Google Scholar]
- 25. Ketelut-Carneiro N, Silva GK, Rocha FA, et al. IL-18 triggered by the Nlrp3 inflammasome induces host innate resistance in a pulmonary model of fungal infection. J Immunol 2015; 194:4507–17. [DOI] [PubMed] [Google Scholar]
- 26. Simmons RP, Scully EP, Groden EE, et al. HIV-1 infection induces strong production of IP-10 through TLR7/9-dependent pathways. AIDS 2013; 27:2505–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Noel N, Boufassa F, Lécuroux C, et al. ; ANRS C021 CODEX Study Group Elevated IP10 levels are associated with immune activation and low CD4⁺ T-cell counts in HIV controller patients. AIDS 2014; 28:467–76. [DOI] [PubMed] [Google Scholar]
- 28. Gazendam RP, van de Geer A, Roos D, et al. How neutrophils kill fungi. Immunol Rev 2016; 273:299–311. [DOI] [PubMed] [Google Scholar]
- 29. Othman J, Brown CM. Talaromyces marneffei and dysplastic neutrophils on blood smear in newly diagnosed HIV. Blood 2018; 131:269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kolls JK, Lindén A. Interleukin-17 family members and inflammation. Immunity 2004; 21:467–76. [DOI] [PubMed] [Google Scholar]
- 31. Huang W, Na L, Fidel PL, Schwarzenberger P. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J Infect Dis 2004; 190:624–31. [DOI] [PubMed] [Google Scholar]
- 32. Murdock BJ, Huffnagle GB, Olszewski MA, Osterholzer JJ. Interleukin-17A enhances host defense against cryptococcal lung infection through effects mediated by leukocyte recruitment, activation, and gamma interferon production. Infect Immun 2014; 82:937–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Trautwein-Weidner K, Gladiator A, Nur S, Diethelm P, LeibundGut-Landmann S. IL-17-mediated antifungal defense in the oral mucosa is independent of neutrophils. Mucosal Immunol 2015; 8:221–31. [DOI] [PubMed] [Google Scholar]
- 34. Schulte W, Bernhagen J, Bucala R. Cytokines in sepsis: potent immunoregulators and potential therapeutic targets–an updated view. Mediators Inflamm 2013; 2013:165974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kellum JA, Kong L, Fink MP, et al. ; GenIMS Investigators Understanding the inflammatory cytokine response in pneumonia and sepsis: results of the Genetic and Inflammatory Markers of Sepsis (GenIMS) Study. Arch Intern Med 2007; 167:1655–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mege JL, Meghari S, Honstettre A, et al. The two faces of interleukin 10 in human infectious diseases. Lancet Infect Dis 2006; 6:557–69. [DOI] [PubMed] [Google Scholar]
- 37. Koguchi Y, Kawakami K. Cryptococcal infection and Th1-Th2 cytokine balance. Int Rev Immunol 2002; 21:423–38. [DOI] [PubMed] [Google Scholar]
- 38. Mera S, Tatulescu D, Cismaru C, et al. Multiplex cytokine profiling in patients with sepsis. APMIS 2011; 119:155–63. [DOI] [PubMed] [Google Scholar]
- 39. Wu HP, Chen CK, Chung K, et al. Serial cytokine levels in patients with severe sepsis. Inflamm Res 2009; 58:385–93. [DOI] [PubMed] [Google Scholar]