Abstract
Recent research suggests that cancer stem-like cells (CSCs) are the key subpopulation for tumor relapse and metastasis. Due to cancer plasticity in surface antigen and enzymatic activity markers, functional tumorsphere assays are promising alternatives for CSC identification. To reliably quantify rare CSCs (1–5%), thousands of single-cell suspension cultures are required. While microfluidics is a powerful tool in handling single cells, previous works provide limited throughput and lack automatic data analysis capability required for high-throughput studies. In this study, we present the scaling and automation of high-throughput single-cell-derived tumor sphere assay chips, facilitating the tracking of up to ~10 000 cells on a chip with ~76.5% capture rate. The presented cell capture scheme guarantees sampling a representative population from the bulk cells. To analyze thousands of single-cells with a variety of fluorescent intensities, a highly adaptable analysis program was developed for cell/sphere counting and size measurement. Using a Pluronic® F108 (poly(ethylene glycol)-block-poly(propylene glycol)-block-poly(ethylene glycol)) coating on polydimethylsiloxane (PDMS), a suspension culture environment was created to test a controversial hypothesis: whether larger or smaller cells are more stem-like defined by the capability to form single-cell-derived spheres. Different cell lines showed different correlations between sphere formation rate and initial cell size, suggesting heterogeneity in pathway regulation among breast cancer cell lines. More interestingly, by monitoring hundreds of spheres, we identified heterogeneity in sphere growth dynamics, indicating the cellular heterogeneity even within CSCs. These preliminary results highlight the power of unprecedented high-throughput and automation in CSC studies.
Introduction
Cancer is known for its cellular heterogeneity, and it is believed that a small population of “cancer stem-like/initiating cells” (CSCs) is responsible for tumor metastasis and tumor relapse.1–5 Some membrane surface markers and intracellular enzymatic markers have been used to identify CSC populations.6,7 However, due to the heterogeneity and cellular plasticity of cancer, it is possible that CSCs carry distinct expressions from different tumors.8 In addition to the markers, CSCs can also be recognized by their cell behavior.9 When cultured in a suspension environment, CSCs can survive and proliferate into tumorspheres, whereas non-CSCs perform programmed cell death (anoikis) due to the loss of anchorage to substrates.10 Hence, in vitro single-cell-derived sphere formation assays are an attractive alternative to identify CSCs.
Performing in vitro single-cell-derived sphere assays, however, is technically more challenging than the traditional bulk assays. To ensure a single-cell culture, researchers have used limiting dilution methods with low-attachment 96/384 well plates to isolate single-cells in each well for sphere culture.11–13 However, without a robotic system, this method is labor intensive and limited in throughput because the capture rate is limited by Poisson distribution (10–30%). Fluorescence-activated cell sorting (FACS) can automate the single-cell dispensing process and achieve higher single-cell seeding rate; however, high shear stress during the sorting can potentially affect cell viability and influence the results.14 Given the low throughput of conventional approaches, people can barely quantify the sphere formation rate; therefore, it is difficult to investigate the cellular heterogeneity within rare CSC populations. The controversy of the correlation between cancer cell size and stemness is one example. In recent publications, some researchers reported evidence showing that smaller cancer cell size is associated with cancer stem-like cell activity,15–17 whereas other researchers reported higher mitochondria mass and increased cell size correlate with cancer stemness and chemo-resistance.18–20 To study the heterogeneity in the CSC populations, there is an unmet need of an in vitro high-throughput approach for rare cell studies.
Microfluidic culture systems emerge to be a powerful method for single-cell studies.21 Combined with a non-adherent culture substrate, single-cell capture chips were developed for single-cell-derived sphere assay.22,23 However, previous works using hydrodynamic capture schemes required extended meander channels to achieve a high cell capture rate (>80%), which constrained the number of wells per area.23–28 The prolonged time for imaging over a large area limits the assay throughput and could potentially affect cell viability if an environmental chamber is not used during image capture under a microscope. For other capture methods, droplet systems can achieve high-throughput analysis by encapsulating single cells in aqueous droplets.29 However, droplet approaches are limited by short assay time due to the difficulty in media exchange. Clonal sphere assay would typically require 14 days for culture and thus cannot be implemented by droplet systems. Micro-well systems are another simple yet effective tool to isolate single cells for clonal culture.30,31 However, most micro-well systems rely on random seeding with a low cell capture rate around 10–30%. Schemes using dielectrophoresis force or dual-wells can facilitate higher capture rates, but they either require sophisticated active control or exhibit size-dependent capture.32,33 High density and high capture rate can be realized by a filter array structure system.34,35 However, in these works, capture sites were connected in series, resulting in high flow resistance and low flow rate, given that flow rate (2 μL h−1), a high concentration of cells and a long loading time are required. In that case, cell aggregation and clogging may inevitably occur for numerous cancer cell lines. In addition, without automatic cell analysis, it requires manual inspection to read out data from microscope images, which is challenging when hundreds or thousands of single-cells are analyzed.
To address these challenges, we developed a high-throughput single-cell capture device utilizing highly-parallelized structures for single-cell-derived tumorsphere studies. The highly scalable fluidic structure enables reliable single cell capture from 800 wells per chip to up to 12 800 wells per chip. The capture scheme can reliably sample a representative cell population from the bulk. With an automatic analysis program, assay results from thousands of cells and spheres can be analyzed after microscopic imaging. The high-throughput culture system with automatic analysis enables the analysis of heterogeneity within the CSC populations to study cancer stemness and cell size correlation and single-cell-derived sphere growth dynamics.
Experimental
Device design and fabrication
The high-throughput device was built using a PDMS (polydimethylsiloxane) piece with microfluidic patterns bonded to another blank PDMS piece or a glass slide. PDMS was patterned by standard soft lithography. The SU-8 mold used for soft-lithography was created by a 3-layer photolithography process with 10 μm, 40 μm, and 100 μm thick SU-8 (Microchem) following the manufacturer’s protocol. The pattern was designed using a computer-aided design software (AutoCAD 2015, Autodesk®), and the masks were made by a mask-making instrument (μPG 101, Heidelberg Instruments). The SU-8 mold was treated by vaporized trichloro(3,3,3-trifluoropropyl)silane (452807 Aldrich) under vacuum overnight to promote the release of cured PDMS. PDMS (Sylgard 184, Dow Corning) was prepared by mixing with elastomer and curing agent in a (w/w) ratio of 10 : 1, poured on SU-8 molds, and cured at 100 °C overnight before peeling. Inlet and outlet holes are created by biopsy punch cutting. The PDMS with microfluidic channel structures and the blank PDMS (or glass slide) were treated using oxygen plasma (80 W for 60 seconds) and bonded. The devices after bonding were heated at 80 °C overnight to ensure bonding quality.
Cell culture
Different breast cancer cell lines, including MDA-MB-231, SUM-159, SUM-149, MCF-7 and T47D, were cultured in Petri dishes for device testing. MDA-MB-231 cells were used for cell loading test and cell size capture range characterization. SUM-159, SUM-149, MCF-7 and T47D were loaded and cultured in the chip for single-cell-derived sphere experiment. MDA-MB-231 cells were obtained from Dr. Gary Luker’s Lab (University of Michigan, MI, USA). SUM-159, SUM-149, and T47D cells were obtained from Dr. Max Wicha’s Lab (University of Michigan, MI, USA). MCF-7 cells were obtained from Dr. Sofia Merajver’s Lab (University of Michigan, MI, USA). MDA-MB-231 and MCF-7 cells were cultured in DMEM (Gibco 11965) with 10% FBS (Gibco 10082) and 1% penicillin/streptomycin (Gibco 15070). T47D cells were cultured in RPMI (Gibco 11875) with 10% FBS (Gibco 10082) and 1% penicillin/streptomycin (Gibco 15140). SUM-159 and SUM-149 cells were cultured in F-12 based media (Ham’s F12 (Gibco 11765) supplemented with 1% penicillin/streptomycin (Gibco 15070), 5 μg mL−1 (2.5 mg/500 mL), insulin (Sigma I6634), 1 μg mL−1 (0.5 mg/500 mL) hydrocortisone (Sigma H4001), and 5% FBS (Gibco 10082). All the cells were cultured and passaged when the cells reached over 80% confluency in the dish.
Capture rate and cell size capture range characterization
The microfluidic chips were sanitized using UV radiation and primed using a 5% (w/w) PEO-terminated triblock polymer (Pluronic® F108, BASF) in DI water overnight before use. The devices were primed at least 7 days after plasma activation to ensure PDMS restored its hydrophobicity to ensure quality F108 anti-fouling coating.36,37 Before cell loading, channels were washed by phosphate-buffered saline (PBS) for one hour. MDA-MB-231 cells were first stained by 10 μM green CellTracker dye (ThermoFisher C2925) following the manufacturer’s protocol and then suspended using Trypsin-EDTA (Gibco 25200). Cell concentrations were calculated using an automatic cell counter (LUNA-II, Logos Biosystems). Different concentrations, including 25k cells per mL, 50k cells per mL, 100k cells per mL, and 200k cells per mL, were used for test. 500 μL of the cell suspension solution was then pipetted into the chip inlets, and the cells were driven into the chip by gravity flow. The initial flow rate was adjusted to be 20 μL min−1. After cell loading for 15 minutes, the cell suspension solution was replaced by 500 μL of the PBS to stop the loading and the chips were scanned to readout capture results.
High-throughput single-cell-derived sphere assay
The microfluidic chips were prepared as described in the previous section. For experimental replicates, three independent devices were used for each cell line. SUM-159, SUM-149, MCF-7 and T47D were first stained by green CellTracker dye (ThermoFisher C2925) following the manufacturer’s protocol, suspended using Trypsin-EDTA (Gibco 25200), and loaded into the chips. After cell loading, the gravity flow was stopped by removing all the solution in the inlet and outlet. The devices were then flipped so the cells could be seeded onto the bottom of each well. In this flipped configuration, cells would be retained in each well and the residual cells in the channels would not enter the wells as they were blocked by the walls of the wells. The cells were cultured using custom serum-free media for sphere culture. This serum-free media contains MEBM (CC-3151, Lonza) supplemented with B27 (Gibco 17504–044), 20 ng mL−1 bFGF (BD 354060), 20 ng mL−1 EGF (BD 354052), 5 μg mL−1 insulin (Sigma I6634), 1 mM lipid concentrate (Gibco 11905–031), 1 μg mL−1 hydrocortisone (Sigma H4001), 7.8 μg mL−1 mercaptoethanol (Sigma M3148), 3.9 μg mL−1 cholesterol (Sigma C4951), and 1% penicillin/streptomycin (Gibco 15070). The media were exchanged daily. The chips were scanned directly after loading (day 0), 7 days after loading (day 7), and 14 days after loading (day 14). The cells were stained again using green CellTracker dye (ThermoFisher C2925) before imaging on day 7 and day 14 for sphere size analysis. For the assay readout, in order to distinguish spheres with multiple cells from single large cells, only spheres with a diameter larger than 40 μm were included for analysis because very few cells (<0.2%) have a cell diameter larger than 40 μm (Fig. 7(a)).
Fig. 7.
Comparison of sphere formation rate and the initial single cell size: (a) cell size analyzed by μFAST showing different cell size distributions in cell lines (600 cells randomly sampled for each cell line). (b) Sphere formation rate with small (bottom 30%) and large (top 30%) population from each cell line (N = 3 for each cell line).
Image acquisition
The microfluidic devices were imaged using an inverted microscope (Nikon) with a XYZ motorized stage (ProScan II, Prior Scientific). The bright-field and fluorescent images were taken with a 10× objective and a charge-coupled device (CCD) camera (Coolsnap HQ2, Photometrics). A FITC filter cube was used for fluorescent imaging. To ensure optimized image quality, auto-focusing was done after imaging every 3 frames. After scanning, the Nikon NIS-Elements Basic Research software module was used to stitch individual images into a large image for analysis.
Automatic image analysis program
A custom-made program, called μFAST (μFluidics AnalySis Tool), was scripted using MATLAB 2015a (Windows 64-bit version) with an image processing toolbox. A desktop computer with an Intel® Xeon® CPU E3–1245 (3.4 GHz) processor and 16 GB RAM was used to perform the computation for automatic high-throughput calculation and analysis.
Data analysis and processing
For capture rate characterization, at least 10% of the wells were randomly selected and manually checked to verify that the program counting accuracy was higher than 95%. For single-cell-derived sphere assays, all the data were manually checked to ensure all the spheres were started with single cells. Statistical analyses were performed using Microsoft Excel. Two-tailed, unpaired Student’s t-tests were used for all comparisons and significance level of P < 0.05 was used to consider statistical significance. * refers to P < 0.05, ** refers to P < 0.01, and *** refers to P < 0.001. Results are presented as mean ± SD.
Results and discussion
Design of the high-throughput single-cell-derived sphere chip
Single-cells are captured in micro-wells when they flow into the micro-wells and block the capture site. The micro-wells were designed to be 100 μm × 100 μm × 100 μm cubes to provide room for sphere culture. To increase the throughput, it was found that simple duplication of identical micro-wells into a larger array will suffer from low cell capture rate and clogging caused by non-uniformity of cell distribution between the upstream and downstream. To overcome this challenge, the scaling of the chip is achieved by engineering two aspects. First, we investigated how to add more cell capture wells in each branch channel. Second, the throughput can be scaled up by parallelizing the branch channels. A branch channel of 12 800-well chip is shown in Fig. 1(c). In this design, each branch channel comprises an entrance channel and an exit channel with 200 micro-wells connected in parallel. After the entrance channel, a 40 μm high escape channel was added to release the residual cells in the entrance channel after loading. To ensure uniform cell capture at the upstream and downstream of each branch channel, we designed the unit flow resistance of entrance and exit channels to be significantly lower than each capture well by 100 times using multi-layer fabrication (Fig. S1 and S2†). The flow resistance difference was achieved by implementing entrance channels and exit channels with a large channel height (100 μm) compared to the capture sites (10 μm). As the fluid was gradually transferred from entrance channels to exit channels when flowing to downstream, the entrance channels were tapered smaller and the exit channels were tapered larger to maintain the flow velocity in the channel. 40 μm high channels were also used to connect the main channel to the micro-wells; when the chips were flipped after cell capture, these formed a wall barrier around each micro-well to prevent cells from moving in and out of the wells (Fig. S3†). After finalizing the branch channel design, we connected the channels to the same inlet and outlet with branching channels. Using this scheme, chips with throughput from 800 wells per chip to 12 800 wells per chip were fabricated and tested. 800-well devices are composed of 16 branch channels with each containing 50 micro-wells, whereas 3200-well devices are composed of 32 branch channels with each containing 100 micro-wells (Fig. S4†). The 12 800-well array is composed of 64 parallel branch channels with each containing 200 micro-wells, as shown in Fig. 1(a) and (b). This 12 800 single-cell well array covers 24 mm × 27 mm area; therefore, the entire chip with inlet, outlet, and other branching channels can fit on a 3″ × 1″ glass slide. The highly parallel structure also results in low flow resistance, enabling gravity flow (100 Pa) cell loading by simple pipetting. The complete layout of a 12 800-well chip can be found in Fig. 1(d). Fig. 1(e) shows the microscopic image of cells being captured in the array, highlighting the power of high-throughput single-cell capture capability.
Fig. 1.
Overview of the high-throughput single-cell culture chip. (a) Image of a chip with 12 800 single-cell chambers. (b) Close-up image of the branching channels and single cell chambers. (c) Schematic of a single branch channel with 200 single cell chambers. (d) Schematic of an entire device with specified structure height. (e) Microscopic picture of single-cells captured in the well arrays (scale bar: 100 μm).
Automatic image analysis program
A custom-made MATLAB program, called μFAST, was developed to achieve “image-in-result-out” capability with manual sample checking function to ensure analysis quality. First, a user specifies the four corners of a chip, so μFAST can identify the location of each micro-well with a unique address using vector space operation. After well segmentation, μFAST performs image analysis to calculate the parameters of interest such as the number of cells and the size of the cell/sphere in each well. Due to the heterogeneity of cell fluorescent intensity, contrast enhancement should be adjusted before analysis to ensure dim cells are also counted (Fig. 2(a) and (b)). Since most parts of the background fluorescent image were dark, global histogram equalization generates artifact signals from noise in the background, making the image unusable for analysis (Fig. 2(c) and (d)).38 To overcome this problem, contrast-limited adaptive histogram equalization (CLAHE) was used to enhance contrast in a localized patch area, minimizing the noise from a homogenous dark background (Fig. 2(e) and (f)).39 For cell counting, a double k-means clustering algorithm was then applied to find the local peak of the fluorescent intensity to identify the position of cells (Fig. 3 and S5†).40,41 A noise removal algorithm by morphological opening operations in local pixel areas was also applied to remove false positives such as cell debris or local noise. This counting mechanism works reliably for both suspension and adherent cells (Fig. S6 and S7†). To measure the cell size, the Hough transform was used to identify circular cells and their diameters (Fig. S8†).42 For sphere size calculation, intensity thresholding was applied to calculate the pixel area to extract the sphere area (Fig. S9†). The pixel area was then converted to μm2 to calculate the sphere diameter. The presented μFAST program enables high-throughput analysis of thousands of cells with information such as cell size, number of cells, and sphere size.
Fig. 2.
Contrast enhancement for image processing. Global histogram equalization creates artifacts from noise in the background, whereas CLAHE can reliably enhance the image of the fluorescent cells (scale bar: 50 μm).
Fig. 3.
Cell counting with CLAHE contrast enhancement and adaptive k-means clustering to find the center of the cells in the image. Reliable counting results are achieved with examples of images with single cell, double cells, and multiple cells (scale bar: 40 μm).
Cell capture and captured cell size characterization
We achieved a high single-cell capture rate of ~76.5% at the optimal cell concentration of 50k cells per mL. Reliable capture rate of >60% could be attained in a wide range of concentrations from 25–100k cells per mL (Fig. 4(a)). Double and multiple captures have higher occurrence when higher concentrations were used. It was observed that double and multiple captures have two different causes. They could be due to the second cell coming into the micro-well before the first cell completely blocks the capture site. It could also be the consequence of the cells aggregating together in a higher concentration in the cell suspension, resulting in cell cluster captures. Loading a lower concentration of cells helped significantly reduce the double and multiple captures. However, the cell loading time is longer for lower cell concentrations, which can potentially affect cell viability. Given a 15 minute loading time, 50k cells per mL provides the optimal capture rate with consistent results. Due to the size heterogeneity of cancer cells, it is critical that the capture scheme should sample the representative population from bulk cells. The size distributions of cells captured on-chip and in bulk were measured and compared (Fig. 4(b)). This shows the capability to capture cells with a wide range of sizes comparable to bulk cells, as shown in Fig. 4(c–f). The high capture rate with a representative cell size distribution provides robust single-cell isolation to study highly heterogeneous cancer cells.
Fig. 4.
(a) Cell capture result with different loading cell concentrations (N = 5). (b) Size distribution of MDA-MB-231 cells in bulk and captured in chip (N = 600 for each case). (c)–(f) Examples of cells with different sizes captured in the chamber. The cell diameters are 12 μm in (c), 20 μm in (d), 30 μm in (e), and 40 μm in (f) (scale bar: 50 μm).
Single cell sphere formation rate and sphere size comparison
To validate the high-throughput single-cell-derived sphere assay, four different cell lines, including SUM-159, SUM-149, MCF-7, and T47D, were loaded and cultured in the device for 14 days. Since the PDMS was coated with F108, the polyethylene oxide (PEO) group prevents the cells from adhering to the substrate,36,37 enabling single-cell-derived sphere culture on the chip to investigate sphere formation rates and sphere sizes. In a suspension culture, stem-like cells grew into spheres from single-cells, whereas non-stem-like cells died as a result of anoikis (Fig. 5 and 6). After 14 days of culture, the sphere size heterogeneity between cell lines and within cell lines was observed (Fig. 6(a)). SUM-159 has the highest sphere formation rate around 45% and the average sphere size is also higher than the other three cell lines. Although SUM-149 has a similar sphere formation rate to MCF-7 and T47D, the average sphere size is slightly higher than other two cell lines with statistical significance. The experiments reproduced similar sphere formation rates reported in previous in vitro single-cell-derived sphere chips, validating the single-cell-derived sphere assay using this high-throughput platform.22,23 As the sphere formation rate could be as small as 1–5%, this high-throughput system enables tens to hundreds of spheres to be analyzed from thousands of starting cells, allowing investigation of heterogeneity within the sphere populations.
Fig. 5.
Microscopic images of cells in single-cell-derived assay with four breast cancer cell lines. Stem-like cells grew into a sphere from single-cells, whereas non-stem-like cells died of anoikis due to the loss of anchorage (scale bar: 40 μm).
Fig. 6.
Large-scale single-cell-derived sphere assay (N = 3 for each cell line): (a) sphere distribution after 14 days culture (1000 single-cells randomly selected from each cell line). (b) Sphere formation rate after 14 days culture. (c) Sphere size after 14 days culture.
Sphere formation rate from different initial single cell sizes
After validating the high-throughput capability, we applied this technology to study the correlation of cancer stemness and cell sizes. Since the presented microfluidic chip is capable of capturing representative cell size populations, the correlation could be investigated by assessing sphere formation rates of subpopulations with different cell sizes on the same chip. First, we measured the size distribution of the cells captured on-chip and separated them into two subpopulations based on cell size: small cells (bottom 30% in cell size) and large cells (top 30% in cell size) (Fig. 7(a)). Then, the sphere formation rates of the two groups were compared in each cell line (Fig. 7(b)). It was found that smaller cells have a higher sphere-forming potential than large cells in SUM-159, whereas SUM-149 and T47D have the opposite result. There was no significant difference in the sphere formation rate observed between small and large MCF-7 cells. The result indicates that the relationship between cell size and stemness is cell line dependent, which may explain why different conclusions were attained by different groups.
Single-cell-derived sphere growth dynamics
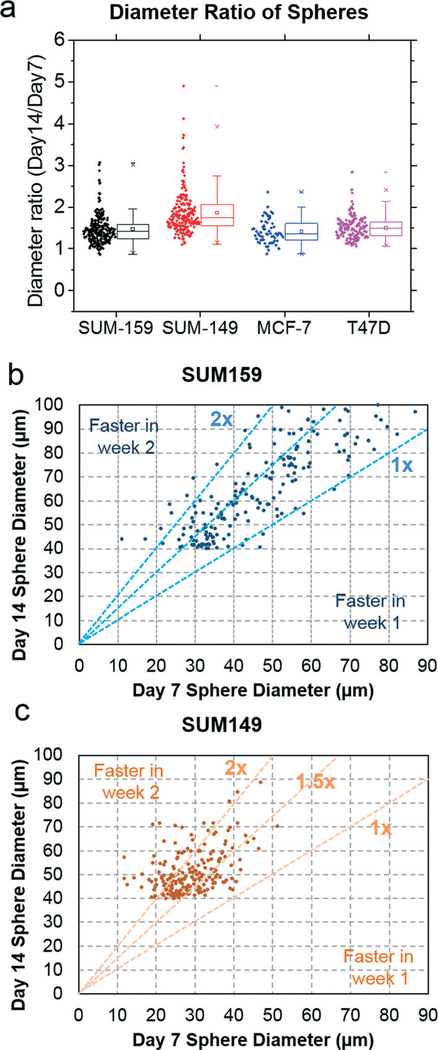
Another intriguing aspect of the presented sphere assay is the capability to track the sphere formation dynamics of each sphere with high-throughput, showing the proliferation patterns of spheres (Fig. 8 and Fig. S10–S13†). For instance, in addition to the spheres growing at a constant rate, some spheres could be more proliferative in the beginning and then become quiescent later or vice versa. When comparing sphere diameter ratios from day 14 to day 7, heterogeneous sphere growth dynamic patterns could be observed (Fig. 9(a)). A ratio close to 1 implies the limited proliferation of the sphere in the second week. When the ratio is larger, it implies faster proliferation in the second week. Among the four cell lines, SUM-149 was observed to be more proliferative in the second week. To look into the difference of growth dynamics in SUM-159 and SUM-149, we plotted the sphere size on day 7 and day 14 on a scatter plot (Fig. 9(b) and (c), respectively). As shown in Fig. 9(b), SUM-159 has wider spread due to the high proliferation rate and sphere size heterogeneity. In contrast, SUM-149 shows a cluster of samples within the 1.5× and 2× lines (Fig. 9(c)). While all sphere-forming cells are considered cancer stem-like based on conventional definition, huge variation of growth dynamics was observed, clearly implicating the cellular heterogeneity even within cancer stem-like cells. This observation can only be enabled by the scaling and automation of single-cell assays. In future, this heterogeneity can be further investigated by using reporters to reflect the regulations within spheres and retrieving individual spheres for gene expression analysis.
Fig. 8.
Microscopic images of cells showing different sphere growth dynamics over 14 days of cultures. In addition to spheres with steady proliferation, some spheres were more proliferative in the first week and became quiescent in the second, whereas others were more quiescent in the first week and became proliferative in the second week (scale bar: 40 μm).
Fig. 9.
Sphere growth dynamics: (a) heterogeneity of sphere growth of each cell line (N = 3, 60 spheres randomly selected from each cell line). (b) Scattering plot showing sphere size on day 7 and day 14 for SUM-159. (c) Scattering plot showing sphere size on day 7 and day 14 for SUM-149 (200 spheres were randomly selected for plots in (b) and (c)).
Conclusion
We presented a high-throughput analysis chip with highly parallel structures for single-cell-derived sphere assays with a wide range of scaling capability from 800 wells per chip to 12 800 wells per chip. For the highest throughput assay chips presented in this work (12 800-well chip), an optimal single cell capture rate (~76.5%) could be achieved and a representative cell size population comparable to bulk (10–40 μm in diameter) could be comprehensively analyzed. With the custom automated analysis software, μFAST, we were able to monitor various parameters of sphere assays in a high-throughput manner, validating the sphere size heterogeneity across different cell lines. Moreover, we observed the correlation between the initial cell sizes and cancer stemness by monitoring sphere formation rates of different size subpopulations. Finally, different sphere growth dynamics were observed in inter- and intra-cell lines. The presented results demonstrate the power of high-throughput single-cell-derived sphere assay chips for functional identification and analysis of cancer stem-like cells.
Supplementary Material
Acknowledgements
This study was supported in part by NIH R21 CA175857 and 1R21 CA195016–01A1. Device fabrication by the Lurie Nanofabrication Facility of the University of Michigan (Ann Arbor, MI) is greatly appreciated. The authors also thank Dr. Gary Luker’s lab, Dr. Sofia Merajver’s lab and Dr. Max Wicha’s lab for providing cells for the experiments.
Footnotes
Competing financial interests
The authors declare no competing financial interests.
Electronic supplementary information (ESI) available. See DOI: 10.1039/c6lc00778c
References
- 1.Clevers H, Nat. Med, 2011, 17, 313–319. [DOI] [PubMed] [Google Scholar]
- 2.Li F, Tiede B, Massagué J and Kang Y, Cell Res, 2007,17, 3–14. [DOI] [PubMed] [Google Scholar]
- 3.Reya T, Morrison SJ, Clarke MF and Weissman IL, Nature, 2001, 414, 105–111. [DOI] [PubMed] [Google Scholar]
- 4.Magee JA, Piskounova E and Morrison SJ, Cancer Cell, 2012, 21, 283–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Almendro V, Marusyk A and Polyak K, Annu. Rev. Pathol.: Mech. Dis, 2012, 8, 121023133009008. [DOI] [PubMed] [Google Scholar]
- 6.Ricardo S, Vieira AF, Gerhard R, Leitao D, Pinto R, Cameselle-Teijeiro JF, Milanezi F, Schmitt F and Paredes J, J. Clin. Pathol, 2011, 64, 937–946. [DOI] [PubMed] [Google Scholar]
- 7.Marcato P, Dean CA, Giacomantonio CA and Lee PW, Cell Cycle, 2011, 10, 1378–1384. [DOI] [PubMed] [Google Scholar]
- 8.Owens TW and Naylor MJ, Front. Physiol, 2013, 4,1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dontu G and Wicha MS, J. Mammary Gland Biol. Neoplasia, 2005, 10, 75–86. [DOI] [PubMed] [Google Scholar]
- 10.Kruyt FAE and Schuringa JJ, Biochem. Pharmacol, 2010, 80, 423–430. [DOI] [PubMed] [Google Scholar]
- 11.Vermeulen L, Todaro M, de Sousa Mello F, Sprick MR, Kemper K, Perez Alea M, Richel DJ, Stassi G and Medema JP, Proc. Natl. Acad. Sci. U. S. A, 2008, 105, 13427–13432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stingl J, J. Pathol, 2009, 217, 229–241. [DOI] [PubMed] [Google Scholar]
- 13.Liao MJ, Cheng CZ, Zhou B, Zimonjic DB, Mani SA, Kaba M, Gifford A, Reinhardt F, Popescu NC, Guo W, Elinor NE, Lodish HF and Weinberg RA, Cancer Res, 2007, 67, 8131–8138. [DOI] [PubMed] [Google Scholar]
- 14.Shapiro E, Biezuner T and Linnarsson S, Nat. Rev. Genet, 2012, 14, 618–630. [DOI] [PubMed] [Google Scholar]
- 15.Li Q, Rycaj K, Chen X and Tang DG, Semin. Cancer Biol, 2015, 35, 191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aravalli RN, Sahin M. Behnan, Cressman ENK and Steer CJ, Biochem. Biophys. Res. Commun, 2010, 391, 56–62. [DOI] [PubMed] [Google Scholar]
- 17.Bortolomai I, Canevari S, Facetti I, De Cecco L, Castellano G, Zacchetti A, Alison MR and Miotti S, Cell Cycle, 2010, 9, 1194–1206. [DOI] [PubMed] [Google Scholar]
- 18.Farnie G, Sotgia F, Lisanti MP and Gillian Farnie FSMPL, Oncotarget, 2015, 6, 30472–30486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Srivastava VK and Nalbantoglu J, Cytometry, Part A, 2008, 73, 940–948. [DOI] [PubMed] [Google Scholar]
- 20.Zhang S, Mercado-Uribe I, Xing Z, Sun B, Kuang J and Liu J, Oncogene, 2014, 33, 116–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Y-C, Ingram P, Luan Y and Yoon E, in Essentials of Single-Cell Analysis: Concepts, Applications and Future Prospects, ed. Tseng F-G and Santra ST, Springer Berlin Heidelberg, Berlin, Heidelberg, 2016, pp. 1–29. [Google Scholar]
- 22.Chen Y-C, Ingram P, Lou X and Yoon E, in International Conference on Miniaturized Systems for Chemistry and Life Sciences (MicroTAS ‘12), 2012, pp. 1241–1244. [Google Scholar]
- 23.Chen Y-C, Patrick NI, Fouladdel S, McDermott SP, Azizi E, Wicha MS and Yoon E, Sci. Rep, 2016, 6, 27301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chung J, Kim YJ and Yoon E, Appl. Phys. Lett, 2011, 98, 123701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Y-C, Cheng Y-H, Kim HS, Ingram PN, Nor JE and Yoon E, Lab Chip, 2014, 14, 2941–2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frimat J-P, Becker M, Chiang Y-Y, Marggraf U, Janasek D, Hengstler JG, Franzke J and West J, Lab Chip, 2011, 11, 231–237. [DOI] [PubMed] [Google Scholar]
- 27.Tan W-H and Takeuchi S, Proc. Natl. Acad. Sci. U. S. A, 2007, 104, 1146–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Y-C, Ingram P and Yoon E, Analyst, 2014,139, 6371–6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huebner A, Bratton D, Whyte G, Yang M, DeMello AJ, Abell C and Hollfelder F, Lab Chip, 2009, 9, 692–698. [DOI] [PubMed] [Google Scholar]
- 30.Lecault V, Vaninsberghe M, Sekulovic S, Knapp DJHF, Wohrer S, Viel F, Mclaughlin T, Jarandehei A, Miller M, Falconnet D, Adam K, Kent DG, Copley MR, Taghipour F, Eaves CJ, Humphries RK, James M and Hansen CL, Nat, Methods, 2011, 8, 9–11. [DOI] [PubMed] [Google Scholar]
- 31.Rettig JR and Folch A, Anal. Chem, 2005, 77, 5628–5634. [DOI] [PubMed] [Google Scholar]
- 32.Kim SH, Antfolk M, Kobayashi M, Kaneda S, Laurell T and Fujii T, Lab Chip, 2015, 15, 4356–4363. [DOI] [PubMed] [Google Scholar]
- 33.Lin C-H, Hsiao Y-H, Chang H-C, Yeh C-F, He C-K, Salm EM, Chen C, Chiu I-M and Hsu C-H, Lab Chip, 2015, 15, 2928–2938. [DOI] [PubMed] [Google Scholar]
- 34.Chung K, Rivet CA, Kemp ML and Lu H, Anal. Chem, 2011, 83, 7044–7052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hong S, Pan Q and Lee LP, Integr. Biol, 2012, 4, 374. [DOI] [PubMed] [Google Scholar]
- 36.Liu VA, Jastromb WE and Bhatia SN, J. Biomed. Mater. Res, 2002, 60, 126–134. [DOI] [PubMed] [Google Scholar]
- 37.Hellmich W, Regtmeier J, Duong TT, Ros R, Anselmetti D and Ros A, Langmuir, 2005, 21, 7551–7557. [DOI] [PubMed] [Google Scholar]
- 38.Hummel R, Comput. Graph. Image Process, 1977, 6, 184–195. [Google Scholar]
- 39.Pizer SM, Amburn EP, Austin JD, Cromartie R, Geselowitz A, Greer T, Romeny BTH and Zimmerman JB, Comput. Vis. Graph. Image Process, 1987, 39, 355–368. [Google Scholar]
- 40.Kanungo T, Mount DM, Netanyahu NS, Piatko CD, Silverman R and Wu AY, IEEE Trans. Pattern Anal. Mach. Intell, 2002, 24, 881–892. [Google Scholar]
- 41.Zhang C, Xiao X, Li X, Chen Y-J, Zhen W, Chang J, Zheng C and Liu Z, Sensors, 2014, 14, 16128–16147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yuen H, Princen J, Illingworth J and Kittler J, Image Vis. Comput, 1990, 8, 71–77. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.