Abstract
Background
Growing political attention to antimicrobial resistance (AMR) offers a rare opportunity for achieving meaningful action. Many governments have developed national AMR action plans, but most have not yet implemented policy interventions to reduce antimicrobial overuse. A systematic evidence map can support governments in making evidence-informed decisions about implementing programs to reduce AMR, by identifying, describing, and assessing the full range of evaluated government policy options to reduce antimicrobial use in humans.
Methods and findings
Seven databases were searched from inception to January 28, 2019, (MEDLINE, CINAHL, EMBASE, PAIS Index, Cochrane Central Register of Controlled Trials, Web of Science, and PubMed). We identified studies that (1) clearly described a government policy intervention aimed at reducing human antimicrobial use, and (2) applied a quantitative design to measure the impact. We found 69 unique evaluations of government policy interventions carried out across 4 of the 6 WHO regions. These evaluations included randomized controlled trials (n = 4), non-randomized controlled trials (n = 3), controlled before-and-after designs (n = 7), interrupted time series designs (n = 25), uncontrolled before-and-after designs (n = 18), descriptive designs (n = 10), and cohort designs (n = 2). From these we identified 17 unique policy options for governments to reduce the human use of antimicrobials. Many studies evaluated public awareness campaigns (n = 17) and antimicrobial guidelines (n = 13); however, others offered different policy options such as professional regulation, restricted reimbursement, pay for performance, and prescription requirements. Identifying these policies can inform the development of future policies and evaluations in different contexts and health systems. Limitations of our study include the possible omission of unpublished initiatives, and that policies not evaluated with respect to antimicrobial use have not been captured in this review.
Conclusions
To our knowledge this is the first study to provide policy makers with synthesized evidence on specific government policy interventions addressing AMR. In the future, governments should ensure that AMR policy interventions are evaluated using rigorous study designs and that study results are published.
Protocol registration
PROSPERO CRD42017067514.
In a systematic review, Susan Rogers Van Katwyk and colleagues assess policy interventions intended to reduce antimicrobial overuse.
Author summary
Why was this study done?
Despite global commitments to reduce antimicrobial resistance and protect the effectiveness of antimicrobials, most countries have not yet started implementing government policies to reduce their overuse and misuse of antimicrobials.
To the best of our knowledge, no evidence syntheses have attempted to identify the policy options available to government policy makers to tackle antimicrobial resistance by reducing antimicrobial use in humans.
What did the researchers do and find?
We searched 7 academic databases to identify impact evaluations of government policy interventions aiming to reduce human antimicrobial use that were published in any language before January 28, 2019.
We found 69 studies that evaluated government policy interventions to reduce antimicrobial use around the world. From these, we were able to describe 17 different types of policies that governments have used to tackle this major driver of antimicrobial resistance in humans.
Commonly used policy strategies included public awareness campaigns and antimicrobial guidelines; however, other policy strategies focused on vaccination, stewardship, and changing regulations around prescribing and reimbursement.
We found 4 randomized controlled trials and 35 studies using rigorous quasi-experimental designs. The remaining 30 studies used uncontrolled and descriptive study designs.
What do these findings mean?
Our systematic evidence map suggests that governments have a variety of policy options at their disposal to respond to the growing threat of antimicrobial resistance.
Unfortunately, most existing policy options have not been rigorously evaluated, which limits their usefulness in planning future policy interventions.
To avoid wasting public resources, governments should ensure that future antimicrobial resistance policy interventions are evaluated using rigorous study designs, and that study results are published.
Introduction
Antimicrobial resistance (AMR) is currently high on the global political agenda. This attention has opened a rare policy window for achieving meaningful action on AMR [1–7]. Although the potential for AMR has been recognized since the earliest days of antibiotics [8], the misuse and overuse of antimicrobials has persisted over decades, contributing to the development of resistance [9]. AMR is now expected to have severe consequences for human health, social well-being, and economic development. AMR has already rendered some infections untreatable using existing antimicrobials [10,11], and global projections suggest that AMR could derail the Sustainable Development Goals, driving an estimated 24 million people into extreme poverty and exacerbating global economic inequality [12], and potentially resulting in tens of millions of deaths [13].
Successfully overcoming the threat posed by AMR will require multi-sectoral and multi-jurisdictional cooperation to protect the effectiveness of existing and future antimicrobials [2,3,5]. Recent political initiatives addressing AMR, including the 2016 United Nations resolution [14] and the 2017 Berlin Declaration of the G20 Health Ministers [15], are promising signs that governments and international agencies are mobilizing to act on AMR. The 194 member states of the World Health Organization (WHO) agreed to develop national AMR action plans by 2017 [16], and countries have largely responded [17].
Despite these positive steps, most countries have not yet started implementing policies to reduce their overuse and misuse of antimicrobials [17]. Evidence from high-income countries suggests that reducing antimicrobial use is associated with lower rates of resistance [9], yet there is limited evidence on what types of government policy interventions effectively reduce antimicrobial use. Typically, government policy changes are useful tools for improving public health when the health threat requires widespread change and uniform compliance with a set of minimum standards [18]. However, research on AMR has principally focused on changing the prescribing behaviours of individual physicians [19], rather than creating large-scale reductions in antimicrobial use through population-wide interventions.
Given that governments are currently grappling with the challenge of implementing AMR policies under their recently developed national action plans, a focus on the potential impact of government policy interventions on antimicrobial use is timely. Governments are currently attempting to weigh the merits of numerous types of policy interventions that could safely reduce antimicrobial use, utilizing policy levers such as legislation, taxation, economic incentives, funding support, public awareness campaigns, and regulation of professionals and businesses whose work might affect AMR [18]. Policy makers would benefit from a tool that catalogues and assesses the government policy responses that have been used in various contexts and health system settings. Thus we undertook a systematic evidence mapping project to support evidence-informed action on AMR at the government level, by identifying, describing, and assessing the full range of government policy interventions aiming to reduce human antimicrobial use that have been implemented and evaluated.
Methods
A protocol describing the full methods of this project was published in advance [20] and registered in PROSPERO (CRD42017067514). Deviations from the protocol are noted below, and the paper has been reported in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines [21]. In brief, we produced an evidence map that identifies government policy interventions aiming to reduce antimicrobial use in humans. To be included in the evidence map, studies had to (1) clearly describe a government policy intervention aiming to reduce human antimicrobial use and (2) apply a quantitative design to measure the impact.
Search strategy and selection criteria
We searched 7 electronic databases from medicine and the social sciences (MEDLINE, CINAHL, EMBASE, PAIS Index, Cochrane Central Register of Controlled Trials, Web of Science, and PubMed [articles not indexed in MEDLINE]) from inception to January 28, 2019, without language or date limits. Targeted web searching was used to identify grey literature, and the ProQuest Dissertations & Theses database was used to identify dissertations. We contacted subject-matter experts in each of WHO’s 6 regions to identify missing studies.
We screened titles and abstracts against 3 inclusion criteria: (1) the evaluated intervention was a policy intervention defined as an intervention enacted by a government or government agency at the federal, state, provincial, or municipal level that aimed to change antimicrobial use through education, restriction, incentivization, coercion, training, persuasion, changing the physical or social context, modelling appropriate behaviour, or reducing barriers to action [22]; (2) the study quantitatively evaluated the effect of the intervention; and (3) the study assessed an outcome measure related to human antimicrobial use such as consumption, dosing, prescribing, or sales of an antibiotic, antiviral, antiparasitic, or antifungal drug. Examples of interventions include regulating the sales of antimicrobials, restricting the use of last-resort antibiotics, and launching public awareness campaigns. Titles and abstracts were each independently screened by 2 reviewers (SRVK and S Jones, A Srivastava, or RN), and disagreements were resolved by consensus. The full text of potentially relevant studies was screened by 2 reviewers (SRVK and MN or RN). Non-English articles were translated using Google Translate, or a translation was requested from the corresponding author.
Data analysis
Data on study characteristics, study participants, interventions, analyses, and measured effects were extracted in duplicate by 2 reviewers (SRVK and MN or RN) using a customized data extraction tool (see S2 Text). In consultation with SJH and JMG, SRVK grouped studies according to the Behaviour Change Wheel framework’s intervention functions and our definition of policy intervention [22]. Where appropriate, studies were coded with multiple Behaviour Change Wheel intervention functions; however, studies were coded with a single Behaviour Change Wheel policy approach. We inductively identified and described policy options based on groupings of similar interventions, and coded studies according to their region, study design, and the intervention functions of the Behaviour Change Wheel. WHO regions were used to group countries; the region of the Americas was subdivided into Canada/US and Latin America.
Results
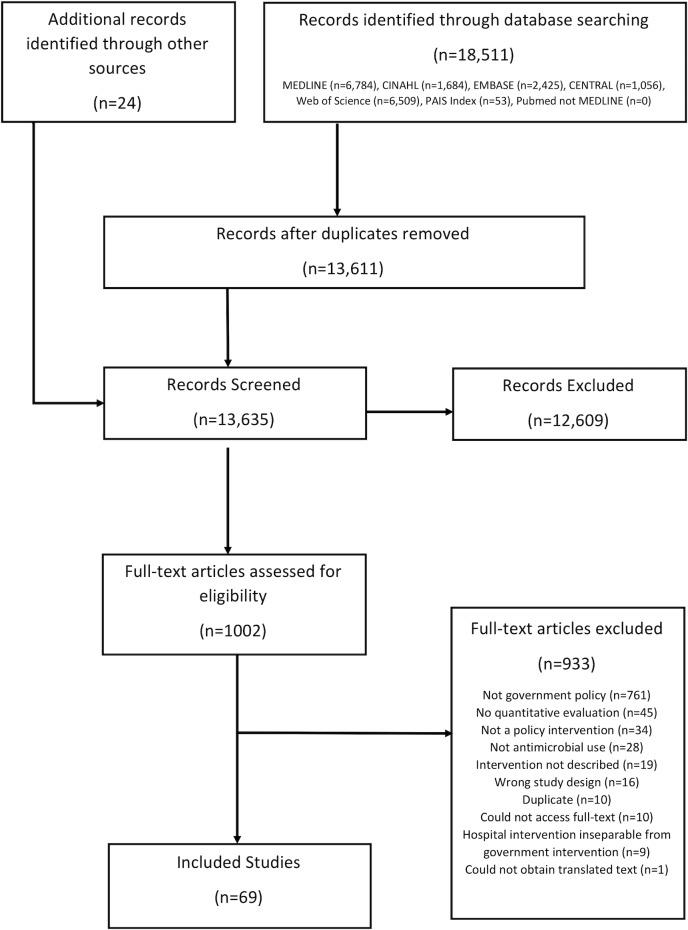
From 13,635 abstracts, we identified 69 evaluations of government policy interventions to reduce human antimicrobial use. Fig 1 shows the full summary of screening and inclusion. Of the 69 included studies, 67 focused on antibiotics and 2 on antimalarials; no studies aimed to reduce the use of other antimicrobial agents. The majority of policies targeted healthcare workers (n = 44) or healthcare workers and the community (n = 13), while the remaining 12 policies exclusively targeted a community audience. We found evaluations in 4 of the 6 WHO regions—the Americas (n = 24), Western Pacific (n = 22), Europe (n = 21), and Africa (n = 2)—but did not identify any evaluations from the South East Asian region or the Eastern Mediterranean region. Of the 69 included studies, 67 were published in English and 2 were published in Spanish.
Fig 1. PRISMA summary flow chart.
CENTRAL, Cochrane Central Register of Controlled Trials.
Using our definition of policy intervention, we organized studies according to the policy categories of the Behaviour Change Wheel framework. Table 1 describes the interventions of the included studies. The largest grouping of policies was regulatory interventions (n = 27), followed by guidelines (n = 18), communication policies (n = 17), legislation (n = 3), and fiscal measures (n = 3). One evaluation was identified for service provision policies; however, we did not identify any social planning policies. Regulatory policies (n = 20/27) and legislation (n = 3/3) were largely organized at the national level. Communication policies were organized at different levels of government; 8 were at the national level, 3 were at the state/provincial level, and 6 were at the regional level (municipality, county, or other geographic unit). Similarly, 12 of the guidelines were at the national level, 2 were at the state/provincial level, and 4 were at the regional level. One of the fiscal measures policies was at the national level, and 2 were at the regional level. The sole service provision policy was organized at the national level.
Table 1. Included studies by policy approach.
| Policy approach | Description* | Studies |
|---|---|---|
| Regulatory interventions | Establishing rules or principles of behaviour or practice | [23–49] |
| Guideline interventions | Creating documents that recommend or mandate practice | [50–67] |
| Communication interventions | Using print, electronic, telephonic, or broadcast media | [68–84] |
| Legislation interventions | Making or changing laws | [85–87] |
| Service provision interventions | Delivering a service | [88] |
| Environmental/social planning interventions | Designing or controlling the physical or social environment | None |
| Fiscal interventions | Using the tax system and other financial measures to reduce or increase the financial cost | [89–91] |
*Policy approach descriptions from the Behaviour Change Wheel framework [22].
The majority of the 69 included studies were retrospective evaluations using routinely collected data from health insurance databases or electronic health records (n = 46), or sales data from IMS Health (n = 14). Four of the included studies were randomized controlled trials, 3 were non-randomized controlled trials, 7 used non-randomized controlled before-and-after designs, 25 used time series designs, 18 used uncontrolled before-and-after designs, 10 used descriptive methods, and 2 used cohort study designs (Box 1). The included studies predominantly used antibiotic consumption measured as defined daily doses (n = 25) or physician prescribing rates (n = 26) as an outcome measure.
Box 1. Definitions of included study designs
Randomized controlled trial: An experimental study in which people are allocated to different interventions using methods that are random.
Non-randomized controlled trial: An experimental study in which people are allocated to different interventions using methods that are not random.
Time series design: A study that uses observations at multiple time points before and after an intervention. The design attempts to detect whether the intervention has had an effect significantly greater than any underlying trend over time.
Non-randomized controlled before-and-after design: A study in which observations are made before and after the implementation of an intervention, both in a group that receives the intervention and in a control group that does not.
Uncontrolled before-and-after design: A study in which observations are made before and after the implementation of an intervention in a single intervention group.
Cohort design: A study in which designated groups of people are followed over time to ascertain the occurrence of an event.
Descriptive design: A study that employs observational, cross-sectional, ecological, or other descriptive methods, to infer or hypothesize the impact of an intervention.
All definitions except “cohort design” and “descriptive design” were taken from Cochrane’s Effective Practice and Organisation of Care Group [92].
Among the 69 included evaluations, we identified 17 distinct policy options that have been evaluated for their ability to reduce antimicrobial use. Table 2 summarizes these policy options and lists the studies that evaluated specific manifestations of them. By far the most common of these policy options were informational strategies, including public awareness campaigns (n = 17), which informed healthcare workers and/or the public about AMR and antimicrobial overuse, and antimicrobial guidelines (n = 13), which provided information to healthcare workers on the preferred use of antimicrobial drugs or preferred treatments for resistant infections. These strategies were widely used across most regions, and in particular represented a large proportion of the interventions evaluated in Canada/US [52,53,59,61,62,69,74,76–78,80,81] and Europe [63,65,67,68,70,71,73,75,79,82,90,91].
Table 2. Description of policy options that have aimed to reduce human antimicrobial consumption.
| Policy option | Description | Studies |
|---|---|---|
| Policies to improve infection prevention and stewardship efforts | ||
| Published antimicrobial guidelines | Information provided to healthcare workers on the preferred use of antimicrobial drugs, or preferred treatment for resistant infections | [50–53,55,57–64] |
| Vaccination guidelines | Guidelines and policies recommending vaccinations likely to reduce antimicrobial use | [90] |
| Committee development | Guidelines encouraging the formation of expert groups on stewardship and resistance | [56] |
| Stewardship | A requirement that specific stewardship policies be introduced | [27,33,65] |
| Disclosure | A requirement for public disclosure of antibiotic use level | [32] |
| Funding | Provision of funding towards a specific stewardship program or goal | [88] |
| Policies to educate health professionals, policy makers, and the public on sustainable antibiotic use | ||
| Public awareness | Public educational campaigns drawing on media and internet to inform healthcare workers and/or the public about antimicrobial resistance | [68–84] |
| Feedback | Audit and feedback to providers about their antimicrobial use habits | [54,66] |
| Policies to change incentives that encourage antibiotic overuse and misuse | ||
| Reimbursement penalty for patients | A reduction in the amount that a patient is reimbursed for a prescription by a drug plan | [41] |
| Reimbursement penalty for prescribers | The prescriber is not paid for their services unless the guidelines for prescribing antimicrobials are met | [26] |
| Restricted reimbursement | Introduces an additional step in the prescribing pathway such as consultation with a specialist or provision of proof of infection in order for the prescription to be reimbursed | [30,31,34,36,38] |
| Restricted use | Introduces an additional step in the prescribing pathway such as consultation with a specialist or provision of proof of infection in order for the prescription to be dispensed | [23,28,35,43,47,48] |
| Pay for performance | Pay-for-performance funding provided to healthcare centres that meet particular antimicrobial-use-related guidelines and targets | [67,89,91] |
| Policies to change features of the health system | ||
| Professional regulation | Changes to codes of practice with regards to what can be done by members of different healthcare professions | [87] |
| Prescription requirement | Requirement of a prescription to purchase antimicrobial drugs | [24,25,39,40,44,85,86] |
| Formulary change | Removal of a drug from the formulary or addition of a drug to the formulary | [37] |
| National essential medicines policies | Introduction of policies in line with WHO’s essential medicines policies | [29,45,46,49] |
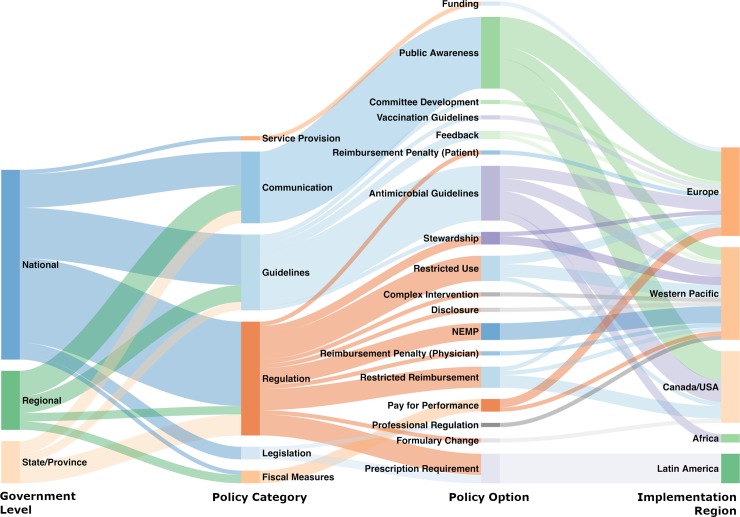
Other policy options were less commonly reported and tended to group regionally, as can be seen in Fig 2. For example, 7 studies were categorized as “prescription requirement” policies, which were regulatory and legislative policies essentially banning the sale of over-the-counter antibiotics by requiring a prescription from a healthcare professional [24,25,39,40,44,85,86]. These policies were implemented starting in the late 1990s in countries or regions in Latin America where over-the-counter antibiotic sales were not previously prohibited, or where existing regulations were not enforced. Countries in WHO’s Western Pacific region tried a diverse range of strategies, most of which were evaluated only once or twice. These policies were largely implemented in China [27,29,33,42,43,45–49,60,66,89], South Korea [32,49,57,87], and Taiwan [26,30], and included disclosure requirements for hospitals to post their antibiotic use rates online, professional regulation strategies that changed the codes of practice around the prescribing and dispensing of antibiotics by different health professions, and reimbursement penalties for physicians, who were not paid for their services unless their prescriptions met the guidelines for antibiotic prescribing. Along similar lines, 1 European country (Denmark) explored using reimbursement penalties targeting patients, where the national health insurance plan reimbursed patients a smaller proportion of the antibiotic cost than was previously reimbursed [41]. Three studies in Canada/US [34,36,38] and 1 each in Europe [31] and the Western Pacific [30] also tried reimbursement restrictions, where the patient was not reimbursed the cost of an antibiotic by their national health insurance plan unless the physician met particular guidelines such as proving the existence of an infection or consulting with an infectious disease specialist. In the African region, we found only 2 studies, both targeting the use of antimalarial drugs, and both employing published guidelines to change prescribing [50,51]. Some national-level interventions were evaluated in multiple studies using different methods, populations, and evaluation time frames; these included the ban on over-the-counter sales of antimicrobials in Chile [24,25,44], the Antibiotics Are Not Automatic campaign in France [70,71,73,82], and the national essential medicines policy in China [29,45,46,49].
Fig 2. Evidence map of the relationships between government level, policy approach (policy category), policy option, and region of implementation.
The thickness of lines represents the proportions of included studies. The colors show the grouping of policies and trace the flow of policies between categories. NEMP, national essential medicines policy.
Discussion
Principal findings
Around the world, governments are currently working to develop policy responses to the growing threat of AMR. In our evidence map, we identified 69 evaluation studies looking at the impact of policy interventions on antimicrobial use across 4 of WHO’s 6 regions. From this search, we were able to identify 17 different policy options and examples of each that governments can use to inform their future AMR policies.
Many of the policy options identified in this map were evaluated in only a few studies. These evaluations were highly regionalized, which likely results from similarities between contexts and health systems within regions of a country, or in neighbouring countries. Policy makers in other parts of the world who operate with similar contextual problems or health systems may find these policies useful models for policy development in their countries. For example, the prescription requirement policies [24,25,39,40,44,85,86] were implemented in 5 Latin American countries where over-the-counter antibiotic sales were formally or informally permitted. While this type of policy would not be useful in Canada and the US, where prescriptions are already required, the regulations and legislation used in Latin America may be a useful model for many other countries, such as those in Africa, the Eastern Mediterranean, South East Asia, and the Western Pacific that currently allow over-the-counter sales of antibiotics, and where overuse of antimicrobials is likely to decrease in response to this restriction.
Similarly, we identified several policies that used electronic medical records and national health insurance systems to change physician and patient behaviours around antimicrobial use. These policies, including restricted reimbursements and reimbursement penalties for patients and prescribers, were used in high-income jurisdictions such as Canada, Sweden, and Taiwan. These policies take a different approach, targeting overuse through restrictive and coercive financial mechanisms.
Policy implications
Given the complexity of AMR, and the need to balance conservation of antimicrobial effectiveness with ensuring access to appropriate antimicrobials for those who need them [2], there is unlikely to be a “silver bullet” intervention that solves the global AMR problem. These 17 government policy interventions offer a starting point for countries to adapt to their local context. Since most of these 17 policies have been evaluated only once or twice and in particular contexts, it would be unwise to draw strong conclusions about their effectiveness. Indeed, many of these interventions were evaluated using low-quality, non-randomized designs; while many systematic reviews would exclude studies on this basis, we retained them in our evidence map to ensure that we captured the widest range of policy options possible. To avoid future waste of public resources, and in line with WHO recommendations for national action on AMR [93], governments should ensure that AMR policy interventions are evaluated using rigorous study designs and that study results are published.
Not surprisingly, our evidence map found that public awareness campaigns and guidelines were commonly used strategies for reducing antimicrobial use across all regions. These educational approaches are traditional public health strategies and have been promoted by both WHO and the UK’s Review on Antimicrobial Resistance [13]. While launched at the government level, many of these programs and policies still focus on changing the practice of individual prescribers, usually physicians, rather than targeting other healthcare professionals or altering healthcare structures to reduce overuse and misuse of antibiotics. Different governments have different policy levers at their disposal, including the ability to implement complex regulatory, legislative, fiscal, and service provision policies, which could potentially bring about more dramatic change than policies focused on individual prescriber behaviour change. Many approaches to reducing antimicrobial consumption can only be implemented by governments, including many of the policies we identified (e.g., professional regulation, restricted reimbursement, and prescription requirements) as well as policies identified by others in academic literature for which we did not find any evaluations (e.g., creating human-only classes of antimicrobials [1], banning direct-to-consumer advertising [3,6], and using tax or fiscal measures [94]). Given that governments can employ a broad range of policy options beyond public awareness campaigns and guidelines, the full range of possible AMR policies should be further explored.
Strengths and limitations
Our evidence map represents the first systematic effort, to our knowledge, to identify government policy interventions and specific policy mechanisms for reducing human antimicrobial use. We worked with 3 research librarians from 3 disciplines and contacted experts around the world to identify published and grey literature on government and AMR policies. However, we recognize that there are implemented policies (e.g., [95]) that have not been captured in this evidence map. We suspect that these studies have not been evaluated, or have not been evaluated with respect to antimicrobial use, or the results of these studies have not been made public.
As with many studies about AMR, we were unable to directly investigate the human health impact of government action on AMR due to the complex relationships among AMR, the use of antimicrobials in humans, animals, and agriculture, and health outcomes. This complexity will continue to be a challenge for AMR research until such a time as “one health” monitoring systems for both antimicrobial use and AMR improve. However, reductions in antimicrobial use are a more immediate measure of policy impact, and large-scale reductions in antimicrobial use are likely to lead to lower levels of resistance [96]. Reducing antimicrobial use is therefore a valuable target for policy makers tackling AMR at the population level.
Conclusions
Our identification of 17 different policy strategies for reducing human antimicrobial use suggests that governments have a variety of policy options at their disposal for mitigating AMR. However, we also note that most existing policy options have not been rigorously evaluated, and some commonly discussed policy options have not been evaluated for their impact on antimicrobial use. To avoid wasting public resources, governments should ensure that future AMR policy interventions are evaluated using rigorous study designs and that study results are published.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We would like to thank librarians Michael Boutet, Catherine McGoveran, and Lindsey Sikora at the University of Ottawa, who provided advice, support, and peer review for the development of our search strategy. We would also like to thank Sara Jones and Archita Srivastava for their assistance with screening and Theresa Tam for her advice on this project.
The corresponding author affirms that this paper is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Abbreviations
- AMR
antimicrobial resistance
- WHO
World Health Organization
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This study was partially supported by the International Collaboration for Capitalizing on Cost‐Effective and Life‐Saving Commodities (i4C) that is funded through the Research Council of Norway's Global Health & Vaccination Programme (SJH; GLOBVAC Project #234608) and contract funding from the Public Health Agency of Canada (SJH). The Canadian funder provided input on the research question, but neither funder had any role in study design, data collection, data analysis, data interpretation, or writing of the report. SJH is additionally funded by the Canadian Institutes of Health Research and the Ontario Government's Ministry of Research, Innovation and Science. SRVK is supported by an Ontario Graduate Scholarship. JG holds a Canada Research Chair in Health Knowledge Transfer and Uptake.
References
- 1.Behdinan A, Hoffman SJ, Pearcey M. Some Global policies for antibiotic resistance depend on legally binding and enforceable commitments. J Law Med Ethics. 2015;43(Suppl 3):68–73. 10.1111/jlme.12277 [DOI] [PubMed] [Google Scholar]
- 2.Hoffman SJ, Caleo GM, Daulaire N, Elbe S, Matsoso P, Mossialos E, et al. Strategies for achieving global collective action on antimicrobial resistance. Bull World Health Organ. 2015;93(12):867–76. 10.2471/BLT.15.153171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoffman SJ, Outterson K, Rottingen JA, Cars O, Clift C, Rizvi Z, et al. An international legal framework to address antimicrobial resistance. Bull World Health Organ. 2015;93(2):66 10.2471/BLT.15.152710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ardal C, Outterson K, Hoffman SJ, Ghafur A, Sharland M, Ranganathan N, et al. International cooperation to improve access to and sustain effectiveness of antimicrobials. Lancet. 2016;387(10015):296–307. 10.1016/S0140-6736(15)00470-5 [DOI] [PubMed] [Google Scholar]
- 5.Hoffman SJ, Outterson K. What will it take to address the global threat of antibiotic resistance? J Law Med Ethics. 2015;43(2):363–8. 10.1111/jlme.12253 [DOI] [PubMed] [Google Scholar]
- 6.Laxminarayan R, Duse A, Wattal C, Zaidi AKM, Wertheim HFL, Sumpradit N, et al. Antibiotic resistance—the need for global solutions. Lancet Infect Dis. 2013;13(12):1057–98. 10.1016/S1473-3099(13)70318-9 [DOI] [PubMed] [Google Scholar]
- 7.Laxminarayan R, Sridhar D, Blaser M, Wang M, Woolhouse M. Achieving global targets for antimicrobial resistance: the UN should promote targets, funding, and governance. Science. 2016;353(6302):874–5. 10.1126/science.aaf9286 [DOI] [PubMed] [Google Scholar]
- 8.Fleming A. Penicillin: Nobel lecture, December 11, 1945. Stockholm: Nobel Media; 1945 [cited 2017 Feb 23]. Available from: http://www.nobelprize.org/nobel_prizes/medicine/laureates/1945/fleming-lecture.html.
- 9.Holmes AH, Moore LSP, Sundsfjord A, Steinbakk M, Regmi S, Karkey A, et al. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet. 387(10014):176–87. 10.1016/S0140-6736(15)00473-0 [DOI] [PubMed] [Google Scholar]
- 10.Liu Y-Y, Wang Y, Walsh TR, Yi L-X, Zhang R, Spencer J, et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis. 2016;16(2):161–8. 10.1016/S1473-3099(15)00424-7 [DOI] [PubMed] [Google Scholar]
- 11.Klopper M, Warren RM, Hayes C, Gey van Pittius NC, Streicher EM, Müller B, et al. Emergence and spread of extensively and totally drug-resistant tuberculosis, South Africa. Emerg Infect Dis. 2013;19(3):449–55. 10.3201/EID1903.120246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Bank. Drug resistant infections: a threat to our economic future Washington (DC): World Bank; 2017. [Google Scholar]
- 13.Review on Antimicrobial Resistance. Tackling drug-resistant infections globally: final report and recommendations. London: Review on Antimicrobial Resistance; 2016 [cited 2019 May 7]. Available from: https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf.
- 14.United Nations General Assembly. Political declaration of the high-level meeting of the General Assembly on antimicrobial resistance. New York: United Nations; 2016. [Google Scholar]
- 15.G20 Health Ministers. Berlin declaration of the G20 Health Ministers: together today for a healthy tomorrow. 2017.
- 16.World Health Organization. Global action plan on antimicrobial resistance. Geneva: World Health Organization; 2015. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization, Food and Agricultural Organization of the United Nations, World Organisation for Animal Health. Monitoring global progress on addressing antimicrobial resistance: analysis report of the second round of results of AMR country self-assessment survey 2018. Geneva: World Health Organization; 2018. [Google Scholar]
- 18.Magnusson R. Advancing the right to health: the vital role of law. Geneva: World Health Organization; 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davey P, Marwick CA, Scott CL, Charani E, McNeil K, Brown E, et al. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev. 2017;(2):1465–858. 10.1002/14651858.CD003543.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rogers Van Katwyk S, Grimshaw JM, Mendelson M, Taljaard M, Hoffman SJ. Government policy interventions to reduce human antimicrobial use: protocol for a systematic review and meta-analysis. Syst Rev. 2017;6(1):256 10.1186/s13643-017-0640-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci. 2011;6(1):42 10.1186/1748-5908-6-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Altunsoy A, Aypak C, Azap A, Ergonul O, Balik I. The impact of a nationwide antibiotic restriction program on antibiotic usage and resistance against nosocomial pathogens in Turkey. Int J Med Sci. 2011;8(4):339–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bavestrello L, Cabello A, Casanova D. Impact of regulatory measures in the trends of community consumption of antibiotics in Chile. Rev Med Chil. 2002;130(11):1265–72. [PubMed] [Google Scholar]
- 25.Bavestrello FL, Cabello MA. Community antibiotic consumption in Chile, 2000–2008. Revista Chilena Infectol. 2011;28(2):107–12. [PubMed] [Google Scholar]
- 26.Chang SC, Chen YC, Hu OY. Antibiotic use in public hospitals in Taiwan after the implementation of National Health Insurance. J Formos Med Assoc. 2001;100(3):155–61. [PubMed] [Google Scholar]
- 27.Fan Q, Li M, Hao J, Jun C. Effects of a restrictive antibiotic policy on antibiotic usage and Staphylococcus aureus resistance In: Chang L, Guiran C, editors. Proceedings of the International Conference on Electronics, Mechanics, Culture and Medicine. Volume 45 Paris: Atlantis Press; 2016. pp. 654–8. 10.2991/emcm-15.2016.97 [DOI] [Google Scholar]
- 28.Furst J, Cizman M, Mrak J, Kos D, Campbell S, Coenen S, et al. The influence of a sustained multifaceted approach to improve antibiotic prescribing in Slovenia during the past decade: findings and implications. Expert Rev Anti Infect Ther. 2015;13(2):279–89. 10.1586/14787210.2015.990381 [DOI] [PubMed] [Google Scholar]
- 29.Gong Y, Yang C, Yin X, Zhu M, Yang H, Wang Y, et al. The effect of essential medicines programme on rational use of medicines in China. Health Policy Plan. 2016;31(1):21 10.1093/heapol/czv008 [DOI] [PubMed] [Google Scholar]
- 30.Ho M, Hsiung CA, Yu HT, Chi CL, Chang HJ. Changes before and after a policy to restrict antimicrobial usage in upper respiratory infections in Taiwan. Int J Antimicrob Agents. 2004;23(5):438–45. 10.1016/j.ijantimicag.2003.10.013 [DOI] [PubMed] [Google Scholar]
- 31.Kurt H, Karabay O, Birengel S, Memikoglu O, Bozkurt GY, Yalci A. effects of legal antibiotic restrictions on consumption of broad-spectrum beta-lactam antibiotics, glycopeptides and amphotericin B. Chemotherapy. 2010;56(5):359–63. 10.1159/000321553 [DOI] [PubMed] [Google Scholar]
- 32.Lee YS, Kwon JW, Oh OH, Sohn HS. Temporal decrease in overall antibiotic consumption accompanying antibiotic prescribing rate disclosure policy: evidence from analysis of national health insurance claims data in South Korea. Arch Pharm Res. 2014;37(10):1295–300. 10.1007/s12272-014-0333-5 [DOI] [PubMed] [Google Scholar]
- 33.Ma XD, Xie JF, Yang Y, Guo FM, Gao ZW, Shao H, et al. Antimicrobial stewardship of Chinese ministry of health reduces multidrug-resistant organism isolates in critically ill patients: a pre-post study from a single center. BMC Infect Dis. 2016;16:704 10.1186/s12879-016-2051-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.MacCara ME, Sketris IS, Comeau DG, Weerasinghe SD. Impact of a limited fluoroquinolone reimbursement policy on antimicrobial prescription claims. Ann Pharmacother. 2001;35(7–8):852–8. 10.1345/aph.10272 [DOI] [PubMed] [Google Scholar]
- 35.Mamdani M, McNeely D, Evans G, Hux J, Oh P, Forde N, et al. Impact of a fluoroquinolone restriction policy in an elderly population. Am J Med. 2007;120(10):893–900. 10.1016/j.amjmed.2007.02.028 [DOI] [PubMed] [Google Scholar]
- 36.Manns B, Laupland K, Tonelli M, Gao S, Hemmelgarn B. Evaluating the impact of a novel restricted reimbursement policy for quinolone antibiotics: a time series analysis. BMC Health Serv Res. 2012;12:290 10.1186/1472-6963-12-290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marra F, Patrick DM, White R, Ng H, Bowie WR, Hutchinson JM. Effect of formulary policy decisions on antimicrobial drug utilization in British Columbia. J Antimicrob Chemother. 2005;55(1):95–101. 10.1093/jac/dkh501 [DOI] [PubMed] [Google Scholar]
- 38.Marshall D, Gough J, Grootendorst P, Buitendyk M, Jaszewski B, Simonyi S, et al. Impact of administrative restrictions on antibiotic use and expenditure in Ontario: time series analysis. J Health Serv Res Policy. 2006;11(1):13–20. 10.1258/135581906775094253 [DOI] [PubMed] [Google Scholar]
- 39.Santa-Ana-Tellez Y, Mantel-Teeuwisse AK, Dreser A, Leufkens HG, Wirtz VJ. Impact of over-the-counter restrictions on antibiotic consumption in Brazil and Mexico. PLoS ONE. 2013;8(10):e75550 10.1371/journal.pone.0075550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Santa-Ana-Tellez Y, Mantel-Teeuwisse AK, Leufkens HGM, Wirtz VJ. Seasonal variation in penicillin use in Mexico and Brazil: analysis of the impact of over-the-counter restrictions. Antimicrobial Agents Chemother. 2015;59(1):105–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steffensen FH, Schonheyder HC, Mortensen JT, Nielsen K, Sorensen HT. Changes in reimbursement policy for antibiotics and prescribing patterns in general practice. Clin Microbiol Infect. 1997;3(6):653–7. [DOI] [PubMed] [Google Scholar]
- 42.Sun J, Shen X, Li M, He L, Guo SY, Skoog G, et al. Changes in patterns of antibiotic use in Chinese public hospitals (2005–2012) and a benchmark comparison with Sweden in 2012. J Glob Antimicrob Resist. 2015;3(2):95–102. 10.1016/j.jgar.2015.03.001 [DOI] [PubMed] [Google Scholar]
- 43.Tao JH, Zhang TT, Xu J, Wu CB. Analysis of the current situation of antibiotics use in china: a hospital-based perspective. Ther Innov Regul Sci. 2013;47(1):23–31. 10.1177/0092861512466397 [DOI] [PubMed] [Google Scholar]
- 44.Wirtz VJ, Herrera-Patino JJ, Santa-Ana-Tellez Y, Dreser A, Elseviers M, Vander Stichele RH. Analysing policy interventions to prohibit over-the-counter antibiotic sales in four Latin American countries. Tropical Med Int Health. 2013;18(6):665–73. [DOI] [PubMed] [Google Scholar]
- 45.Xiao YH, Wang J, Shen P, Zheng BW, Zheng YD, Li LJ. Retrospective survey of the efficacy of mandatory implementation of the Essential Medicine Policy in the primary healthcare setting in China: failure to promote the rational use of antibiotics in clinics. Int J Antimicrob Agents. 2016;48(4):409–14. 10.1016/j.ijantimicag.2016.06.017 [DOI] [PubMed] [Google Scholar]
- 46.Yang L, Liu C, Ferrier JA, Zhou W, Zhang X. The impact of the National Essential Medicines Policy on prescribing behaviours in primary care facilities in Hubei province of China. Health Policy Plan. 2013;28(7):750–60. 10.1093/heapol/czs116 [DOI] [PubMed] [Google Scholar]
- 47.Zou XX, Fang Z, Min R, Bai X, Zhang Y, Xu D, et al. Is nationwide special campaign on antibiotic stewardship program effective on ameliorating irrational antibiotic use in China? Study on the antibiotic use of specialized hospitals in China in 2011–2012. J Huazhong Univ Sci Technolog Med Sci. 2014;34(3):456–63. 10.1007/s11596-014-1300-6 [DOI] [PubMed] [Google Scholar]
- 48.Tang YQ, Liu CJ, Zhang ZN, Zhang XP. Effects of prescription restrictive interventions on antibiotic procurement in primary care settings: a controlled interrupted time series study in China. Cost Eff Resour Alloc. 2018;16:1 10.1186/s12962-018-0086-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wei X, Yin J, Walley JD, Zhang Z, Hicks JP, Zhou Y, et al. Impact of China’s essential medicines scheme and zero-mark-up policy on antibiotic prescriptions in county hospitals: a mixed methods study. Trop Med Int Health. 2017;22(9):1166–74. 10.1111/tmi.12922 [DOI] [PubMed] [Google Scholar]
- 50.Bastiaens GJH, Schaftenaar E, Ndaro A, Keuter M, Bousema T, Shekalaghe SA. Malaria diagnostic testing and treatment practices in three different Plasmodium falciparum transmission settings in Tanzania: before and after a government policy change. Malar J. 2011;10:76 10.1186/1475-2875-10-76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.D’Acremont V, Kahama-Maro J, Swai N, Mtasiwa D, Genton B, Lengeler C. Reduction of anti-malarial consumption after rapid diagnostic tests implementation in Dar es Salaam: a before-after and cluster randomized controlled study. Malar J. 2011;10:107 10.1186/1475-2875-10-107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dowell D, Tian LH, Stover JA, Donnelly JA, Martins S, Erbelding EJ, et al. Changes in fluoroquinolone use for gonorrhea following publication of revised treatment guidelines. Am J Public Health. 2012;102(1):148–55. 10.2105/AJPH.2011.300283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Duval M, Desrosiers M. Guidelines for management of acute bacterial rhinosinusitis: impact on Quebec physicians’ prescriptions for antibiotics. Otolaryngol Head Neck Surg. 2007;136(2):258–60. 10.1016/j.otohns.2006.11.002 [DOI] [PubMed] [Google Scholar]
- 54.Hallsworth M, Chadborn T, Sallis A, Sanders M, Berry D, Greaves F, et al. Provision of social norm feedback to high prescribers of antibiotics in general practice: a pragmatic national randomised controlled trial. Lancet. 2016;387(10029):1743–52. 10.1016/S0140-6736(16)00215-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hernandez-Santiago V, Marwick CA, Patton A, Davey PG, Donnan PT, Guthrie B. Time series analysis of the impact of an intervention in Tayside, Scotland to reduce primary care broad-spectrum antimicrobial use. J Antimicrob Chemother. 2015;70:2397–404. 10.1093/jac/dkv095 [DOI] [PubMed] [Google Scholar]
- 56.Molstad S, Cars O. Major change in the use of antibiotics following a national programme: Swedish strategic programme for the rational use of antimicrobial agents and surveillance of resistance (STRAMA). Scand J Infect Dis. 1999;31(2):191–5. [DOI] [PubMed] [Google Scholar]
- 57.Shin JY, Kim MH, Shin SM, Lee SH, Park BJ. Dramatic decrease in fluoroquinolones in the pediatric population in Korea. Pharmacoepidemiol Drug Saf. 2014;23(12):1320–4. 10.1002/pds.3696 [DOI] [PubMed] [Google Scholar]
- 58.Thornhill MH, Dayer MJ, Forde JM, Corey GR, Chu VH, Couper DJ, et al. Impact of the NICE guideline recommending cessation of antibiotic prophylaxis for prevention of infective endocarditis: before and after study. BMJ. 2011;342:d2392 10.1136/bmj.d2392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weiss K, Blais R, Fortin A, Lantin S, Gaudet M. Impact of a multipronged education strategy on antibiotic prescribing in Quebec, Canada. Clin Infect Dis. 2011;53(5):433–9. 10.1093/cid/cir409 [DOI] [PubMed] [Google Scholar]
- 60.Zhang W, Shen X, Wang Y, Chen Y, Huang M, Zeng Q, et al. Antibiotic use in five children’s hospitals during 2002–2006: the impact of antibiotic guidelines issued by the Chinese Ministry of Health. Pharmacoepidemiol Drug Saf. 2008;17(3):306–11. 10.1002/pds.1544 [DOI] [PubMed] [Google Scholar]
- 61.Kelly AA, Jones MM, Echevarria KL, Kralovic SM, Samore MH, Goetz MB, et al. A Report of the efforts of the Veterans Health Administration National Antimicrobial Stewardship Initiative. Infect Control Hosp Epidemiol. 2017;38(5):513–20. 10.1017/ice.2016.328 [DOI] [PubMed] [Google Scholar]
- 62.Long MJ, LaPlant BN, McCormick JC. Antimicrobial stewardship in the Federal Bureau of Prisons: approaches from the national and local levels. J Am Pharm Assoc (2003). 2017;57(2):241–7. 10.1016/j.japh.2016.11.012 [DOI] [PubMed] [Google Scholar]
- 63.Ouldali N, Bellettre X, Milcent K, Guedj R, de Pontual L, Cojocaru B, et al. Impact of implementing national guidelines on antibiotic prescriptions for acute respiratory tract infections in pediatric emergency departments: an interrupted time series analysis. Clin Infect Dis. 2017;65(9):1469–76. 10.1093/cid/cix590 [DOI] [PubMed] [Google Scholar]
- 64.Song SY, Shin JH, Hyeon SY, Kim D, Kang WK, Choi SH, et al. Pediatric fluoroquinolone prescription in South Korea before and after a regulatory intervention: a nationwide study, 2007–2015. PLoS ONE. 2017;12(5):e0176420 10.1371/journal.pone.0176420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Walker AJ, Curtis HJ, Goldacre B. Impact of Chief Medical Officer activity on prescribing of antibiotics in England: an interrupted time series analysis. J Antimicrob Chemother. 2019;74(4):1133–6. 10.1093/jac/dky528 [DOI] [PubMed] [Google Scholar]
- 66.Zhen L, Jin C, Xu H-N. The impact of prescriptions audit and feedback for antibiotic use in rural clinics: interrupted time series with segmented regression analysis. BMC Health Serv Res. 2018;18(1):777 10.1186/s12913-018-3602-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ellegard LM, Dietrichson J, Anell A. Can pay-for-performance to primary care providers stimulate appropriate use of antibiotics? Health Econ. 2018;27(1):e39–54. 10.1002/hec.3535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bauraind I, Lopez-Lozano J, Beyaert A, Marchal J, Seys B, Yane F, et al. Association between antibiotic sales and public campaigns for their appropriate use. JAMA. 2004;292(20):2468–70. 10.1001/jama.292.20.2468-b [DOI] [PubMed] [Google Scholar]
- 69.Belongia EA, Knobloch MJ, Kieke BA, Davis JP, Janette C, Besser RE. Impact of statewide program to promote appropriate antimicrobial drug use. Emerg Infect Dis. 2005;11(6):912–20. 10.3201/eid1106.050118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bernier A, Delarocque-Astagneau E, Ligier C, Vibet MA, Guillemot D, Watier L. Outpatient antibiotic use in France between 2000 and 2010: after the nationwide campaign, It Is Time To Focus on the Elderly. Antimicrob Agents Chemother. 2014;58(1):71–7. 10.1128/AAC.01813-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chahwakilian P, Huttner B, Schlemmer B, Harbarth S. Impact of the French campaign to reduce inappropriate ambulatory antibiotic use on the prescription and consultation rates for respiratory tract infections. J Antimicrob Chemother. 2011;66(12):2872–9. 10.1093/jac/dkr387 [DOI] [PubMed] [Google Scholar]
- 72.Curry M, Sung L, Arroll B, Goodyear-Smith F, Kerse N, Norris P. Public views and use of antibiotics for the common cold before and after an education campaign in New Zealand. N Z Med J. 2006;119(1233):U1957 [PubMed] [Google Scholar]
- 73.Dommergues MA, Hentgen V. Decreased paediatric antibiotic consumption in France between 2000 and 2010. Scand J Infect Dis. 2012;44(7):495–501. 10.3109/00365548.2012.669840 [DOI] [PubMed] [Google Scholar]
- 74.Finkelstein JA, Huang SS, Kleinman K, Rifas-Shiman SL, Stille CJ, Daniel J, et al. Impact of a 16-community trial to promote judicious antibiotic use in Massachusetts. Pediatrics. 2008;121(1):e15–23. 10.1542/peds.2007-0819 [DOI] [PubMed] [Google Scholar]
- 75.Formoso G, Paltrinieri B, Marata AM, Gagliotti C, Pan A, Moro ML, et al. Feasibility and effectiveness of a low cost campaign on antibiotic prescribing in Italy: community level, controlled, non-randomised trial. BMJ. 2013;347:f5391 10.1136/bmj.f5391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fuertes EI, Henry B, Marra F, Wong H, Patrick DM. Trends in antibiotic utilization in Vancouver associated with a community education program on antibiotic use. Can J Public Health. 2010;101(4):304–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gonzales R, Corbett KK, Leeman-Castillo BA, Glazner J, Erbacher K, Darr CA, et al. The “minimizing antibiotic resistance in Colorado” project: impact of patient education in improving antibiotic use in private office practices. Health Serv Res. 2005;40(1):101–16. 10.1111/j.1475-6773.2005.00344.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hennessy TW, Petersen KM, Bruden D, Parkinson AJ, Hurlburt D, Getty M, et al. Changes in antibiotic-prescribing practices and carriage of penicillin-resistant Streptococcus pneumoniae: a controlled intervention trial in rural Alaska. Clin Infect Dis. 2002;34(12):1543–50. 10.1086/340534 [DOI] [PubMed] [Google Scholar]
- 79.Lambert MF, Masters GA, Brent SL. Can mass media campaigns change antimicrobial prescribing? A regional evaluation study. J Antimicrob Chemother. 2007;59(3):537–43. 10.1093/jac/dkl511 [DOI] [PubMed] [Google Scholar]
- 80.McKay RM, Vrbova L, Fuertes E, Chong M, David S, Dreher K, et al. Evaluation of the Do Bugs Need Drugs? program in British Columbia: can we curb antibiotic prescribing? Can J Infect Dis Med Microbiol. 2011;22(1):19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Perz JF, Craig AS, Coffey CS, Jorgensen DM, Mitchel E, Hall S, et al. Changes in antibiotic prescribing for children after a community-wide campaign. JAMA. 2002;287(23):3103–9. [DOI] [PubMed] [Google Scholar]
- 82.Sabuncu E, David J, Bernede-Bauduin C, Pepin S, Leroy M, Boelle PY, et al. Significant reduction of antibiotic use in the community after a nationwide campaign in France, 2002–2007. PLoS Med. 2009;6(6):e1000084 10.1371/journal.pmed.1000084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wutzke SE, Artist MA, Kehoe LA, Fletcher M, Mackson JM, Weekes LM. Evaluation of a national programme to reduce inappropriate use of antibiotics for upper respiratory tract infections: effects on consumer awareness, beliefs, attitudes and behaviour in Australia. Health Promot Int. 2007;22(1):53–64. 10.1093/heapro/dal034 [DOI] [PubMed] [Google Scholar]
- 84.Wu J, Taylor D, Ovchinikova L, Heaney A, Morgan T, Dartnell J, et al. Relationship between antimicrobial-resistance programs and antibiotic dispensing for upper respiratory tract infection: an analysis of Australian data between 2004 and 2015. J Int Med Res. 2018;46(4):1326–38. 10.1177/0300060517740813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kliemann BS, Levin AS, Moura ML, Boszczowski I, Lewis JJ. Socioeconomic determinants of antibiotic consumption in the state of Sao Paulo, Brazil: the effect of restricting over-the-counter sales. PLoS ONE. 2016;11(12):e0167885 10.1371/journal.pone.0167885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Moura ML, Boszczowski I, Mortari N, Barrozo LV, Neto FC, Lobo RD, et al. The impact of restricting over-the-counter sales of antimicrobial drugs preliminary analysis of national data. Medicine. 2015;94(38):e1605 10.1097/MD.0000000000001605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Park S, Soumerai SB, Adams AS, Finkelstein JA, Jang S, Ross-Degnan D. Antibiotic use following a Korean national policy to prohibit medication dispensing by physicians. Health Policy Plan. 2005;20(5):302–9. 10.1093/heapol/czi033 [DOI] [PubMed] [Google Scholar]
- 88.Lambert ML, Bruyndonckx R, Goossens H, Hens N, Aerts M, Catry B, et al. The Belgian policy of funding antimicrobial stewardship in hospitals and trends of selected quality indicators for antimicrobial use, 1999–2010: a longitudinal study. BMJ Open. 2015;5(2):e006916 10.1136/bmjopen-2014-006916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yip W, Powell-Jackson T, Chen W, Hu M, Fe E, Hu M, et al. Capitation combined with pay-for-performance improves antibiotic prescribing practices in rural China. Health Aff (Millwood). 2014;33(3):502–10. [DOI] [PubMed] [Google Scholar]
- 90.Eythorsson E, Sigurdsson S, Hrafnkelsson B, Erlendsdottir H, Haraldsson A, Kristinsson KG. Impact of the 10-valent pneumococcal conjugate vaccine on antimicrobial prescriptions in young children: a whole population study. BMC Infect Dis. 2018;18:10 10.1186/s12879-017-2925-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bou-Antoun S, Costelloe C, Honeyford K, Mazidi M, Hayhoe BWJ, Holmes A, et al. Age-related decline in antibiotic prescribing for uncomplicated respiratory tract infections in primary care in England following the introduction of a national financial incentive (the Quality Premium) for health commissioners to reduce use of antibiotics in the community: an interrupted time series analysis. J Antimicrob Chemother. 2018;73(10):2883–92. 10.1093/jac/dky237 [DOI] [PubMed] [Google Scholar]
- 92.Effective Practice and Organization of Care. What study designs should be included in an EPOC review and what should they be called? Oslo: Norwegian Knowledge Centre for the Health Services; 2016.
- 93.World Health Organization, World Organisation for Animal Health, Food and Agriculture Organization of the United Nations. Antimicrobial resistance: a manual for developing national action plans. Geneva: World Health Organization; 2016. [Google Scholar]
- 94.Laxminarayan R, Howard D, Smith DL. Extending the cure: policy responses to the growing threat of antibiotic resistance. Washington (DC): Resources for the Future; 2007. [Google Scholar]
- 95.Rogers Van Katwyk S, Jones SL, Hoffman SJ. Mapping educational opportunities for healthcare workers on antimicrobial resistance and stewardship around the world. Human Resour Health. 2018;16(1):9 10.1186/s12960-018-0270-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bell BG, Schellevis F, Stobberingh E, Goossens H, Pringle M. A systematic review and meta-analysis of the effects of antibiotic consumption on antibiotic resistance. BMC Infect Dis. 2014;14:13 10.1186/1471-2334-14-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.