Abstract
Background:
Previous studies showed that the quantity of the left atrial (LA) periatrial fat tissue predicts recurrence after catheter ablation of atrial fibrillation (AF). We hypothesized that the quality of the LA periatrial fat tissue, measured by the mean computed tomography (CT) attenuation, predicts recurrence after AF ablation independent of the quantity of the LA periatrial fat tissue.
Methods:
We included 143 consecutive patients with drug-refractory AF referred for the first catheter ablation of AF (62.2±10 years, 40% nonparoxysmal AF). All participants had a pre-ablation cardiac CT. We measured the quantity of the LA periatrial fat tissue by the area (mm2) and the quality by the mean CT attenuation (Hounsfield unit, HU) in a standard four-chamber view.
Results:
Patients with AF recurrence after ablation (n=57) had a significantly larger fat area (167.6 [interquartile range 124.1–255] vs. 145.4 [95.6 – 229.3] mm2, p=0.018) and a higher fat attenuation (−92.0±9.8 vs. −96.5±9.4 HU, p=0.006) than those without recurrence (controls). LA fat attenuation was correlated with LA fat volume and LA bipolar voltage by invasive mapping, and was associated with AF recurrence after adjusting for clinical risk factors, including BMI, AF type, LA dimension, and fat area (HR:2.65, p=0.001).
Conclusions:
The quality of the LA periatrial fat tissue is an independent predictor of recurrence after the first AF ablation. Assessment of LA periatrial fat attenuation can improve AF ablation outcomes by refining patient selection.
Journal Subject Terms: Left atrial, Fat, Atrial fibrillation
Keywords: Pulmonary vein isolation, Left atrium, Cardiac CT, Atrial fibrillation, Periatrial fat
CLINICAL SUMMARY
AF ablation is the indicated treatment for individuals with drug-refractory AF. However, the rate of recurrence after the procedure is relatively high, in part, due to poor patient selection before the intervention. Thus, identifying tools that assist the physician in the selection of adequate candidates is imperative. Our study examined the value of periatrial fat quality in predicting the success of AF ablation beyond traditional risk factors, and other indices of LA structure and function. Starting from present observations, further prospective studies are needed to determine the direct implications of this novel index in the process of selecting the best candidates to be referred to AF ablation.
INTRODUCTION
Obesity is a worldwide epidemic and constitutes a strong risk factor for atrial fibrillation (AF) 1, the most prevalent arrhythmia in humans. Obesity is also independently associated with AF recurrence after catheter ablation 2. However, the exact mechanism by which obesity is causally related to AF remains unknown. Atrial stretch, oxidative stress, dysregulation of signaling pathways, pro-inflammatory cytokines, adipocytokines, fat infiltration, and autonomic modulation have been proposed as mechanisms that link obesity with AF 3,4,5,6.
The volume of the epicardial fat tissue is independently associated with AF recurrence after catheter ablation 7,8,9,10, suggesting that local interactions between the fat tissue and the heart may account for the mechanism of AF. In fact, the volume11 and the thickness12 of the periatrial fat tissue are larger in individuals with AF than those of heathy subjects. The quality of the fat tissue, measured by the mean computed tomography (CT) attenuation [ranges between −45 and −195 Hounsfield Unit (HU)]13, is associated with cardiovascular adverse outcomes. For instance, visceral fat attenuation predicts adverse cardiovascular events independently of the fat volume 14. In addition, higher fat attenuation in the perivascular cardiac tissue is a strong marker of tissue inflammation by histology and PET scan, and is associated with coronary plaque obstruction and plaque instability independently of the fat volume 15. Furthermore, subcutaneous fat attenuation is associated with incident metabolic risk factors beyond overall adiposity16.
The aim of the present work was to investigate the relationship between the quality of the periatrial fat tissue and AF. We hypothesized that the quality of the left atrial (LA) periatrial fat tissue is an independent predictor of recurrence after catheter ablation of AF. To test the hypothesis, we studied the association between the quality of the LA periatrial fat tissue in the pre-ablation cardiac CT and clinical outcomes in patients with either paroxysmal or persistent AF who were referred for the first catheter ablation of AF.
METHODS
Because of the sensitive nature of the data collected for this study, requests to access the dataset will not be allowed given the fact that this request is not included in the protocol that was approved by the Institutional Review Board of our institution.
Study population.
Consecutive patients with symptomatic, drug-refractory AF referred for catheter ablation of AF at the Johns Hopkins Hospital between January 2012 and January 2017 who underwent pre-procedural CT were included. Patients who had prior AF ablation or surgical procedure in the left atrium were excluded. A total of 143 patients were included in the final analysis. Table 1 summarizes the baseline characteristics. The protocol was approved by the Institutional Review Board and all the patients provided written informed consent.
Table 1.
Baseline characteristics
| Recurrence (n = 57) |
Control (n =86) |
p | |
|---|---|---|---|
| Clinical | |||
| Age, years | 62.2 ± 9.5 | 62.2 ± 11.1 | 0.997 |
| Body mass index, Kg/m2 | 33.3 ± 8.2 | 31.7 ± 6.5 | 0.195 |
| Male, n (%) | 36 (63.2) | 55 (64.0) | 0.923 |
| Persistent AF, n (%) | 25 (43.9) | 33 (38.4) | 0.513 |
| Heart failure, n (%) | 12 (21.1) | 8 (9.3) | 0.047 |
| Coronary artery disease/vascular disease, n (%) | 11 (19.3) | 8 (9.3) | 0.085 |
| Diabetes, n (%) | 15 (26.3) | 14 (16.3) | 0.144 |
| Hypertension, n (%) | 43 (75.4) | 53 (61.6) | 0.085 |
| History of Stroke/TIA, n (%) | 8 (15.4) | 13 (14.3) | 0.858 |
| CHA2DS2-VASC | 2.5 ± 1.6 | 2.1 ± 1.4 | 0.143 |
| Obstructive sleep apnea, n (%) | 13 (22.8) | 21 (24.4) | 0.825 |
| Ablation strategy (Cryoablation), n (%) | 15 (26.3) | 22 (25.6) | 0.529 |
| LVEF, % | 58.9 ± 9.3 | 57.8 ± 10.9 | 0.534 |
| Medication | |||
| ACEI/ARBS, n (%) | 23 (40.4) | 32 (37.2) | 0.705 |
| Beta-Blockers, n (%) | 38 (66.7) | 48 (55.8) | 0.194 |
| Calcium-channel blockers, n (%) | 32 (56.1) | 33 (38.4) | 0.037 |
| Anticoagulant use, total (%) | 53 (93.0) | 80 (93.0) | 0.993 |
| Number of antiarrhythmic drugs | 1.3 ± 0.8 | 1.1 ± 0.7 | 0.153 |
Data are presented as mean ± standard deviation, n (%), or median. ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blockers.
Please add the abbreviations of AF, TIA, LVEF, and CHA2DS2-VASC
CT protocol.
Images were acquired using a 320-detector CT scanner (Aquilion ONE, Toshiba Medical Systems, Otawara, Japan) up to one week before the procedure. Slice thickness was 0.5 mm, and tube voltage was 80, 100, or 120 kV, depending on the body habitus. Tube current ranged from 320 to 580 mA, depending on the body habitus and heart rate. Image acquisition was ECG-gated at 40% R-R interval during a breath-hold. The contrast protocol included a total volume of 60 mL (70 mL if BMI >30 kg/m2) of the nonionic low-osmolar iodinated contrast material iopamidol (Isovue 370, Bracco Diagnostics, Princeton, NJ) administered at a rate of 5 mL/s. First pass images were used for segmentation and analysis.
Image analysis.
The CT images were reconstructed with an in-plane resolution of 0.5 mm x 0.5 mm and a through-plane resolution of 3.0 mm with a 1.5-mm gap between slices. The images were transferred to a workstation (Vitrea Advanced Visualization, version 7.7.0, Vital Images) for offline fat analysis. The operator responsible for the imaging analysis was blinded to AF outcomes. The LA periatrial fat measurements were derived from manually segmenting the left atrium on a single two-dimensional (2-D) slice in the standard four- (4-CH) and two-chamber (2-CH) views (Figure 1). Contiguous voxels between the HU limits of (−200, −50) were defined as fat voxels, and the mean attenuation of each fat depot was calculated14,15,16. In addition, the total area of fat (mm2) in each region of interest was also calculated. To study the correlation between two- (2D) and three-dimensional (3D) fat measurements, the LA periatrial fat was also manually segmented in 3D from the pulmonary artery bifurcation to the diaphragm (Vitrea Advanced Visualization, version 7.7.0, Vital Images) (Figure S1, Supplemental Material).
Figure 1. Quantification of LA periatrial fat area and attenuation using CT.
A, 4-CH view with red contours surrounding the LA. B, 2-CH view with red contours surrounding the LA. CT, computed tomography; CH, chamber. Yellow circle, the left atrial – periesophageal fat pad region captured by the 4-CH view.
Mapping and ablation.
Mapping and ablation of AF were performed using an electroanatomical mapping (EAM) system with an image integration module to merge pre-procedural CT(CARTO and CartoMerge®, Biosense Webster, Diamond Bar, CA). A detailed description of the procedure have been described elsewhere17. Briefly, all patients underwent the standard pulmonary vein isolation (PVI), where electrical isolation of the pulmonary veins were confirmed by a circular multipolar electrode mapping catheter (Lasso, Biosense Webster). In cases of persistent AF, the ablation procedure usually included additional ablation lesions, as described in the Results section. Ablation was performed with either an open-irrigated radiofrequency (RF) ablation catheter with or without force sensing (NaviStar, ThermoCool or ThermoCool SmartTouch, Biosense Webster Inc., Diamond Bar, CA), or a cryoballoon ablation catheter (Arctic Front Advance, Medtronic Inc., Minneapolis, MN). A subgroup of patients underwent bipolar voltage mapping during sinus rhythm prior to PVI using the EAM system and the ablation catheter with a 3.5-mm distal tip and 2-mm inter-electrode spacing. Endocardial contact during point acquisition was validated by recording a stable signal for >2 beats.
Follow-up.
AF recurrence was defined as any documented episodes of AF, atrial flutter, or atrial tachycardia lasting more than 30 seconds after a three-month blanking period18. All medications, including antiarrhythmic and anticoagulation drugs, were continued in all patients during the blanking period. Patients were seen in clinic 10 to 12 weeks following ablation. At the clinic visit, antiarrhythmic and anticoagulation drugs were discontinued at the discretion of the physician according to the patient’s stroke risk. Then the patients were followed by the referring physician on a regular basis. In addition, the patients received direct phone interviews or emails with a standardized questionnaire to report AF symptoms, if any, in detail. Patients were excluded if they were not followed up for at least one year after ablation. When recurrence was suspected, the patients underwent a 24-hour Holter monitor or a 30-day cardiac event monitor depending on the symptom frequency.
Statistical analysis.
Baseline patient demographics are presented as mean+SD or percentage, and are compared using Student’s t-test, X2, and Fisher’s, as appropriate. Multivariable Cox proportional hazards models were used to evaluate the effects of LA total fat area and attenuation on AF recurrence after ablation. Three models are presented: Model 1: unadjusted; Model 2: Adjusted for clinical characteristics (age, sex [female and male], type of AF [paroxysmal and persistent], body mass index [BMI], history of heart failure [as categorical variable], antiarrhythmic drug [AAD] use, hypertension [as categorical variable], and obstructive sleep apnea [OSA, as categorical variable]); Model 3: Model 2+ LA dimension and LA area of fat. Correlation between LA attenuation, fat and voltage map were defined by Pearson’s correlation coefficient. Final models goodness of fit was evaluated by by using the Cox-Snell residuals on the e Nelson-Aalen cumulative hazard function. The model fit was established by was stablished by visually plotting the function and analyzing if the hazard function follows the 45 degree line. Our final models mostly followed the 45 degree angle except for the very large values of time which is mostly due to the censoring effect.Time-to-first recurrence is presented using Kaplan-Meier curves. A log-rank test was conducted to detect differences in the survival distributions for two curves. The two curves were generated based on Receiver Operating Characteristic (ROC) best cut point using Liu’s criteria. In a subset of randomly selected patients (n =15), intra- and inter-observer reproducibility was performed, and the intra-class correlation coefficient (ICC) with a two-way random model (ICC, <0.40, poor; ICC >0.40–0.75, fair to good; and ICC >0.75, excellent agreement) was evaluated. The 0.05 significance level was used for all hypothesis tests, and all t- tests were 2-sided. The statistical computations were performed using STATA (version 12.0, StataCorp).
RESULTS
Patient demographics.
A total of 357 patients were selected from our database, who underwent to first catheter ablation to treat AF, of those 208 underwent to MRI to prior the procedure, one patient (#04) had poor contrast opacification of the left atrium, and one patient’s (#02) images were unavailable; those individuals were excluded from our study.
There were 91 (63.6%) male patients, and the average age was 62.2+10.4 years. Acute PVI was achieved in all subjects. Seventeen patients (11.9%) had additional ablation lesions beyond PVI. Thirteen patients (9.1%) had a linear ablation lesion connecting the right and the left superior pulmonary veins (the roof line), and one (0.7%) had a linear ablation lesion connecting the coronary sinus and the left inferior pulmonary vein (the mitral isthmus line). One patient (0.8%) underwent both the roof line and the mitral isthmus line. No ablation was performed in the right atrium in any of the patients.
A total of 57 patients developed AF recurrence after ablation (recurrence group), and the remaining 86 patients were free of AF recurrence (control group). Among the subjects who had recurrence, 15 underwent a second procedure and 37 started using AAD, 7 subjects without recurrence started using AAD. Compared with the control group, more patients in the recurrence group were on calcium-channel blockers prior to ablation (p=0.037). The patients in the recurrence group were also more likely to have a history of hypertension, heart failure, OSA, and persistent AF than those of the control group. A total of 48 patients (33.5%) in the final analysis had bipolar voltage mapping available during sinus rhythm. Except for the age and history of paroxysmal AF, which were both higher in the voltage mapping subgroup, all other clinical characteristics were similar to the entire cohort.
LA fat attenuation and recurrence.
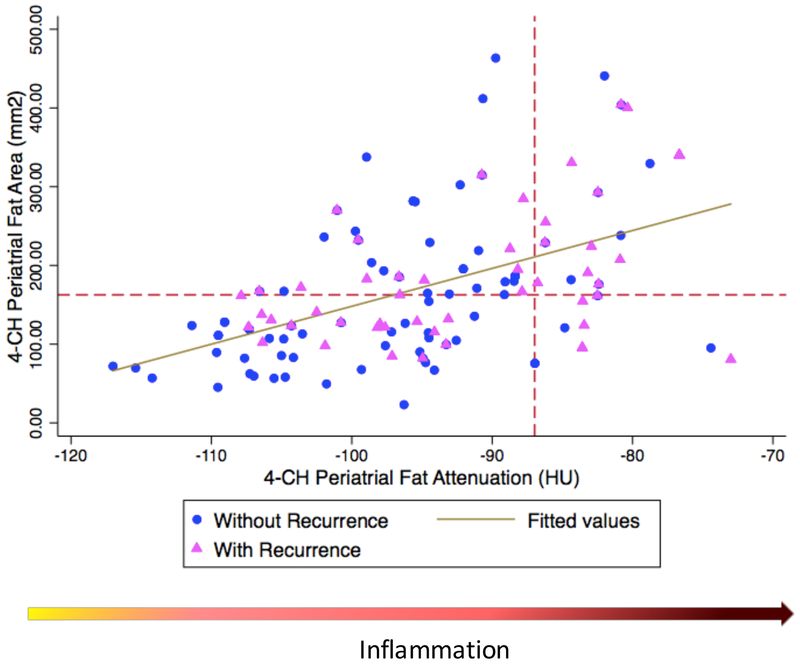
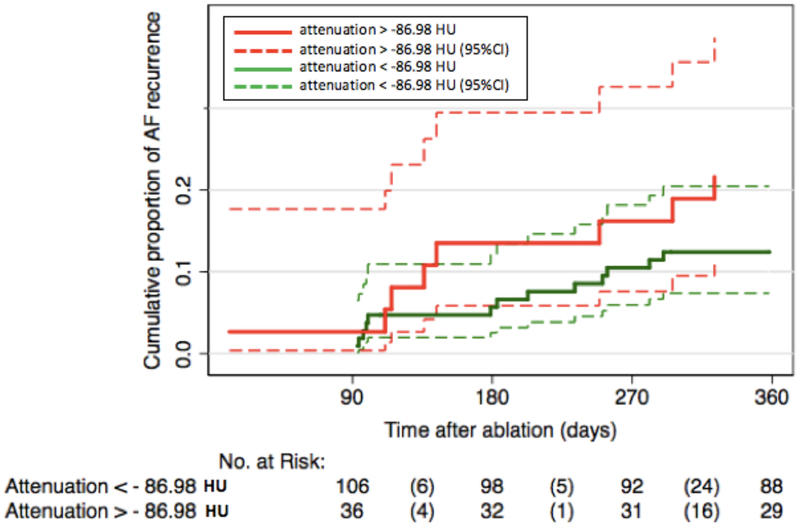
Our analysis showed an excellent intra-reader and inter-reader reproducibility. The intra-reader reproducibility ranged from 0.92 (area, IC: 0.89 – 0.94, p=0.003) to 0.96 (attenuation, IC: 0.95 – 0.97, p <0.001) and the inter-reader reproducibility ranged from 0.87 (area, IC: 0.84 – 0.90, p =0.005) to 0.91 (attenuation, IC: 0.87 – 0.93, p =0.005). Patients in the recurrence group had significantly larger LA dimension (5.8 vs. 5.2 cm, p =0.038), larger 4-CH LA periatrial fat area (167.6 vs. 145.4 mm2, p =0.018) and lower attenuation (−96.5 vs. −92.0 HU, p =0.006) compared with control (Table 2). There was no significant difference in the 2-CH LA periatrial fat area or attenuation between the recurrence and the control groups (p=0.328). The 4-CH LA periatrial fat area ([mm2], y-axis) and the 4-CH LA periatrial fat attenuation ([HU], x-axis) were significantly correlated with the correlation coefficient of 0.51 (p <0.001, Figure 2). The 4-CH LA periatrial fat attenuation was associated with AF recurrence (Figure 3). The correlation coefficient was 0.40 (p=0.001) between the 4-CH LA periatrial fat area and the 3D LA periatrial fat volume, and 0.38 (p = 0.004) between the 4-CH LA periatrial fat attenuation and the 3D LA periatrial fat attenuation (Figure S2-3, Supplemental Material). The correlation coefficient was 0.28 (p =0.02) between the 4-CH LA periatrial fat area and the mean LA bipolar voltage, and 0.22 (p=0.03) between the 4-CH LA periatrial fat attenuation and the mean LA bipolar voltage.
Table 2.
LA parameters by groups
| Recurrence, n = 57 | Control, n = 86 | ||||
|---|---|---|---|---|---|
| Mean | CI | Mean | CI | p | |
| 4CH – LA periatrial fat attenuation, HU | −92.0 ± 9.8 | −94.6 – −89.3 | −96.5 ± 9.4 | −98.5 – −94.5 | 0.006 |
| 2CH – LA periatrial fat attenuation, HU | −79.7 ± 10.3 | −82.5 – −76.9 | −81.5 ± 11.3 | −84.0 – −79.1 | 0.328 |
| Median | IQR | Median | IQR | P | |
| 4CH – LA periatrial fat area, mm2 | 167.6 | 124.1 – 255 | 145.4 | 95.6 – 229.3 | 0.018 |
| 4CH – LA periatrial fat area index, mm2/mm2 | 83.1 | 57.7 – 109.0 | 65.0 | 45.8 – 102.7 | 0.042 |
| 2CH – LA periatrial fat area, mm2 | 137.6 | 70.2 – 230.6 | 129.1 | 81.7 – 251.4 | 0.979 |
| 2CH – LA periatrial fat are index, mm2/mm2 | 62.9 | 30.8 – 104.3 | 60.3 | 39.1 – 120.2 | 0.687 |
| LA dimension (axial), cm | 5.8 | 5.5 – 6.5 | 5.2 | 4.5 – 5.8 | 0.038 |
Data are presented as median (IQR - interquartile range), or mean ± standard deviation (SD).
CI – Confidence interval, CH, chamber; LA, left atrium.
Figure 2. Correlation between 4-CH LA periatrial fat area and 4-CH LA periatrial fat attenuation.
Red dotted lines, the best discriminatory point by Liu criteria for left atrial (LA) fat area and attenuation; Blue circles, patients in the AF Free group; Pink triangle, patients in the recurrence group. Blue line, linear regression line.
Figure 3. Symptomatic Recurrence Based on Fat Attenuation.
Solid red line, attenuation > −86.98 HU; Broken red line, 95% confidence interval (CI) of attenuation > −86.98 HU; Solid green line, attenuation < −86.98 HU; Broken green line, 95%CI of attenuation < −86.98 HU.
Multivariable analyses.
In Model 1, univariable (unadjusted) analysis identified BMI, AF type, LA periatrial fat area and attenuation as contributors of AF recurrence (Table 3). Patients with 4-CH LA periatrial fat attenuation > −86.98 HU had an increased hazard for AF recurrence compared to those with <−86.98 HU (HR 2.04, p = 0.009). After adjusting for age, sex, BMI, AF type, ADD use, history of heart failure, OSA, hypertension, LA dimension, and 4-CH LA periatrial fat area, the 4-CH LA periatrial fat attenuation remained significantly associated with recurrence (HR 2.65, p=0.008; Model 3 in Table 3). We also performed a sensitivity analysis excluding the subjects who underwent additional procedures n=17 and the values of HR for the unadjusted (HR: 1.03, p: 0.023) and adjusted (HR: 1.05, p: 0.015) models were still significant for the 4-CH view.
Table 3.
Multivariable analyses
| Model 1 Unadjusted |
Model 2 Clinical variables |
Model 3 Model 2 + LA dimension + LA fat volume |
||||
|---|---|---|---|---|---|---|
| Total cohort, n = 143 | HR | p | HR | p | HR | p |
| Clinical variables | ||||||
| Age | 0.99 | 0.781 | ||||
| Sex (Male) | 1.11 | 0.692 | ||||
| Body mass index, Kg/m2 | 1.02 | 0.027 | ||||
| AF type (Persistent) | 1.37 | 0.024 | ||||
| Hypertension | 1.01 | 0.835 | ||||
| History of heart failure | 1.00 | 0.986 | ||||
| History of diabetes | 1.35 | 0.309 | ||||
| Obstructive sleep apnea | 0.80 | 0.490 | ||||
| CT variables | ||||||
| 4CH – LA Fat area, mm2 | 1.01 | 0.035 | 1.00 | 0.640 | ||
| 2CH – LA Fat area, mm2 | 1.00 | 0.430 | ||||
| 4CH – LA Fat attenuation, HU | 1.05 | 0.002 | 1.03 | 0.030 | 1.04 | 0.031 |
| Attenuation > - 86.98 HU vs. <- 86.98 | 2.04 | 0.009 | 2.02 | 0.019 | 2.65 | 0.008 |
| 2CH – LA Fat attenuation, HU | 1.01 | 0.313 | ||||
CT, computed tomography; HR, Hazard ratio.. Abbreviations as in Table 2.
Model 2, adjusted for age, sex, type of atrial fibrillation (AF), body mass index, history of heart failure, hypertension, obstructive sleep apnea.
Model 3, co-variables included in model 2 in addition to minimum LA dimension and fat area.
DISCUSSION
Main findings.
To our knowledge, this is the first study to investigate the association between the quality of the LA periatrial fat tissue and clinical outcomes after AF ablation. We found that the LA periatrial fat attenuation was an independent predictor of AF recurrence. In addition, we demonstrate that the simple measurement of LA periatrial fat in a single 4-CH view alone is sufficient to predict the clinical outcome.
LA Fat inflammation and ablation outcomes.
Several studies have demonstrated the heterogeneity of fat characteristics and distribution through the body4,5,13,15,19. Indeed, periatrial epicardial fat may be an atypical adipose tissue with distinct characteristics compared with periventricular or pericoronary epicardial fat19. In experimental models, only the periatrial fat was able to express genes implicated in oxidative phosphorylation, muscular contraction, and calcium signaling19. The fat attenuation by CT reflects the tissue quality and has been demonstrated to be correlated with the degree of inflammation (by histology and PET scan) and adipocyte differentiation in pericoronary tissue15. An emerging concept of the bidirectional interaction between the endothelial and fat tissue highlights the effect of the endothelium in promoting fat inflammation by releasing paracrine factors such as IL-6, TNF-α, and IFN-γ, which alters the adipocyte differentiation and induces lymphocyte migration. In Individuals with AF, periatrial epicardial fat showed a significant positive association with increased levels of intercellular adhesion molecule 1 (sICAM-1) and von Willebrand Factor (vWF), which are biomarkers of endothelial dysfunction. Interestingly, BMI and total pericardial fat were not associated with inflammatory factors in the same population, suggesting that the local fat instead of the total fat content modulates the endothelial dysfunction in patients with AF. 12 In fact, atrial biopsies from patients with AF have shown the infiltration of inflammatory cells20. Furthermore, individuals with AF presented higher levels of inflammatory cytokines in the LA than in the periphery21. It is possible that level of attenuation by CT represents a marker of tissue activity and unfavorable outcomes due to elevated inflammation, edema which precludes proper ablation lesions formation by modifying the tissue intrinsic characteristics including impedance and conductivity.
Obesity, periatrial fat attenuation, and atrial fibrillation recurrence.
In our recent work, we found that BMI is an independent predictor of AF recurrence following ablation22 In contrast, our results indicate that BMI was not significantly associated with AF outcomes after adjusting for other clinical risk factors (Table 3). This apparent contradiction is explained by an equal distribution of obesity in both the recurrence (BMI=33.3+ 8.2) and the control groups (BMI=31.7+ 6.5), and there was no significant difference between those two groups (p=0.195, Table 1). The present paper complements the paper by Sivasambu et al. in three aspects. First, our results confirm that LA periatrial fat area is not necessarily associated with BMI. This finding is consistent with our previous report that BMI was not associated with the volume of the periatrial fat tissue23. Those findings can be explained by the focused distribution of the periatrial fat tissue out of proportion to the total fat in the body 12,20,21,24. The clinical significance of the local distribution, rather than the total amount, of the fat tissue in predicting adverse events, has been recognized as the ‘obese mortality paradox’ in the ORBIT-AF25 and ARISTOTLE26, where BMI represented a protector factor for the mortality in individuals with AF. Second, our results show that the quality, or attenuation, of the periatrial fat tissue is more important than the quantity. Previous studies showed that the volume of the periatrial fat tissue is important in predicting AF recurrence after catheter ablation10. In our study, although the LA periatrial fat area was higher in the recurrence group, multivariable analysis showed that the LA periatrial fat area was not an independent predictor of recurrence. This finding suggests that the quality of the fat tissue may accurately reflect the level of stress to which the chamber is exposed, therefore representing a better surrogate of LA remodeling. Third, our results indicate that LA periatrial fat quantification identifies a high-risk subgroup who is resistant to AF ablation even among the obese individuals.
4-CH vs. 2-CH views and ablation outcomes.
A previous study showed that the periatrial fat pad between the esophagus and the mid-LA (LA-ESO) was significantly associated with AF and AF burden (paroxysmal vs. persistent AF)24. However, the same study did not show a significant association of AF or AF burden with periatrial fat between mid-LA and pulmonary artery (LA-PA) or between mid-LA and thoracic aorta (LA-TA). This study highlights the critical importance of the periatrial fat pad in the LA-ESO region, which is captured by the 4-CH view (green arrow in Figure 1). This finding likely explains why the fat pad quantification in the 4-CH view is a better marker of recurrence than that of the 2-CH view. Another potential reason to account for the difference in our findings between the 2-CH and the 4-CH views is the partial volume effect. In our reconstructed image, the 2-CH view is more prone to the partial volume effect due to the poorer through-plane resolution compared with the in-plane resolution. In contrast, the 4-CH view has the minimal partial volume effect due to the through-plane resolution, because it is essentially a slightly off-axis transverse image. This partial volume effect may also explain, at least partially, why the LA fat attenuation in the 2-CH view was higher than that of the 4-CH view.
Clinical implications.
Catheter ablation of AF remains far from curative, with recurrence rates up to 40%27 Measurement of the quantity and the quality of the LA periarial fat tissue by cardiac CT is a relatively simple method to quantify the degree of ‘inflamed’ fat and to identify candidates who are responsive to catheter ablation. Our results clearly indicate that the periatrial fat attenuation value over −86.98 HU is a significant predictor of AF recurrence independent of clinical risk factors and LA structure. This simple parameter could improve patient selection and thus reduce recurrence by saving candidates with higher degrees of LA remodeling from futile procedures and potential procedural complications. LA periatrial fat attenuation measurements on a single 2-D slice in the standard 4-CH view is simple, and thus incorporating it into routine clinical practice is relatively straightforward, unlike LA fibrosis quantification by MRI which requires extensive post-processing28.
Limitations.
This study represents a single-center analysis of patients referred for catheter ablation of AF; therefore, there is a non-negligible chance of selection bias. Our definition of recurrence was strongly influenced by symptoms. Therefore, it is possible that we could have missed recurrence that is completely asymptomatic. For fat analysis, we used only two- and four-chamber views. Therefore, it is possible that our analysis underestimated the degree of LA fat by missing regions that were not covered by those two views. The current study evaluated the outcomes up to 12 months after ablation. Further studies will be necessary to evaluate the impact of periatrial fat on late recurrence beyond 12 months. Despite these potential causes of underestimation, our analysis demonstrated a significant association between LA quality and AF recurrence. Moreover, 2D LA fat was significantly correlated with 3D fat. Therefore, we believe that the advantage of our approach outweighs the disadvantage of including more views to assess the whole LA fat, which would increase the scan time and post-processing burden. Furthermore, our study lacks external validation outside the population that was included in our analysis. Last, this is an observational study and, despite rigorous statistical adjustments, unmeasured confounding cannot be excluded.
Conclusions.
The quality of the LA periatrial fat tissue, measured by CT attenuation, is an independent predictor of recurrence after the first catheter ablation of paroxysmal or persistent AF. The quality of the LA periatrial fat tissue is a relatively simple metric to quantify the degree of underlying LA remodeling to identify candidates who are responsive to catheter ablation. Therefore, assessment of LA periatrial fat attenuation can improve ablation outcomes by refining patient selection.
Supplementary Material
Acknowledgments
SOURCES OF FUNDING
This work was supported by research grants from NIH R56 HL138429 (to HA), NIH T32 5T32HL007227–42 (to KNA), the Edward St. John Foundation for AF Research (to HC), The Roz and Marvin H Weiner and Family Foundation (to HC), The Dr. Francis P. Chiaramonte Foundation (to HC), The Marilyn and Christian Poindexter Arrhythmia Research Fund (to HC), and The Norbert and Louise Grunwald Cardiac Arrhythmia Research Fund (to HC).
Footnotes
DISCLOSURE
No authors have any potential conflict of interest to disclose.
References
- 1.Healey JS, Connolly SJ, Gold MR, Israel CW, Van Gelder IC, Capucci A, Lau CP, Fain E, Yang S, Bailleul C, Morillo CA, Carlson M, Themeles E, Kaufman ES, Hohnloser SH. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med. 2012;366:120–129. [DOI] [PubMed] [Google Scholar]
- 2.Huxley RR, Lopez FL, Folsom AR, Agarwal SK, Loehr LR, Soliman EZ, MacLehose R, Konety S, Alonso A. Absolute and attributable risks of atrial fibrillation in relation to optimal and borderline risk factors: The atherosclerosis risk in communities (ARIC) study. Circulation. 2011;123:1501–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lau DH, Schotten U, Mahajan R, Antic NA, Hatem SN, Pathak RK, Hendriks JML, Kalman JM, Sanders P. Novel mechanisms in the pathogenesis of atrial fibrillation: Practical applications. Eur Heart J. 2016;37:1573–1581. [DOI] [PubMed] [Google Scholar]
- 4.Mahajan R, Lau DH, Sanders P. Impact of obesity on cardiac metabolism, fibrosis, and function. Trends Cardiovasc Med. 2015;25:119–126. [DOI] [PubMed] [Google Scholar]
- 5.Mahajan R, Lau DH, Brooks AG, Shipp NJ, Manavis J, Wood JPM, Finnie JW, Samuel CS, Royce SG, Twomey DJ, Thanigaimani S, Kalman JM, Sanders P. Electrophysiological, Electroanatomical, and Structural Remodeling of the Atria as Consequences of Sustained Obesity. J Am Coll Cardiol. 2015;66:1–11. [DOI] [PubMed] [Google Scholar]
- 6.Wong CX, Ganesan AN, Selvanayagam JB. Epicardial fat and atrial fibrillation: Current evidence, potential mechanisms, clinical implications, and future directions. Eur Heart J. 2017;38:1294–1302. [DOI] [PubMed] [Google Scholar]
- 7.Wong CX, Abed HS, Molaee P, Nelson AJ, Brooks AG, Sharma G, Leong DP, Lau DH, Middeldorp ME, Roberts-Thomson KC, Wittert GA, Abhayaratna WP, Worthley SG, Sanders P. Pericardial fat is associated with atrial fibrillation severity and ablation outcome. J Am Coll Cardiol. 2011;57:1745–1751. [DOI] [PubMed] [Google Scholar]
- 8.Tsao H-M, Hu W-C, Wu M-H, Tai C-T, Lin Y-J, Chang S-L, Lo L-W, Hu Y-F, Tuan T-C, Wu T-J, Sheu M-H, Chang C-Y, Chen S-A. Quantitative analysis of quantity and distribution of epicardial adipose tissue surrounding the left atrium in patients with atrial fibrillation and effect of recurrence after ablation. Am J Cardiol. 2011;107:1498–1503. [DOI] [PubMed] [Google Scholar]
- 9.Nagashima K, Okumura Y, Watanabe I, Nakai T, Ohkubo K, Kofune T, Kofune M, Mano H, Sonoda K, Hirayama A. Association between epicardial adipose tissue volumes on 3-dimensional reconstructed CT images and recurrence of atrial fibrillation after catheter ablation. Circ J. 2011;75:2559–2565. [DOI] [PubMed] [Google Scholar]
- 10.Kocyigit D, Gurses KM, Yalcin MU, Turk G, Evranos B, Yorgun H, Sahiner ML, Kaya EB, Hazirolan T, Tokgozoglu L, Oto MA, Ozer N, Aytemir K. Periatrial epicardial adipose tissue thickness is an independent predictor of atrial fibrillation recurrence after cryoballoon-based pulmonary vein isolation. J Cardiovasc Comput Tomogr. 2015;9:295–302. [DOI] [PubMed] [Google Scholar]
- 11.Nakamori S, Nezafat M, Ngo LH, Manning WJ, Nezafat R. Left Atrial Epicardial Fat Volume Is Associated With Atrial Fibrillation: A Prospective Cardiovascular Magnetic Resonance 3D Dixon Study. J Am Heart Assoc. 2018;7:7–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Girerd N, Scridon A, Bessière F, Chauveau S, Geloen A, Boussel L, Morel E, Chevalier P. Periatrial Epicardial Fat Is Associated with Markers of Endothelial Dysfunction in Patients with Atrial Fibrillation. PLoS One. 2013;8:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maurovich-Horvat P, Massaro J, Fox CS, Moselewski F, O’Donnell CJ, Hoffmann U. Comparison of anthropometric, area- and volume-based assessment of abdominal subcutaneous and visceral adipose tissue volumes using multi-detector computed tomography. Int J Obes (Lond). 2007;31:500–506. [DOI] [PubMed] [Google Scholar]
- 14.Rosenquist KJ, Pedley A, Massaro JM, Therkelsen KE, Murabito JM, Hoffmann U, Fox CS. Visceral and Subcutaneous Fat Quality is Associated with Cardiometabolic Risk. Int J Cardiovasc Imaging. 2013;6:762–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Antonopoulos AS, Sanna F, Sabharwal N, Thomas S, Oikonomou EK, Herdman L, Margaritis M, Shirodaria C, Kampoli A-M, Akoumianakis I, Petrou M, Sayeed R, Krasopoulos G, Psarros C, Ciccone P, Brophy CM, Digby J, Kelion A, Uberoi R, Anthony S, Alexopoulos N, Tousoulis D, Achenbach S, Neubauer S, Channon KM, Antoniades C. Detecting human coronary inflammation by imaging perivascular fat. Sci Transl Med. 2017;9: 114–120. [DOI] [PubMed] [Google Scholar]
- 16.Abraham TM, Pedley A, Massaro JM, Hoffmann U, Fox CS. Association Between Visceral and Subcutaneous Adipose Depots and Incident Cardiovascular Disease Risk Factors. 2016;132:1639–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang E, Ipek EG, Balouch M, Mints Y, Chrispin J, Marine JE, Berger RD, Ashikaga H, Rickard J, Calkins H, Nazarian S, Spragg DD. Factors impacting complication rates for catheter ablation of atrial fibrillation from 2003 to 2015. Eur Eur pacing, arrhythmias, Card Electrophysiol J Work groups Card pacing, arrhythmias, Card Cell Electrophysiol Eur Soc Cardiol. 2017;19:241–249. [DOI] [PubMed] [Google Scholar]
- 18.Calkins H, Hindricks G, Cappato R, Kim Y-H, Saad EB, Aguinaga L, Akar JG, Badhwar V, Brugada J, Camm J, Chen P-S, Chen S-A, Chung MK, Nielsen JC, Curtis AB, Davies DW, Day JD, d’Avila A, de Groot NMSN, Di Biase L, Duytschaever M, Edgerton JR, Ellenbogen KA, Ellinor PT, Ernst S, Fenelon G, Gerstenfeld EP, Haines DE, Haissaguerre M, Helm RH, Hylek E, Jackman WM, Jalife J, Kalman JM, Kautzner J, Kottkamp H, Kuck KH, Kumagai K, Lee R, Lewalter T, Lindsay BD, Macle L, Mansour M, Marchlinski FE, Michaud GF, Nakagawa H, Natale A, Nattel S, Okumura K, Packer D, Pokushalov E, Reynolds MR, Sanders P, Scanavacca M, Schilling R, Tondo C, Tsao H-M, Verma A, Wilber DJ, Yamane T. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Hear Rhythm. 2017;14:e275–e444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaborit B, Venteclef N, Ancel P, Pelloux V, Gariboldi V, Leprince P, Amour J, Hatem SN, Jouve E, Dutour A, Clement K. Human epicardial adipose tissue has a specific transcriptomic signature depending on its anatomical peri-atrial, peri-ventricular, or peri-coronary location. Cardiovasc Res. 2015;108:62–73. [DOI] [PubMed] [Google Scholar]
- 20.Frustaci A, Chimenti C, Bellocci F, Morgante E, Russo MA, Maseri A. Histological substrate of atrial biopsies in patients with lone atrial fibrillation. Circulation. 1997;96:1180–1184. [DOI] [PubMed] [Google Scholar]
- 21.Marcus GM, Smith LM, Ordovas K, Scheinman MM, Kim AM, Badhwar N, Lee RJ, Tseng ZH, Lee BK, Olgin JE. Intracardiac and extracardiac markers of inflammation during atrial fibrillation. Hear Rhythm. 2010;7:149–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sivasambu B, Balouch MA, Zghaib T, Bajwa RJ, Chrispin J, Berger RD, Ashikaga H, Nazarian S, Marine JE, Calkins H, Spragg DD. Increased rates of atrial fibrillation recurrence following pulmonary vein isolation in overweight and obese patients. J Cardiovasc Electrophysiol. 2018;29:239–245. [DOI] [PubMed] [Google Scholar]
- 23.Zghaib T, Ipek EG, Zahid S, Balouch MA, Misra S, Ashikaga H, Berger RD, Marine JE, Spragg DD, Zimmerman SL, Zipunnikov V, Trayanova N, Calkins H, Nazarian S. Association of left atrial epicardial adipose tissue with electrogram bipolar voltage and fractionation: Electrophysiologic substrates for atrial fibrillation. Hear Rhythm. 2016;13:2333–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Batal O, Schoenhagen P, Shao M, Ayyad AE. NIH Public Access. 2011;3:230–236. [Google Scholar]
- 25.Pandey A, Gersh BJ, McGuire DK, Shrader P, Thomas L, Kowey PR, Mahaffey KW, Hylek E, Sun S, Burton P, Piccini J, Peterson E, Fonarow GC. Association of Body Mass Index With Care and Outcomes in Patients With Atrial Fibrillation: Results From the ORBIT-AF Registry. JACC Clin Electrophysiol. 2016;2:355–363. [DOI] [PubMed] [Google Scholar]
- 26.Sandhu RK, Ezekowitz J, Andersson U, Alexander JH, Granger CB, Halvorsen S, Hanna M, Hijazi Z, Jansky P, Lopes RD, Wallentin L. The “obesity paradox” in atrial fibrillation: observations from the ARISTOTLE (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation) trial. Eur Heart J. 2016;37:2869–2878. [DOI] [PubMed] [Google Scholar]
- 27.Verma A, Jiang C, Betts TR, Chen J, Deisenhofer I, Mantovan R, Macle L, Morillo CA, Haverkamp W, Weerasooriya R, Albenque J-P, Nardi S, Menardi E, Novak P, Sanders P. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med. 2015;372:1812–1822. [DOI] [PubMed] [Google Scholar]
- 28.Khurram IM, Beinart R, Zipunnikov V, Dewire J, Yarmohammadi H, Sasaki T, Spragg DD, Marine JE, Berger RD, Halperin HR, Calkins H, Zimmerman SL, Nazarian S. Magnetic resonance image intensity ratio, a normalized measure to enable interpatient comparability of left atrial fibrosis. Hear Rhythm. 2014;11:85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.