Abstract
Objective
GlycA, a novel NMR biomarker of inflammation, has been associated with incident cardiovascular disease (CVD) in the general population, but its association with CVD among HIV-infected individuals is unknown. We examined the associations between GlycA and subclinical coronary plaque among HIV-infected and HIV-uninfected men participating in MACS.
Design
Cross-sectional analysis of 935 men with plasma measurement of GlycA and non-contrast cardiac CT and/or coronary CT angiography.
Methods
We used multivariable Poisson and linear regression to assess associations of GlycA with prevalent coronary atherosclerosis and plaque extent, respectively.
Results
Mean±SD age was 54±7 years; 31% were black; 63% HIV-infected. GlycA levels were higher in HIV-infected compared to HIV-uninfected men (397±68 vs 380±60 μmol/L, p=0.0001), and higher for men with detectable viral load vs. undetectable (413±79 vs 393±65 μmol/L, p=0.004). After adjusting for HIV serostatus, demographic and CVD risk factors, every one SD increment in GlycA level was associated with a higher prevalence of coronary artery calcium (CAC>0) [prevalence ratio 1.09 (95% CI 1.03–1.15)] and coronary stenosis ≥50% [1.20 (1.02–1.41)]. These associations did not significantly differ after adjusting for traditional inflammatory biomarkers or by HIV serostatus. Among men with plaque, GlycA was positively associated with the extent of CAC and total plaque.
Conclusion
HIV-infection was associated with higher GlycA levels. In both HIV-infected and -uninfected individuals, GlycA was significantly associated with several measures of subclinical coronary atherosclerosis, independent of other CVD risk factors and inflammatory biomarkers. These findings suggest the potential role of GlycA in CVD risk stratification among HIV patients.
Keywords: GlycA, HIV-infection, coronary artery calcium, coronary atherosclerosis, cardiac CT, inflammation
INTRODUCTION
With the use of combination antiretroviral therapy (ART), individuals infected with human immunodeficiency virus (HIV) are now living longer and have higher rates of cardiovascular disease (CVD) events[1, 2] and increased prevalence of subclinical coronary atherosclerosis compared to HIV-uninfected individuals of similar age and risk factors.[3, 4] Atherosclerosis is an inflammatory process[5, 6] and chronically elevated systemic inflammation conferred by HIV infection may explain this increased CVD risk.[7–9] Traditional CVD risk stratification tools often inadequately depict CVD risk in HIV infection.[10] In the general population, biomarkers of inflammation, especially high sensitivity C-reactive protein (hsCRP), have been shown to independently predict incident CVD events.[11, 12] However, hsCRP may predict risk less well among HIV-infected individuals compared to other inflammatory markers.[13]
There is a further need to identify biomarkers that can more accurately capture the inflammatory risk among individuals with HIV-infection. The Multicenter AIDS Cohort Study (MACS) previously reported associations of biomarkers of monocyte activation, systemic inflammation and coagulation with subclinical coronary artery disease (CAD) in men with and without HIV infection.[8, 9] GlycA is a novel composite biomarker of systemic inflammation measured by nuclear magnetic resonance (NMR) spectroscopy and may be may be less prone to day to day fluctuations.[14] It reflects mainly the serum concentrations and glycosylation states of 5 abundant acute-phase reactants–α1-acid glycoprotein, haptoglobin, α1-antitrypsin, α1-antichymotrypsin and transferrin.[14] In the general population, higher levels of GlycA have been was independently associated with incident CVD events,[15–19] diabetes mellitus,[20] and cardiovascular and all-cause mortality.[15, 21, 22] GlycA levels were associated subclinical coronary atherosclerosis independent of traditional CVD risk factors and hsCRP in patients with psoriasis[23] but not among patients with lupus.[24] Whether GlycA is associated with increased subclinical coronary atherosclerosis among HIV-infected individuals is unknown.
The aims of this study were twofold: first, we investigated whether GlycA levels are associated with the presence and burden of subclinical coronary atherosclerosis as ascertained by cardiac computed tomography (CT) imaging. Second, we determined whether such associations differed by HIV serostatus. We hypothesized that: 1) GlycA levels will be higher among HIV-infected participants, 2) GlycA will be associated with subclinical coronary atherosclerosis measures beyond traditional CVD risk factors and traditional inflammatory markers, and 3) the magnitude of the association of GlycA with subclinical coronary atherosclerosis will be more pronounced among HIV-infected individuals compared to HIV-uninfected individuals.
METHODS
Population
Detailed description of the Multicenter AIDS Cohort Study (MACS) has been previously published.[25] Briefly, MACS is an ongoing multi-center prospective cohort study of the natural and treated histories of HIV-1 infection in men who have sex with men. MACS enrolled a total of 7,352 HIV-infected and HIV-uninfected participants during three periods (1984–85, 1987–91, and 2001–03) from 4 U.S. sites: Baltimore, Maryland/ Washington, DC; Chicago, Illinois; Pittsburgh, Pennsylvania; and Los Angeles, California.[25]
Between the years of 2010 to 2013, 1,006 MACS participants aged 40 to 70 years, weighing less than 136 kg, without a history of cardiac surgery or percutaneous coronary intervention, participated in a MACS Cardiovascular Ancillary Study.[3] These participants underwent non-contrast cardiac CT scanning to measure coronary artery calcium (CAC) scores. Among men who completed a non-contrast cardiac CT, 759 also met the inclusion criteria to undergo contrast coronary CT angiography (CTA) to measure total plaque burden, plaque composition and coronary stenosis. Exclusion criteria for CTA were contrast allergy, atrial fibrillation, or chronic kidney disease (estimated glomerular filtration rate [eGFR]<60 mL/min/1.73m2 within 30 days of imaging).
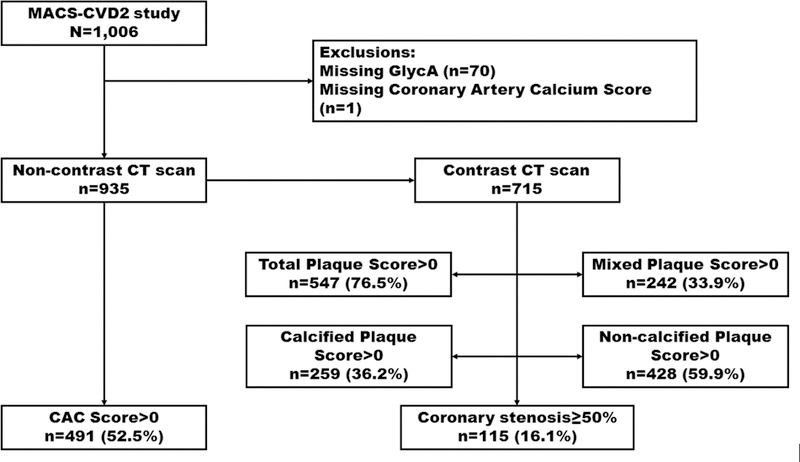
Of the 1,006 MACS-CVD2 participants enrolled, we excluded participants with missing GlycA levels (n=70) and CAC measurement (n=1), resulting in 935 eligible participants for assessment of GlycA with CAC, 715 of whom also underwent CTA (Figure 1). The Institutional Review Boards of all participating centers approved the studies and all participants provided written informed consent.
Figure 1.
Flow chart illustrating our exclusions
Measurement of GlycA
Blood was drawn on the day of cardiac imaging after a 12-hour fast. All samples were stored at – 80˚C until they were subsequently thawed for NMR analysis. GlycA levels (μmol/L) in ethylenediaminetetraacetic acid (EDTA) plasma samples were quantified from NMR LipoProfile test spectra collected at LipoScience (now LabCorp, Raleigh, NC) as previously described.[14] The intra‐assay and inter-assay coefficients of variability for GlycA measurement were 1.9% and 2.6%, respectively.[15] Previous work by Otvos et al found that GlycA levels were similar when measured in serum vs. plasma samples, fasting vs non-fasting states, and after short-term vs long-term storage.[14]
Cardiac CT and assessment of subclinical coronary atherosclerosis
Primary outcomes of interest included: (1) presence of CAC (Agatston score >0), (2) presence of any coronary plaque on CTA (total plaque (TP) score >0), and (3) moderate-to-severe coronary artery stenosis (≥50%). Secondary outcomes included measures of plaque composition: non-calcified plaque score >0, mixed plaque score >0, and calcified plaque score >0.
Cardiac CT scans were obtained following procedures as previously described.[26] Briefly, participants received a beta-blocker or calcium channel blocker if needed for heart rate control just before the time of scanning, followed by sublingual nitroglycerin before administration of IV contrast unless contraindicated. Coronary CTA was performed with electrocardiogram-triggered protocols and median radiation dose of 1.9 mSv (interquartile range, 1.7–2.7 mSv). CT images were analyzed at the core CT reading center (Los Angeles Biomedical Research Institute at Harbor-UCLA) by trained, experienced readers blinded to participant clinical information.
CAC scores obtained from non-contrast CTs were calculated using the Agatston method.[27] Coronary CTA images were examined to characterize coronary plaque burden (presence, size, and composition of plaque) and degree of coronary stenosis in all segments following the modified 15-segment model of the American Heart Association.[28]
Total plaque score was calculated as the sum of individual plaque size scores across all coronary segments that showed any plaque, with a maximum score of 45.[29] Non-calcified plaque was defined as any discernable structure clearly assignable to the vessel wall with a CT density less than the contrast-enhanced coronary lumen but greater than the surrounding connective tissue in at least 2 independent planes. Calcified plaque was defined as any structure with CT attenuation >130 Hounsfield Units visualized as distinct from the intravascular lumen in at least 2 independent planes. The total non-calcified plaque, mixed plaque, and calcified plaque scores were calculated as the sum of the respective scores across all coronary segments.
Covariates
As part of routine MACS visits, study participants are seen every 6 months and data are collected regarding CVD risk factors and HIV clinical variables by questionnaires, physical examination, and laboratory tests using standard protocols.[3, 25] For this analysis, we used clinical data collected at the MACS visit closest to the CT date. The laboratory measures were obtained from blood samples drawn at same visit as the CT scan.
Covariates included in our models were age, race/ethnicity, scanning center, HIV serostatus, study cohort (pre-/post-2001 to account for recruitment cohort effect), measured systolic blood pressure, measured body mass index (BMI), fasting blood glucose, total cholesterol, HDL cholesterol, pack-years of smoking, use of antihypertensive, lipid-lowering or antidiabetic medications, eGFR, hepatitis C virus (HCV) infection status, and serum levels of traditional inflammatory biomarkers [hsCRP, IL-6, D-dimer and fibrinogen). We also chose to evaluate levels of monocyte activation markers soluble CD14 (sCD14), soluble CD163 (sCD163), and chemokine (C-C motif) ligand 2 (CCL2) because they have previously been shown to be elevated in highly active antiretroviral therapy (HAART)-treated HIV-infected men and to be associated with atherosclerosis.[9] Among the HIV-infected men, the HIV disease characteristics evaluated included plasma HIV viral load (VL) (determined by the Roche ultrasensitive assay with a lower limit detection of 50 copies/mL),[30] CD4+ T-lymphocyte cell counts/mm3 (CD4) measured by flow cytometry, history of AIDS-defining illness, and use and duration of HAART. The detailed measurement protocols and definitions for all of these covariates have been previously published.[3, 8, 31]
Statistical analysis
Baseline characteristics were stratified by quartiles of GlycA levels for the whole cohort and by HIV serostatus. Continuous variables were presented as means (standard deviation [SD]) and categorical variables as frequencies (percentage). Skewed variables were presented as medians (25th – 75th percentiles).
We used multivariable Poisson regression models with robust variance estimation to assess the association between GlycA levels, presented per SD increment, with prevalent subclinical coronary atherosclerosis (CAC, coronary stenosis ≥50%, any plaque, calcified plaque, non-calcified plaque, and mixed plaque). Among men with plaque present, a multivariable linear regression model was used to assess for associations of GlycA with plaque burden (extent). Since plaque scores were not normally distributed, they were natural-log transformed.
Models were progressively adjusted as follows: Model 1 was adjusted for age, race/ethnicity, scanning center, HIV serostatus, and study cohort (pre-/post-2001). Model 2, our primary analytical model, was further adjusted for established CVD risk factors including systolic blood pressure, BMI, physical activity level, use of hypertension medications, diabetes medications, and lipid lowering medications, fasting glucose, total and HDL cholesterol, pack-years of tobacco smoking, eGFR and HCV infection status. We then performed two exploratory models to determine whether GlycA levels might be associated with subclinical coronary atherosclerosis independent of other established inflammatory marker levels. Model 3 was additionally adjusted for levels of hsCRP, D-dimer, IL-6 and fibrinogen (i.e. traditional markers of systemic inflammation). Model 4 was additionally adjusted for sCD163, sCD14, and CCL2 levels (i.e. markers of monocyte activation).
All results were presented for the overall cohort, and then stratified by HIV sero-status. In the stratified analyses for HIV-infected individuals only, a supplemental Model 5 additionally adjusted for HIV-related factors (CD4 cell count, detectable HIV VL, nadir CD4, history of AIDS, duration of HAART, and use of protease inhibitors) among HIV-infected men.
Effect modification (i.e. interaction testing) by HIV serostatus was evaluated with the Wald test. Statistical significance was established at a two-tailed p-value less than 0.05.
RESULTS
Characteristics of the study population
The characteristics of the MACS participants included in our study, overall and by quartiles of GlycA, are summarized in Table 1. The mean (SD) age of participants was 54 (7) years. The sample included a large proportion of blacks (31.2%) and HIV-infected individuals (63.0%). Higher quartiles of plasma GlycA were significantly positively associated with HCV infection, HIV infection, levels of hsCRP, IL-6, fibrinogen, sCD163, sCD14, CCL2 and CAC (Table 1). GlycA levels were higher in HIV-infected compared to HIV-uninfected men (397±68 vs 380±60 μmol/L, p=0.0001), and higher for men with detectable viral load vs. undetectable (413±79 vs 393±65 μmol/L, p=0.004) (Supplemental Figure 1). The characteristics of the study population by HIV serostatus is shown in Supplemental Table 1.
Table 1.
Characteristics of participants Undergoing Cardiac CT by GlycA quartiles, the MACS-CVD2 study (2010–2013) *,†
| Characteristics | Overall | GlycA (μmol/L) | ||||
|---|---|---|---|---|---|---|
| Q1 230.7 – 346.6 |
Q2 346.7 – 381.7 |
Q3 381.8 – 426.1 |
Q4 426.3 – 732.0 |
P trend | ||
| N | 935 | 234 | 234 | 234 | 233 | |
| Demographics | ||||||
| Age, y | 53.7 ± 6.9 | 53.2 ± 6.7 | 53.9 ± 7.2 | 53.9 ± 6.9 | 53.7 ± 7.0 | 0.88 |
| Race | ||||||
| Non-Hispanic white | 539 (57.7) | 140 (59.8) | 151 (64.5) | 133 (56.8) | 115 (49.4) | 0.16 |
| Non-Hispanic black | 292 (31.2) | 62 (26.5) | 56 (23.9) | 73 (31.2) | 101 (43.4) | 0.002 |
| Others | 104 (11.1) | 32 (13.7) | 27 (11.5) | 28 (12.0) | 17 (7.3) | 0.07 |
| Lifestyle and clinical factors | ||||||
| Systolic BP, mmHg | 127 ± 15 | 125 ± 15 | 126 ± 15 | 128 ± 14 | 130 ± 16 | 0.12 |
| BMI, kg/m2 | 27 ± 5 | 26 ± 4 | 27 ± 5 | 27 ± 5 | 27 ± 5 | 0.046 |
| Fasting glucose, mg/dL | 102 ± 30 | 96 ± 13 | 105 ± 37 | 105 ± 38 | 103 ± 26 | 0.25 |
| Total cholesterol, mg/dL | 191 ± 40 | 186 ± 38 | 192 ± 38 | 191 ± 39 | 192 ± 45 | 0.39 |
| HDL cholesterol, mg/dL | 50 ± 16 | 52 ± 16 | 51 ± 16 | 49 ± 15 | 50 ± 18 | 0.035 |
| Pack-years of tobacco smoking‡ | 12.5 (3.4 – 30) | 10.0 (2.1 – 27.0) | 12.2 (1.1 – 26.6) | 15.7 (5.4 – 30) | 15.5 (5.1 – 32.7) | 0.001 |
| eGFR, ml/min/1.73 m2 | ||||||
| <60 | 61 (6.5) | 13 (5.6) | 10 (4.3) | 10 (4.3) | 28 (12.0) | 0.013 |
| 60–<90 | 416 (44.5) | 104 (44.4) | 105 (44.9) | 99 (42.3) | 108 (46.4) | 0.89 |
| ≥90 | 404 (43.2) | 102 (43.6) | 109 (46.6) | 112 (47.9) | 81 (34.8) | 0.27 |
| Physical activity status | ||||||
| Low | 227 (24.3) | 44 (18.8) | 50 (21.4) | 64 (27.4) | 69 (29.6) | 0.017 |
| Medium | 233 (24.9) | 48 (20.5) | 61 (26.1) | 70 (29.9) | 54 (23.2) | 0.46 |
| High | 348 (37.2) | 106 (45.3) | 98 (41.9) | 73 (31.2) | 71 (30.5) | 0.008 |
| HCV infection | 121 (12.9) | 23 (9.8) | 22 (9.4) | 32 (13.7) | 44 (18.9) | 0.005 |
| HIV infection | 589 (63.0) | 134 (57.3) | 143 (61.1) | 149 (63.7) | 163 (70.0) | 0.17 |
| Medications | ||||||
| BP lowering | 312 (33.4) | 65 (27.8) | 70 (29.9) | 83 (35.5) | 94 (40.3) | 0.027 |
| Glucose lowering | 81 (8.7) | 8 (3.4) | 20 (8.6) | 20 (8.6) | 33 (14.2) | <0.001 |
| Lipid lowering | 308 (32.9) | 71 (30.3) | 77 (32.9) | 78 (33.3) | 82 (35.2) | 0.44 |
| Inflammatory markers | ||||||
| hsCRP, μg/mL‡ | 1.18 (0.59 – 2.51) | 0.77 (0.35 – 1.30) | 0.91 (0.56 – 1.79) | 1.33 (0.7 – 3.11) | 2.09 (1.02 – 4.88) | <0.001 |
| D-dimer, μg/mL‡ | 0.19 (0.11 – 0.29) | 0.17 (0.09 – 0.26) | 0.19 (0.11 – 0.29) | 0.18 (0.11 – 0.26) | 0.20 (0.13 – 0.35) | 0.05 |
| IL-6, pg/mL‡ | 1.45 (0.94 – 2.32) | 1.06 (0.68 – 1.71) | 1.34 (0.90 – 2.02) | 1.52 (1.06 – 2.51) | 1.94 (1.27 – 3.36) | <0.001 |
| Fibrinogen, mg/dL | 336 ± 94 | 303 ± 102 | 322 ± 98 | 340 ± 75 | 377 ± 82 | <0.001 |
| sCD163, ng/mL | 666 ± 254 | 641 ± 233 | 628 ± 242 | 667 ± 248 | 729 ± 280 | 0.26 |
| sCD14, ng/mL | 1531 ± 450 | 1421 ± 393 | 1470 ± 388 | 1541 ± 388 | 1691 ± 559 | 0.003 |
| CCL2, pg/mL‡ | 264 (199 – 336) | 248 (199 – 314) | 251 (196 – 331) | 270 (199 – 331) | 278 (219 – 375) | 0.43 |
| CT measures | ||||||
| Total CAC score, Agatston unitsठ| 72 (23 Р209) | 44 (19 Р156) | 50 (20 Р194) | 108 (30 Р239) | 91 (28 Р242) | 0.004 |
| Total Plaque Score‡§‖ | 3 (2 – 6) | 3 (2 – 5) | 3 (2 – 5) | 5 (2 – 8) | 4 (2 – 8) | 0.001 |
| Non-calcified Plaque Score‖ | 2 (1 – 3) | 2 (1 – 3) | 2 (1 – 3) | 2 (1 – 4) | 2 (1 – 3) | 0.136 |
| Mixed Plaque Score‡§‖ | 2 (1 – 3) | 1 (1 – 3) | 1 (1 – 2) | 2 (1 – 4) | 2 (2 – 3) | <0.001 |
| Calcified Plaque Score‡§‖ | 2 (1 – 4) | 2 (1 – 4) | 1 (1 – 3) | 2 (1 – 4) | 2 (1 – 4) | 0.24 |
| Coronary stenosis ≥50%‖ | 115 (16.1) | 22 (11.8) | 22 (12.6) | 37 (19.5) | 34 (20.9) | 0.043 |
Abbreviations: BMI = body mass index; BP = blood pressure; CAC = coronary artery calcium; eGFR = estimated glomerular filtration rate; HCV= hepatitis C virus; HDL= high density lipoprotein; HIV = human immunodeficiency virus; hsCRP = high-sensitivity C-reactive protein; IL-6 = interleukin 6; MACS=Multicenter AIDS Cohort Study; sCD14 = soluble CD14; sCD163 = soluble CD163; CCL2 = chemokine (C-C motif) ligand 2
Data are presented as Mean ± Standard Deviation for continuous variables and frequency (percentage) for categorical variables unless otherwise specified.
Data presented as median (interquartile interval).
Results are for participants with values greater than zero.
Coronary CT angiography data measured in a subsample of 715 participants.
Correlation of GlycA with demographic, clinical, inflammatory markers and HIV-related factors
For lifestyle and clinical parameters, plasma GlycA levels positively correlated with smoking pack-years and HCV infection status and inversely correlated with HDL cholesterol levels and physical activity level among HIV-infected participants (Table 2). Among HIV-uninfected participants, significant correlations included positive correlations with BMI, fasting blood glucose, and smoking pack-years, and inverse correlation with HDL cholesterol (Table 2). The use of protease inhibitors was positively correlated, and nadir CD4 was inversely correlated with GlycA levels among HIV-related factors examined. Among inflammatory markers, GlycA was significantly correlated with hsCRP, D-dimer, IL-6, fibrinogen and sCD14 levels among both HIV-infected and HIV-uninfected participants (Table 2).
Table 2.
Spearman correlations of GlycA levels with demographic, cardiometabolic, other inflammatory biomarkers and HIV-related factors in the MACS-CVD2.*,†
| Total cohort (N=935) | HIV-infected (N=589) | HIV-uninfected (N=346) | |
|---|---|---|---|
| Demographics | |||
| Age, y | −0.005 (NS) | 0.01 (NS) | 0.01 (NS) |
| Lifestyle and clinical factors | |||
| Systolic blood pressure | 0.09 (0.02) | 0.09 (NS) | 0.11 (NS) |
| Body mass index | 0.07 (NS) | −0.01 (NS) | 0.22 (0.001) |
| Fasting glucose | 0.10 (0.01) | 0.03 (NS) | 0.19 (0.002) |
| Total cholesterol | 0.02 (NS) | 0.01 (NS) | 0.06 (NS) |
| HDL cholesterol | −0.12 (0.003) | −0.10 (0.04) | −0.12 (0.05) |
| Pack-years of tobacco smoking | 0.17 (<0.0001) | 0.13 (0.01) | 0.20 (0.002) |
| eGFR | 0.009 (NS) | −0.003 (NS) | 0.03 (NS) |
| Physical activity status | −0.15 (0.0002) | −0.16 (0.001) | −0.11 (NS) |
| HCV infection | 0.14 (0.0002) | 0.15 (0.002) | 0.10 (NS) |
| Medications | |||
| BP lowering | 0.12 (0.001) | 0.15 (0.002) | 0.07 (NS) |
| Glucose lowering | 0.12 (0.002) | 0.11 (0.03) | 0.13 (0.04) |
| Lipid lowering | 0.05 (NS) | 0.04 (NS) | 0.07 (NS) |
| Inflammatory markers | |||
| hsCRP | 0.37 (<0.0001) | 0.36 (<0.0001) | 0.39 (<0.0001) |
| D-dimer | 0.14 (0.0004) | 0.14 (0.01) | 0.14 (0.02) |
| IL-6 | 0.32 (<0.0001) | 0.27 (<0.0001) | 0.41 (<0.0001) |
| Fibrinogen | 0.40 (<0.0001) | 0.40 (<0.0001) | 0.41 (<0.0001) |
| sCD163 | 0.09 (0.02) | 0.08 (NS) | 0.06 (NS) |
| sCD14 | 0.24 (<0.0001) | 0.24 (<0.0001) | 0.26 (<0.0001) |
| CCL2 | 0.14 (0.0004) | 0.17 (0.001) | 0.08 (NS) |
| HIV related factors | |||
| CD4 cell count | - | −0.02 (NS) | - |
| HIV RNA ≥ 50 copies per ml | - | 0.09 (0.03) | - |
| Nadir CD4 cell count | - | −0.12 (0.004) | - |
| History of AIDS | - | 0.08 (0.05) | - |
| Duration of HAART | - | −0.08 (NS) | - |
| Use of protease inhibitors | - | 0.14 (0.001) | - |
Abbreviations: BMI = body mass index; BP = blood pressure; CAC = coronary artery calcium; eGFR = estimated glomerular filtration rate; HCV= hepatitis C virus; HDL= high density lipoprotein; HIV = human immunodeficiency virus; hsCRP = high-sensitivity C-reactive protein; IL-6 = interleukin 6; MACS=Multicenter AIDS Cohort Study; sCD14 = soluble CD14; sCD163 = soluble CD163; CCL2 = chemokine (C-C motif) ligand 2; AIDS = acquire immunodeficiency syndrome; HAART = highly active antiretroviral therapy
Values are expressed as ρ (p value) for all variables. NS = not significant (p >0.05)
GlycA and the prevalence of subclinical coronary atherosclerosis
Table 3 and Supplemental Figure 2 summarize the associations of GlycA levels with the prevalence of subclinical coronary atherosclerosis by non-contrast cardiac CT and CTA. In demographic-adjusted models (model 1), one SD increment in plasma GlycA was significantly associated with greater prevalence of CAC (Agatston score >0), coronary artery stenosis ≥50%, mixed plaque and calcified plaque with prevalence ratios (95% CI) of 1.12 (1.07–1.18), 1.33 (1.13–1.56), 1.12 (1.01–1.24) and 1.14 (1.04–1.26), respectively (Table 3). For CAC, coronary stenosis, and calcified plaque, these associations remained significant even after further adjustments for traditional CVD risk factors in our main model (Model 2) and after adjustment for traditional inflammatory marker levels (Model 3). However, the association for coronary stenosis was attenuated after adjusted for monocyte activation markers (Model 4). We did not find an association with non-calcified plaque in any model. In sensitivity analyses when we compared the top quartile of GlycA to the bottom quartile, findings were similar to the main analyses using GlycA as a continuous measure (Supplemental Table 2).
Table 3.
Prevalence ratios (95% CI) for the presence of coronary atherosclerosis per one SD increment in GlycA levels; the MACS-CVD2 study*,†, ‡,§,‖
| Whole cohort (935) | HIV-infected (589) | HIV-uninfected (346) | |
|---|---|---|---|
| Coronary artery calcium | |||
| Model 1 | 1.12 (1.07 – 1.18) | 1.10 (1.04 – 1.17) | 1.18 (1.07 – 1.30) |
| Model 2 | 1.09 (1.03 – 1.15) | 1.08 (1.01 – 1.15) | 1.11 (1.00 – 1.22) |
| Model 3 | 1.09 (1.03 – 1.16) | 1.08 (1.01 – 1.16) | 1.13 (1.00 – 1.28) |
| Model 4 | 1.09 (1.03 – 1.15) | 1.07 (1.00 – 1.15) | 1.14 (1.01 – 1.28) |
| Model 5 | - | 1.07 (0.99 – 1.15) | - |
| p-for-interaction=0.11 | |||
| Coronary stenosis | |||
| Model 1 | 1.33 (1.13 – 1.56) | 1.28 (1.06 – 1.54) | 1.41 (1.04 – 1.93) |
| Model 2 | 1.20 (1.02 – 1.41) | 1.18 (0.97 – 1.45) | 1.26 (0.93 – 1.71) |
| Model 3 | 1.18 (1.00 – 1.39) | 1.12 (0.90 – 1.38) | 1.37 (1.02 – 1.84) |
| Model 4 | 1.15 (0.98 – 1.36) | 1.06 (0.86 – 1.32) | 1.53 (1.13 – 2.05) |
| Model 5 | - | 1.05 (0.83 – 1.31) | - |
| p-for-interaction=0.15 | |||
| Any plaque | |||
| Model 1 | 1.02 (0.98 – 1.07) | 1.01 (0.96 – 1.07) | 1.04 (0.97 – 1.11) |
| Model 2 | 1.00 (0.96 – 1.04) | 1.00 (0.95 – 1.05) | 1.00 (0.93 – 1.07) |
| Model 3 | 0.98 (0.94 – 1.03) | 0.97 (0.92 – 1.03) | 1.01 (0.94 – 1.10) |
| Model 4 | 0.98 (0.94 – 1.02) | 0.97 (0.92 – 1.02) | 1.01 (0.94 – 1.10) |
| Model 5 | - | 0.97 (0.92 – 1.03) | - |
| p-for-interaction=0.71 | |||
| Noncalcified plaque | |||
| Model 1 | 1.05 (0.99 – 1.12) | 1.05 (0.98 – 1.13) | 1.04 (0.93 – 1.18) |
| Model 2 | 1.03 (0.97 – 1.09) | 1.03 (0.96 – 1.11) | 1.00 (0.88 – 1.14) |
| Model 3 | 1.03 (0.96 – 1.10) | 1.04 (0.96 – 1.13) | 1.00 (0.87 – 1.14) |
| Model 4 | 1.02 (0.95 – 1.09) | 1.03 (0.95 – 1.12) | 1.00 (0.86 – 1.15) |
| Model 5 | - | 1.04 (0.96 – 1.13) | - |
| p-for-interaction=0.93 | |||
| Mixed plaque | |||
| Model 1 | 1.12 (1.01 – 1.24) | 1.11 (0.98 – 1.25) | 1.12 (0.94 – 1.34) |
| Model 2 | 1.05 (0.95 – 1.17) | 1.06 (0.93 – 1.21) | 1.07 (0.89 – 1.27) |
| Model 3 | 1.03 (0.92 – 1.15) | 1.01 (0.88 – 1.17) | 1.12 (0.93 – 1.35) |
| Model 4 | 1.02 (0.92 – 1.15) | 1.00 (0.86 – 1.15) | 1.12 (0.93 – 1.36) |
| Model 5 | - | 0.97 (0.83 – 1.13) | - |
| p-for-interaction=0.29 | |||
| Calcified plaque | |||
| Model 1 | 1.14 (1.04 – 1.26) | 1.10 (0.97 – 1.25) | 1.22 (1.05 – 1.42) |
| Model 2 | 1.12 (1.02 – 1.23) | 1.07 (0.94 – 1.22) | 1.19 (1.02 – 1.40) |
| Model 3 | 1.15 (1.04 – 1.27) | 1.11 (0.97 – 1.26) | 1.22 (1.03 – 1.46) |
| Model 4 | 1.15 (1.04 – 1.27) | 1.08 (0.95 – 1.24) | 1.22 (1.03 – 1.46) |
| Model 5 | - | 1.11 (0.96 – 1.28) | - |
| p-for-interaction=0.35 | |||
SD GlycA = 65.4 μmol/L
Prevalence ratios (95% CI) are from Poisson regression models that were progressively adjusted as follows:
Model 1: adjusted for age, race, scanning center, pre/post 2001-cohort, and HIV sero-status (in whole cohort analysis).
Model 2 (Main Model): Model 1 + systolic BP, BMI, physical activity level, use of hypertension medications, use of diabetes medications, fasting glucose, total and HDL cholesterol, use of lipid lowering medications, pack-years of tobacco smoking, eGFR and hepatitis C virus (HCV) - infection status.
Model 3: Model 2 + ln hsCRP, ln D-dimer, ln IL-6 and Fibrinogen.
Model 4: Model 3 + sCD163, sCD14, and ln CCL2.
Model 5: Model 4 + CD4 cell count, presence of detectable HIV RNA, nadir CD4 cell count, history of AIDS, duration of HAART, and use of protease inhibitors.
p-for-interaction adjusted for model 2
Bolded items are statistically significant (p≤0.05)
GlycA and plaque burden
Among participants with plaque present on cardiac CT and CTA (respective scores >0), each SD increment in plasma GlycA was significantly associated with greater burden of log-transformed CAC, total plaque, and mixed plaque scores (Table 4 Model 1). Again, these associations remained significant even after further adjustments for traditional CVD risk factors in our primary model (Model 2) and after additional adjustment for hsCRP, D-dimer, IL-6 and fibrinogen levels (Model 3). The associations with total plaque and mixed plaque remained significant after adjustment for monocyte activation markers (Model 4). In sensitivity analyses when we compared to the top quartile of GlycA levels to the bottom quartile, findings were similar to the main analyses using GlycA as a continuous measure (Supplemental Table 3).
Table 4.
Difference of plaque extent per 1 SD increment in GlycA levels among individuals with plaque present (respective scores >0): the MACS-CVD2 study*,†,‡,§,‖
| Whole cohort (935) | HIV-infected men (589) | HIV-uninfected men (346) | |
|---|---|---|---|
| ln CAC score | |||
| Model 1 | 0.21 (0.07, 0.36) | 0.17 (−0.003, 0.34) | 0.30 (0.02, 0.57) |
| Model 2 | 0.21 (0.07, 0.36) | 0.19 (0.02, 0.37) | 0.24 (−0.07, 0.56) |
| Model 3 | 0.18 (0.02, 0.35) | 0.17 (−0.02, 0.36) | 0.21 (−0.16, 0.58) |
| Model 4 | 0.15 (−0.02, 0.31) | 0.11 (−0.09, 0.30) | 0.22 (−0.15, 0.59) |
| Model 5 | - | 0.10 (−0.10, 0.29) | - |
| p-for-interaction=0.29 | |||
| ln TP score | |||
| Model 1 | 0.19 (0.12, 0.26) | 0.12 (0.04, 0.21) | 0.30 (0.18, 0.42) |
| Model 2 | 0.15 (0.07, 0.22) | 0.09 (−0.002, 0.18) | 0.23 (0.11, 0.35) |
| Model 3 | 0.17 (0.09, 0.25) | 0.11 (0.01, 0.21) | 0.21 (0.07, 0.36) |
| Model 4 | 0.16 (0.08, 0.24) | 0.10 (0.002, 0.20) | 0.22 (0.07, 0.36) |
| Model 5 | - | 0.10 (−0.0003, 0.21) | - |
| p-for-interaction=0.003 | |||
| ln NCP score | |||
| Model 1 | 0.06 (−0.01, 0.13) | 0.02 (−0.06, 0.11) | 0.13 (0.01, 0.25) |
| Model 2 | 0.02 (−0.05, 0.09) | −0.01 (−0.10, 0.08) | 0.05 (−0.07, 0.17) |
| Model 3 | 0 (−0.08, 0.08) | −0.05 (−0.15, 0.06) | 0.05 (−0.09, 0.18) |
| Model 4 | 0 (−0.08, 0.07) | −0.03 (−0.13, 0.07) | 0.05 (−0.09, 0.19) |
| Model 5 | - | −0.03 (−0.14, 0.08) | - |
| p-for-interaction= 0.13 | |||
| ln MP score | |||
| Model 1 | 0.14 (0.05, 0.24) | 0.07 (−0.04, 0.18) | 0.28 (0.09, 0.46) |
| Model 2 | 0.12 (0.02, 0.22) | 0.06 (−0.06, 0.18) | 0.22 (0.002, 0.44) |
| Model 3 | 0.15 (0.03, 0.27) | 0.14 (−0.01, 0.28) | 0.25 (−0.03, 0.53) |
| Model 4 | 0.15 (0.02, 0.27) | 0.12 (−0.03, 0.27) | 0.25 (−0.05, 0.54) |
| Model 5 | - | 0.13 (−0.03, 0.29) | - |
| p-for-interaction=0.03 | |||
| ln CP score | |||
| Model 1 | 0.05 (−0.05, 0.14) | 0.03 (−0.09, 0.15) | 0.10 (−0.08, 0.27) |
| Model 2 | 0.01 (−0.08, 0.11) | −0.01 (−0.14, 0.12) | 0.07 (−0.11, 0.25) |
| Model 3 | 0.02 (−0.09, 0.13) | −0.02 (−0.17, 0.12) | 0.10 (−0.11, 0.30) |
| Model 4 | 0.02 (−0.09, 0.13) | −0.02 (−0.17, 0.13) | 0.12 (−0.09, 0.33) |
| Model 5 | - | −0.03 (−0.19, 0.13) | - |
| p-for-interaction=0.34 | |||
Table Abbreviations: CAC = coronary artery calcium; CP = calcified plaque; MP = mixed plaque; NCP = non-calcified plaque; TP = total plaque
SD for GlycA = 65.4 μmol/L
Associations are presented in beta-coefficients (95% CI) from linear regression models which were progressively adjusted as follows:
Model 1: adjusted for age, race, scanning center, pre/post 2001-cohort and HIV sero-status (in whole cohort analysis).
Model 2 (Main Model): Model 1 + systolic BP, BMI, physical activity level, use of hypertension medications, use of diabetes medications, fasting glucose, total and HDL cholesterol, use of lipid lowering medications, pack-years of tobacco smoking, eGFR and hepatitis C virus (HCV) -infection status.
Model 3: Model 2 + ln hsCRP, ln D-dimer, ln IL-6 and Fibrinogen.
Model 4: Model 3 + sCD163, sCD14, and ln CCL2.
Model 5: Model 4 + CD4 cell count, presence of detectable HIV RNA, nadir CD4 cell count, history of AIDS, duration of HAART, and use of protease inhibitors.
p-for-interaction adjusted for model 2
Bolded items are statistically significant (p <0.05)
GlycA with subclinical coronary atherosclerosis by HIV serostatus
Overall, the association of GlycA with subclinical atherosclerosis did not significantly differ by HIV serostatus, with p-values for interaction being non-significant (Supplemental Figure 2). Results were generally similar although associations of GlycA with total plaque and mixed plaque seemed weaker among HIV-infected men (Table 4).
Discussion
In this well-characterized cohort of HIV-infected and HIV-uninfected men, we demonstrated that: (1) GlycA levels were higher in HIV-infected (especially with higher viral loads) than uninfected men. (2) GlycA levels were significantly and positively correlated with levels of hsCRP, D-dimer, IL-6, fibrinogen and sCD14 among both HIV-infected and HIV-uninfected men (3) GlycA was significantly associated with multiple CT measures of subclinical coronary atherosclerosis, and results were generally similar among HIV-infected and -uninfected men. Specifically, higher GlycA levels were significantly associated with the presence of CAC, coronary stenosis and calcified plaque (but not with non-calcified plaque) even after controlling for levels of other markers of systemic inflammation including hsCRP, D-dimer, IL-6, and fibrinogen, as well as markers of monocyte activation including sCD163, sCD14, and CCL2. Also, higher GlycA levels were significantly associated with higher burden of CAC, total plaque, and mixed plaque, independent of hsCRP, D-dimer, IL-6 and fibrinogen. Taken together, our findings suggest that GlycA is a promising marker of subclinical coronary atherosclerosis regardless of HIV serostatus.
Since the two-hit hypothesis of atherosclerosis was proposed by Ross[5] –consisting of an inflammatory response following an initial endothelial injury mediated by cholesterol– robust epidemiologic studies have underscored the potential role of inflammation in atherogenesis. Yet this inflammatory hypothesis remained largely unproven until recently. In the CANTOS randomized clinical trial, Ridker et al. reported that using the anti-inflammatory therapy canakinumab to target the interleukin-1β innate immunity pathway significantly lowered the rate of recurrent CVD events compared to placebo, independent of lipid-level lowering.[32] This opens the door for future preventive strategies targeting inflammation for CVD prevention.
Inflammatory biomarkers, especially hsCRP have been shown to predict future risk of incident CVD in the general population beyond traditional CVD risk factor assessment.[11, 12] Thus, inflammatory biomarkers have been shown to be useful for the early identification of high risk individuals who may benefit from targeted preventive strategies such as statin therapy in order to reduce CVD morbidity and mortality. However, these biomarker levels have been shown to be prone to fluctuations by many variables and have also been shown not to consistently predict both subclinical and clinical CVD events in populations with chronic inflammatory conditions such as HIV infection, psoriasis and rheumatoid arthritis.[23] GlycA is a composite inflammatory biomarker that may bridge this gap. Indeed, GlycA has been shown to be a biomarker of chronic inflammation[33] and its utility as a CVD risk assessment tool has been explored in some chronic inflammatory conditions.[23, 24, 34, 35] For example, Joshi et al found an association of GlycA levels with subclinical coronary atherosclerosis among psoriatic patients beyond traditional CVD risk factors and hsCRP.[23] GlycA may be a useful marker of disease severity among patients with rheumatoid arthritis[35] and is elevated in systemic lupus erythematosus.[24] However, unlike our findings noted in a cohort enriched with individuals living with HIV-infection, Chung et al did not find any significant association of GlycA with CAC among lupus patients, but their sample size was small.[24] To our knowledge, our present study is the first to test the relationship of GlycA with subclinical coronary atherosclerosis among HIV-infected individuals and compared with similar HIV-uninfected individuals in the MACS cohort. GlycA was associated with multiple measures of subclinical atherosclerosis in this population, with generally similar results by HIV serostatus.
Although hsCRP is a validated CVD risk stratification tool in the general population, it may not be as useful in predicting the risk of future CVD events among HIV infected individuals.[13] In a prior analysis from the MACS study, a significant association of IL-6, ICAM-1, sTNFR I and sTNFR II, but not hsCRP, were found with subclinical coronary atherosclerosis among HIV-infected men.[8] Higher levels of IL-6 and D-dimer may be stronger markers of CVD risk in HIV-infection than hsCRP,[13, 36] although we did additionally adjust for those markers in our supplemental models. In sum, these findings highlight the complex nature of the interactions between HIV infection, antiretroviral medication use, and systemic inflammation that may work in synergy to promote atherogenesis.[37] While we recognize that these biomarkers reflect different domains in the inflammatory cascade, GlycA integrates multiple inflammatory pathways and thus may better capture the degree of systemic inflammation.[38] Kelesidis et al found that GlycA was the only biomarker of inflammation among hsCRP, IL-6, and D-dimer that decreased in all groups after treatment with atazanavir-, raltegravir-, darunavir-based initial ART.[37] Collectively, these findings support the notion that GlycA may be a reliable biomarker of subclinical CVD risk and treatment response in HIV.
This study benefited from detailed phenotyping of subclinical coronary atherosclerosis including plaque composition and burden using both non-contrast cardiac CT scans and CTA in a large, well-characterized cohort. Nonetheless, the findings of our study should be considered in the context of several limitations. First, by study design, the MACS enrolled only men who have sex with men, so the present findings may not apply to women or to individuals who acquired HIV infection through other behaviors. Second, we utilized a cross-sectional study design and lack temporality to determine the direction of the observed associations between the contemporaneously measured GlycA and subclinical coronary atherosclerosis. Future studies are needed to see if GlycA levels are predictive of incident CVD events among HIV-infected individuals. Our study is observational, and residual confounding may in part explain associations seen despite our rigorous adjustment for numerous traditional CVD risk factors and other common inflammatory markers of risk. We did not have measures of immunoglobulins (IgG) to consider in our analysis. Finally, false positive results associated with simultaneously testing multiple outcomes are possible. However, this is less likely given the level of significance and consistency of most of the associations highlighted in this paper.
In conclusion, GlycA levels, integrating multiple inflammatory pathways, are higher among HIV-infected men than HIV-uninfected men and correlate well with other inflammatory and monocyte activation biomarker levels as well as with multiple measures of CT subclinical coronary atherosclerosis. Our study suggests the potential use of GlycA in CVD risk stratification in HIV patients that is similar to HIV-uninfected persons, but further study is needed in this area.
Supplementary Material
Acknowledgements
The authors thank the staff and participants of the MACS for their important contributions. The MACS website is located at http://aidscohortstudy.org/.
Funding sources: This study is funded by the National Heart, Lung and Blood Institute (NHLBI) grant R01 HL095129 to Dr. Post. The MACS is funded by the National Institute of Allergy and Infectious Diseases (NIAID), with additional supplemental funding from the National Cancer Institute (NCI), the National Institute on Drug Abuse (NIDA), the National Institute of Mental Health (NIMH), and the NHLBI as follows: UO1-AI-35042, UL1-RR025005, UM1-AI-35043, UO1-AI-35039, UO1-AI-35040, UO1-AI-35041. Drs. Michos and Zhao are supported by the Blumenthal Scholars Program in Preventive Cardiology. Dr. Brown is supported in part by the National Institute for Allergy and Infectious Diseases (K24 AI120834). Dr Mora is supported by the NHLBI (R01HL134811 and K24 HL136852), and the National Institute of Diabetes and Digestive and Kidney Diseases (DK112940).
Role of the Sponsor: This work was funded by grants from the NIH, but the funding source had no input in writing or design of manuscript.
Footnotes
Disclosures: Dr. Brown has served as a consultant to Gilead Sciences, Merck, BMS, EMD-Serono, and Theratechnologies. Dr. Budoff has received research funds from GE Healthcare. Dr. Otvos is employed by LabCorp (formally LipoScence). Dr. Mora has received research grant support from Atherotech Diagnostics, and has received consulting fees from Quest Diagnostics for work outside the current study. Dr. Mora has a patent application on the use of GlycA for predicting risk of colorectal cancer. No other authors report any conflicts.
References
- 1.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab 2007; 92(7):2506–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freiberg MS, Chang CC, Kuller LH, Skanderson M, Lowy E, Kraemer KL, et al. HIV infection and the risk of acute myocardial infarction. JAMA internal medicine 2013; 173(8):614–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Post WS, Budoff M, Kingsley L, Palella FJ Jr., Witt MD, Li X, et al. Associations between HIV infection and subclinical coronary atherosclerosis. Annals of internal medicine 2014; 160(7):458–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lo J, Abbara S, Shturman L, Soni A, Wei J, Rocha-Filho JA, et al. Increased prevalence of subclinical coronary atherosclerosis detected by coronary computed tomography angiography in HIV-infected men. AIDS 2010; 24(2):243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ross R Atherosclerosis--an inflammatory disease. The New England journal of medicine 1999; 340(2):115–126. [DOI] [PubMed] [Google Scholar]
- 6.Libby P, Ridker PM, Hansson GK, Leducq Transatlantic Network on A. Inflammation in atherosclerosis: from pathophysiology to practice. Journal of the American College of Cardiology 2009; 54(23):2129–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Margolick JB, Bream JH, Martinez-Maza O, Lopez J, Li X, Phair JP, et al. Frailty and Circulating Markers of Inflammation in HIV+ and HIV- Men in the Multicenter AIDS Cohort Study. J Acquir Immune Defic Syndr 2017; 74(4):407–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bahrami H, Budoff M, Haberlen SA, Rezaeian P, Ketlogetswe K, Tracy R, et al. Inflammatory Markers Associated With Subclinical Coronary Artery Disease: The Multicenter AIDS Cohort Study. Journal of the American Heart Association 2016; 5(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McKibben RA, Margolick JB, Grinspoon S, Li X, Palella FJ Jr., Kingsley LA, et al. Elevated levels of monocyte activation markers are associated with subclinical atherosclerosis in men with and those without HIV infection. J Infect Dis 2015; 211(8):1219–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Triant VA, Perez J, Regan S, Massaro JM, Meigs JB, Grinspoon SK, et al. Cardiovascular Risk Prediction Functions Underestimate Risk in HIV Infection. Circulation 2018; 137(21):2203–2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. The New England journal of medicine 2000; 342(12):836–843. [DOI] [PubMed] [Google Scholar]
- 12.Ridker PM, Glynn RJ, Hennekens CH. C-reactive protein adds to the predictive value of total and HDL cholesterol in determining risk of first myocardial infarction. Circulation 1998; 97(20):2007–2011. [DOI] [PubMed] [Google Scholar]
- 13.Borges AH, O’Connor JL, Phillips AN, Neaton JD, Grund B, Neuhaus J, et al. Interleukin 6 Is a Stronger Predictor of Clinical Events Than High-Sensitivity C-Reactive Protein or D-Dimer During HIV Infection. J Infect Dis 2016; 214(3):408–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Otvos JD, Shalaurova I, Wolak-Dinsmore J, Connelly MA, Mackey RH, Stein JH, et al. GlycA: A Composite Nuclear Magnetic Resonance Biomarker of Systemic Inflammation. Clinical chemistry 2015; 61(5):714–723. [DOI] [PubMed] [Google Scholar]
- 15.Duprez DA, Otvos J, Sanchez OA, Mackey RH, Tracy R, Jacobs DR Jr. Comparison of the Predictive Value of GlycA and Other Biomarkers of Inflammation for Total Death, Incident Cardiovascular Events, Noncardiovascular and Noncancer Inflammatory-Related Events, and Total Cancer Events. Clinical chemistry 2016; 62(7):1020–1031. [DOI] [PubMed] [Google Scholar]
- 16.Akinkuolie AO, Buring JE, Ridker PM, Mora S. A novel protein glycan biomarker and future cardiovascular disease events. Journal of the American Heart Association 2014; 3(5):e001221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akinkuolie AO, Glynn RJ, Padmanabhan L, Ridker PM, Mora S. Circulating N-Linked Glycoprotein Side-Chain Biomarker, Rosuvastatin Therapy, and Incident Cardiovascular Disease: An Analysis From the JUPITER Trial. Journal of the American Heart Association 2016; 5(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Otvos JD, Guyton JR, Connelly MA, Akapame S, Bittner V, Kopecky SL, et al. Relations of GlycA and lipoprotein particle subspecies with cardiovascular events and mortality: A post hoc analysis of the AIM-HIGH trial. Journal of clinical lipidology 2018; 12(2):348–355 e342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gruppen EG, Riphagen IJ, Connelly MA, Otvos JD, Bakker SJ, Dullaart RP. GlycA, a Pro-Inflammatory Glycoprotein Biomarker, and Incident Cardiovascular Disease: Relationship with C-Reactive Protein and Renal Function. PloS one 2015; 10(9):e0139057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akinkuolie AO, Pradhan AD, Buring JE, Ridker PM, Mora S. Novel protein glycan side-chain biomarker and risk of incident type 2 diabetes mellitus. Arterioscler Thromb Vasc Biol 2015; 35(6):1544–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chandler PD, Akinkuolie AO, Tobias DK, Lawler PR, Li C, Moorthy MV, et al. Association of N-Linked Glycoprotein Acetyls and Colorectal Cancer Incidence and Mortality. PloS one 2016; 11(11):e0165615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lawler PR, Akinkuolie AO, Chandler PD, Moorthy MV, Vandenburgh MJ, Schaumberg DA, et al. Circulating N-Linked Glycoprotein Acetyls and Longitudinal Mortality Risk. Circ Res 2016; 118(7):1106–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joshi AA, Lerman JB, Aberra TM, Afshar M, Teague HL, Rodante JA, et al. GlycA Is a Novel Biomarker of Inflammation and Subclinical Cardiovascular Disease in Psoriasis. Circ Res 2016; 119(11):1242–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chung CP, Ormseth MJ, Connelly MA, Oeser A, Solus JF, Otvos JD, et al. GlycA, a novel marker of inflammation, is elevated in systemic lupus erythematosus. Lupus 2016; 25(3):296–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaslow RA, Ostrow DG, Detels R, Phair JP, Polk BF, Rinaldo CR Jr. The Multicenter AIDS Cohort Study: rationale, organization, and selected characteristics of the participants. Am J Epidemiol 1987; 126(2):310–318. [DOI] [PubMed] [Google Scholar]
- 26.Hacioglu Y, Gupta M, Choi TY, George RT, Deible CR, Jacobson LP, et al. Use of cardiac CT angiography imaging in an epidemiology study - the Methodology of the Multicenter AIDS Cohort Study cardiovascular disease substudy. Anadolu Kardiyol Derg 2013; 13(3):207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr., Detrano R Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 1990; 15(4):827–832. [DOI] [PubMed] [Google Scholar]
- 28.Austen WG, Edwards JE, Frye RL, Gensini GG, Gott VL, Griffith LS, et al. A reporting system on patients evaluated for coronary artery disease. Report of the Ad Hoc Committee for Grading of Coronary Artery Disease, Council on Cardiovascular Surgery, American Heart Association. Circulation 1975; 51(4 Suppl):5–40. [DOI] [PubMed] [Google Scholar]
- 29.Pagali SR, Madaj P, Gupta M, Nair S, Hamirani YS, Min JK, et al. Interobserver variations of plaque severity score and segment stenosis score in coronary arteries using 64 slice multidetector computed tomography: a substudy of the ACCURACY trial. J Cardiovasc Comput Tomogr 2010; 4(5):312–318. [DOI] [PubMed] [Google Scholar]
- 30.Erali M, Hillyard DR. Evaluation of the ultrasensitive Roche Amplicor HIV-1 monitor assay for quantitation of human immunodeficiency virus type 1 RNA. J Clin Microbiol 1999; 37(3):792–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Korada SKC, Zhao D, Tibuakuu M, Brown TT, Jacobson LP, Guallar E, et al. Frailty and subclinical coronary atherosclerosis: The Multicenter AIDS Cohort Study (MACS). Atherosclerosis 2017; 266:240–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. The New England journal of medicine 2017; 377(12):1119–1131. [DOI] [PubMed] [Google Scholar]
- 33.Ritchie SC, Wurtz P, Nath AP, Abraham G, Havulinna AS, Fearnley LG, et al. The Biomarker GlycA Is Associated with Chronic Inflammation and Predicts Long-Term Risk of Severe Infection. Cell systems 2015; 1(4):293–301. [DOI] [PubMed] [Google Scholar]
- 34.Bartlett DB, Connelly MA, AbouAssi H, Bateman LA, Tune KN, Huebner JL, et al. A novel inflammatory biomarker, GlycA, associates with disease activity in rheumatoid arthritis and cardio-metabolic risk in BMI-matched controls. Arthritis research & therapy 2016; 18:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ormseth MJ, Chung CP, Oeser AM, Connelly MA, Sokka T, Raggi P, et al. Utility of a novel inflammatory marker, GlycA, for assessment of rheumatoid arthritis disease activity and coronary atherosclerosis. Arthritis Res Ther 2015; 17:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nordell AD, McKenna M, Borges AH, Duprez D, Neuhaus J, Neaton JD. Severity of cardiovascular disease outcomes among patients with HIV is related to markers of inflammation and coagulation. J Am Heart Assoc 2014; 3(3):e000844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kelesidis T, Tran TT, Stein JH, Brown TT, Moser C, Ribaudo HJ, et al. Changes in Inflammation and Immune Activation With Atazanavir-, Raltegravir-, Darunavir-Based Initial Antiviral Therapy: ACTG 5260s. Clin Infect Dis 2015; 61(4):651–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lawler PR, Mora S. Glycosylation Signatures of Inflammation Identify Cardiovascular Risk: Some Glyc It Hot. Circ Res 2016; 119(11):1154–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.